Abstract
Normal adult uninjured nerve is unable to support axonal regeneration. We have studied the mechanisms underlying the regeneration of peripheral nerve by culturing adult mouse dorsal root ganglia (DRG) explants on unfixed, longitudinal cryosections of either the uninjured sciatic nerve or the distal segment of the transected sciatic nerve. We found that, initially, DRG grew vigorously on cryosections of both uninjured and postinjury sciatic nerves. However, the neurites began to degenerate shortly after contact with the uninjured nerve, whereas those growing on postinjury nerve substrate remained healthy for up to 9 days in culture. This ability to support stable outgrowth peaked at 8 days, gradually decreased by 10 days, and disappeared by 20 days after injury. Macrophages appeared in the distal segment by 4 days postinjury and had infiltrated its entire length by 8 days. Uninjured nerve cryosections could be rendered supportive of stable outgrowth by preincubation with macrophage-conditioned medium or by brief trypsinization. The activity of the macrophage-conditioned medium was augmented upon activation of macrophages. Together these findings suggest that the environment of the sciatic nerve undergoes a transformation during Wallerian degeneration such that it becomes transiently supportive of the stable outgrowth of neurites. This transformation may be mediated by a proteolytic activity, generated by activated macrophages, that removes a putative “degeneration signal” protein normally present in the adult nerve and thus contributes to the maintenance of stable regenerating neurites.
Keywords: peripheral nerve regeneration, peripheral nerve injury, Wallerian degeneration, macrophage, neurite outgrowth
The mammalian peripheral nervous system (PNS) is remarkable in its ability to repair itself after injury. The key to this success is related to a series of cellular and molecular events associated with Wallerian degeneration occurring in the distal nerve segment shortly after peripheral nerve injury (Fawcett and Keynes, 1990). The onset of Wallerian degeneration is marked by the invasion of a large number of macrophages (Perry and Brown, 1992; LaFleur et al., 1996; Fu and Gordon, 1997), probably recruited by tumor necrosis factor-α (TNF-α) derived from denervated Schwann cells (Liefner et al., 2000). These macrophages induce degeneration and fragmentation of the axons. In addition, they also secrete interleukin-1 (LaFleur et al., 1996; Gulati, 1998), which induces Schwann cells to dedifferentiate and shed their myelin sheaths (Beuche and Friede, 1984). The dedifferentiated Schwann cells then proliferate (Salzer and Bunge, 1980), highly express several growth factors (Tofaris et al., 2002) and extracellular matrix (ECM) components (Agius and Cochard, 1998), and act as a scaffold for the incoming, regenerating axons (Torigoe et al., 1996). The bulk of the axonal and myelin debris is subsequently phagocytized by macrophages.
Macrophages are essential for successful regeneration in the PNS. In addition to phagocytosis of myelin debris, they clear the damaged ECM, indirectly support the growth of neurites by inducing Schwann cells to proliferate and to secrete neurotrophic factors such as nerve growth factor (NGF; Heuman et al., 1987), and physically recraft the undamaged ECM of the distal segment (LaFleur et al., 1996).
The importance of macrophages has also been established in the injured central nervous system (CNS), where nerve regeneration is quite limited (Avellino et al., 1995; Hirschberg and Scwartz, 1995; Perry et al., 1993). Implantation of macrophages, cocultured with PNS tissue, into the lesion site of the spinal cord promotes CNS regeneration and partial functional recovery in rats (Schwartz et al., 1999). Similarly, optic nerve cryosections, which are nonpermissive for embryonic dorsal root ganglia (DRG) neurite outgrowth, can become permissive when preincubated with macrophages derived from injured brain (David et al., 1990).
Although Wallerian degeneration is a prerequisite for the successful regeneration of the injured peripheral nerve, the molecular signals that favor regrowth of axons are not well understood. To understand better the changes in the peripheral nerve environment during and after Wallerian degeneration, an in vitro system has been developed in which dissociated DRG neurons are cultured on unfixed, longitudinal cryosections of sciatic nerve. Comparisons of neurites growing on nerve substrates derived from different sources or biochemically modified have yielded significant insights into the molecular events that influence neurite plasticity (summarized in Bedi et al., 1992). It has been shown that the sciatic nerve after injury becomes increasingly more permissive for neurite growth (Bedi et al., 1992) and that the action of matrix metalloproteinase-2 (MMP-2) at the level of the growth cones plays a role in overcoming the neurite-inhibiting effect of chondroitin sulfate proteoglycan in the postinjury sciatic nerve (Zuo et al., 1998).
This in vitro PNS model has traditionally consisted of dissociated DRG neurons derived from embryonic animals and/or an animal species different from that used for the sciatic nerve preparation. We have therefore modified this model to reflect more accurately the plasticity in the adult PNS by using adult, nondissociated DRG explants that are derived from the same species as the sciatic nerve preparation. In the present study, we have used this modified model to compare the extent to which the uninjured and injured sciatic nerve supports neurite growth and maintenance as well as the molecular events whereby macrophages play a role in the stabilization of neurite outgrowth.
MATERIALS AND METHODS
Preparation of Sciatic Nerve Cryosections
CF1 adult female mice between 2 and 4 months of age were anesthetized by intraperitoneal injection of Avertin (2% tribromoethanol and 2.5% tertiary amyl alcohol in water; 0.13 ml/10 g body weight). Both sciatic nerves were transected with a pair of iris scissors through a small incision at the upper to midthigh level. The two stumps were gently positioned such that the ends closely apposed each other. The skin was closed with a single wound clip, and the animal was allowed to recover under monitoring for the next 2 hr. After a specific period postinjury, the sciatic nerves were removed from the animals after cervical dislocation. The tissue was placed in a plastic mold, snap frozen in liquid nitrogen for 2 min, and then stored at − 80°C. The frozen sciatic nerve was embedded in frozen section embedding medium (Histoprep from Fisher Scientific, Fair Lawn, NJ). Ten-micrometer-thick cryosections were cut with a cryostat and mounted on 12-mm round glass coverslips (Fisher Scientific) that had been acid washed, autoclaved, and precoated with 0.1 mg/ml poly-D-lysine hydrobromide (43–100 kd; Sigma, St. Louis, MO) in sodium borate solution. The cryosections were air dried at room temperature for 5 min and stored for up to 2 days at −20°C until plating of DRG explants.
Preparation of Adult Mouse DRG Explants
CF1 adult female mice between 2 and 4 months old were euthanized by cervical dislocation. The vertebral column was completely removed, and the dorsal spine and lamina were excised. After the spinal cord was gently lifted, approximately 25–30 DRG were removed by cutting their roots and placed in 2 ml Leibovitz-15 (L-15) culture medium at room temperature. The DRG explants were treated with 0.125% collagenase D (with specific activity of 0.5 U/mg; Boehringer Mannheim, Indianapolis, IN) in L-15 medium at 37°C for 1.0 hr, with one change of collagenase L-15 medium at the midpoint of the incubation, and then washed three times with 5 ml calcium- and magnesium-free phosphate-buffered saline (PBS) with 0.4% EDTA at room temperature. For batches of collagenase D with weaker or stronger activity, the time of incubation was proportionally prolonged or shortened. The DRG were resuspended in “DRG culture medium” [DME-H21, 10% fetal bovine serum (FBS), 50 ng/ml NGF (7s), 20 μM cytosine arabinoside, penicillin, streptomycin, and gentamycin], transferred to a petri dish, and stored in a 37°C incubator for up to 3 hr until plating.
Culturing of DRG Explants on Sciatic Nerve Cryosections
Sciatic nerve cryosections on glass coverslips were placed in Nunc four-well plates (one coverslip per well) and washed three times with PBS. Then, 165 μl of “DRG culture medium” were added to each well. Connective tissue was removed from the DRG explant with the aid of a dissecting microscope. The DRG was then placed in the middle and on top of the sciatic nerve cryosection on the coverslip. The plates were incubated in a moisture-saturated, 37°C incubator with 5% carbon dioxide for the standard duration of 6 days, unless otherwise stated. One hundred microliters of the 165 μl culture medium in each well were replaced with fresh medium once every 3 days. All experiments were repeated at least three times.
Immunostaining of DRG Neurites
Neurite outgrowths on the cryosection substrate were visualized by immunostaining against GAP-43, a marker for rapidly growing neurites. The DRG cultures were first fixed with 250 μl of a mixture of 4% paraformaldehyde, 0.075% saponin, and 5% sucrose in PBS for 20 –30 min at room temperature. The DRG were rinsed with PBS, treated with 0.1 M glycine in PBS for 30 min, permeabilized with 0.1% saponin in PBS for 10 min, and preblocked with DME with 10% FBS for 15 min. Primary staining was performed with mouse IgG against rat GAP-43 (1:500 dilution in DME with 10% FBS; mouse monoclonal, clone 91E12; Boehringer Mannheim) inside a moistened chamber overnight at 4°C. The samples were rinsed with PBS, and secondary staining was initiated with lissamine rhodamine (LRSC)-conjugated donkey anti-mouse IgG antibodies (1:100 dilution in DME with 10% FBS; Jackson Immunoresearch Laboratories, West Grove, PA) inside a moistened chamber for 2 hr at room temperature. Finally, the samples were rinsed with PBS, and the coverslips were mounted on a glass microscope slide with Gelvatol [8 g polyvinyl alcohol (Monsanto) dissolved in 40 ml of 0.2 M Tris, pH 8.5, by warming to 50 – 60°C, then mixing with 20 ml glycerol after cooling]. A Zeiss Axiophot compound microscope equipped with epifluorescence was used to view the samples.
Antimicrotubule-associated proteins (MAPs; rabbit polyclonal; Sigma), anti-NCAM (rabbit polyclonal; Chemicon, Temecula, CA), antisynaptophysin (mouse monoclonal, clone SY 38; Boehringer Mannheim), and antineurofilament-200 (rabbit polyclonal IgG; Sigma) were visualized as described in the preceding paragraph at a 1:200 dilution. Macrophages were identified using an F4/80 monoclonal antibody (a mouse macrophage marker; prepared from undiluted hybridoma supernatant). Fixed sections were incubated in primary antibody overnight at 4°C, rinsed in DME with 10% FBS, and incubated in alkaline phosphatase-conjugated rabbit anti-rat IgG antibodies (at 1:100 dilution in DME with 10% FBS) for 2 hr at room temperature. Nonimmune sera or IgGs were used as controls. Color reaction was performed with 4-nitroblue tetrazolium chloride (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP).
Semiquantitative Neurite Scoring System
Immunostained neurite outgrowths were graded in a blinded fashion using a scoring system of 1 (highly degenerative outgrowth) to 6 (highly stable outgrowth). The scoring criteria and examples are detailed in Figure 1. In general, one DRG explant, plated on the middle of an uninjured nerve cryosection, yielded two separate neurite scores, each representing the outgrowth in either direction of the cryosection. However, because the full length of the postinjury distal segment was needed to provide enough space for neurite outgrowth, one DRG explant plated on a postinjury nerve cryosection yielded only one neurite score.
Fig. 1.
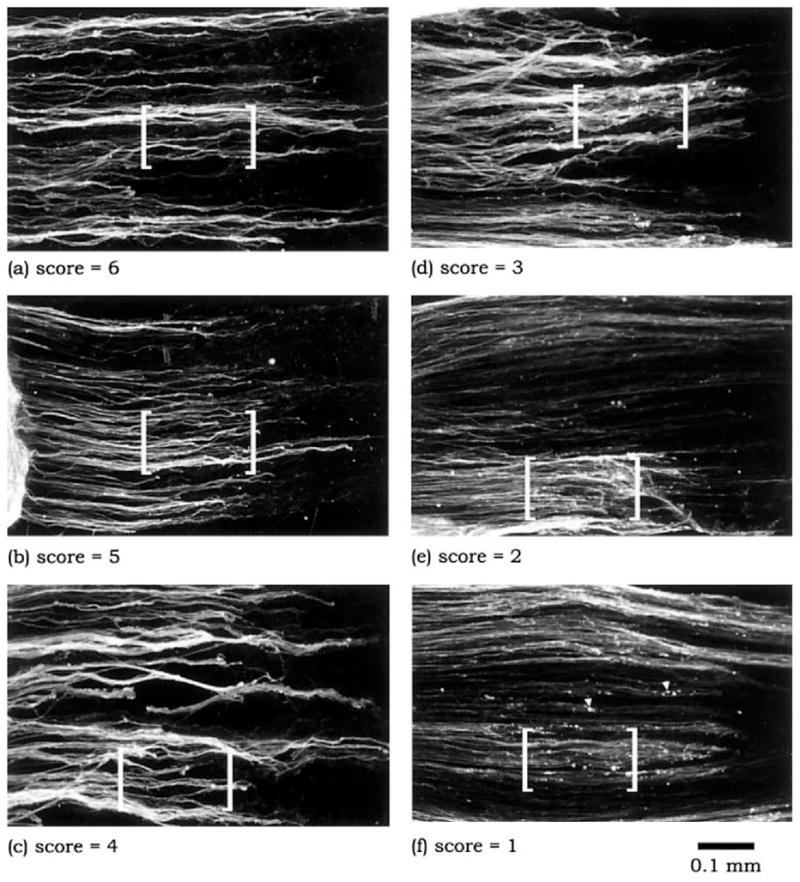
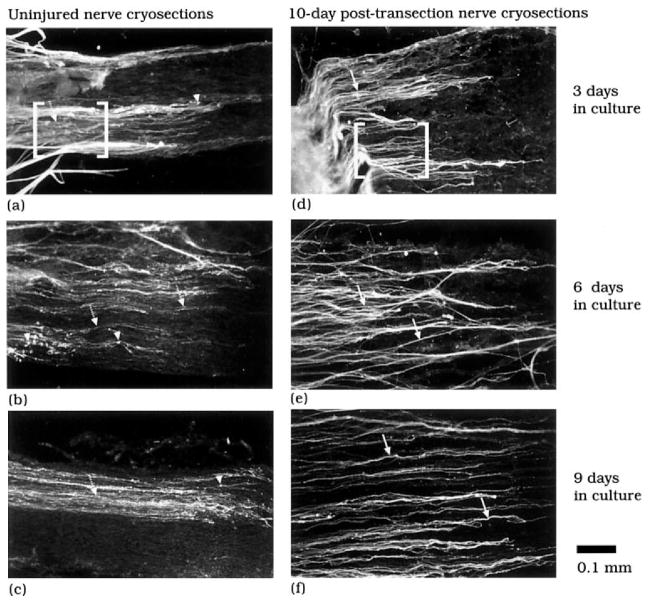
Neurites, derived from adult DRG explants that had been plated on cryosections of adult sciatic nerve, were immunostained for GAP-43 and then categorized using a semiquantitative scoring method, with neurite scores ranging from 1 (highly degenerative outgrowth) to 6 (highly stable outgrowth). The scoring was performed in a blinded fashion. It should be emphasized that the neurite score represents the overall impression of the condition of all the neurites present in an outgrowth. For purposes of illustration, characteristic distinctions between each of the scores are demonstrated within the bracketed regions of a–f. A score of “6” is assigned to neurites that exhibit uniform and robust immunostaining, whereas a score of “1” is characterized by uneven, lightly immunostained processes and an abundance of discrete focal areas of intense staining, referred to as neuritic elements (arrowheads). Intermediate scores are defined on the basis of consistency of immunostaining for neurites and the presence of neuritic elements. Thus, the progression from a score of “5” to a score of “2” reflects increasing heterogeneity in GAP-43 immunostaining of neuritic processes as well as an increasing presence of neuritic elements.
Neurite scores, obtained from two different conditions, were compared using Wilcoxon’s two-sample test (two-tailed) for nonparametric data. Statistical significance was set at P ≤ .05.
Preparation of Conditioned Media
Longitudinal nerve cryosections, mounted on glass coverslips, were incubated overnight at 37°C in conditioned media prepared as described below.
Macrophage-conditioned media
Two-month-old CF1 mice were injected intraperitoneally with 1 ml of 3% Brewer’s thioglycollate broth. Three days later, the mice were euthanized. After injection of 5 ml calcium- and magnesium-free PBS into the peritoneum, the peritoneum was gently massaged and the calcium- and magnesium-free PBS was withdrawn with a sterile Pasteur pipet. The cells in the collected PBS were then spun down at 700 rpm for 5 min and resuspended in DME with 10% FBS to a concentration of 1 million cells/ml. Two milliliters of cells were plated per T75 cell culture flask. Two to three hours after plating, the medium was replaced with DME containing 0.2% lactalbumin hydrolysate (LH) with or without 10 ng/ml lipopolysaccharide (LPS; Werb and Chin, 1983). The conditioned media were collected after 4 days of incubation, except in one experiment (depicted in Fig. 8e,f) in which they were collected after only 1 day of incubation. The conditioned medium was stored frozen in 1-ml aliquots until use.
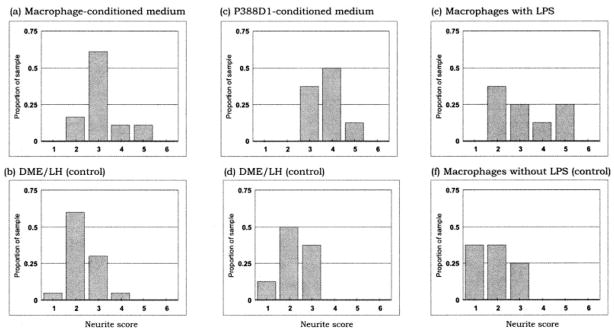
Fig. 8.
Analysis of DRG neuritic stability on uninjured sciatic nerve cryosections pretreated with macrophage-conditioned medium (Mac CM), P388D1 (a mouse monocyte/macrophage cell line)-conditioned medium (P388D1 CM), or conditioned media from LPS-activated macrophages. Neurites are significantly more stable when the substrate has been pretreated with Mac CM (a) compared with control medium (b; P < .002). A similar stabilizing effect is observed with pretreatment with P388D1 CM (c) compared with control medium (d; P < .003), and with pretreatment with conditioned medium from LPS-activated macrophages (e) compared with conditioned medium from nonactivated macrophages (P < .02). It should be noted that, to overcome the effect of LPS activation in e and f, the Mac CMs were produced by incubating macrophages for 1 day only, by which time the CM from nonactivated macrophages had little or no stabilizing effect (e), whereas CM from activated macrophages had a significantly greater stabilizing effect (f). Had we prolonged the incubation period to 4 days, the standard duration of incubation for all other instances, we would have generated moderate to high levels of activity with and without LPS, making it difficult to discern the effect of LPS activation.
Schwann cell-conditioned medium
Schwann cells were collected from 1-day-old neonatal mouse sciatic nerves, placed in 2 ml of DME, and incubated with 0.1% bacterial collagenase for 30 min at 37°C, then in 2 ml of DME with 0.1% collagenase and 0.25% trypsin for 30 min at 37°C. After the medium was replaced with 5 ml of DME with 10% FBS, the tissue was centrifuged at 1,500 rpm for 5 min. The pellet was resuspended in 2 ml of DME with 10% FBS and triturated. The cells were then plated overnight in a T75 flask. The medium was replaced with DME with Bottenstein’s N2 supplement (Gibco BRL, Grand Island, NY) and 10 μM cytosine arabinoside. The conditioned medium was collected 4 days later.
Fibroblast-conditioned medium
Fibroblasts were collected from the synovium of a rabbit and cultured in DME with tetradecanoylphorbol acetate (TPA) at a density of 1 million cells/ml. The conditioned medium was collected 4 days after culture.
P388D1-conditioned medium
P388D1 cells were incubated for 4 days at near-confluence in T-75 flasks with 10 ml of DME with 0.2% LH, 100 ng/ml LPS, and antibiotics.
Pretreatment of Nerve Cryosections With Trypsin
Longitudinal nerve cryosections mounted on glass cover-slips were incubated at 37°C with trypsin for 3 min (from 0.002% to 0.05% in calcium- and magnesium-free PBS; purified crystalline), followed by four washes of calcium- and magnesium-free PBS with 0.04% EDTA.
RESULTS
Comparison of Adult DRG Neurite Outgrowths on Uninjured and Postinjury Nerve Substrates
We first investigated the ability of adult DRG explants to extend neurites on uninjured vs. postinjury sciatic nerve substrates by placing DRG explants on 10-μm-thick cryosections of these two types of tissues and incubating for various durations (3, 6, or 9 days) in medium containing serum and NGF. The neurites of the DRG were then visualized by GAP-43 immunostaining, followed by epifluorescence. DRG grew extensive neurites both on uninjured sciatic nerve cryosections (Fig. 2a– c) and on the distal segments of the 10-day-postinjury sciatic nerve cryosections (Fig. 2d–f), indicating that both tissues were permissive for neurite regeneration. There was no significant difference between the average lengths of the two groups of neurites (16.5 ± 2.3 mm vs. 14.8 ± 1.4 mm, mean ± SE, respectively; Student’s t-test, P < .05) after 6 days in culture. There were, however, distinctive qualitative differences between the neurite outgrowths on each of these substrates. Neurites of DRG plated on uninjured nerve exhibited a characteristic uneven pattern of GAP-43 immunostaining. Whereas some neurites were intensely stained, others exhibited very faint immunostaining. In addition to a lack of uniformity of staining along the length of neurites, there were distinctive GAP-43-positive, bead-like “neuritic elements.” These neuritic elements, which were littered along and near the neurites, were immunoreactive for a number of neural markers besides GAP-43, including NCAM, MAPs, and synaptophysin (Fig. 3). These findings suggest that neuritic elements are the result of beading and fragmentation of degenerating neurites. In contrast, neurites of DRG plated on 10-day-posttransection nerve cryosections were characterized by a uniform pattern of immunostaining for GAP-43, and each neurite appeared individually distinct and relatively free of neuritic elements throughout the first 9 days in culture.
Fig. 2.
Typical appearance of GAP-43-labeled neurites derived from adult DRG explants (positioned to the left of each panel and out of view) that had been plated on cryosections of either uninjured (a– c) or 10-day-postinjury sciatic nerve (d–f). Neurite outgrowths on the uninjured nerve exhibit inconsistent immunostaining. Lightly stained neurites (dotted arrows, a– c) are interspersed with more intensely stained processes, and there are varying numbers of neuritic elements (arrowheads, a– c). These observations are in contrast to the more robust staining of neurites (solid arrows, d–f) from DRG plated on injured nerve cryosections (compare bracketed areas in a and d) and the relative absence of neuritic elements.
Fig. 3.
The neural markers GAP-43 (a), NCAM (b), MAPs (c), and synaptophysin (d) are consistently immunolocalized in both neuritic processes and neuritic elements (arrowheads) of DRG explants that have been plated on the uninjured sciatic nerve and maintained for 6 days in culture.
Neurites typically emerged from the DRG explant by 2–3 days after plating on the sciatic nerve. Thus, neurites of a 3-day culture assumed contact with the cryosections at about day 3. Upon this initial contact, the neurites of DRG plated on uninjured nerve substrate lacked crisp, clearly defined margins and were associated with globular neuritic elements (Fig. 2a). These morphologic findings suggest that the degeneration of neurites and the formation of neuritic elements had taken place soon after the neurites made contact with the cryosections. Between 3 and 6 days in culture, the abundance of neuritic elements increased proportionately with the appearance of lightly stained neurites (Fig. 2b). By 9 days after plating (Fig. 2c), the vast majority of the neurites appeared highly degenerated, as evidenced by their beaded and fragmented appearance and the abundance of neuritic elements distributed parallel to their axes. Together these observations demonstrate that the ability to maintain neuritic extensions on the injured nerve substrate is related to specific properties unique to that substrate.
Support of Stable Neurite Outgrowth on Injured Nerve Substrate Is Optimal at 8 Days Postinjury
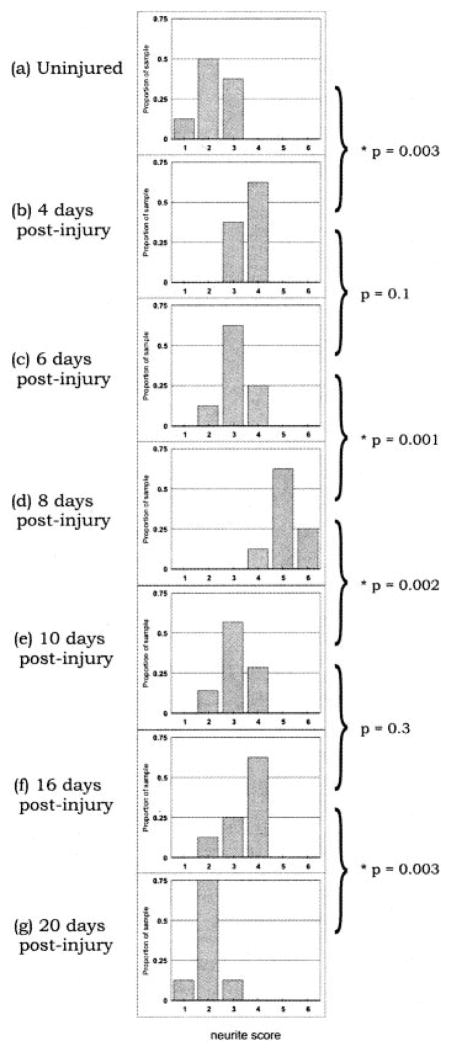
We next determined the temporal changes in the ability of the nerve to support stable neurite outgrowth after injury. We first addressed the length of time after injury that is needed for the nerve environment to become supportive. Adult mouse DRG were cultured on cryosections from mouse sciatic nerves that were harvested 0 (uninjured), 4, 6, 8, 10, 16, and 20 days after transection. After 6 days of culturing, the condition of the neuritic extensions was determined by using a semiquantitative neurite scoring system (Fig. 4). The uninjured sciatic nerves (t = 0 day) yielded a median neurite score of 2, indicative of unstable neuritic extension. In contrast, the median neurite score at 4 and 6 days posttransection was between 3 and 4, implying that the nerve environment had become substantially more supportive of stable outgrowth (Fig. 4b,c). By 8 days posttransection, the median neurite score was 5 (Fig. 4d), an indication that the distal segment was significantly more supportive than at 6 days posttransection.
Fig. 4.
Adult DRG explants were cultured on cryosections of sciatic nerves that were prepared at 4 –20 days postinjury. The stability of the neurite outgrowths was analyzed by using the semiquantitative graded scale. Note the rightward shifting of histogram bars from a to d, indicative of an increase in the stability of the neurites on the 0- to 8-day-postinjury substrates. Conversely, the leftward shifting of the histogram bars from d to g is indicative of a decrease in the stability of the neurites on the 8- to 20-day-postinjury substrates. Eight samples were scored for each time point. The P values of Wilcoxon’s test for each of the bracketed pairs of graphs are indicated (★P < .05). In addition, P was <.04 for each of the groups from 4 to 16 days postinjury (b–f) compared with the uninjured group (a). Scores from the 20-day-postinjury group (g), however, were not significantly different from those of the uninjured group (a; P = .4). Finally, P was <.0001 for overall differences in scores across the seven groups (Kruskall-Wallis test).
We then addressed how long the nerve environment remains supportive of stable neurite outgrowth. It is notable that the ability to support stable neurite outgrowth was transient. At 10 days after injury, the median neurite score was 3 (Fig. 4e), a value significantly lower than that at 8 days after injury. The supportive environment of the sciatic nerve was maintained at this same intermediate level until about 16 days posttransection (Fig. 4f). Thereafter, by 20 days after transection, the distribution of neurite scores decreased to control (uninjured) values (Fig. 4g). These results demonstrate that the distal nerve segment transiently supports stable neurite outgrowth, with optimal support at 8 days after injury.
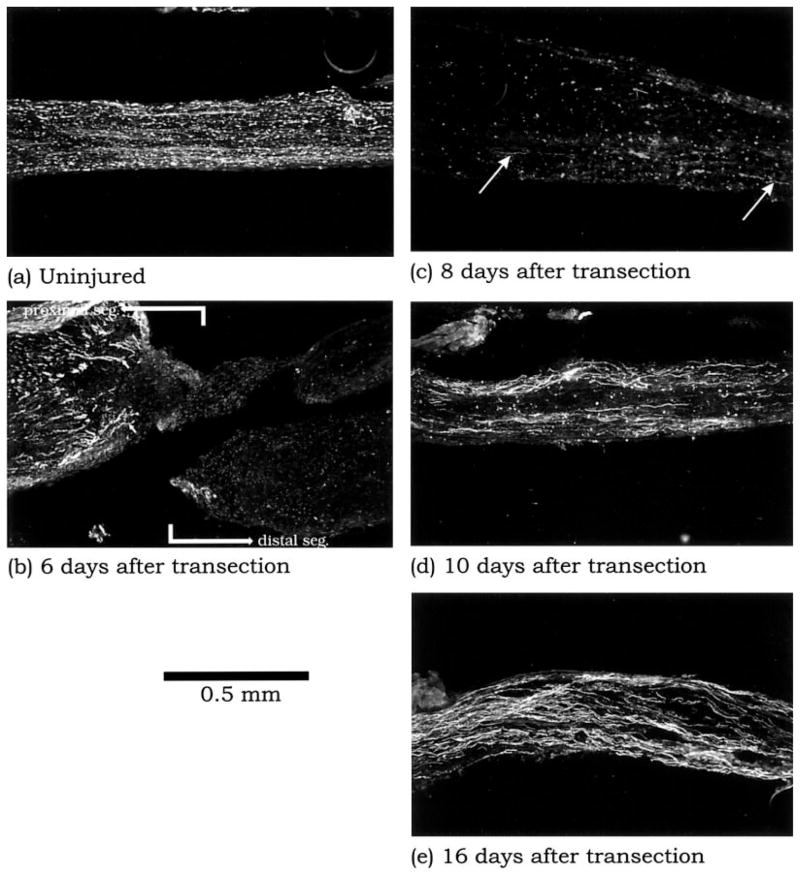
We next examined the biological basis that defines this receptivity to neurite outgrowth. Because neurofilament staining was not evident in the distal segment by 1–2 days after transection (data not shown), we visualized the progress of the natural nerve regeneration at different time points by immunolocalization of neurofilaments (NF-200) on adjacent longitudinal sections of the same sciatic nerves used in the previous time-course experiment. By 4 and 6 days after transection, abundant regenerating neurites had amassed amidst thick, mature neurite bundles at the proximal segment of the injured nerve (Fig. 5b). However, there was still no evidence of regenerating neurites in the distal segment. Regenerating neurites in the distal segment were apparent at 8 days after transection (Fig. 5c). Thus, this time point corresponds to the early successful penetration of neurites into the distal segment. As the regeneration process continued to 10 and 16 days after transection, neurofilament-positive neurites became more abundant in the distal segment (Fig. 5d,e).
Fig. 5.
The uninjured sciatic nerve (a) and the sciatic nerve at 6 –16 days postinjury (b– e) immunostained for neurofilaments (NF-200). The uninjured nerve exhibits abundant NF-200 along its length (a). In contrast, NF-200 immunostaining has been transiently lost in the distal segment by 6 days postinjury (b). Many regenerating neurites, which appear as slender processes (small arrows) amass at the proximal stump, but none appears to have accessed the distal segment. There is a gradual restoration of neurofilament-stained processes from 8 (large arrows, c) to 16 days (e) postinjury in the distal segment.
Macrophage Infiltration Coincides With Stable Neurite Outgrowth
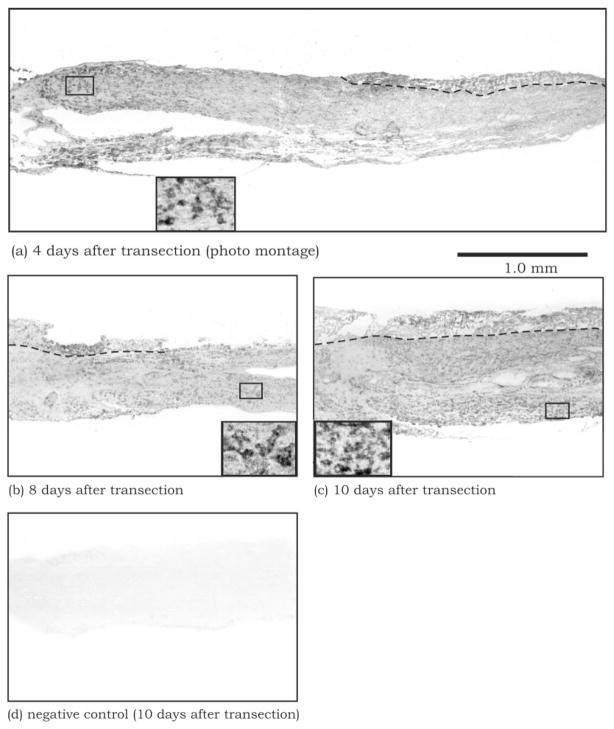
Wallerian degeneration is a postinjury process characterized by massive infiltration of macrophages, which may establish a favorable environment for neurite regeneration (Fawcett and Keynes, 1990). We therefore examined the progress of macrophage infiltration during Wallerian degeneration. Macrophages first began to infiltrate the distal segment of the transected nerve by 4 days after injury, but only at the region immediately distal to the injury site (Fig. 6a). They appeared more distally by 6 days after injury (not shown) and were present throughout the entire length of the distal segment by 8 days after injury (Fig. 6b). At 10 days and beyond (Fig. 6c), the macrophages persisted, and their density appeared to increase steadily throughout the distal segment. These findings demonstrate that, although inflammatory macrophages begin infiltration of the distal segment by 4 days after injury, this process of invading the entire segment is not completed until 8 days after injury, coinciding with the time point of optimal support of stable neurites by the distal nerve environment.
Fig. 6.
Macrophages, defined by F4/80 immunostaining, gradually infiltrate into the entire distal segment of the sciatic nerve over the period from 4 (a) to 8 (b) days posttransection. By 10 days post-transection (c), the distal segment appears to have amassed considerable numbers of macrophages compared with the earlier time points. Dashed lines denote the boundary between the endoneurium (connective tissue) and the core of the sciatic nerve (axon bundles). The cellular localization of F4/80 staining is shown at a higher magnification in the insets. No staining is observed in the control, in which the F4/80 antibody was replaced by fetal calf serum (d).
Macrophages Secrete a Diffusible Factor That Enhances the Ability of Uninjured Nerve Substrate To Support Stable Neurite Outgrowth
We next investigated whether any of the major cell types present during Wallerian degeneration—fibroblasts, Schwann cells, and macrophages—were involved in modifying the responsiveness of the postinjury distal nerve environment to neurite extension. Uninjured sciatic nerve cryosections were incubated overnight in conditioned media prepared from each of these cell types. DRG explants were then plated on these cryosections. We found that neurites growing on cryosections pretreated with Schwann cell-conditioned medium (Fig. 7b) or fibroblast-conditioned medium (Fig. 7c) degenerated after 6 days of culture, as did neurites growing on cryosections pretreated with unconditioned medium (negative controls, Fig. 7d). However, neurites growing on cryosections pretreated with macrophage-conditioned medium did not degenerate and remained stable after 6 days of culture (Figs. 7a, 8a,b). The appearance of such stable outgrowth was similar to that of neurites growing on cryosections of 8-day-posttransection sciatic nerve. The stable neurite growth on uninjured nerve cryosections preincubated with macrophage-conditioned medium was likewise replicated using conditioned medium of P388D1, a mouse monocyte-macrophage cell line (Fig. 8c,d). These findings suggest that cells of the macrophage lineage may be responsible for secreting a diffusible factor that alters the properties of the postinjury nerve environment.
Fig. 7.
DRG neurite outgrowths, defined by GAP-43 immunostaining, on uninjured sciatic nerve cryosections that were pretreated with various conditioned media. The neurites appear healthy when grown on cryosections pretreated with macrophage-conditioned medium (a). However, the neurites exhibit features of degeneration, as evidenced by uneven immunostaining and presence of neuritic elements, when grown on cryosections pretreated with Schwann cell-conditioned medium (b), fibroblast-conditioned medium (c), or nonconditioned medium (control; d).
Macrophages enter an activated stage following infiltration of the injured sciatic nerve, as exemplified by their multivacuolated phenotype and increased ability to phagocytize myelin (Dailey et al., 1998). We therefore determined whether the enhancement effect of the conditioned medium could be altered by the activation of macrophages. Peritoneal macrophages were incubated for 1 day in culture media with or without LPS, a broad-spectrum, bacteria-derived activating agent (Werb and Chin, 1983). The resulting conditioned media were then used to pretreat uninjured sciatic nerve cryosections before plating DRG explants on the cryosections. The conditioned medium of LPS-activated macrophages exhibited a significantly greater enhancement of neurite stabilization than that of unactivated macrophages (Fig. 8e,f). These observations suggest that the active component produced by macrophages is dependent on the activation state of macrophages.
The Active Molecule in Macrophage-Conditioned Medium Is Likely a Protein and Can Be Mimicked by a Protease
We next treated LPS-activated macrophage conditioned-medium in various ways and assessed its activity posttreatment (using the semiquantitative scoring assay as before) to define the chemical properties of the active component. The activity of the conditioned medium was destroyed by heating for 15 min in a boiling water bath (P < .01, heat-treated vs. nontreated). Second, the activity of the conditioned medium was greatly reduced by treatment with purified crystalline trypsin (at 0.05% in calcium- and magnesium-free PBS for 30 min at 37°C and then stopped by 1% soybean trypsin inhibitor and 10% serum; P < .01 compared with control without trypsin). Third, the activity was retained after the macrophage-conditioned medium was dialyzed (14-kd cutoff) against a large volume of PBS overnight at 4°C. The activity was not significantly different from that of nondialyzed conditioned medium but significantly higher than that of the DME control (P < .01). Thus the active molecule of the macrophage-conditioned medium has a size above that of typical soluble carbohydrates and lipids, is heat labile, and is degradable by proteolytic digestion. Together these findings suggest that this molecule very likely is, or at least is associated with, a protein.
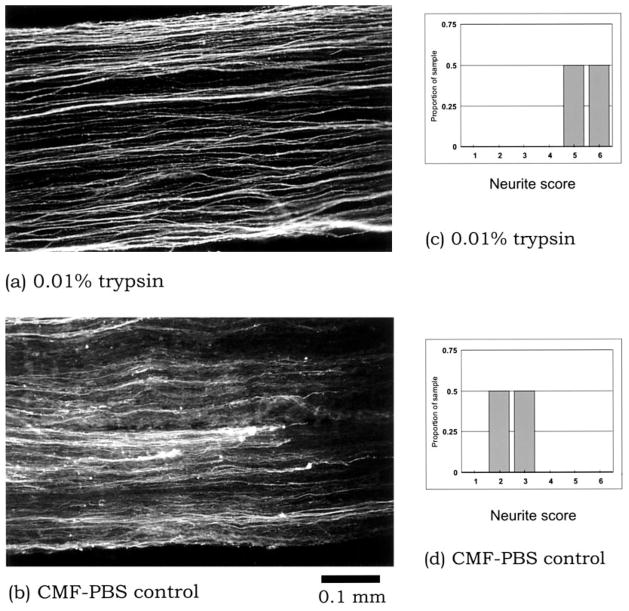
We hypothesized that the macrophage-secreted molecule acts by removing or inactivating a “degeneration signal” molecule putatively present in the uninjured sciatic nerve, thus rendering the nerve environment supportive of stable neurite outgrowth. A precedent for a similar “degeneration signal” molecule was reported by Ichijo and Bonhoeffer (1998), who characterized an as yet unidentified protein “withdrawal factor” produced by the chick posterior tectum. To test this hypothesis, uninjured sciatic nerve cryosections were briefly treated with trypsin, and the ability of the cryosections to support stable neurite outgrowth was then assayed. DRG neurites growing on the trypsin-treated cryosections appeared healthy, exhibited robust immunostaining for GAP-43, and were free of debris (Fig. 9a). These features were reminiscent of neurites of DRG plated on 8-day-postinjury nerve cryosections. This enhancement effect could be seen with trypsin concentrations as low as 0.002% (P < .05 compared with calcium- and magnesium-free PBS control) and was highly replicable among all the samples in multiple experiments (Fig. 9c,d). These findings suggest that macrophage-conditioned medium acts by removing an “inhibitory protein” that is native to the uninjured nerve.
Fig. 9.
Uninjured sciatic nerve cryosections, pretreated with 0.01% trypsin, are more supportive of stable outgrowth of DRG neurites (a) compared with pretreatment with the control consisting of calcium- and magnesium-free phosphate-buffered saline (CMF-PBS; b). These qualitative findings are further supported by semiquantitative analysis of the neurite outgrowths (compare c and d, P < .05).
DISCUSSION
We report that, although neurites initially extend on both uninjured and postinjury nerve substrates, they are not able to maintain their structures on the former substrate. The ability of the nerve substrate to support stable outgrowth is optimal at 8 days after transection injury, coinciding with the arrival of the first neurites in the distal segment and the completion of macrophage infiltration into the entire length of the distal segment. We further demonstrate that macrophages secrete a protein that effectively transforms the uninjured nerve substrate to one that supports the maintenance of stable neurites. Taken together these results support the hypothesis that macrophages are responsible for altering the postinjury nerve to a substrate favorable for neurite maintenance.
Degeneration Following Axonal Extension
We demonstrate that the uninjured and postinjury nerves are two fundamentally different substrates, as evidenced by their difference in the ability to support the growth of stable neurites. This observation contrasts with other studies, which demonstrate that dissociated neurons are not able to extend neurites of any significant length on uninjured nerve substrate (Bedi et al., 1992). It is likely that our observation of extensive initial neurite outgrowth is related to the use of intact adult DRG explants rather than dissociated neurons. Our experimental system is a more enriched model that likely reflects the group behavior of the regenerating neurites and the effect of nonneuronal cell types that normally reside in the DRG.
The degeneration of neurites in response to contact with uninjured sciatic nerve may be attributable to molecular guidance cues that are unique to the peripheral nerve. In the mature CNS, there exist many inhibitors of neurite outgrowth, such as Nogo (Grandpre and Strittmatter, 2001), myelin-associated glycoprotein (Tang et al., 2001), collapsin (semaphorin 3; Pasterkamp and Verhaagen, 2001), and chondroitin sulfate proteoglycan (Snow et al., 2001), which are believed to play a role in the maintenance of the established neural circuitry by restricting inappropriate rewiring and by preventing excessive sprouting (Goldberg and Barres, 2000). For example, it has been shown that, if Nogo is neutralized in the rat cerebellum by application of the monoclonal antibody IN-1, the Purkinje cells will spread profuse neurites that cover much of the granular layer within 1 week (Buffo et al., 2000). These aberrant neurites, however, eventually disappear within 1 month as the effect of the antibody disappears.
In the PNS, however, the extracellular matrix is composed primarily of molecules that are favorable for neurite outgrowth, such as laminin and collagen IV (Lander, 1987). Therefore, a mechanism similar to that in the CNS is not likely to be functioning in the PNS. It is tempting to speculate that the degenerative response we observed may function as an innate mechanism of the mature PNS to eliminate inappropriate axonal sprouting after it occurs. If this is the case, it is notable that such a mechanism does not operate by the immediate repulsion or collapsing of growth cones, as conventional inhibitors and negative guidance cues do. Instead, the response is one of delayed withdrawal: Neurites transiently extend into the nerve environment but are later induced to degenerate if in an inappropriate location. The ultimate effect, similar to that of CNS inhibitors, is still the maintenance of the established neural configurations between the sensory (or motor) neurons and their peripheral targets.
There is evidence that neurite trimming indeed functions as a normal process, such as in the development of the retinocollicular projection in rat (Simon et al., 1994). In this system, the retinal ganglion cells (RGC) first extend long neurites to establish topographically specific, but overlapping and diffuse, connections with the superior colliculus. However, many of the axons make initial targeting errors, and the final map is attained after birth as a result of retraction of the aberrant branches (O’Leary et al., 1986; Mark et al., 1993). This process of neurite trimming is mediated by a secreted protein factor that selectively causes withdrawal of temporal, but not nasal, RGC in a delayed manner (Ichijo and Bonhoeffer, 1998). Perhaps a molecule functionally similar to this secreted protein is also present in the uninjured PNS, causing the withdrawal of aberrant DRG neurites in the adult normal sciatic nerve.
Molecular Models of Macrophage-Mediated Transformation of Postinjury Nerve
Our results with macrophage-conditioned medium have further extended the list of the many roles that macrophages play in Wallerian degeneration, namely, the transformation of the postinjury nerve environment into one that supports stable neurite outgrowth. This enhancement effect can be accounted for by at least two possible molecular models, the “degeneration signal model” and the “permissive signal model.” The first model involves the removal or inactivation of a negative “degeneration signal” molecule that innately resides within an adult uninjured nerve, which causes neurites to degenerate on contact. When this degeneration signal molecule is removed by the macrophage-derived secretory protein, the incoming neurites are no longer induced to degenerate and thus continue to outgrow stably. The second model involves the laying down of a macrophage-derived “permissive signal” molecule in the nerve environment that the incoming neurites require in order to remain stable. Thus, the normal nerve cannot support stable neurite outgrowth, because it lacks this permissive signal and the neurites proceed to degenerate.
Our current data appear to favor the “degeneration signal model.” First, we demonstrate that trypsin, a protease, is capable of rendering uninjured sciatic nerve cryosections supportive of stable neurite outgrowth. This implies that the transformation of the nerve environment does not necessarily require the addition of any extra “permission signal” molecule. Rather, the proteolytic processing of a certain protein, presumably a “degeneration signal” molecule that preexists in the normal nerve, is sufficient to allow stable neurite outgrowth in the nerve substrate. Second, although macrophages do not secrete trypsin, they typically secrete a wide range of other proteases during tissue remodeling events, some of which can possibly perform the same proteolytic processing of the nerve substrate exhibited by trypsin in vitro. Certainly MMPs are present in injured peripheral nerve (LaFleur et al., 1996; Misko et al., 2002). In a study using sections, enzymatic pretreatment of adult peripheral nerve tissue with MMP-2 and MMP-9 created a growth-permissive environment for neurite elongation (Ferguson and Muir, 2000), suggesting that the cleavage of a protein facilitates neurite progression. However, in our study, neurite outgrowth rates were unabated on sections of uninjured nerve. Instead, it was stabilization that was affected. Our preliminary data with MMP inhibitors and MMP-deficient mice do not support a major role for these proteases in conditioning of the nerve.
Finally, one may argue in favor of the degeneration signal model based on the fact that many of the matrix molecules abundant in the peripheral nerve, such as laminin, fibronectin, and type I and type IV collagen, are known to be highly permissive substrates (Lander, 1987). It follows that the environment of a normal nerve should favor stable neurite outgrowth, unless a special degeneration signal is present to cause the neurites to abort.
Nonetheless, the actual forces that shape the transformation of postinjury nerve may be a combination of both the removal of degeneration signal and the addition of permissive signal. One possible source of such a permissive signal is the activated macrophages themselves. Hikawa and Takenaka (1996; see also Hikawa et al., 1993) reported the presence of neurotrophic factors in the conditioned medium of myelin-stimulated macrophages, which are capable of enhancing both survival and regeneration of adult DRG neurons. Interestingly, this neurotrophic activity is not produced by LPS-stimulated macrophages, raising the probability that it is important in a later stage of Wallerian degeneration, when macrophages are actively ingesting myelin debris. On the other hand, it is also possible that a previously masked permissive signal within the nerve substrate becomes exposed as a result of the removal of the putative degeneration signal. This permissive signal is then available to induce the stability of regenerating neurites.
Why does the sciatic nerve begin to lose its ability to support stable neurite outgrowth at 10 days? Are macrophages initially beneficial to neurite outgrowth, but, by 10 days, are they beginning to degrade the injured sciatic nerve, reducing the nerve’s ability to act as a supportive substrate? Macrophages are capable of exerting both beneficial and neurotoxic effects after injury to either the peripheral nerve or the CNS. Therefore, it is possible that a temporal change in the phenotype of macrophages leads to a decline in the supportive nature of the nerve tissue. However, it is equally possible that the incoming regenerating neurites may be modifying the substrate as they enter the distal nerve environment, perhaps to signal a “slowdown” of regeneration as new neurites repopulate the tissue. If this neurite-mediated modification cannot be overcome by activated macrophages, this would satisfactorily explain the apparent paradox. The analysis of these possibilities is a subject for future studies.
Timing of Neurite Regeneration Relative to Changes in the Nerve Environment
The ability to support stable neurite outgrowth by the distal nerve environment, as determined by the in vitro assay, gradually increases after injury, reaching an optimum at about 8 days postinjury. We found that regenerating axons arrive at the tip of the proximal stump of the injured sciatic nerve by 4 days after injury but not at the distal segment until 8 days after injury. We can only speculate on what factors determine when neurites make their initial entry into the distal segment. It is quite possible that the timing is programmed to coincide with the period when optimal support for stable outgrowth is afforded by the distal nerve environment. Moreover, the matrix molecules in injured nerve are different from those in uninjured nerve (Agius and Cochard, 1998), implying that both permissive and degeneration signals may exist. Thus, the transformation of the postinjury nerve environment may be a rate-limiting step in the progress of neurite regeneration at this early stage. It has been shown that, when Wallerian degeneration is delayed, such as in the C57BL/Wlds (also known as C57BL/Ola) mutant mouse (Brown et al., 1994), the progress of sensory neurite regeneration into the distal segment is also delayed. The latter may reflect a perturbation in the transformation of the nerve environment.
The supportive nature of the distal segment is transient. Two days after the first neurites arrive, the ability of the distal nerve environment to support stable outgrowth has significantly declined, and, by 20 days after injury, no stable outgrowth is apparent. Although neurites are still undergoing active regeneration during this period, such a decline may serve an important function in the later stage of regeneration. Some insights can be gained from studies of regeneration of injured motor neurons toward their targets in skeletal muscle. Schwann cells near the neuromuscular junctions are induced to express the chemorepellent semaphorin 3A (De Winter et al., 1999, 2000). It has been suggested that semaphorin 3A may be critical in slowing the growth of regenerating axons as they approach the proximity of their targets and eventually acts as a stop signal. By analogy, the decline in the ability to support stable outgrowth by the injured sciatic nerve may also be a result of the distal segment generating a “stop signal” to the incoming neurites.
It is conceivable that the pioneering neurites may have only a narrow window of time in which to establish themselves successfully in the distal segment. However, once established, even though the nerve environment is no longer optimal, the neurites remain stable and do not degenerate, perhaps because the growth cones are no longer sensitive to the nonsupportive nature of the environment. It is also possible that these established neurites may be used by late-coming neurites as the substrate for outgrowth.
In conclusion, our results recognize that the maintenance of stable neurites, in addition to the initial axonal extension, is an important determinant of successful neurite regeneration. Furthermore, the maintenance of stable neurites is a function of the postinjury nerve environment, which undergoes a transformation process mediated by macrophages.
Acknowledgments
We thank Dr. Alex McMillan, UCSF Comprehensive Cancer Center, for assistance in the statistical analysis of data and Drs. David W. Sretavan and Louis F. Reichardt for their thoughtful input to this study.
Contract grant sponsor: NIH NINDS; Contract grant number: NS 39278; Contract grant number: NS 39847; Contract grant sponsor: Academic Senate, University of California.
References
- Agius E, Cochard P. Comparison of neurite outgrowth induced by intact and injured sciatic nerves: a confocal and functional analysis. J Neurosci. 1998;18:328–338. doi: 10.1523/JNEUROSCI.18-01-00328.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during Wallerian degeneration of axons. Exp Neurol. 1995;136:183–198. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Bedi KS, Winter J, Berry M, Cohen J. Adult rat dorsal root ganglion neurons extend neurites on predegenerated but not on normal peripheral nerves in vitro. Eur J Neurosci. 1992;4:193–200. doi: 10.1111/j.1460-9568.1992.tb00867.x. [DOI] [PubMed] [Google Scholar]
- Beuche W, Friede RL. The role of non-resident cells in Wallerian degeneration. J Neurocytol. 1984;13:767–796. doi: 10.1007/BF01148493. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. Eur J Neurosci. 1994;6:420–428. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Buffo A, Zagrebelsky M, Huber AB, Skerra A, Schwab ME, Strata P, Rossi F. Application of neutralizing antibodies against NI-35/250 myelin-associated neurite growth inhibitory proteins to the adult rat cerebellum induces sprouting of uninjured purkinje cell axons. J Neurosci. 2000;20:2275–2286. doi: 10.1523/JNEUROSCI.20-06-02275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci. 1998;18:6713–6722. doi: 10.1523/JNEUROSCI.18-17-06713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Bouchard C, Tsatas O, Giftochristos N. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990;5:463–469. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- De Winter F, Pasterkamp RJ, Verhaagen J. Semaphorin 3A is expressed in terminal Schwann cells during postnatal development and regeneration of adult rat neuromuscular junctions. Soc Neurosci Abstr. 1999;25:241. [Google Scholar]
- De Winter F, Pasterkamp RJ, Stam F, Cozijnsen M, Holtmaat AJGD, Verhaagen J. Injury-induced regulation of semaphorin 3A expression in the neuromuscular system. Soc Neurosci Abstr. 2000;26:579. [Google Scholar]
- Fawcett JW, Keynes RJ. Peripheral nerve regeneration. Annu Rev Neurosci. 1990;13:43–60. doi: 10.1146/annurev.ne.13.030190.000355. [DOI] [PubMed] [Google Scholar]
- Ferguson TA, Muir D. MMP-2 and MMP-9 increase the neurite-promoting potential of Schwann cell basal laminae and are upregulated in degenerated nerve. Mol Cell Neurosci. 2000;16:157–67. doi: 10.1006/mcne.2000.0859. [DOI] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Barres BA. Nogo in nerve regeneration. Nature. 2000;403:369–370. doi: 10.1038/35000309. [DOI] [PubMed] [Google Scholar]
- Grandpre T, Strittmatter SM. Nogo: a molecular determinant of axonal growth and regeneration. Neuroscientist. 2001;7:377–386. doi: 10.1177/107385840100700507. [DOI] [PubMed] [Google Scholar]
- Gulati AK. Immune response and neurotrophic factor interactions in peripheral nerve transplants. Acta Haematol. 1998;99:171–174. doi: 10.1159/000040832. [DOI] [PubMed] [Google Scholar]
- Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci USA. 1987;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikawa N, Takenaka T. Myelin-stimulated macrophages release neurotrophic factors for adult dorsal root ganglion neurons in culture. Cell Mol Neurobiol. 1996;16:517–528. doi: 10.1007/BF02150231. [DOI] [PubMed] [Google Scholar]
- Hikawa N, Horie H, Takenaka T. Macrophage-enhanced neurite regeneration of adult dorsal root ganglia neurones in culture. Neuroreport. 1993;5:41–44. doi: 10.1097/00001756-199310000-00010. [DOI] [PubMed] [Google Scholar]
- Hirschberg DL, Schwartz M. Macrophage recruitment to acutely injured central nervous system is inhibited by a resident factor: a basis for an immune-brain barrier. J Neuroimmunol. 1995;61:89–96. doi: 10.1016/0165-5728(95)00087-i. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Bonhoeffer F. Differential withdrawal of retinal axons induced by a secreted factor. J Neurosci. 1998;18:5008–5018. doi: 10.1523/JNEUROSCI.18-13-05008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFleur M, Underwood JL, Rappolee DA, Werb Z. Basement membrane and repair of injury to peripheral nerve: defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J Exp Med. 1996;184:2311–2326. doi: 10.1084/jem.184.6.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander AD. Molecules that make axons grow. Mol Neurobiol. 1987;1:213–245. doi: 10.1007/BF02936609. [DOI] [PubMed] [Google Scholar]
- Liefner M, Siebert H, Sachse T, Michel U, Kollias G, Bruck W. The role of TNF-alpha during Wallerian degeneration. J Neuroimmunol. 2000;108:147–152. doi: 10.1016/s0165-5728(00)00262-9. [DOI] [PubMed] [Google Scholar]
- Mark RF, Freeman TC, Ding Y, Marotte LR. Two stages in the development of a mammalian retinocollicular projection. Neuroreport. 1993;5:117–120. doi: 10.1097/00001756-199311180-00005. [DOI] [PubMed] [Google Scholar]
- Misko A, Ferguson T, Notterpek L. Matrix metalloproteinase mediated degradation of basement membrane proteins in Trembler J neuropathy nerves. J Neurochem. 2002;83:885–94. doi: 10.1046/j.1471-4159.2002.01200.x. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Fawcett JW, Cowan WM. Topographic targeting errors in the retinocollicular projection and their elimination by selective ganglion cell death. J Neurosci. 1986;6:3692–3705. doi: 10.1523/JNEUROSCI.06-12-03692.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Emerging roles for semaphorins in neural regeneration. Brain Res Brain Res Rev. 2001;35:36–54. doi: 10.1016/s0165-0173(00)00050-3. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC. Macrophages and nerve regeneration. Curr Opin Neurobiol. 1992;2:679–682. doi: 10.1016/0959-4388(92)90038-m. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Andersson PB. Macrophage responses to central and peripheral nerve injury. Adv Neurol. 1993;59:309–314. [PubMed] [Google Scholar]
- Salzer JL, Bunge RP. Studies of Schwann cell proliferation. I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980;84:739–752. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Lazarov-Spiegler O, Rapalino O, Agranov I, Velan G, Hadani M. Potential repair of rat spinal cord injuries using stimulated homologous macrophages. Neurosurgery. 1999;44:1041–1045. doi: 10.1097/00006123-199905000-00057. discussion 1045–1046. [DOI] [PubMed] [Google Scholar]
- Simon DK, Roskies AL, O’Leary DD. Plasticity in the development of topographic order in the mammalian retinocollicular projection. Dev Biol. 1994;162:384–393. doi: 10.1006/dbio.1994.1095. [DOI] [PubMed] [Google Scholar]
- Snow DM, Mullins N, Hynds DL. Nervous system-derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: a light, epifluorescence, and electron microscopy study. Microsc Res Techniq. 2001;54:273–286. doi: 10.1002/jemt.1140. [DOI] [PubMed] [Google Scholar]
- Tang S, Qiu J, Nikulina E, Filbin MT. Soluble myelin-associated glycoprotein released from damaged white matter inhibits axonal regeneration. Mol Cell Neurosci. 2001;18:259–269. doi: 10.1006/mcne.2001.1020. [DOI] [PubMed] [Google Scholar]
- Tofaris GK, Patterson PH, Jessen KR, Mirsky R. Denervated Schwann cells attract macrophages by secretion of leukemia inhibitory factor (LIF) and monocyte chemoattractant protein-1 in a process regulated by interleukin-6 and LIF. J Neurosci. 2002;22:6696–6703. doi: 10.1523/JNEUROSCI.22-15-06696.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp Neurol. 1996;137:301–308. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- Werb Z, Chin JR. Endotoxin suppresses expression of apoprotein E by mouse macrophages in vivo and in culture. A biochemical and genetic study. J Biol Chem. 1983;258:10642–10648. [PubMed] [Google Scholar]
- Zuo J, Ferguson TA, Hernandez YJ, Stetler-Stevenson WG, Muir D. Neuronal matrix metalloproteinase-2 degrades and inactivates a neurite-inhibiting chondroitin sulfate proteoglycan. J Neurosci. 1998;18:5203–5211. doi: 10.1523/JNEUROSCI.18-14-05203.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]