Abstract
There is strong interest to study the involvement of brain cannabinoid subtype-1 (CB1) receptors in neuropsychiatric disorders with single photon emission computed tomography (SPECT) and a suitable radioligand. Here we report the synthesis of a novel high-affinity radioiodinated CB1 receptor ligand ([125I]8, [125I]1-(2-iodophenyl)-4-cyano-5-(4-methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxylate, [125I]SD7015). By autoradiography in vitro, [125I]8 showed selective binding to CB1 receptors on human brain postmortem cryosections and now merits labeling with iodine-123 for further evaluation as a SPECT radioligand in non-human primate.
Keywords: SPECT, PET, CB1 receptors, radioiodination, radioligand
Cannabis sativa (marijuana) is one of the oldest known plant derived-therapeutics, owing mainly to its anti-nociceptive properties.1 Other beneficial effects of cannabis intake may include anti-emesis and appetite stimulation. Conversely, the accompanying psychological “high” and memory impairment have limited its therapeutic value. The most abundant and psychoactive cannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC, 1), binds with high affinity to two known body receptor systems. These receptors have been named cannabinoid subtype-1 (CB1) and cannabinoid subtype-2 (CB2).2–4 CB1 receptors have high-densities in brain and are implicated in a number of neuropsychiatric disorders5 such as, schizophrenia6,7 and depression8,9. CB2 receptors are located mainly in the periphery and are of less interest to neuropsychiatric research.10,11
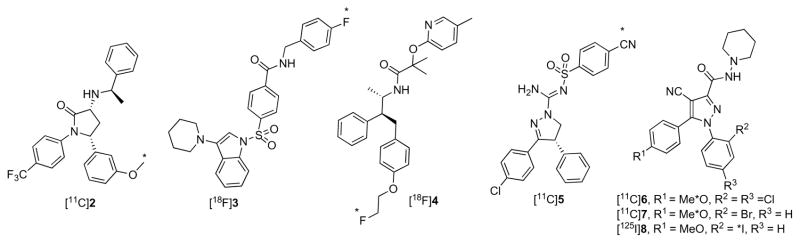
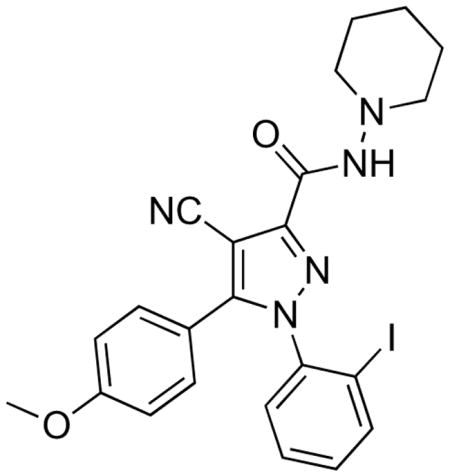
Ability to image and measure brain CB1 receptors non-invasively with radiation computed tomography would assist neuropsychiatric research and drug development. The development of radioligands suitable for imaging brain CB1 receptors is therefore of strong importance. Recently, there has been a great deal of progress in the development of useable radioligands for positron emission tomography (PET). Some of the more notable PET radioligands (Figure 1) are [11C]MePPEP ([11C]2)12,13, [18F]PipISB ([18F]3)14,15, [18F]MK-9470 ([18F]4)16,17, (−)-[11C]SD5014 ((−)-[11C]5)18, [11C]OMAR ([11C]6)19,20, and [11C]JHU75575 ([11C]7)20,21. In comparison, the development of radioligands for more widespread single-photon computed tomography (SPECT) is much less advanced. Due to the longer half-life of radioiodine for SPECT imaging (e.g., 123I, t1/2 = 13.2 h), an on-site cyclotron is not required for radiolabeling, as with PET, which allows for more widespread use in pharmaceutical, academic and hospital settings. Here we report the synthesis, receptor screening, radioiodination and in vitro autoradiographic evaluation of a novel radioiodinated CB1 receptor radioligand ([125I]4-cyano-1-(2-iodophenyl)-5-(4-methoxyphenyl)-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide, [125I]8, [125I]SD7015). Our results strongly suggest that [123I]8 would merit evaluation as a SPECT radioligand in vivo.
Figure 1.
Structures of [11C]2, [18F]3, [18F]4, [11C]5– 7, and [125I]8. Asterisks denote positions of radiolabels.
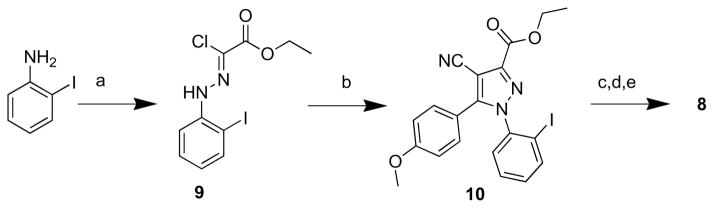
Compound 8 was synthesized according to the procedure depicted in Scheme 1. Conversion of 2-iodoaniline into a diazonium salt, followed by treatment with ethyl 2-chloroacetoacetate in ethanol-water solution under basic conditions gave chloro[(2-iodophenyl)hydrazono]ethyl acetate (9). Heating of a solution of 9, 4-methoxybenzoylacetonitrile and N,N-diisopropylethylamine in tert-butanol to reflux gave 10 in low but adequate yield. Hydrolysis of 10 with LiOH in aq-tetrahydrofuran gave the corresponding carboxylic acid, which was then converted into the acyl chloride with oxalyl chloride and a catalytic amount of N,N-dimethylformamide. The synthesis of 8 was completed by coupling the acyl chloride with 1-aminopiperidine under basic conditions.
Scheme 1.
Synthesis of 8. Reagent, conditions and yields: a) concentrated HCl, NaNO2, ethyl 2-chloroacetoacetate, NaOAc, EtOH– H2O, 16 h, 72%; b) 4-methoxybenzoylacetonitrile, DIPEA, tert-BuOH, reflux, 16 h, 6%; c) aq-LiOH, THF, 65 °C, 4 h; d) DMF(cat), (COCl)2, DCM; e) 1-aminopiperidine, DIPEA, DCM, 2 h, 73%.
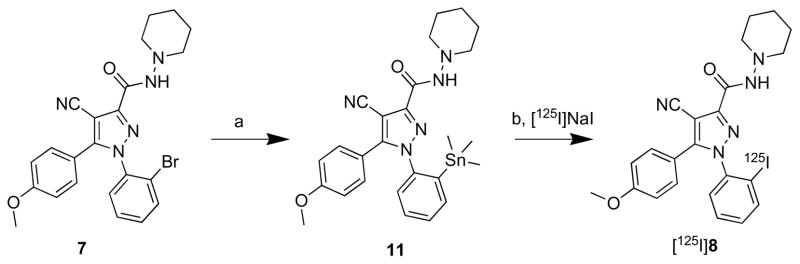
The trimethylstannylated precursor, 11, required for radiolabeling, was synthesized by refluxing a solution of the known bromo compound 720 and hexamethylditin in the presence of palladium catalyst (Scheme 2)
Scheme 2.
Radiosynthesis of [125I]8. Reagents, conditions and yields: a) hexamethylditin, Pd(PPh3)4, toluene, 20 h, 32%; b) [125I]NaI, chloramine- T, aq-HCl, 5 min, 48– 59%.
A radioligand for imaging brain CB1 receptors would ideally possess adequately high affinity and moderate lipophilicity to promote rapid development of a high ratio of receptor-specific binding to non-specific binding in brain in vivo, that might then allow accurate computation of output measures, such as binding potential.22,23 CB1 receptors are one of the most abundant G protein-coupled receptors in brain, reaching a concentration (Bmax) of 1752 fmol/mg protein (175 nM) in rat cerebellum.24 Generally, a SPECT radioligand should show a substantial Bmax/Kd value (> 5) to be successful. Hence, an effective CB1 receptor radioligand might require only a moderately high affinity (Ki or Kd < 35 nM). Ligand 8 was found to have about ten-fold higher affinity (3.4 nM; Table 1) than perhaps needed. Calculated ligand lipophilicity (cLogD7.4) can be an important predictor of blood–brain barrier penetration and brain non-specific binding. Moderate lipophilicity (cLogD7.4 in the range 2.0 – 3.5) is usually preferred for adequate brain entry without incurring excessive non-specific binding in brain.23 However, when target binding sites exist in high concentration, higher lipophilicity may be tolerated. For example, [11C]MePPEP is a successful PET radioligand for brain CB1 receptors despite having a high cLogD7.4 value of 5.42.12,13 The cLogD7.4 value of 8 is 4.14 and appears more favorable for a radioligand than that of [11C]MePPEP. Futhermore, the physiochemical and pharmacological properties of 8 compare well with other successful PET radioligands.20,21 Therefore, ligand 8 presents acceptable CB1 receptor affinity and lipophilicity for development as a SPECT radioligand (Table 1).
Table 1.
Ki values for CB1 and CB2 receptors, selectivities for CB1 versus CB2 receptors and calculated lipophilicities.
 | ||||
|---|---|---|---|---|
| Ligand | CB1Ki (nM)a | CB2Ki (nM)a | CB1 versus CB2 selectivitya | cLogD7.4b |
| rimonabant | 1.38 ± 0.17 | 927 ± 66 | 672 ± 94 | 6.01 |
| 8 | 3.40 ± 0.43 | 548 ± 23 | 161 ± 47 | 4.14 |
Values represent mean ± SD of three determinations.
cLogD7.4 values were calculated using Advanced Chemistry Development (ACD) 9.2.
In addition to acceptable binding affinity and lipophilicity a candidate SPECT radioligand should be selective for binding to the target protein. Ligand 8, at 10 μM concentration, showed < 50% inhibition (n = 4) of radioligand binding to the sites: 5-HT1B –E, 5-HT2A– C, 5-HT3, 5-HT5A, 5-HT6, 5-HT7, α1A,B, α2A,B,β1–3, D1– 4, DOR, H1– 4, M1–5, NET, SERT,σ1,2, V1A,1B,2. Ki values (n = 3) of 93.5 ± 20.4 nM (5-HT1A), > 10,000 nM (α2C), 1,477 ± 148 nM (KOR), 1,496 ± 216 nM (MOR) and 3,166 ± 586 nM (TSPO) were found. Details of the employed binding assays may be found at the NIMH PDSP web site: http://pdsp.med.unc.edu. Hence, 8 was found to have excellent CB1 receptor selectivity for development as a SPECT radioligand.
[125I]8 was prepared by [125I]iodo-destannylation of the corresponding trimethylstannyl precursor (11) with [125I]NaI, aq-HCl and chloramine-T (oxidizer) in methanol (Scheme 2). The crude product was purified with high-performance liquid chromatography (HPLC) as described in Supplementary Information. The decay-corrected radiochemical yield of [125I]8 ranged from 48 to 59%. The specific radioactivity of [125I]8 was 81.4 GBq/μmol and the radiochemical purity > 98%. [125I]8 was thus obtained in adequate yield and purity for further evaluation with sensitive post mortem autoradiography in vitro. Furthermore, these conditions would be applicable to labeling 8 with iodine-123 for evaluation in SPECT imaging.
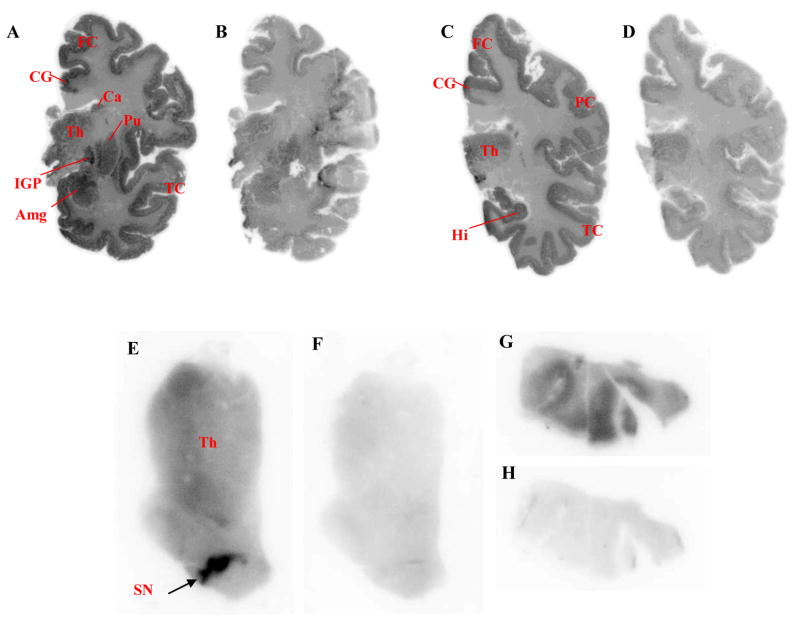
CB1 receptors are spread heterogeneously in brain, with high-densities appearing in substantia nigra, globus pallidus, amygdala, cortical regions and striatum.25,26 Brain regions with low CB1 receptor densities are thalamus, pons and white matter. In post mortem autoradiography in vitro, [125I]8 bound substantially to human brain regions with high CB1 receptor densities (Figure 2; Panels A, C, E & G) with highest binding in globus pallidus and substantia nigra (Figure 2; Panels A & E). Additionally [125I]8 showed much lower binding in brain regions with low CB1 receptor density, including thalamus (Figure 2; Panels C & E). Under conditions in which the CB1 receptors were blocked with the selective CB1 ligand, rimonabant (10 μM), the binding of [125I]8 in CB1 rich regions was greatly reduced and the distribution of radioligand became more homogeneous (Figure 2, Panels B, D, F & H). Therefore, the regional selectivity of [125I]8 indicated that 123I-labeled 8 would be promising for imaging brain CB1 receptors with SPECT.
Figure 2.
Autoradiographs from whole-hemisphere cryosections (Panels A– D; 100 m thickness) and cryosections (20 μm thickness) covering thalamus and brainstem (Panels E & F) and temporal cortex (Panels G & H) incubated with [125I]8 under baseline (Panels A, C, E & G) and blocked (rimonabant, 10 μM; Panels B, D, F & H) conditions. Abbreviations: Amg, amygdala; Ca, caudate nucleus; CG, cingulate gyrus; FC, frontal cortex; Hi, hippocampus; IGP, globus pallidus, internal segment; PC, parietal cortex; Pu, putamen; SN, substantia nigra; TC, temporal cortex; Th, thalamus.
Ligand 8 demonstrated high affinity and good selectivity for CB1 receptors. [125I]8 was obtained in acceptable radiochemical yield, specific radioactivity and purity for evaluation in vitro. Autoradiographs of human brain obtained with [125I]8 showed radioactivity distribution according to known regional CB1 receptor densities. Future research evaluating [123I]8 with SPECT imaging is therefore warranted.
Supplementary Material
Acknowledgments
SRD and VWP were supported by the Intramural Research Program of the National Institutes of Health, specifically the National Institute of Mental Health (NIMH), project number ZO1MH002793. We thank the NIMH Psychoactive Drug Screening Program (PDSP) for performing binding assays and Siv Eriksson for the excellent technical assistance with autoradiographic experiments. The PDSP is directed by Bryan L. Roth, PhD, with project officer Jamie Driscoll (NIMH) at the University of Carolina at Chapel Hill (contract NO1MH32004)
Footnotes
Supplementary Information Available: Experimental details of chemistry, labeling and autoradiography. This material is available free of charge via the Internet at www.sciencedirect.com.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lambert DM. J Pharm Belg. 2001;56:111. [PubMed] [Google Scholar]
- 2.Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Mol Pharmacology. 1988;34:605. [PubMed] [Google Scholar]
- 3.Munro S, Thomas KL, Abushaar M. Nature. 1993;365:61. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 4.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. Pharmacol Rev. 2002;54:161. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 5.Van Laere K. Eur J Nucl Med Mol Imaging. 2007;34:1719. doi: 10.1007/s00259-007-0505-3. [DOI] [PubMed] [Google Scholar]
- 6.Dean B, Sundram S, Bradbury R, Scarr E, Copolov D. Neurosci. 2001;103:9. doi: 10.1016/s0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 7.Eggan SM, Hashimoto T, Lewis DA. Arch Gen Psychiatry. 2008;65:772. doi: 10.1001/archpsyc.65.7.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serra G, Fratta W. Clin Pract Epidemol Ment Health. 2007;3:25. doi: 10.1186/1745-0179-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zavitsanou K, Garrick T, Huang XF. Prog Neuropschyopharmacol Biol Psychiatry. 2004;28:355. doi: 10.1016/j.pnpbp.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Lynn AB, Herkenham M. J Pharmacol Exp Ther. 1994;268:1612. [PubMed] [Google Scholar]
- 11.Gong JP, Onaivi ES, Ishiguro H, Liu QR, Tagliaferro PA, Brusco A, Uhl GR. Brain Res. 2006;1071:10. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 12.Yasuno F, Brown AK, Zoghbi SS, Krushinski JH, Chernet E, Tauscher J, Schaus JM, Phebus LA, Chesterfield AK, Felder CC, Gladding RL, Hong J, Halldin C, Pike VW, Innis RB. Neuropsychopharmacology. 2008;33:259. doi: 10.1038/sj.npp.1301402. [DOI] [PubMed] [Google Scholar]
- 13.Donohue SR, Krushinski JH, Pike VW, Chernet E, Chesterfield AK, Felder CC, Halldin C, Schaus JM. J Med Chem. 2008;51:5833. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donohue SR, Halldin C, Schou M, Hong J, Phebus LA, Chernet E, Hitchcock SA, Gardinier KM, Ruley KM, Krushinski JH, Schaus JM, Pike VW. J Labelled Compd Radiopharm. 2008;51:149. [Google Scholar]
- 15.Finnema SJ, Donohue SR, Zoghbi SS, Brown AK, Gulyás B, Innis RB, Halldin C, Pike VW. Synapse. 2009;63:22. doi: 10.1002/syn.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, Jewell JP, Lin LS, Liu P, Goulet MT, Gottesdiener K, Wagner JA, de Hoon J, Mortelmans L, Fong TM, Hargreaves RJ. Proc Natl Acad Sci USA. 2007;104:9800. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P, Lin LS, Hamill TG, Jewell JP, Lanza TJ, Gibson RE, Krause SM, Ryan C, Eng WS, Sanabria S, Tong XC, Wang JY, Levorse DA, Owens KA, Fong TM, Shen CP, Lao JL, Kumar S, Yin WJ, Payack JF, Springfield SA, Hargreaves R, Burns HD, Goulet MT, Hagmann WK. J Med Chem. 2007;50:3427. doi: 10.1021/jm070131b. [DOI] [PubMed] [Google Scholar]
- 18.Donohue SR, Pike VW, Finnema SJ, Truong P, Andersson J, Gulyás B, Halldin C. J Med Chem. 2008;51:5608. doi: 10.1021/jm800329z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP, Alexander M, Kumar A, Rahmim A, Scheffel U, Wong DF, Dannals RF. J Nucl Med. 2006;47:1689. [PubMed] [Google Scholar]
- 20.Fan H, Ravert HT, Holt DP, Dannals RF, Horti AG. J Labelled Compd Radiopharm. 2006;49:1021. [Google Scholar]
- 21.Donohue SR, Zoghbi S, Yasuno F, Terry G, Gourley J, Innis RB, Halldin C, Pike VW. Curr Radiopharmaceuticals. 2008;1:93. [Google Scholar]
- 22.Laruelle M, Slifstein M, Huang Y. Mol Imaging Biol. 2003;5:363. doi: 10.1016/j.mibio.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Waterhouse RN. Mol Imaging Biol. 2003;5:376. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Hirst RA, Almond SL, Lambert DG. Neurosci Lett. 1996;220:101. doi: 10.1016/s0304-3940(96)13233-x. [DOI] [PubMed] [Google Scholar]
- 25.Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. J Neurosci. 1991;11:563. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glass M, Dragunow M, Faull RL. Neuroscience. 1997;77:299. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.