Abstract
Performance of a unimanual motor task often induces involuntary mirror electromyographic (EMG) activity in the opposite, resting hand. In spite of the ubiquitous presence of mirroring, little is known regarding the underlying cortical contributions. Here, we used functional magnetic resonance imaging (fMRI) to study brain regions activated in association with parametric increases in right isometric wrist flexion force (10%, 20%, 30%, and 70%) in 12 healthy volunteers. During scanning, EMG activity was recorded bilaterally from flexor carpi radialis (FCR), extensor carpi radialis (ECR), biceps brachii (BB), and triceps brachii (TB). Mirror EMG was observed in left FCR during 20%, 30%, and 70% of force. Left ECR, BB, and TB showed mirror EMG only at 70% of force. Increasing force was associated with a linear increase of blood-oxygen-level–dependent (BOLD) signal in bilateral primary motor cortex (M1), supplementary motor area (SMA), caudal cingulate, and cerebellum. Mirroring in the left FCR correlated with activity in bilateral M1, SMA, and the cerebellum. Overall, our results suggest that activity in these regions might reflect sensorimotor processes operating in association with mirroring and suggest caution when interpreting fMRI activity in studies that involve unilateral force generation tasks in the absence of simultaneous bilateral EMG/kinematics measurements.
Keywords: caudal cingulate, fMRI, mirror EMG, primary motor cortex, supplementary motor area
During unimanual motor tasks, muscle activity may not be restricted to the contracting arm, but has also been reported in the contralateral resting arm, referred to as mirror electromyographic (EMG) activity (Mayston et al. 1999; Leocani et al. 2000; Hoy et al. 2004; Carson 2005; Giovannelli et al. 2006; Cincotta and Ziemann 2008). Mirror EMG activity has been reported during performance of simple (Uttner et al. 2007; Ottaviani et al. 2008) and complex (Armatas et al. 1994; Cincotta et al. 2006) motor tasks as well as during strong unimanual voluntary contractions (Muellbacher et al. 2000; Zijdewind et al. 2006) in healthy individuals and in patients with motor disorders.
Previous studies demonstrated a possible role for the primary motor cortex (M1) ipsilateral to a voluntary contraction in the generation of mirror EMG activity (Mayston et al. 1999; Hoy et al. 2004, Carson 2005, Zijdewind et al. 2006). Accordingly, functional magnetic resonance imaging (fMRI) studies have shown an increase in activity in M1 ipsilateral to a contracting arm during strong unimanual force generation (Dettmers et al. 1995; Thickbroom et al. 1998; Dai et al. 2001; van Duinen et al. 2008), when mirror EMG activity has been reported. Furthermore, studies in patients with movement disorders have demonstrated a decrease in intracortical inhibition in the M1 ipsilateral to the moving arm in the presence of mirror EMG activity (Cincotta et al. 2006; Cincotta and Ziemann 2008) supporting the view that mirroring is related to functional changes in corticospinal projections originating in the M1 ipsilateral. Although these studies characterized detailed physiological and blood-oxygen-level–dependent (BOLD) changes in ipsilateral M1 (Dettmers et al. 1995; Thickbroom et al. 1998; Dai et al. 2001), others have evaluated simultaneously changes in BOLD signal and EMG activity (Post et al. 2008), suggesting that simultaneous recording of BOLD signal and EMG would be necessary to identify the possible involvement of brain regions other than M1 in mirroring.
To address this issue, we used fMRI to study brain regions activated during performance of a parametric unimanual force generation task with simultaneous EMG recordings from 8 muscles in both arms. We chose this combined fMRI/EMG design to be able to relate accurately activation in different cortical areas engaged in force generation with mirror EMG activity. The main finding of our study was that during performance of a unimanual force generation task, in addition to M1 ipsilateral to the active hand, mirroring correlated with increased activity in the contralateral M1 and the medial premotor structures including SMA and the cerebellum.
Methods
Subjects
Fifteen healthy volunteers (6 women, 9 male; 29 ± 9.5 years) participated in the study. All subjects gave their informed consent to the experimental procedure, which was approved by the National Institutes of Neurological Disorders and Stroke (NINDS) ethics committee. The study was performed in accordance with the Declaration of Helsinki. All the subjects were right handed as tested by the Edinburgh handedness inventory (Oldfield 1971). All subjects participated in a single session carried out in the MRI scanner. In this session, subjects performed 10%, 20%, 30%, and 70% of their maximal right wrist isometric flexion force in a pseudo-randomized order with their right arm. Two subjects were excluded from data analysis because of excessive head movement (>5 mm) and 1 for not respecting the behavioral instructions during scanning. The other subjects showed no significant movements during the different levels of right wrist flexion force (>2 mm; range 0.1–2.6 mm, mean = 0.84 ± 0.67 mm). Therefore, data from 12 subjects were included in the analysis.
Motor Task and Procedures
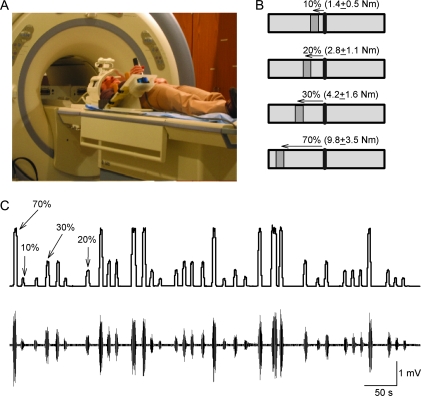
During testing, subjects lay in the MRI scanner. A custom 6-axis load cell (35-E15A; Woodland, CA) was attached to the right arm to measure wrist flexion force (Fig. 1A). Custom software was written to acquire signals from the load cell and to display visual feedback (Fig. 1B) corresponding to rest, 10%, 20%, 30%, and 70% of each subject maximal right wrist flexion force in real-time (Matlab R14SP3, Mathworks, Natick, MA; Hidler et al. 2006). Subjects were instructed to respond to the GO (target) signal presented on a computer monitor by moving a cursor to a target box located at different distances according to their maximal right wrist flexion force (i.e., larger distances at larger levels of force generation) and to maintain the cursor in the target box for 2 s by performing isometric right wrist flexion force (Fig. 1B). The visual target was displayed on a rear-projection screen using a color liquid crystal display (LCD) projector. The subject viewed the display through a mirror fixed to the top of the head coil. The instruction for the subject was “When you see the GO signal bend the right wrist.” The maximal right wrist flexion force was measured 3 times at the beginning of each session and the measurements were averaged. The experiment was conducted in an event-related design using all force conditions (10%, 20%, 30%, and 70%). The gap between movements was 6 and 14 s. The stimulus-onset-asynchrony between trials was jittered with an average of 9 s (range = 6–14 s). Subjects performed 2 experimental runs. Each run consisted of 64 trials with 16 trials for each force level. EMG measurements (see below EMG recordings and analysis) were acquired from both arms at rest and during 10%, 20%, 30%, and 70% of maximal right wrist flexion force (Fig. 1C). During testing, the left arm was oriented parallel to the trunk and supported from the forearm to the radial side of the hand with cushions. It is important to consider that during force generation in the lying position, stabilization of the trunk might be achieved by contracting the other extremity or other segments of the same arm. To minimize this possibility, the segments proximal to the wrist were supported by 4 padded adjustable bumpers. The contact point of the bumpers along the forearm and the width of the bumpers were adjusted to accommodate forearms of different subjects. The configuration of the bumpers secured form closure, which prevented the forearm from rotating and also minimized the forces generated at the hand and wrist from propagating up the arm and trunk (Hidler et al. 2006).
Figure 1.
Experimental setup. (A) Schematics of the experimental setup. Subjects lay in the MRI scanner with their right arm attached to a custom device during performance of different levels of right wrist flexion force. (B) Diagram showing the visual display presented to all subjects during testing. The black vertical line in the center shows the cursor that subjects were instructed to move by performing right isometric wrist flexion force over the manipulandum. The “GO” signal (dark gray box located to the left of the cursor) was also the target to where subjects had to move the cursor, maintaining it in position for 2 s. The distance between cursor and target related to the magnitude of force required to accomplish each task, normalized to the maximal wrist flexion force determined in each participant. (C) Traces showing force and EMG recordings after MRI artifact correction in the right FCR (primary mover) on a representative subject. Note the randomized presentation of the force trials during testing.
EMG Recordings and Analysis
EMG recordings were acquired using the BrainAmp MR Plus recorder and software (Brainproducts GmbH, Munich, Germany; van Duinen et al. 2005). Surface electrodes were positioned bilaterally on the skin overlying the flexor carpis radialis (FCR), extensor carpi radialis (ECR), biceps brachii (BB), and triceps brachii (TB) muscles in a bipolar montage (interelectrode distance, 2 cm). To avoid EMG movement artifact, the EMG leads were secured with adhesive tapes on the electrodes and cohesive bandages around the arms. The EMG signals were filtered (band-pass, 10–400 Hz), sampled at 5 kHz, and stored on a PC for off-line analysis. Brain Vision Analyzer software was used to remove scanner artifacts according to the method described by Allen et al. (2000). After the scanner artifact was removed, EMG data (ASCII format) were imported into Spike2 (Cambridge Electronic Design, Cambridge, UK) for further analysis. EMG signals were rectified, and the mean EMG activity was obtained, trial-by-trial, from all recording muscles. The onset of each EMG burst was defined as the time point when the mean EMG activity exceeded the baseline activity (BL) by 3 standard deviations (SD) of the baseline (BL ± 3SD). The offset of each EMG burst was defined as the time point when the EMG signal fell below this value. EMG data were expressed as percent of baseline (BL) EMG. A total of 32 trials (trials from 2 fMRI runs combined) per condition were averaged. Kolmogorov–Smirnov and Mauchly's tests were initially used to characterize the distribution and sphericity of data, respectively. Repeated measures analysis of variance (ANOVARM) was used to determine the effect of FORCE (Baseline, 10%, 20%, 30%, and 70%) on each muscle EMG. Tukey post hoc test was used for multiple comparisons (SigmaStat, Version 2.03, Systat Software Inc., San Jose, CA). To assess the presence of mirror EMG activity, we tested the effect of FORCE (Baseline, 10%, 20%, 30%, and 70%) on EMG activity on each muscle. Significance was set at P< 0.05. Variance is expressed as mean ± SD.
fMRI Data Acquisition and Analysis
The fMRI scans were performed using a GE 3-T scanner (GE Medical, Waukesha, WI) with an 8-channel receiving head coil. The functional images were acquired in 2 separated runs (274 volumes each) using a T2*-weighted interleaved echo-planar imaging (EPI) sequence covering the whole brain (TR = 2 s, TE = 30 ms, flip angle = 90°, NEX = 1, field of view = 220 × 220, number of slices = 32, slice thickness = 4 mm, gap = 0, and matrix size = 64 × 64). T1-weighted structural images with 1 × 1 × 4-mm resolution were also acquired for each subject.
fMRI data preprocessing and analysis were performed with SPM2 and SPM5 (for second level group analysis) software (the Wellcome Department of Imaging Neuroscience, University College London, London, UK). The first 5 volumes (scans) of images were automatically discarded prior to data processing. All images of each time series (i.e., a scan run) were first slice-time corrected and then realigned to the first image of the first run with a 6-parameter rigid-body transformation for each subject. Resulting spatially realigned images were normalized to the template MNI brain. Therefore, the coordinates used to report the locations of activation are all in the MNI space. The image volumes were subsequently spatially smoothed (FWHM = 8 mm3) and high-pass filtered with a cut-off frequency of 1/128 s to remove low frequency drift. Statistical analyses were carried out with the SPM2 and SPM5 software using the general linear model (Friston et al. 1995; Turner et al. 1998). The SPM matrix modeled an implicit baseline that included the resting baseline between trials in the fMRI design.
For the first level fixed-effects analysis, statistical contrasts (t-tests) of MR signals for each level of force (10%, 20%, 30%, and 70%) relative to the baseline were performed for each individual subject. Anatomical identification was carefully performed by superimposing activation foci on the MNI brain and on the normalized structural T1-weighted images of each subject. The resulting contrast images were used as input for the random-effects analysis at the group level using SPM5. Task-related changes in BOLD signal between and across force levels were examined using 1-sample t-tests over the voxels in the whole brain. A voxel is considered to be significantly different from the baseline or in a contrast if it survived the False Discovery Rate (FDR) correction for multiple comparisons at the threshold of P < 0.05 or otherwise specified. To characterize changes in BOLD signal across conditions as the force level increased, a linear trend analysis was also performed at the second level group analysis. The trend analysis was done using the output of a within-subject ANOVA in SPM5 by assigning linear weights to the force condition (using a “Flexible Factorial” design with force level as a within-subject factor). The purpose of the linear trend analysis was to identify cortical regions that showed a significant linear increase in BOLD signal with increasing levels of force. The activated areas were defined with a probabilistic cytoarchitectonic map (Eickhoff et al. 2005). We used the atlas of Schmahmann et al. (1999) for a specific neuroanatomical differentiation of cerebellar activations and the nomenclature of Larsell and Jansen (1972) to label cerebellar lobules. The SPM anatomy toolbox (Eickhoff et al. 2005) was used to estimate cytoarchitectonic probabilities when possible. In order to evaluate the relationship between EMG activity and BOLD signal, Pearson correlation analysis was performed between mean EMG signals and the activity of the peak voxel of the areas showing a significant linear increase across force levels depicted in Figure 3 using SPM5. In addition, we performed multiple regression analyses of BOLD signal change at the group level using 2 regressors: 1) right FCR EMG and 2) left FCR EMG. The 2 EMG regressors were orthogonalized to the mean EMG of the 2 muscles (i.e., the constant) in 2 regression models using the Gram–Schimdt orthogonalization algorithm. With the orthogonalized regressors, the 2 models accounted for (i.e., subtracted) the mean effect of force/task from the EMG regressors and allowed estimation of BOLD signal change uniquely associated with the right and the left FCR EMG across force levels (van Duinen at al. 2008; van Rootselaar et al. 2008). In model 1, right FCR EMG was entered as the first regressor and left FCR as the second regressor. This model provides estimate for the BOLD signal change associated with the right FCR and of the variance of the activation uniquely associated with the left FCR. In model 2, left FCR EMG was entered as the first regressor and right FCR as the second regressor. This model provides estimate for the BOLD signal change associated with the left FCR and of the variance of the activation uniquely associated with the right FCR. All significant correlations survived the threshold of P = 0.05 (FDR corrected).
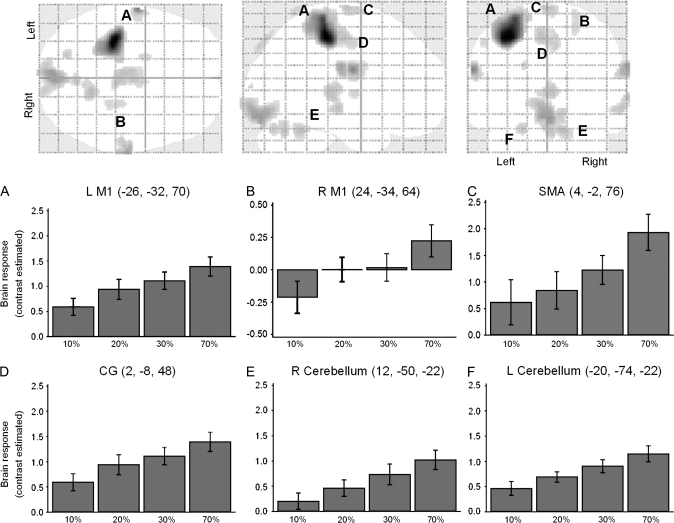
Figure 3.
Force-related brain network. Brain areas that showed a significant linear increase in BOLD signal activity at all force levels (random-effects analysis, within-subjects ANOVA, FDRcorr. P < 0.05; glass-brain figures). The graphs show the main effects of force in the peak voxel of these force-related brain areas. In all graphs, the abscissa shows the conditions tested (10%, 20%, 30%, and 70%) and the ordinate shows the contrast estimate (average Beta values for each condition). Note that in all displayed areas, there is a linear increase of BOLD signal activity with increasing levels of force. Abbreviations: L M1 (left primary motor cortex), R M1 (right primary motor cortex), SMA (supplementary motor area), and CG (caudal cingulate). Cluster threshold ≥5.
Results
EMG Activity in the Right Arm
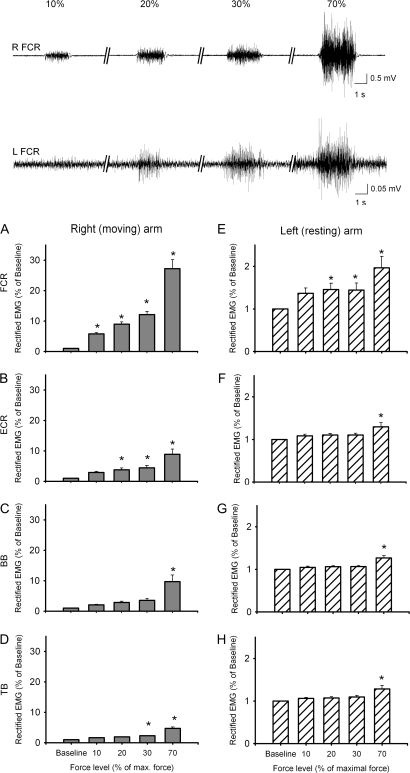
Figure 2 (upper traces) illustrates the right FCR EMG activity recorded in a single subject during 10%, 20%, 30%, and 70% of force. The group data are shown in Figure 2A–D. We found a significant effect of FORCE on EMG activity (% of baseline) in all muscles tested in the right arm (FCR, F(4,44) = 76.9, P ≤ 0.001, Fig. 2A; ECR, F(4,44) = 22.0, P ≤ 0.001, Fig. 2B; BB, F(4,44) = 16.9, P ≤ 0.001, Fig. 2C; TB, F(4,28) = 34.1, P ≤ 0.001, Fig. 2D). In the right FCR, EMG activity was increased at 70% (P ≤ 0.001), 30% (P ≤ 0.001), 20% (P ≤ 0.001), and 10% (P = 0.036) of force compared with baseline. In the right ECR, EMG activity was increased at 70% (P ≤ 0.001), 30% (P ≤ 0.01), and 20% (P ≤ 0.02) of force compared with baseline. In the right BB, increased EMG activity was observed at 70% (P≤ 0.001) but not at 30% (P = 0.2), 20% (P = 0.5), and 10% (P = 0.9) of force compared with baseline. In the right TB, increased EMG activity was observed at 70% (P ≤ 0.001) and 30% (P ≤ 0.01) but not at 20% (P = 0.08) and 10% (P = 0.3) of force compared with baseline. Similar effect of FORCE was observed on raw EMG data in all muscles tested in the right arm (FCR, F(4,44) = 27.1, P ≤ 0.001; ECR, F(4,44) = 42.8, P ≤ 0.001; BB, F(4,44) = 6.6, P ≤ 0.001; TB, F(4,28) = 6.2, P ≤ 0.001).
Figure 2.
EMG data in left and right arm muscles. Traces show EMG activity after MRI artifact correction in a representative subject in the right (upper traces, R FCR) and left (lower traces, L FCR) FCR muscle during 10%, 20% 30%, and 70% of maximal right wrist force. Note the presence of mirror EMG activity in the left FCR during 20%, 30%, and 70% of maximal right wrist force. The bar graphs show group EMG data after MRI artifact correction. In all graphs, the abscissa shows the conditions tested (10%, 20%, 30%, and 70%) and the ordinate shows the mean rectified EMG activity (expressed as a percent of baseline mean rectified EMG activity) in all muscles tested in the right arm: (A) FCR, (B) ECR, (C) BB, and (D) TB and left arm: (E) FCR, (F) ECR, (G) BB, and (H) TB. Error bars indicate standard errors; *P < 0.05. Abbreviations: flexor carpis radialis (FCR), extensor carpi radialis (ECR), biceps brachii (BB), and triceps brachii (TB) muscles.
EMG Activity in the Left Arm (Mirror EMG)
Figure 2 (lower traces) illustrates the left FCR EMG activity recorded in a single subject while the right FCR was performing 10%, 20%, 30%, and 70% of force. The group data are shown in Figure 2E–H. ANOVARM showed a significant effect of FORCE on EMG activity (% of baseline) in all muscles tested in the left arm (FCR, F(4,44) = 11.64, P ≤ 0.001, Fig. 2E; ECR, F(4,44) = 10.23, P ≤ 0.001, Fig. 2F; BB, F(4,44) = 11.38, P ≤ 0.001, Fig. 2G; TB, F(4,28) = 16.77, P≤ 0.001, Fig. 2H). In the left FCR, post hoc testing showed an increase in EMG activity at 70% (P ≤ 0.001), 30% (P = 0.02), 20% (P = 0.02) but not at 10% (P = 0.09) of force compared with baseline, indicating the presence of mirror activity. Mirror EMG activity was also observed at 70% in the left ECR (P ≤ 0.001), left BB (P ≤ 0.001) and TB (P ≤ 0.001). Similar effect of FORCE was observed on raw EMG data in all muscles tested in the left arm (FCR, F(4,44) = 7.6, P ≤ 0.001; ECR, F(4,44) = 7.7, P < 0.001; BB, F(4,44)=11.4, P < 0.001; TB, F(4,28)=12, P ≤ 0.001).
Functional MRI
The results of the linear trend analysis showed a significant (FDR corrected P < 0.05) linear trend of the BOLD signal increase primarily in 6 regions as the force level increased (Fig. 3). The 6 cortical areas that exhibited a linear increasing trend in BOLD activity with force were bilateral M1 (Fig. 3A,B), SMA (Fig. 3C), caudal cingulate (Fig. 3D), and 2 cerebellar areas (left hemisphere, lobule IV and right hemisphere, lobules IV–V: Fig. 3E,F). A second order quadratic trend analysis showed no voxels activated in these regions. The bar graphs of Figure 3 show beta values of the contrast estimates (relative to the baseline) of the peak voxels of each condition. Additional activation, relative to the baseline, was also observed in the occipital cortex (BA 17 and BA 18) and bilateral somatosensory cortex (BA3b and BA1).
In addition to the linear trend analysis, second level (group) random-effects analysis showed significant effect of FORCE. One-sample t-tests showed significant (P <0.05, FDR corrected) differences between 70% and 30% relative to 10% of force. There was no significant difference between the higher force levels (70% and 30%), and between the lower force conditions (20% and the 10%). A separate 1-sample t-test, combining the higher force levels (70% and 30%) to contrast the 10% (lower force), showed additional activation at the higher force level (P < 0.005, uncorrected) in the SMA, bilateral M1, somatosensory, caudal cingulate, caudate nucleus, and bilateral cerebellum (Table 1). Reverse contrasts (t-tests comparing lower force level minus higher force level) did not show any significant differences except for the contrast (1-tailed t-test) 10–20%, which showed significantly more activation in the right inferior parietal cortex (BA40).
Table 1.
Brain regions significantly activated at the higher force (70% and 30%) compared with the lower force (10%) level during the right wrist force
| Anatomy label | BA | Laterality | MNI coordinate |
Voxel (Z) | Cluster size | ||
| x | y | z (mm) | |||||
| Primary motor cortex | 4 | L | −34 | −28 | 54 | 5.36 | 275 |
| R | 20 | −32 | 56 | 3.25 | 22 | ||
| SMA | 6 | L/R | −4 | −12 | 74 | 3.44 | 213 |
| Somatosensory cortex | 1, 2, 3, 5 | L | −36 | −28 | 54 | 4.98 | 427 |
| R | 22 | −32 | 56 | 3.24 | 51 | ||
| Cingulate | 24 | L | −8 | −10 | 50 | 3.43 | 36 |
| 24 | R | 4 | −8 | 46 | 3.27 | 68 | |
| Caudate nuclei | L | −18 | −4 | 24 | 3.48 | 58 | |
| R | 20 | 4 | 20 | 4.64 | 161 | ||
| Thalamus | L/R | 2 | −22 | 4 | 4.04 | 552 | |
| Occipital cortex | 17/18 | LR | −4 | −92 | −10 | 2.88 | 50 |
| Cerebellum | L | −22 | −72 | −18 | 2.67 | 8 | |
| R | 12 | −48 | −20 | 3.11 | 137 | ||
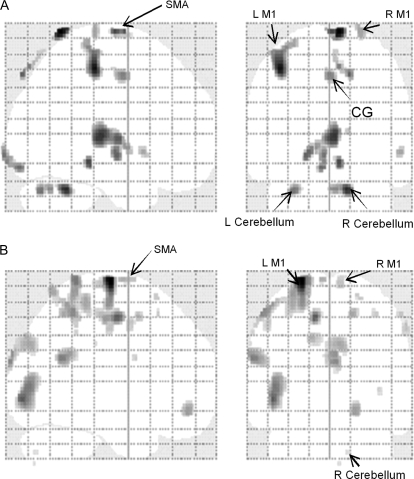
The results of the multiple regression analyses with orthogonalized regressors showed that when the mean effect of force was removed significant activation was observed in the bilateral M1, SMA, somatosensory cortex, and the cerebellum (Fig. 6 and Table 2). These areas are consistent with the clusters seen in the linear trend analysis (Fig. 3).
Figure 6.
Multiple regression analyses. The figure shows activation in brain regions that were uniquely accounted for by the right FCR EMG (A) and the left FCR EMG (B). These areas included bilateral M1, SMA, caudal cingulate, and the cerebellum. All activation results were FDR corrected at P > 0.05. Note that all displayed areas are within the clusters seen in the linear trend analysis (Fig. 3). Abbreviations: L M1 (left primary motor cortex), R M1 (right primary motor cortex), SMA (supplementary motor area), and CG (caudal cingulate).
Table 2.
BOLD signal change in brain regions common and unique to the variability in the right and the left FCR EMG
| EMG | Anatomy label | BA | Laterality | MNI coordinate |
Voxel (Z) | Cluster size | ||
| x | Y | z (mm) | ||||||
| Right FCR | Primary motor cortex | 4 | L | −38 | −28 | 54 | 4.95 | 219 |
| R | 30 | −32 | 70 | 4.20 | 6 | |||
| SMA | 6 | L/R | 2 | −10 | 76 | 4.50 | 73 | |
| L | −10 | 8 | 52 | 3.85 | 18 | |||
| R | 26 | −26 | 74 | 4.00 | 72 | |||
| Somatosensory cortex | 1, 2 ,3 | L | −34 | −32 | 66 | 4.57 | 474 | |
| Cingulate | 24 | L/R | −4 | −10 | 52 | 3.90 | 166 | |
| Temporal pole | 44 | R | 58 | 12 | −2 | 4.00 | 39 | |
| Thalamus | L/R | 4 | −20 | 10 | 4.14 | 475 | ||
| Occipital cortex | 17/18 | LR | −4 | −100 | −4 | 4.21 | 125 | |
| Cerebellum | L | −28 | −72 | −28 | 3.87 | 80 | ||
| R | 14 | −50 | −26 | 4.56 | 316 | |||
| Left FCR | Primary motor cortex | 4 | L | −30 | −30 | 60 | 4.73 | 19 |
| SMA | 6 | L | −4 | −4 | 76 | 4.31 | 25 | |
| −22 | −14 | 76 | 6.50 | 90 | ||||
| R | 8 | −20 | 74 | 3.99 | 65 | |||
| Somatosensory cortex | 1, 2, 3 | L | −28 | −42 | 68 | 4.64 | 58 | |
| R | 58 | −20 | 54 | 3.83 | 41 | |||
| Superior parietal | 7 | L | −18 | −60 | 68 | 4.30 | 60 | |
| R | 18 | −52 | 54 | 4.37 | 51 | |||
| Supra marginal | 39 | L | −62 | −54 | 24 | 4.59 | 218 | |
| Occipital cortex | 17/18 | L/R | −4 | −94 | −28 | 4.48 | 420 | |
| Cerebellum | R | −16 | −74 | −36 | 3.93 | 35 | ||
| Right FCR (unique) | Primary motor cortex | 4 | L | −36 | −30 | 64 | 3.89 | 42 |
| R | 26 | -26 | 76 | 3.76 | 25 | |||
| SMA | 6 | L/R | 2 | −10 | 76 | 4.21 | 36 | |
| Somatosensory cortex | 1, 2, 3 | L | −28 | −34 | 68 | 3.86 | 109 | |
| R | 22 | −32 | 56 | 3.24 | 51 | |||
| Cingulate | 24 | L/R | 0 | −4 | 48 | 3.92 | 57 | |
| Thalamus | L/R | 4 | −22 | 8 | 4.33 | 378 | ||
| Occipital cortex | 17/18 | LR | −6 | −88 | −12 | 4.04 | 48 | |
| Cerebellum | L | −28 | −70 | −28 | 3.99 | 44 | ||
| R | 6 | −64 | −26 | 3.89 | 32 | |||
| Left FCR (unique) | Primary motor cortex | 4 | L | −30 | −34 | 62 | 4.10 | 10 |
| R | 10 | −26 | 68 | 3.79 | 10 | |||
| 62 | −14 | 46 | 3.79 | 17 | ||||
| SMA | 6 | L | −6 | −4 | 76 | 3.94 | 14 | |
| −10 | −8 | 52 | 5.09 | 18 | ||||
| R | 8 | −20 | 74 | 3.79 | 90 | |||
| Somatosensory cortex | 1, 2, 3, 5 | L | −18 | −42 | 76 | 4.71 | 273 | |
| R | 58 | −20 | 54 | 3.69 | 6 | |||
| Inferior parietal | 39/40 | L | −52 | −44 | 56 | 4.45 | 75 | |
| Superior parietal | 7 | R | 18 | −52 | 54 | 4.07 | 23 | |
| Occipital cortex | 18 | LR | 4 | −96 | −22 | 4.45 | 212 | |
| 19 | L | −46 | −86 | −2 | 5.09 | 280 | ||
| Cerebellum | R | 16 | −74 | −36 | 3.39 | 3 | ||
Note: Unique = variability in the BOLD signal that was uniquely accounted for by the left or the right FCR EMG above and beyond the mean effect of force and the effect of either EMG as the first regressor in the orthogonalized multiple regression analyses. L = left, R = right.
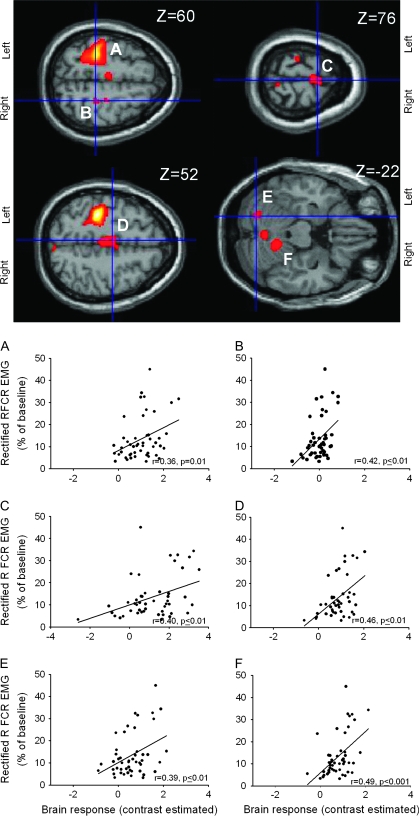
Correlations between Brain Activity and EMG
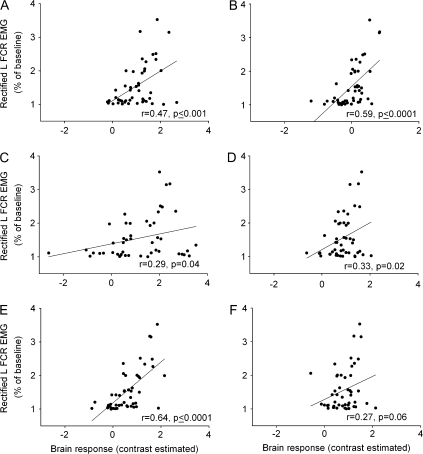
EMG activity in the right FCR correlated with BOLD signal increases in the following regions integrating the network in Figure 3: left M1 (r = 0.36, P = 0.01; Fig. 4A), right M1 (r = 0.42; P ≤ 0.01, Fig. 4B), SMA (r = 0.40, P ≤ 0.01; Fig. 4C), caudal cingulate (r = 0.46, P ≤ 0.01; Fig. 4D) and the cerebellum (left cerebellum, r = 0.39, P ≤ 0.01, Fig. 4E; right cerebellum, r = 0.49, P ≤ 0.001, Fig. 4F). The results of model 1 whole-brain regression analysis with the right FCR EMG as the first regressor also showed bilateral M1, SMA, somatosensory cortex, caudal cingulate, and cerebellum (Table 2). The regression model 2 with the right FCR as the second regressor that allowed estimation of a unique association between the right FCR EMG and the BOLD signal change (i.e., above and beyond the activation accounted for by the mean effect of force and the left FCR) revealed again significant activation in these brain regions (Fig. 6A, Table 2). EMG activity in the left FCR (mirror EMG activity) showed a significant correlation with BOLD signal change in left M1 (r = 0.47, P≤ 0.001; Fig. 5A), right M1 (r = 0.59, P ≤ 0.0001; Fig. 5B), SMA (r = 0.29, P = 0.04, Fig. 5C), caudal cingulate (r = 0.33, P = 0.02; Fig. 5D) and left cerebellum (r = 0.64, P ≤ 0.0001; Fig. 5E). A correlational trend was observed between mirror EMG and activity in the right cerebellum (r = 0.27, P = 0.06; Fig. 5F). Consistently, the results of model 1 whole-brain regression analysis with the left FCR EMG as the second regressor showed activation in bilateral M1, SMA, and cerebellum (Fig. 6B, Table 2). No correlations were found between mirror EMG activity at the high levels of force in the ECR, BB, and TB and the regions involved in mirror EMG activity in the left FCR.
Figure 4.
Correlation between BOLD signal and right FCR EMG activity (primary mover). Force-related brain areas shown in Figure 3 were chosen for correlations with EMG activity present in the right FCR (primary mover). In all graphs, the abscissa shows the contrast estimate (average Beta values for each condition) and the ordinate shows the mean rectified right FCR EMG activity across force levels. Note that the brain regions showing a significant correlation with EMG activity in the right FCR were left (A) and right (B) M1, SMA (C), caudal cingulate (D), left (E) and right (F) cerebellum. The functional maps are displayed on the canonical T1-weighted single subject MNI brain. R-values represent Pearson's correlation coefficient.
Figure 5.
Correlation between BOLD signal and mirror EMG activity. Force-related brain areas shown in Figure 3 were chosen for correlations with mirror EMG activity present in the left FCR. In all graphs, the abscissa shows the contrast estimate (average Beta values for each condition) and the ordinate shows the mean rectified left FCR EMG activity during all force levels. Note that a significant correlation between mirror EMG activity in the left FCR and brain activity in the left M1 (A), right M1 (B), SMA (C), caudal cingulate (D), and left cerebellum (E). A correlational trend was found between mirror EMG in the left FCR and the right cerebellum (F). R-values represent Pearson's correlation coefficient.
Discussion
In the present study, we combined fMRI/EMG to investigate BOLD changes associated with mirror EMG activity during parametric increase of unimanual force generation. We found mirror EMG activity in the left FCR during 20%, 30%, and 70% of right wrist force. Left ECR, BB, and TB showed mirror EMG activity only at 70% of right wrist force. Mirror EMG activity in the left FCR correlated with BOLD changes in bilateral M1, SMA, and the cerebellum.
Cortical Network Involved in Force Generation
We found a linear increase in BOLD signal activity in bilateral M1, SMA, caudal cingulate, and the cerebellum with increasing levels of right wrist force consistent with previous results (Dettmers et al. 1995; Thickbroom et al. 1998; Dai et al. 2001; Peck et al. 2001; Cramer et al. 2002; Halder et al. 2007; van Duinen et al. 2008). EMG activity in all muscles tested in the right arm linearly increased with increasing right force generation. We also observed a linear relationship between force and BOLD activation, as reported in previous studies (Dettmers et al. 1995; Ward and Frackowiak 2003; van Duinen et al. 2008). A number of studies have addressed simultaneously the relation between muscle output (EMG) and fMRI-measured brain activity (Liu et al. 2000; Dai et al. 2001; Oga et al. 2002; van Duinen et al. 2005, 2008) without a detailed description of the brain regions that show activity associated to mirroring in different muscle groups in both arms, which was a goal of our study. Here, using an artifact correction paradigm similar to that of van Duinen et al. 2005, we found increasing activity in bilateral M1, SMA, caudal cingulate, and cerebellum correlated with increasing EMG activity of the right FCR (primary mover), consistent with the direct involvement of these brain regions in the force generation task (see Fig. 4) and in agreement with previous studies (Dettmers et al. 1995; van Duinen et al. 2008). It is less likely that the increased activity on these areas is related to muscle fatigue, because we observed a parallel increase in EMG and brain activity, whereas during muscle fatigue brain activity increases and EMG activity decreases (Post et al. 2008). The use of visual targets in the experimental setup (Sestieri et al. 2007) and visual feedback (Hadler et al. 2007) could have contributed to the significant activation identified in the visual cortex (Fig. 3).
Cortical Network Involved in Mirroring
We found mirror EMG activity in all muscles tested in the left arm during 70% of right wrist force, in agreement with previous results in more proximal and distal muscles (Zijdewind and Kernell 2001; Carson 2005; Zijdewind et al. 2006; van Duinen et al. 2008). Mirror EMG in the left FCR was also present during 20% and 30% of right wrist force. These results are in line with previous studies that demonstrated the presence of mirror activity during similar low levels of force generation by the contralateral hand (Armatas et al. 1994; Mayston et al. 1999; Shinohara et al. 2003). The main novel finding in our study was the correlation between changes in BOLD signal in bilateral M1, SMA, and the cerebellum and mirror EMG in the left FCR when using force as a covariate. The unique involvement of bilateral M1, SMA, and the cerebellum in mirroring was further supported by the results of the whole-brain multiple regression analyses in which the mean effect of force was accounted for and the right and left EMG regressors were orthogonalized. The finding that BOLD signal activity in the CG correlated with mirror EMG activity in the left FCR only when force was not accounted for, does not allow us to exclude the possibility that the linear increase in activation in the CG (reported in Fig. 3) was partially related to the force level.
Primary Motor Cortices
Our results show that activity in both M1s was associated with mirror EMG activity in the left FCR. The finding of a linear correlation between BOLD changes in right M1 and mirror EMG supports the proposal that descending pathways originated in the right M1 are at least partially involved in the generation of mirror EMG activity (Carson 2005). Consistent with this view, excitability in the M1 ipsilateral to a moving arm increases in the presence of mirror EMG in healthy subjects (Zijdewind et al. 2006) and in patients with motor disorders (Cincotta et al. 2006). It has been proposed that by increasing the functional demands of the motor task (in this case force) transcallosal pathways originating in the voluntarily active M1 exert a disinhibitory effect on the excitability of intracortical circuits measured in the resting hemisphere, which may partially contribute to control excitability in the ipsilateral M1 (Li 2007; Perez and Cohen 2008). Therefore, our results of a correlation between activity in the left M1 and mirror EMG suggest that the contribution of the left M1 to mirror might theoretically occur throughout ipsilateral descending pathways or disinhibitory influences on the right M1.Because previous evidence suggest that ipsilateral descending pathways play a less fundamental contribution to the generation of mirror EMG activity (Cincotta and Ziemann 2008), we would like to suggest that their involvement may occur to some extent through the right M1. However, it is important to keep in mind that from our experimental design it is not possible to determine if the contribution of the left M1 to mirroring occurs through their inputs on the right M1, direct descending ipsilateral pathways or both. Although the pattern of BOLD signal activity observed in the right M1 was similar to previous studies (Dettmers et al. 1995; van Duinen et al. 2008), at 20% and 30% of force, very small increases in BOLD signal were observed in the presence of mirror EMG activity in the left FCR. A possible explanation is that lower levels of force do not induce significant change in the magnitude of BOLD signal activity (van Duinen et al. 2008). This result might also indicate that the right M1 is more likely to contribute to the mirror EMG at the higher levels of force generation. Furthermore, we cannot exclude the possibility that afferent input contributed partially to changes in BOLD signal in the right M1 (Naito et al. 2002; Bestmann et al. 2004).
Supplementary Motor Area (SMA)
Our results also indicate that the SMA is active in association with left FCR mirror EMG activity. Studies in monkeys have shown that neuronal activity in the SMA encodes movement dynamics (such as force) during movement execution (Smith 1979; Padoa-Schioppa et al. 2004). In addition, neuroimaging studies in humans found force-related BOLD signal activity changes in the SMA (Dettmers et al. 1995; Cramer et al. 2002). The involvement of the SMA in mirror EMG activity is supported by their role in bimanual coordination (Wenderoth et al. 2005), movement lateralization (Brinkman 1984; Chan and Ross 1988), and in the performance of mirror motor tasks (Kraft et al. 2007). It is possible that the SMA contributes to modulate activity in the M1 ipsilateral to the moving hand during the presence of mirroring. This proposal is also supported by the strong connections between SMA and M1 (Civardi et al. 2001; Liu et al. 2003) and by the involvement of the SMA in online control of voluntary movements (Tanji and Shima 1994). Alternatively, the SMA, which projects directly to interneurons in the spinal cord (Picard and Strick 1996), could theoretically influence EMG activity in the mirror hand through the recruitment of direct descending corticospinal projections. This is in agreement with the view that, in addition to the classical serial hierarchical organization of the motor system, there is also a parallel involvement of primary and secondary motor areas in the control of mirroring (Cincotta and Ziemann 2008).
Caudal Cingulate
The BOLD activity in the caudal cingulate was not correlated with the mirror EMG activity in the left FCR when the variability in the BOLD signal was accounted for by the right EMG and the mean effect of force (Fig. 6B). Therefore, we cannot exclude the possibility that the linear increase in activation in the CG (reported in Fig. 3) was partially related to the generation of wrist force in general. Previous studies have demonstrated that cingulate motor areas play a substantial role in different aspects of motor behaviors, such as in the preparation, execution, and error detection of movements (Shima et al. 1991; Shima and Tanji 1998; O'Connell et al. 2007). It is also known that activity in the caudal cingulate is modulated by force production in humans (Dettmers et al. 1995; Ehrsson et al. 2007) and monkeys (Cadoret and Smith 1995, 1997; Richardson et al. 2008). Therefore, a possibility is that caudal cingulate contributes to force adaptation-related changes in directional tuning (Richardson et al. 2008). Because neurons in the caudal cingulate project to M1s and the SMA (Picard and Strick 1996), these connections may provide possible pathways for this contribution. On the other hand, studies in monkeys have shown substantial projections from caudal cingulate neurons to the spinal cord as the SMA (Picard and Strick 1996), which may provide another potential pathway for the observed effects.
Cerebellum
We found that the cerebellum is active in association with left FCR mirror EMG activity. What was striking in our results is that the correlation between mirroring and BOLD signal activity in the cerebellum was stronger compared with the SMA. The involvement of the cerebellum in mirroring is in agreement with previous evidence showing that cerebellar neurons encode force in a linear fashion (Smith and Bourbonnais 1981; Townsend et al. 2006), but note that this correlation remained present when using force as a covariate. More specifically, activity in the cerebellum has been related to force prediction (Kawato et al. 2003; Boecker et al. 2005). One of the major projections from the cerebellum is to the M1, most corticopontine and cerebellar projections to the cerebral cortex are contralateral (Middleton and Strick 1998). Therefore, a possibility is that one of the pathways mediating cerebellar influence on mirror EMG activity involves the right M1. Neuroimaging studies have suggested that the cerebellum is involved in the storage (Imamizu et al. 2000), retrieval (Kawato et al. 2003), and switching (Imamizu et al. 2003) of internal models. Because internal models are considered to be mechanisms that can mimic the input–output in the motor system (Kawato et al. 2003), it is possible that the cerebellum contributes to monitor the involuntary output (mirror EMG) in the left resting arm. Our results support the hypothesis of the “generalization capability” of the cerebellum (Boecker et al. 2005), which may be one of the features involved in the generation of mirror activity in the resting arm during movement of the contralateral arm.
Functional Considerations
The presence of mirror EMG activity has been reported in healthy individuals and in patients with motor disorders (for reviews see Carson 2005; Cincotta and Ziemann 2008). Our results provide novel physiological insights suggesting that activation in nonprimary brain regions might reflect sensorimotor processes operating in association with mirror EMG activity. It should also be kept in mind that activation in these regions might point to distant influences from other interconnected areas (Picard and Strick 1996) and reflect either excitatory or inhibitory processes (Waldvogel et al. 2000; Almeida and Stetter 2002) engaged in mirroring. In addition, a spinal contribution to mirroring cannot be ruled out because patients with lesion of the corpus callosum show some level of mirror motor activity to a contralateral resting limb (Meyer et al. 1995).
On the one hand, our results suggest the need for monitoring of EMG activity from multiple muscle groups in both upper limbs of stroke patients performing a motor task to control for the possibility that abnormal contralesional activation observed in M1 ipsilateral (Ward et al. 2006) might be related to the presence of mirror EMG activity. It is tempting to speculate that modulation of excitability in any of these activated areas (i.e., left and right M1, SMA, and cerebellum) by noninvasive brain stimulation might contribute to the balance of unwanted mirroring during performance of unimanual motor tasks in patients with motor disorders. On the other hand, our results might explain previous findings of activation in SMA, ipsilateral M1, and/or the cerebellum in association with performance of unilateral hand movements when EMG activity in the resting hand was not monitored, emphasizing the need for careful control of bilateral upper extremity EMG activity in the scanner.
Conclusions
We found that mirror EMG activity in the left FCR during parametric increases in right isometric force was associated with increased activity in both M1s, the SMA and the cerebellum. Additionally, our results suggest caution when interpreting fMRI activity in studies that involve unilateral force generation tasks in the absence of simultaneous bilateral EMG/kinematics measurements.
Funding
Intramural Research Program of the NIH, NINDS; Alfried Krupp von Bohlen und Halbach-Foundation (to Bernhard Sehm).
Acknowledgments
Conflict of Interest: None declared.
References
- Allen PJ, Josephs O, Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12:230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Almeida R, Stetter M. Modeling the link between functional imaging and neuronal activity: synaptic metabolic demand and spike rates. Neuroimage. 2002;17:1065–1079. [PubMed] [Google Scholar]
- Armatas CA, Summers JJ, Bradshaw JL. Mirror movements in normal adult subjects. J Clin Exp Neuropsychol. 1994;16:405–413. doi: 10.1080/01688639408402651. [DOI] [PubMed] [Google Scholar]
- Bestmann S, Baudewig J, Siebner HR, Rothwell JC, Frahm J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur J Neurosci. 2004;19:1950–1962. doi: 10.1111/j.1460-9568.2004.03277.x. [DOI] [PubMed] [Google Scholar]
- Boecker H, Lee A, Muhlau M, Ceballos-Baumann A, Ritzl A, Spilker ME, Marquart C, Hermsdörfer J. Force level independent representations of predictive grip force–load force coupling: a PET activation study. Neuroimage. 2005;25:243–252. doi: 10.1016/j.neuroimage.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Brinkman C. Supplementary motor area of the monkey's cerebral cortex: short- and long-term deficits after unilateral ablation and the effects of subsequent callosal section. J Neurosci. 1984;4:918–929. doi: 10.1523/JNEUROSCI.04-04-00918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret G, Smith AM. Input–output properties of hand-related cells in the ventral cingulate cortex in the monkey. J Neurophysiol. 1995;73:2584–2590. doi: 10.1152/jn.1995.73.6.2584. [DOI] [PubMed] [Google Scholar]
- Cadoret G, Smith AM. Comparison of the neuronal activity in the SMA and the ventral cingulate cortex during prehension in the monkey. J Neurophysiol. 1997;77:153–166. doi: 10.1152/jn.1997.77.1.153. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Chan JL, Ross ED. Left-handed mirror writing following right anterior cerebral artery infarction: evidence for nonmirror transformation of motor programs by right supplementary motor area. Neurology. 1988;38:59–63. doi: 10.1212/wnl.38.1.59. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Borgheresi A, Balestrieri F, Giovannelli F, Ragazzoni A, Vanni P, Benvenuti F, Zaccara G, Ziemann U. Mechanisms underlying mirror movements in Parkinson's disease: a transcranial magnetic stimulation study. Mov Disord. 2006;21:1019–1025. doi: 10.1002/mds.20850. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Civardi C, Cantello R, Asselman P, Rothwell JC. Transcranial magnetic stimulation can be used to test connections to primary motor areas from frontal and medial cortex in humans. Neuroimage. 2001;14:1444–1453. doi: 10.1006/nimg.2001.0918. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Weisskoff RM, Schaechter JD, Nelles G, Foley M, Finklestein SP, Rosen BR. Motor cortex activation is related to force of squeezing. Hum Brain Mapp. 2002;16:197–205. doi: 10.1002/hbm.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai TH, Liu JZ, Sahgal V, Brown RW, Yue GH. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res. 2001;140:290–300. doi: 10.1007/s002210100815. [DOI] [PubMed] [Google Scholar]
- Dettmers C, Fink GR, Lemon RN, Stephan KM, Passingham RE, Silbersweig D, Holmes A, Ridding MC, Brooks DJ, Frackowiak RS. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. doi: 10.1152/jn.1995.74.2.802. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH, Fagergren A, Ehrsson GO, Forssberg H. Holding an object: neural activity associated with fingertip force adjustments to external perturbations. J Neurophysiol. 2007;97:1342–1352. doi: 10.1152/jn.01253.2005. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Turner R, Frackowiak RS. Characterizing evoked hemodynamics with fMRI. Neuroimage. 1995;2:157–165. doi: 10.1006/nimg.1995.1018. [DOI] [PubMed] [Google Scholar]
- Giovannelli F, Borgheresi A, Balestrieri F, Ragazzoni A, Zaccara G, Cincotta M, Ziemann U. Role of the right dorsal premotor cortex in “physiological” mirror EMG activity. Exp Brain Res. 2006;175:633–640. doi: 10.1007/s00221-006-0581-9. [DOI] [PubMed] [Google Scholar]
- Halder P, Brem S, Bucher K, Boujraf S, Summers P, Dietrich T, Kollias S, Martin E, Brandeis D. Electrophysiological and hemodynamic evidence for late maturation of hand power grip and force control under visual feedback. Hum Brain Mapp. 2007;28:69–84. doi: 10.1002/hbm.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidler J, Hodic T, Xu B, Dobkin B, Cohen LG. MR compatible force sensing system for real-time monitoring of wrist moments during fMRI testing. J Neurosci Methods. 2006;155:300–307. doi: 10.1016/j.jneumeth.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Res Brain Res Rev. 2004;46:315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Kuroda T, Miyauchi S, Yoshioka T, Kawato M. Modular organization of internal models of tools in the human cerebellum. Proc Natl Acad Sci USA. 2003;100:5461–5466. doi: 10.1073/pnas.0835746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Kawato M, Kuroda T, Imamizu H, Nakano E, Miyauchi S, Yoshioka T. Internal forward models in the cerebellum: fMRI study on grip force and load force coupling. Prog Brain Res. 2003;142:171–188. doi: 10.1016/S0079-6123(03)42013-X. [DOI] [PubMed] [Google Scholar]
- Kraft E, Chen AW, Flaherty AW, Blood AJ, Kwong KK, Jenkins BG. The role of the basal ganglia in bimanual coordination. Brain Res. 2007;1151:62–73. doi: 10.1016/j.brainres.2007.01.142. [DOI] [PubMed] [Google Scholar]
- Larsell O, Jansen J. The comparative anatomy and histology of the cerebellum. The human cerebellum, cerebellar connections, and cerebellar cortex. Minneapolis: University Minnesota Press; 1972. [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Li S. Movement-specific enhancement of corticospinal excitability at subthreshold levels during motor imagery. Exp Brain Res. 2007;179:517–524. doi: 10.1007/s00221-006-0809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JZ, Dai TH, Elster TH, Sahgal V, Brown RW, Yue GH. Simultaneous measurement of human joint force, surface electromyograms, and functional MRI-measured brain activation. J Neurosci Methods. 2000;101:49–57. doi: 10.1016/s0165-0270(00)00252-1. [DOI] [PubMed] [Google Scholar]
- Liu JZ, Shan ZY, Zhang LD, Sahgal V, Brown RW, Yue GH. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: an FMRI study. J Neurophysiol. 2003;90:300–312. doi: 10.1152/jn.00821.2002. [DOI] [PubMed] [Google Scholar]
- Mayston MJ, Harrison LM, Stephens JA. A neurophysiological study of mirror movements in adults and children. Ann Neurol. 1999;45:583–594. doi: 10.1002/1531-8249(199905)45:5<583::aid-ana6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 1995;118:429–440. doi: 10.1093/brain/118.2.429. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. The cerebellum: an overview. Trends Neurosci. 1998;21:367–369. doi: 10.1016/s0166-2236(98)01330-7. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Facchini S, Boroojerdi B, Hallett M. Changes in motor cortex excitability during ipsilateral hand muscle activation in humans. Clin Neurophysiol. 2000;111:344–349. doi: 10.1016/s1388-2457(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Naito E, Roland PE, Ehrsson HH. I feel my hand moving: a new role of the primary motor cortex in somatic perception of limb movement. Neuron. 2002;36:979–988. doi: 10.1016/s0896-6273(02)00980-7. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. Eur J Neurosci. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- Oga T, Honda M, Toma K, Murase N, Okada T, Hanakawa T, Sawamoto N, Nagamine T, Konishi J, Fukuyama H, et al. Abnormal cortical mechanisms of voluntary muscle relaxation in patients with writer's cramp: an fMRI study. Brain. 2002;125:895–903. doi: 10.1093/brain/awf083. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ottaviani D, Tiple D, Suppa A, Colosimo C, Fabbrini G, Cincotta M, Defazio G, Berardelli A. Mirror movements in patients with Parkinson's disease. Mov Disord. 2008;23:253–258. doi: 10.1002/mds.21825. [DOI] [PubMed] [Google Scholar]
- Padoa-Schioppa C, Ray Li CS, Bizzi E. Neuronal activity in the supplementary motor area of monkeys adapting to a new dynamic environment. J Neurophysiol. 2004;91:449–473. doi: 10.1152/jn.00876.2002. [DOI] [PubMed] [Google Scholar]
- Peck KK, Sunderland A, Peters AM, Butterworth S, Clark P, Gowland PA. Cerebral activation during a simple force production task: changes in the time course of the haemodynamic response. Neuroreport. 2001;12:2813–2816. doi: 10.1097/00001756-200109170-00012. [DOI] [PubMed] [Google Scholar]
- Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex cortex ipislateral to a moving hand. J Neurosci. 2008;28:5631–5640. doi: 10.1523/JNEUROSCI.0093-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick P. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Post M, Steens A, Renken R, Maurits NM, Zijdewind I. Voluntary activation and cortical activity during a sustained maximal contraction: An fMRI study. Hum Brain Mapp. 2009;30:1014–1027. doi: 10.1002/hbm.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Lassi-Tucci G, Padoa-Schioppa C, Bizzi E. Neuronal activity in the cingulate motor areas during adaptation to a new dynamic environment. J Neurophysiol. 2008;99:1253–1266. doi: 10.1152/jn.01096.2007. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Doyon J, McDonald D, Holmes C, Lavoie K, Hurwitz AS, Kabani N, Toga A, Evans A, Petrides M. Three-dimensional MRI atlas of the human cerebellum in proportional stereotaxic space. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- Sestieri C, Pizzella V, Cianflone F, Luca Romani G, Corbetta M. Sequential activation of human oculomotor centers during planning of visually-guided eye movements: a combined fMRI-MEG study. Front Hum Neurosci. 2007;1:1–8. doi: 10.3389/neuro.09.001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K, Aya K, Mushiake H, Inase M, Aizawa H, Tanji J. Two movement-related foci in the primate cingulate cortex observed in signal-triggered and self-paced forelimb movements. J Neurophysiol. 1991;65:188–202. doi: 10.1152/jn.1991.65.2.188. [DOI] [PubMed] [Google Scholar]
- Shima K, Tanji J. Role for cingulate motor area cells in voluntary movement selection based on reward. Science. 1998;282:1335–1338. doi: 10.1126/science.282.5392.1335. [DOI] [PubMed] [Google Scholar]
- Shinohara M, Keenan KG, Enoka RM. Contralateral activity in a homologous hand muscle during voluntary contractions is greater in old adults. J Appl Physiol. 2003;94:966–974. doi: 10.1152/japplphysiol.00836.2002. [DOI] [PubMed] [Google Scholar]
- Smith AM. The activity of supplementary motor area neurons during a maintained precision grip. Brain Res. 1979;172:315–327. doi: 10.1016/0006-8993(79)90541-9. [DOI] [PubMed] [Google Scholar]
- Smith AM, Bourbonnais D. Neuronal activity in cerebellar cortex related to control of prehensile force. J Neurophysiol. 1981;45:286–303. doi: 10.1152/jn.1981.45.2.286. [DOI] [PubMed] [Google Scholar]
- Tanji J, Shima K. Role for supplementary motor area cells in planning several movements ahead. Nature. 1994;371:413–416. doi: 10.1038/371413a0. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Phillips BA, Morris I, Byrnes ML, Mastaglia FL. Isometric force-related activity in sensorimotor cortex measured with functional MRI. Exp Brain Res. 1998;121:59–64. doi: 10.1007/s002210050437. [DOI] [PubMed] [Google Scholar]
- Townsend BR, Paninski L, Lemon RN. Linear encoding of muscle activity in primary motor cortex and cerebellum. J Neurophysiol. 2006;96:2578–2592. doi: 10.1152/jn.01086.2005. [DOI] [PubMed] [Google Scholar]
- Turner R, Howseman A, Rees GE, Josephs O, Friston K. Functional magnetic resonance imaging of the human brain: data acquisition and analysis. Exp Brain Res. 1998;123:5–12. doi: 10.1007/s002210050538. [DOI] [PubMed] [Google Scholar]
- Uttner I, Kraft E, Nowak DA, Muller F, Philipp J, Zierdt A, Hermsdorfer J. Mirror movements and the role of handedness: isometric grip forces changes. Motor Control. 2007;11:16–28. [PubMed] [Google Scholar]
- van Duinen H, Zijdewind I, Hoogduin H, Maurits N. Surface EMG measurements during fMRI at 3T: accurate EMG recordings after artifact correction. Neuroimage. 2005;27:240–246. doi: 10.1016/j.neuroimage.2005.04.003. [DOI] [PubMed] [Google Scholar]
- van Duinen H, Renken R, Maurits NM, Zijdewind I. Relation between muscle and brain activity during isometric contractions of the first dorsal interosseus muscle. Hum Brain Mapp. 2008;29:281–299. doi: 10.1002/hbm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rootselaar AF, Maurits NM, Renken R, Koelman JH, Hoogduin JM, Leenders KL, Tijssen MA. Simultaneous EMG-functional MRI recordings can directly relate hyperkinetic movements to brain activity. Hum Brain Mapp. 2008;29:1430–1441. doi: 10.1002/hbm.20477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldvogel D, van Gelderen P, Muellbacher W, Ziemann U, Immisch I, Hallett M. The relative metabolic demand of inhibition and excitation. Nature. 2000;406:995–998. doi: 10.1038/35023171. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, Lee L, Thompson AJ, Greenwood RJ, Rothwell JC, Frackowiak RS. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129:809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci. 2005;22:235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006;175:526–535. doi: 10.1007/s00221-006-0570-z. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol. 2001;85:1907–1913. doi: 10.1152/jn.2001.85.5.1907. [DOI] [PubMed] [Google Scholar]