INTRODUCTION
The endocrine pancreas is organized in islets of Langerhans comprising five original cell subtypes, α-, β-, δ-, ε-, and PP-cells, secreting glucagon, insulin, somatostatin, ghrelin, and pancreatic polypeptide (PP), respectively (Collombat et al., 2006). The identification and characterization of the genetic determinants underlying endocrine pancreas morphogenesis and regeneration may potentially aid designing cell replacement therapies to treat type 1 and 2 diabetes. In this context, a number of studies have demonstrated that, during development, the cooperation of several transcription factors successively specifies progenitor cells towards the pancreatic-, endocrine- and ultimately islet-cell fates. Hence, Pdx1 is required for pancreatic epithelium determination (Ahlgren et al., 1996; Ahlgren et al., 1998; Grapin-Botton et al., 2001; Jonsson et al., 1994; Offield et al., 1996) and subsequently Neurogenin3 (Ngn3) for endocrine lineage specification (Gradwohl et al., 2000; Gu et al., 2002; Jensen et al., 2000; Johansson et al., 2007). Next to Ngn3 induction, a complex network of transcription factors progressively and differentially promotes the particular endocrine fates, including Arx and Pax4 (Collombat et al., 2003; Sosa-Pineda et al., 1997). In mice lacking Arx, the β- and δ-cell fates were found favored at the expense of α-cell genesis, while the total endocrine cell content remained normal (Collombat et al., 2003). Conversely, in the absence of Pax4, the opposite phenotype was observed (Sosa-Pineda et al., 1997), indicating an inhibitory, cross-regulatory circuit between Arx and Pax4 (Collombat et al., 2005). Additional findings supported these conclusions and suggested that, firstly, Arx and Pax4 instruct endocrine precursor cells towards either an α-cell or a β-/δ-cell fate, respectively. Next, through the analysis of double-mutant mice, a secondary function of Pax4 in specifying the β-cell lineage in β-/δ-precursor-cells was uncovered (Collombat et al., 2005). Recent evidence have demonstrated that the forced expression of Arx in early pancreatic cells drives endocrine progenitors towards either an α- or, surprisingly, a PP-cell fate (Collombat et al., 2007). It was therefore concluded that Arx is not only necessary, but also sufficient to instruct the α- and PP-cell lineages.
Of particular interest was the finding that the forced expression of Arx triggered into adult β-cells induced their conversion into cells exhibiting α- or PP-cell phenotypes (Collombat et al., 2007). This discovery was of fundamental importance in the context of β-cell-based therapy and implied that the opposite conversion might be achieved, that is, to generate β-cells from other endocrine cells. To test this hypothesis, we generated mice conditionally and ectopically expressing the Pax4 gene. Our data indicates that the ectopic expression of Pax4 in early pancreatic cells, but also in α-cells, induces their respecification towards a β-cell fate or identity. As a consequence of the ensuing glucagon deficiency, an ongoing neogenesis of α-cells occurs. However, such α-cells are continuously converted into β-cells upon Pax4 ectopic expression, resulting in the development of oversized islets of Langerhans. Importantly, a prominent expression of the proendocrine transcription factor Ngn3 in the pancreas of such animals is highlighted. Our results are consistent with the recently reported notion of facultative adult stem cells that reactivate Ngn3 expression in injured pancreas (Xu et al., 2008). Finally, following streptozotocin-induced depletion of β-cells in young mice ectopically expressing Pax4, an α-cell-mediated regeneration of the β-cell mass, a progressive normalization of the glycemia and an extended lifespan are observed.
RESULTS
Pax4 ectopic expression in the developing pancreas promotes the genesis of oversized islets mainly composed of β-cells
Taking advantage of the Cre-LoxP system, we generated transgenic mice able to conditionally express the Pax4 gene (POE). Briefly, the construct used included the cytomegalovirus enhancer/human β-actin (CAG) promoter driving the expression of the eGFP gene followed by translation stop codons in all three frames and flanked by LoxP sites (Figure S1A). A Pax4 cDNA-IRES-β-galactosidase sequence was subsequently cloned downstream of eGFP. Using pronuclear injection, five independent transgenic mouse lines were derived. The resulting mice constitutively expressed eGFP, but not β-galactosidase, at all ages examined (Figure S1B-E).
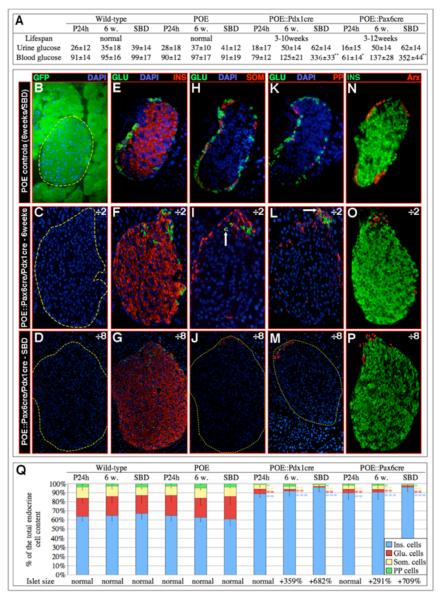
POE animals were bred with Pdx1cre or Pax6cre animals to target Pax4 expression to the pancreatic epithelium or the endocrine tissue, respectively (Ashery-Padan et al., 2004; Gu et al., 2002). Double-transgenic POE::Pdx1cre and POE::Pax6cre mice were born and developed normally. However, animals of both genotypes eventually died at the age of 3 to 12 weeks, a majority after 9 weeks (Figure 1A and data not shown). To assess whether the ectopic expression of Pax4 altered glucose homeostasis, blood sugar levels were measured. One day postpartum, a decrease in glycemia was evidenced in both POE::Pdx1cre and POE::Pax6cre genotypes (Figure 1A). Notably, at six weeks of age, blood glucose levels were found normal in these same animals compared to controls, whereas shortly before death (SBD), a dramatic hyperglycemia was uncovered. This indicates that POE::Pdx1cre and POE::Pax6cre mice initially present a hypoglycemic condition that progressively evolves towards a hyperglycemic one.
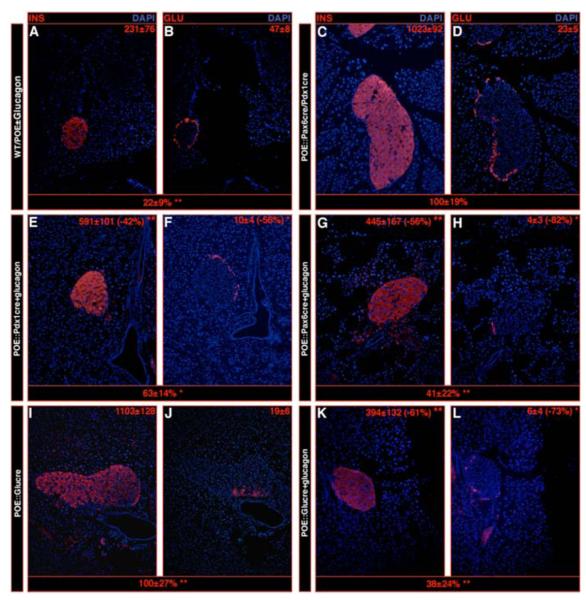
Figure 1. The overexpression of Pax4 in Pdx1 or Pax6 expression domains promotes the genesis of oversized islets mainly containing insulin-expressing cells.
(A) Characterization of the lifespan and glycemia (in mg/dl) of POE::Pdx1cre and POE::Pax6cre mice 1 day and 6 weeks postpartum, as well as short before death (SBD). Note that initially, these animals are hypo- and subsequently hyperglycemic. (B-P) Immunohistochemical analysis of the islets of double-transgenic mice. A dramatic increase in islet size and insulin-expressing cell mass is evident 6 weeks postpartum (C, F, I, L, O) and is even more pronounced SBD (D, G, J, M, P) as compared to representative control islets (B, E, H, K, N). Concurrently, a loss of α- (E-M), δ- (H-J), PP- (K-M), and Arx-labeled (N-P) cells is evident, but, interestingly, few of these remain detectable at one pole of the islet, some co-expressing the glucagon and somatostatin (arrow in I) or PP (arrow in L) hormones. (Q) A quantification of the endocrine cell alterations ascertains these observations (also see Table S2). For the purpose of clarity, the magnification of double-transgenic islets is twice (C, F, I, L, O) or eight times (D, G, J, M, P) reduced compared to controls. (n>11, * P<0.05, ** P<0.01, *** P<0.001, all values expressed as means ± standard error of the mean).
To assess the consequences of the forced expression of Pax4 in Pdx1 and Pax6 expression domains, immunohistochemical analyses of double-transgenic pancreata were performed. As similar results were obtained in POE::Pax6cre and POE::Pdx1cre mice, pictures representative of both genotypes are displayed for 6-week-old animals (Figure 1C, F, I, L, O) and SBD (Figure 1D, G, J, M, P). We did not observe any change in endocrine cell composition in POE animals as compared to their wild-type counterparts (Figure 1B, E, H, K, N and data not shown). The analysis of both POE::Pax6cre and POE::Pdx1cre pancreata showed a dramatic increase in islet size (note the 2 and 8-fold scale reduction in magnification in Figure 1C, F, I, L, O and D, G, J, M, P, respectively - Figure S2-3) containing mainly insulin-expressing cells (Figure 1F-G, O-P, S2-3) and only few δ- and Arx-labeled α- and PP-cells (Figure 1F-G, I-J, L-M, O-P). Notably, the remaining α-, δ- and PP- cells were consistently located at one pole of the islet of Langerhans (Figure 1F-G, I-J, L-M, O-P), a subset of these co-expressing the glucagon and somatostatin or PP hormones (arrows in Figure 1I, L, respectively). A quantification of these alterations (Figure 1Q - Table S1) ascertained (1) an age-dependent increase in islet size and β-cell mass, concurrently with a reduction in non-β-cell numbers (relative as well as absolute numbers), and (2) an abnormal location of the few remaining α-, δ- and PP-cells at one pole of the islet.
Pax4 expression was assayed with a newly-developed antibody that detected Pax4 in wild-type embryonic pancreata (Wang et al., 2008) and specifically in adult β-cells (Figure S4A-B and S5A-D), as previously reported (Theis et al., 2004). Neither in Pax4-depleted, nor in Ngn3-deficient islets, did we find any expression of Pax4 (Figure S4C-D and data not shown). Importantly, in POE::Pax6cre mice, most insulin-producing cells were found positive for Pax4 (Figure S5E-H), whereas the vast majority of pancreatic cells were labeled in POE::Pdx1cre animals (Figure S5I-L). These results were confirmed by RT-PCR analysis (Figure S6) and assessment of β-galactosidase activity (Figure S1F-G).
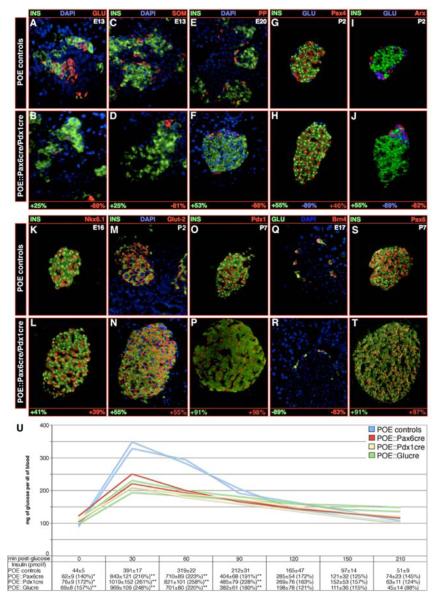
Further examination of POE::Pax6cre and POE::Pdx1cre mice during the development of the endocrine pancreas indicated that α-, δ, and PP-cell lineages were disfavored to the benefit of an insulin-producing cell fate (Figure 2A-H). Most insulin-labeled cells present in double-transgenic mice correctly expressed the β-cell specific markers Nkx6.1, Pdx1, Glut2 and HB9 (Figure 2K-P and data not shown). Accordingly, they lacked Arx (Figure 2I-J) and Brn4 (Figure 2Q-R), labeling α-/PP- and α-cells, respectively, whereas Pax6 and Isl1 were detected in all islet cell types, as expected (Figure 2S-T and data not shown). To further assess the function of these insulin-producing cells postnatally, glucose tolerance tests associated with systemic insulin measurements were performed. Consistent with the increased β-cell mass we previously noted, three-week-old POE::Pdx1cre and POE::Pax6cre mice displayed an improved glucose clearance associated with a more than two-fold increase in circulating insulin levels (Figure 2U). Altogether, our findings suggest that the ectopic expression of the Pax4 gene drives endocrine precursor cells almost exclusively towards an insulin-expressing cell fate, and that these cells exhibit a functional β-cell phenotype.
Figure 2. Insulin-expressing cells detected in POE::Pdx1cre and POE::Pax6cre pancreata exhibit a β-cell identity.
(A-T) Characterization of POE::Pdx1cre and POE::Pax6cre hormone-expressing cells. Sections of double-transgenic pancreata (of the indicated ages) were stained using the mentioned antibodies, the number of labeled cells counted and reported to the count obtained in POE control animals. The values are expressed in percentage of change in labeled cell number compared to controls. All values are statistically significant with P values lower than or equal to 0.05. Note that the numbers of insulin-expressing cells are dramatically increased in double-transgenic animals at all examined stages (A-P, S-T) and this, as early as E13 (A-B), compared to controls. Concurrently, the mean contents of α- (A-B, G-J, Q-R), δ- (C-D), PP- (E-F) and Arx- (I-J) marked cells appear drastically reduced. A thorough analysis of endocrine cell-specific markers demonstrates that, all insulin-producing cells clearly express Pax4 (G-H), as well as the β-cell markers Nkx6.1 (K-L), Glut-2 (M-N) and Pdx1 (O-P). These are negative for the α-cell specific factor Brn-4 (Q-R), whereas, all endocrine cells express Pax6 (S-T). (U) An improved glucose tolerance and insulin release are highlighted in 3-week-old double-transgenic mice as compared to controls POE mice (n>3, ***P<0.001, **P<0.01, * P<0.05). Each picture is representative of at least 4 independent animals; note that, for the purpose of clarity, a POE::Pax6cre islet is displayed in H.
Conversion from an α- to a β-cell phenotype
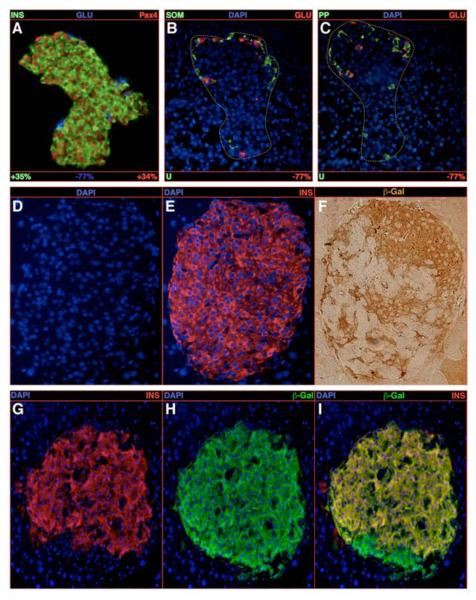
As Pax4 specifically favors the β-cell fate and identity throughout the morphogenesis of the endocrine tissue, we wondered whether this might also apply to mature endocrine cells. Therefore, animals conditionally expressing Pax4 in glucagon-producing cells (Herrera, 2000 - Figure S8) were generated (POE::Glucre) and endocrine cell numbers were monitored. As early as one week postpartum, a 50% enlargement in islet size was evidenced, these containing increased numbers of insulin+/Pax4+ cells compared to controls (Figure 3A). Notably, the content of glucagon-producing cells was found reduced by 77% and most of the remaining glucagon+ cells were located at one pole of the islet (Figure 3A-C). As expected, δ- and PP-cell numbers were unchanged (Figure 3B-C). It is worth noticing that the expression of Pax4 in glucagon-producing cells was accompanied by the production of the β-galactosidase protein (Figure S1A) allowing lineage-tracing experiments. One week following birth, this otherwise glucagon-cell-specific labeling was detected in the majority of insulin-producing cells (Figure 3D-F) expressing a typical β-cell complement of transcription factors (Table S2). Very few cells were found positive for both insulin and glucagon (Figure S9G). Besides, we observed an age-dependent increase in islet size and in the number of insulin-/β-galactosidase-producing cells, the latter exhibiting most β-cell features (Table S2-3, Figure 3G-I, 2U). This suggests that, upon Pax4 ectopic expression, adult glucagon-expressing cells are continuously converted into cells exhibiting a β-cell phenotype.
Figure 3. Conversion of glucagon-expressing cells into insulin-producing cells upon Pax4 ectopic expression.
(A-C) Quantification of the endocrine cell content alterations upon ectopic expression of the Pax4 gene in glucagon-producing cells in 1-week-old animals. A clear increase in the number of insulin-/Pax4-labeled cells at the expense of glucagon-expressing cells is highlighted (A), whereas the δ- and PP-cell contents are found unchanged (B-C). Note the accumulation of the remaining glucagon-marked cells at one pole of the islet. (D-F) The detection of insulin- and β-galactosidase-expressing cells on serial sections demonstrates that numerous insulin-labeled cells do express the β-galactosidase gene that normally marks glucagon-positive cells. (G-I) The same observation is made in 6-week old animals using co-immunofluorescence. All reported values are statistically significant (P<0.05, n=5). See Table S2 for a detailed analysis.
Pax4 ectopic expression in β-cells does not induce their proliferation
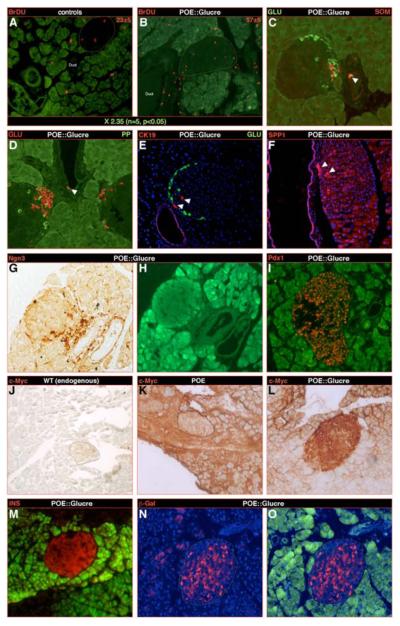
To further characterize the origin of the supernumerary β-cells, we assayed proliferating cells combining bromodeoxyuridine (BrdU) incorporation and Ki67 staining. Following a BrdU pulse in 5-week old POE::Glucre mice, pancreata were quantitatively analyzed a week later: a significant 2.35-fold increase in the number of BrdU-labeled cells was evidenced as compared to controls (Figure 5A-B, Table S4). Interestingly, most BrdU+ (Figure 5B-S9F), but also Ki67+ (Figure S9D) cells were located outside the islets, within or adjacent to the neighboring duct epithelium.
Figure 5. Progenitor cells may be induced and converted into glucagon and subsequently into insulin-expressing cells.
Next to a pulse of BrdU at three weeks of age and examination a week later, a quantitative analysis established a 2.35 increase in the number of proliferating cells in POE::Glucre pancreas compared to POE controls (A-B). Importantly, most BrdU-labeled cells are not detected within the islets, but rather near or within the duct epithelium (B). It is worth noticing that this location corresponds to the cluster of glucagon-producing cells consistently observed in this genotype. Within the duct epithelium, scattered endocrine cells could be detected, some co-expressing the glucagon and sometimes somatostatin hormones (arrowheads in C-D). Along the same line, islet cells positive for the duct marker genes CK19 and SPP1 are observed adjacent to the duct epithelium (arrowheads in E-F). Notably, the duct lining also appears to contain numerous cells positive for the proendocrine marker gene Ngn3 (G-H), but negative for Pdx1 (I). (J-L) Infection of controls (K) and double-transgenic (L-O) pancreata using a construct containing a CMV promoter driving the constitutive expression of a c-Myc tag (the latter being not expressed in wild-type tissues - J). Due to the method used, two weeks post-infection, most exocrine cells are labeled by the virus, whereas only very few islet cells are (islet underlined in K). Importantly, a majority of double-transgenic endocrine cells appear marked by the virus, suggesting their ductal or acinar origin (L). These cells express the insulin hormone (M) and are β-galactosidase-positive (N-O), indicating that they once expressed the glucagon hormone. (H-I, M, O: Green fluorescence corresponding to residual GFP expression after bleaching of the sections).
The cell-autonomous effect of an augmentation of the Pax4 dose in β-cells was analyzed by crossing POE with Inscre mice expressing the cre recombinase under the control of the insulin promoter (Herrera, 2000). The quantification of the endocrine cell numbers in 3-week old POE::Inscre pancreata did not reveal any increase in β-cell numbers, nor in islet size (Figure S10 and data not shown), despite the use of the POE mouse line inducing the highest Pax4 expression (Table S5, line 5). Thus, although Pax4 may promote a modest proliferation of β-cells in rat islet cultures (Brun et al., 2004), alternative mechanisms clearly act to boost the robust expansion of the β-cell mass consistently observed in POE::Pdx1cre, POE::Pax6cre and POE::Glucre mice (from hereon termed “double-transgenic animals”). One issue of concern to our approach was related to the overexpression of Pax4. However, through the use of different POE transgenic founder mice, we demonstrated that the ectopic expression of Pax4 at a dose lower than found in normal β-cells, is sufficient to induce a loss of the α-cell phenotype to the benefit of β-cell features (Table S5).
Rescuing glucagon deficiency prevents the generation of oversized islets
As lineage-tracing experiments demonstrated that glucagon-expressing cells were converted into cells displaying a β-cell phenotype, a depletion of α-cells was expected. However, we consistently detected clusters of glucagon+ cells in our double-transgenic mice (Figures 1-3), suggesting that α-cell neogenesis occurred. We therefore hypothesized that the latter might be driven by the hypoglucagonemia resulting from the α- to β-cell conversion. To test this theory, we injected 3-week-old POE::Pdx1Cre, POE::Glucre, POE::Pax6cre, and control animals twice daily with glucagon during three weeks. A significant decrease in islet size was observed in glucagon-treated animals as compared to controls (Figure 4). This was mainly attributed to a reduction in the β-cell mass (Figure 4E, G compared to C, and K compared to I, Table S3-4). Importantly, the number of α-cells was also found significantly diminished (Figure 4F, H, L and Table S3-4). These results thus indicate that decreased glucagon levels are responsible for the continuous replenishment of α-cells that acquire a β-cell phenotype in double transgenic animals.
Figure 4. Exogenous glucagon supplementation prevents islet overgrowth.
Six-week old pancreata of the indicated genotypes were sectioned and stained using anti-insulin (A, C, E, G, I, K) or glucagon (B, D, F, H, J, L) antibodies. The values at the top right corner of each photograph represent the number of hormone-producing cells per square centimeter of pancreas. The counts reported underneath each picture set represent variations in islet surface (estimated in silico) using POE::Pax6cre/Pdx1cre pancreata (C-D) as reference for photographs A-H and POE::Glucre pancreata (I-J) as reference for photographs I-L. Note the overall diminution in islet size and glucagon+ cell content, as well as the drastic decrease in insulin-expressing cell number (E-H compared to C-D and K-L compared to I-J) in animals supplemented with glucagon for 3 weeks compared to untreated ones (*P<0.05, **P<0.01, n=3).
Requirement of Ngn3 re-expression for α-cell neogenesis and ensuing acquisition of a β-cell phenotype
The location of endocrine non-β-cells at one pole of the islet of Langerhans of double-transgenic animals was remarkable. This observation was confirmed through z-stack analysis of POE::Pdx1Cre, POE::Glucre and POE::Pax6cre whole islets stained with anti-glucagon antibodies (Movie S1A). Most importantly, these glucagon-labeled cell clusters were consistently found adjacent to the ductal lining (Movie S1B, in blue). The very detection of such glucagon+ cells was intriguing as their conversion into β-cells was expected upon Pax4 expression. A likely explanation may be the continuous generation of glucagon-labeled cells, their detection indicating a transitional state prior to their conversion into insulin-expressing cells. This hypothesis was supported by the active cell proliferation observed near and within the duct epithelium (Figure 5B, S9D, F), close to the pole of glucagon+ cells. In addition, scattered glucagon+ cells were found within the lining of the duct (arrowheads in Figure 5C-D), whereas the duct markers cytokeratin-19-(CK19) and SPP1 were detected not only within the duct epithelium, but also in few adjacent islet cells (arrowheads in Figure 5E-F). The existence of facultative progenitor cells in adult mice that, under specific injury conditions, reactivate Ngn3 expression and develop into all four endocrine cell types has been recently reported (Xu et al., 2008). Interestingly, in pancreata ectopically expressing Pax4, we also noticed a reactivation of the islet cell progenitor marker Ngn3 (Figure 5G-H, S9A, S11-12, Table S3-4), while Pax4 expression was never detected in duct cells (Figure S9B). However, due to the difficulties classically encountered to detect Ngn3 in adult tissues, the definitive location and counts of Ngn3-producing cells will await the generation of better antibodies/in situ probes. We therefore asked whether Ngn3 reactivation was involved in the generation of supernumerary insulin+ cells by tracing exocrine- and Ngn3-producing cells, or inhibiting Ngn3 expression in the pancreas of double transgenic mice. Because the expression of Cre, GFP and β-galactosidase in our double-transgenic animals precluded the use of duct-Cre, Ngn3-Cre, Ngn3-GFP or Ngn3-LacZ mouse lines for tracing purposes, a lentiviral approach was preferred. Specifically, recombinant lentiviruses constitutively expressing a c-Myc-tagged DsRED2 reporter were injected into the pancreatic duct of POE::Glucre mice, as previously described (Xu et al., 2008). Due to the high intensity of the GFP signal present in some animals, native DsRED2 fluorescence could not be unambiguously ascertained. We therefore employed immunohistochemistry to detect the exogenous c-Myc epitope-tag fused to DsRED2 (the endogenous c-Myc being unrecognized - Figure 5J). Two weeks post-infection, Myc-tagged cells were evidenced in the vast majority of duct and acinar cells of control animals, whereas less than 5% of endocrine cells were labeled, as reported previously (Xu et al., 2008 - Figure 5K). In the pancreas of double-transgenic mice, however, 71% of endocrine cells were positive for the Myc tag (Figure 5L), most of them expressing insulin (Figure 5M) and β-Galactosidase (Figure 5N-O), the latter labeling cells that previously expressed glucagon. This result suggests that exocrine cells were converted into endocrine cells in the double transgenic mice. Double transgenic mice were also infected with recombinant lentiviruses expressing either the c-Myc-tagged reporter under the control of the Ngn3 promoter or a Ngn3-specific short hairpin (sh) interfering RNA controlled by the CMV promoter, as in Xu et al. (2008). Transduction with the first construct both confirmed the re-expression of Ngn3 and the specificity of Ngn3 promoter employed (Figure S13-15). Interestingly, Ngn3 knockdown caused a dramatic reduction in the number of Ngn3+ (Figure 6A,E), insulin+ (Figure 6B,F,D,H) and glucagon+ (Figure 6C,G) cells, as well as in the level of transcripts encoding Ngn3, insulin and glucagon (Table S6).
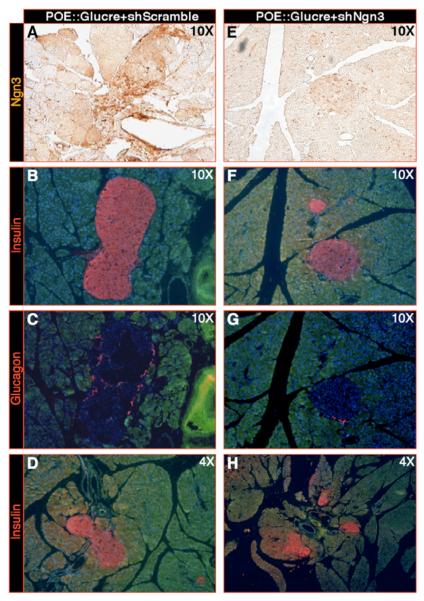
Figure 6. Knockdown of Ngn3 prevents the Pax4-mediated β-cell hyperplasia.
(A-H) Infection of POE::Glucre animals using lentiviruses producing either an shRNA targeting Ngn3 transcripts (E-H - Xu et al., 2008) or producing a scrambled shRNA (based on the former; A-D). Two weeks post-infection, Ngn3 knockdown pancreata (at the indicated magnifications) display an efficient 61% diminution in Ngn3 production (Xu et al., 2008, A, E and Table S6), but also a 69% decrease in insulin- (B, F, D, H) or glucagon- (C, G) expressing cell numbers (Table S6) compared to scramble-infected counterparts.
To test whether glucagon signaling deficiency may also induce the reactivation of Ngn3, in addition to POE::Glucre animals, glucagon receptor-deficient mice (characterized by an α-cell hyperplasia - Gelling et al., 2003) were analyzed. Combining Real-time RT-PCR and counts of immunostained sections (Table S3-4, Figure S16), an increase in Ngn3, but also in the β-cell regeneration-associated Reg3b (or INGAP - Rosenberg, 1998) gene expression was observed in both genotypes. Interestingly, Ngn3 and Reg3b transcripts as well as Ngn3+ cell numbers appeared dramatically reduced in POE::Glucre animals supplemented with glucagon for three weeks (Table S3-4). Thus, our data supports the notion that the glucagon deficiency mediated by the ectopic expression of Pax4 results in Ngn3 expression and in the successive conversion of progenitor cells into glucagon- and thereafter insulin-producing cells.
Pax4 ectopic expression rescues streptozotocin-induced diabetes
Thus far, our data suggested that the forced expression of Pax4 in either Pax6, Pdx1 or glucagon expression domains ultimately led to the development of oversized islets of Langerhans mostly composed of seemingly differentiated and functional β-cells. We reasoned that such cells might be capable of replacing lost β-cells in diabetic mice. Hence, POE::Glucre mice of different ages were injected with a single dose of the β-cell toxin streptozotocin (STZ), and both blood glucose levels as well as viability were monitored for 8 weeks (Figure 7A). Shortly after STZ injection, we often observed a decrease in glycemia, most likely caused by the discharge of insulin from killed β-cells (Figure 7A). A higher mortality rate was found among animals older than 4 weeks, as compared to younger mice. It should be noticed that these animals were already hyperglycemic and weakened prior to STZ injection due to a combined insulin insentivity and β-cell failure to optimally respond to a glucose challenge (Figure S17). In contrast, in 4-week-old and younger STZ-treated animals, we noted a normalization in blood glucose levels following a peak in glycemia and survival rates reaching 41% two months post-injection (Figure 7A), while all controls died (data not shown). Immunohistochemical analyses demonstrated that following the rapid obliteration of the β-cell mass (Figure 7B-E), a massive neogenesis of glucagon-expressing cells occurred, again near the duct lining (Figure 7F-I). Ten days post-injection, only few insulin-producing cells were detected (Figure 7F-G). However, a steady increase in glucagon- and insulin-expressing cell numbers was observed in the following days (Figure 7H). Approximately two months post-injection, POE::Glucre animals exhibited almost normally-sized islets of Langerhans and normoglycemia (Figure 7I). Interestingly, at that age, the β-galactosidase lineage tracer was found uniformly distributed in islet cells (Figure 7J), demonstrating that the insulin-producing cells in these animals derived from cells that previously expressed glucagon. These insulin+ cells displayed a β-cell phenotype and expressed a β-cell-specific complement of transcription factors (data not show). Altogether, our analyses provide direct evidence that, in STZ-treated mice, the ectopic expression of Pax4 in α-cells continuously converts them into β-cells and counters diabetes in animals younger than four weeks of age.
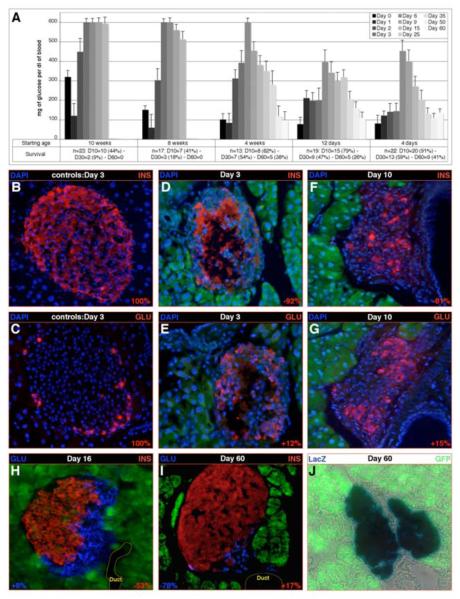
Figure 7. Pax4 ectopic expression promotes the reconstitution of the insulin-expressing cell mass upon β-cell depletion.
(A) Following streptozotocin injection at the indicated age (starting age), the glycemia and survival of the treated animals was followed for two months. Note that animals older than four weeks of age become diabetic and die as a consequence of the obliteration of β-cells, the same being true for all controls (data not shown). Importantly, in younger animals, a steady recovery leading to normoglycemia is observed next to a peak in glucose levels (all values are expressed as means ± standard error of the mean). (B-J) Islet cell contents in 4-week-old POE::Glucre streptozotocin- (D-J) or sham- (B-C) treated animals were quantified (see at the bottom of the concerned pictures) 3 days (B-E), 10 days (F-G), 16 days (H) and 60 days (I-J) days post-injection. Three days post-injection, the β-cell mass present in controls (B-C) is almost entirely lost in streptozotocin-treated mice (D-E), the only insulin labeling being observed in areas devoid of nuclei, suggesting a detection of hormone released from killed β-cells. A 15% increase in glucagon-producing cells is highlighted 10 days post-injection compared to controls, most of these cells neighboring duct structures (G). Importantly, few insulin-labeled cells are detected (F). This trend is ascertained at day 16 (H) with a major increase in the insulin-expressing cell number. A co-detection with the glucagon hormone does not indicate any co-expression. Finally, at day 60, the β-cell mass appears statistically normal as compared to control animals (I compared to B-C), most of the cells present in the islet expressing the β-galactosidase enzyme marking glucagon-producing cells (J).
DISCUSSION
In this study, we report that the forced expression of the Pax4 gene in the mouse endocrine pancreas results in oversized islets composed mainly of cells displaying a β-cell phenotype. Our findings are consistent with the induction of progenitor cells that adopt an α-cell identity as a consequence of decreased glucagon levels, and subsequently acquire β-cell features upon Pax4 ectopic expression, Ngn3 reactivation being instrumental in this processes. The resulting β-cells are functional at least at an early age, and can repopulate the islets of diabetic mice to normalize blood sugar levels.
Pax4 promotes the β-cell fate specification during embryogenesis and induces an α-cell-mediated β-cell neogenesis postpartum
The forced expression of Pax4, either in Pdx1+ pancreatic progenitor cells (and ultimately in all pancreatic cells) or in Pax6+ endocrine precursor cells (and ultimately in all islet cells) results in the nearly exclusive specification of cells exhibiting a β-cell identity at the expense of the α-, δ- and PP-cell lineages. At birth, these animals display normal pancreas morphology, unaffected exocrine tissue and well-sized islets of Langerhans, indicating that the main alterations observed are solely related to the allocation of the different endocrine cell lineages during embryonic development. This also suggests that Pax4 does not alter the pancreatic exocrine differentiation program, but rather acts on the specification of endocrine progenitor cells by promoting the acquisition of the β-cell fate. Interestingly, following birth, an age-dependent increase in islet size is evidenced in pancreata ectopically expressing Pax4 that was attributed to the continuous generation of cells displaying a β-cell phenotype. In an effort to ascertain the origin of such cells, lineage-tracing experiments were performed using POE::Glucre mice. Our initial goal was to trigger the ectopic expression of Pax4 in adult glucagon-producing cells, but an inducible glucagon-cre line is hitherto unavailable. Hence, the classical glucagon-cre mouse line (Herrera, 2000) was used to induce Pax4 expression in glucagon-producing cells. It is important to notice that during embryogenesis, the glucagon gene is initially expressed in early endocrine cells often co-expressing additional hormones, including insulin. However, by irreversibly tagging the progeny of cells using the Cre/LoxP system, Herrera (2000) demonstrated that mature glucagon- and insulin-producing cells do not derive from cells that previously expressed insulin or glucagon, respectively. Interestingly, lineage-tracing experiments performed in POE::Glucre mice revealed that the vast majority of newly formed β-galactosidase+/insulin+ cells originate from cells that previously expressed glucagon (note that endogenous β-galactosidase−/insulin+ cells remain detectable). Based on these results, but also on the age-dependent increase of β-galactosidase+ β-cell numbers and the concomitant decrease in α-cell contents, the dramatic expansion of the β-cell mass throughout the postnatal lifespan of double transgenic mice was attributed to a continuous neo-formation of β-cells through α-cell redifferentiation rather than to the slow self-renewal capacity of β-cells (Dor et al., 2004). Although our findings are in agreement with a putative neogenesis/conversion of somatostatin- or PP-positive cells into β-cells, testing this hypothesis will have to await the generation of somatostatin-cre and PP-cre mice to allow lineage-tracing experiments.
Ngn3 is required for the continuous neogenesis of α-cells ultimately acquiring a β-cell phenotype upon Pax4 ectopic expression
We demonstrate that glucagon supplementation reduces the β-cell hyperplasia in double-transgenic mice, most likely by compensating the deficiency in circulating glucagon resulting from the loss of α-cells through Pax4-induced redifferentiation. Interestingly, compromised glucagon signaling has previously been associated with α-cell neogenesis: both Glucagon receptor (Gcgr)- and prohormone convertase 2 (Pcsk2)-deficient animals display oversized islets, mainly composed of glucagon-producing cells (Furuta et al., 1997; Gelling et al., 2003). Similar to the present report, exogenous glucagon treatment significantly reduced the endocrine cell hyperplasia in Pcsk2-deficient animals (Blume et al., 1995; Petersson and Hellman, 1963; Webb et al., 2002). Pcsk2 mutants were also found to contain glucagon+ cells near the duct epithelium and their contribution to the neogenesis of the supernumerary glucagon+ cells was suggested. Accordingly, our findings indicate that, upon Pax4 ectopic expression, a physiologically significant glucagon deficiency activates a continuous compensatory response resulting in α-cell neogenesis, as seen in Gcgr and Pcsk2 mutant mice. However, these cells are subsequently converted into β-cells upon Pax4 ectopic expression.
In animals ectopically expressing Pax4, but also in Gcgr mutants, a reactivation of Ngn3, a gene normally exclusively expressed during embryonic development of the pancreas and required for the endocrine differentiation program, was observed. However, due to the difficulties encountered to detect Ngn3 transcripts or protein, the determination of the origin of such Ngn3+ cell will require more elaborated lineage-tracing experiments and/or more specific antibodies/in situ probes. Interestingly, lentivirus-mediated cell tracing and knockdown experiments showed that Ngn3 re-expression is in fact crucial for the α-cell-mediated β-cell neogenesis. As important was a recent report demonstrating that, upon pancreatic duct ligation, facultative adult stem cells are activated along the lining of duct epithelium (Dor and Melton, 2008; Xu et al., 2008). It was also established that these cells reactivate Ngn3, differentiate into endocrine cells and contribute to the formation of oversized islets. In mice ectopically expressing Pax4, our results suggest that duct-lining cells may represent the source of the neogenerated glucagon-expressing cells. Although we cannot exclude the contribution of acinar cells to this process, additional evidence favor a duct-to-islet cell conversion mechanism: (1) the continuous detection of neo-generated glucagon+ islet cells adjacent to duct structures, (2) the active proliferation within the duct epithelium and near the islet pole where non-β endocrine cells accumulate, (3) the presence of glucagon+ endocrine cells in the ductal lining, and (4) the detection of cells expressing duct markers within the islet. Our findings are supported by recent reports demonstrating the plasticity of pancreatic cells. For instance, D. Melton and coworkers proved that acinar cells could be reprogrammed into β-cells upon the ectopic expression of selected genes in vivo (Zhou et al., 2008), whereas Inada et al. (2008) provided evidence that duct cells may give rise to endocrine and acinar cells in the adult pancreas. In agreement with these, our analysis underlines that hypoglucagonemia activates compensatory mechanisms provoking the conversion of progenitor cells into α-cells that subsequently acquire a β-cell phenotype upon Pax4 ectopic expression, these Ngn3-dependent processes ultimately leading to the generation of oversized islets of Langerhans.
Pax4 ectopic expression rescues from streptozotocin-induced diabetes
We reasoned that the continuous β-cell neogenesis observed in double-transgenic animals might be able to rescue experimentally-induced diabetes. Therefore, the function of the newly formed β-cells was assessed in diabetic mice with more than 95% β-cell loss following STZ treatment. No insulin supplement was used to counter the sudden β-cell loss, as the consequences of exogenous insulin treatment on islet cells formation are hitherto unclear. Accordingly, a high lethality was expected, especially in older hyperglycemic and weakened mice. However, while all control and double transgenic mice older than 10 weeks died, 41% of the younger animals survived during two months post STZ injection. Closer examination revealed a progressive reconstitution of the islets and normalization of the glycemia and indicated that the new insulin+ cells behaved as true β-cells. Also under these conditions, clusters of glucagon+ cells were detected at one pole of the islets, adjacent to duct structures, such cells subsequently adopting a β-cell phenotype. Furthermore, the endocrine tissue in animals that died during the course of these experiments showed significant β-cell neogenesis, but, most likely, not sufficient to allow survival. The observation that only double transgenic animals younger than four weeks of age could survive STZ treatment was intriguing. Interestingly, this age corresponds to the period when such animals, initially hypoglycemic, progressively develop a hyperglycemic condition. While the low blood glucose levels observed after birth can be easily explained by the β-cell hyperplasia, the development of a diabetic condition was unexpected: the steady increase in insulin-producing cell content and the reduced number of glucagon-expressing cells are in contrast with the observed high glucose levels. In the light of the data reported in this study, it appears that the newly-formed insulin-expressing cells are functional for at least four weeks following birth and respond normally to a glucose challenge. During this time, they express all the β-cell markers we tested and are negative for α-, δ- and PP-cell marker genes. However, glycemia, glucose tolerance, insulin secretion and insulin sensitivity deteriorate in older animals despite a normal expression of a typical β-cell complement of transcription factors, suggesting that older β-cells fail to trigger an optimal response upon glucose challenge. Interestingly, these retain some capacity to secrete insulin when challenged with arginine or a long-acting GLP-1 analog. The reasons for the establishment of such a condition are unclear, but may result from progressive (1) β-cell exhaustion, (2) β-cell alterations, (3) desensitization of the insulin receptor/pathway as a consequence of increased insulin levels, and/or (4) unknown effects induced by the decreased glucagon/somatostatin/PP hormone contents. Defining the mechanisms involved would require further work in which the impact of β-cell hyperplasia, but also of the decrease in α-, δ- and PP-cells contents, could be modulated and analyzed independently. It is interesting to notice that younger double-transgenics subjected to streptozotocin treatment display an extended lifespan in comparison to untreated counterparts (data not shown). This indicates that the development of the diabetic condition in these mice is not age-related, but may rather depend on the islet hyperplasia state. Clearly, using different activation times of exposure to Pax4 would allow us to determine its long-term effect on islet function. Together, our results suggest that the sole ectopic expression of Pax4 in glucagon+ cells can, in younger animals, reverse the consequences of streptozotocin-mediated diabetes through the induction of α-cell neogenesis and their ensuing conversion into functional β-cells. Based on these findings, we suggest that drug-mediated modulation of Pax4 and/or its targets may open new avenues for treatment of diabetes and, in addition, may contribute to strategies aiming to differentiate β-cells from stem, progenitor or other cell types.
EXPERIMENTAL PROCEDURES
Mouse Manipulations
The strategy used to generate the POE mouse line is depicted is Figure S1. These mice were crossed with Pdx1-, Pax6-, Glucagon and Insulin-cre lines (Ashery-Padan et al., 2004; Gu et al., 2002; Herrera, 2000) and genotyped using a combination of fluorescence microscopy for GFP examination and genotyping PCR for cre and β-galactosidase genes.
To assess the effects of glucagon on islet size, mice were injected intraperitoneally twice daily (every 12h) with 5μg of glucagon and sacrificed after 3 weeks of treatment. For STZ-mediated diabetes induction, a freshly prepared 50mg/ml solution in 0.1mol/l sodium citrate, pH 4.5 was injected intraperitoneally (200mg/kg). Lentivirus production and injection were performed as described previously (Xu et al., 2008). Lastly, BrdU was injected intraperitoneally (200μl of 100μg/ml) and was detected by immunohistochemistry (Invitrogen).
Immunohistochemistry
Tissues were fixed in 4% PFA overnight at 4°C, embedded in paraffin and 8-μm sections applied to slides. These sections were assayed as described previously (Collombat et al. 2003). In order to perform co-immunofluorescence, the GFP signal was bleached when necessary, as described (Collombat et al., 2007). The primary antibodies used were the following: mouse monoclonal anti-insulin, anti-glucagon (1/1000 - Sigma), anti-Ngn3 (1/2000- 1F25A1B3, BCBC Antibody Core), anti-c-Myc (1/100 - Abcam); guinea pig anti-insulin, anti-glucagon (1/1000 - Sigma); rabbit anti-somatostatin (1/600 - Dako), anti-PP (1/200 - Dako), anti-Nkx6.1 (1/3000), anti-Nkx2.2 (1/1000 - kindly provided by T. Jessell), anti-Pax6 (1/500 - kindly provided by S. Saule), anti-Pax4 (1/500), and anti-Arx (1/1000); chicken anti-β-galactosidase (1/500 - Biozol); goat anti-Ngn3 (1/1000 - kindly provided by M. Sander). The secondary antibodies (1/1000 - Molecular Probes) used were: 594-alexa anti-mouse; 488-alexa anti-mouse; 594-alexa anti-rabbit; 488-alexa anti-rabbit; 594-alexa anti-guinea pig; 488-alexa anti-guinea pig. Pictures were proceeded using confocal microscopy. For quantification purpose, stained cells were counted manually on every tenth section and the count reported to the pancreatic area estimated in silico.
Glucose Challenge and Circulating Glucose or Insulin Level Measurements
For glucose challenge tests, 6 mice per genotype were fasted for 24h and injected intraperitoneally with glucose (2g/kg) and blood glucose levels measured 0, 30, 60, 90, 120, 150, and 210min afterwards. At each time point, one animal per genotype was sacrificed immediately after glycemia assessment for serum insulin level determination using RIA (Linco). Glucose levels (mg/dl) were determined with the One Touch Glucose monitoring kit (Johnson & Johnson).
Data Analysis
All values are depicted as mean ± standard error of the mean (SEM) from at least three independent experiments and considered significant if p < 0.05. All data were statistically analyzed by multivariate comparison (two-way ANOVA) with Bonferroni correction or one-way ANOVA with Newman-Keuls correction.
Supplementary Material
ACKNOWLEDGEMENTS
We are most grateful to J. Hecksher-Sørensen, G. Gradwohl, G. Melitzer, A. Stoykova, D. Pipeleers, and P. Gruss for discussion. We are indebted to D. A. Melton, C. V. Wright, P. Herrera, M. Sander, B. Ahrén, and L. Kvist for providing us with mice and/or antibodies. We also thank M. Van de Casteele, J. Hald, T. Rabe, S. Niemann-Seyde, T. Mundinger, R. Faubel, O. Jäckle, T. Schulz, U. Franke, and S. Schrötter for excellent technical assistance, as well as the BTL crew for their support with the mice. The authors are supported by the Max-Planck Society, the Dr. H. Storz and Alte Leipziger foundation, the Inserm, the Inserm-Avenir Program, the Juvenile Diabetes Research foundation (26-2008-639) and the NIH Beta Cell Biology Consortium (U19 DK 072495-01).
REFERENCES
- Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery-Padan R, Zhou X, Marquardt T, Herrera P, Toube L, Berry A, Gruss P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev Biol. 2004;269:479–488. doi: 10.1016/j.ydbio.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Blume N, Skouv J, Larsson LI, Holst JJ, Madsen OD. Potent inhibitory effects of transplantable rat glucagonomas and insulinomas on the respective endogenous islet cells are associated with pancreatic apoptosis. J Clin Invest. 1995;96:2227–2235. doi: 10.1172/JCI118278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun T, Franklin I, St-Onge L, Biason-Lauber A, Schoenle EJ, Wollheim CB, Gauthier BR. The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol. 2004;167:1123–1135. doi: 10.1083/jcb.200405148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Serup P, Mansouri A. Specifying pancreatic endocrine cell fates. Mech Dev. 2006;123:501–512. doi: 10.1016/j.mod.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Broccoli V, Krull J, Ponte I, Mundiger T, Smith J, Gruss P, Serup P, Mansouri A. The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development. 2005;132:2969–2980. doi: 10.1242/dev.01870. [DOI] [PubMed] [Google Scholar]
- Collombat P, Hecksher-Sorensen J, Krull J, Berger J, Riedel D, Herrera PL, Serup P, Mansouri A. Embryonic endocrine pancreas and mature beta cells acquire alpha and PP cell phenotypes upon Arx misexpression. J Clin Invest. 2007;117:961–970. doi: 10.1172/JCI29115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat P, Mansouri A, Hecksher-Sorensen J, Serup P, Krull J, Gradwohl G, Gruss P. Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev. 2003;17:2591–2603. doi: 10.1101/gad.269003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- Dor Y, Melton DA. Facultative endocrine progenitor cells in the adult pancreas. Cell. 2008;132:183–184. doi: 10.1016/j.cell.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Furuta M, Yano H, Zhou A, Rouille Y, Holst JJ, Carroll R, Ravazzola M, Orci L, Furuta H, Steiner DF. Defective prohormone processing and altered pancreatic islet morphology in mice lacking active SPC2. Proc Natl Acad Sci U S A. 1997;94:6646–6651. doi: 10.1073/pnas.94.13.6646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, Obici S, Tang B, Holst JJ, Fledelius C, et al. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Proc Natl Acad Sci U S A. 2003;100:1438–1443. doi: 10.1073/pnas.0237106100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci U S A. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grapin-Botton A, Majithia AR, Melton DA. Key events of pancreas formation are triggered in gut endoderm by ectopic expression of pancreatic regulatory genes. Genes Dev. 2001;15:444–454. doi: 10.1101/gad.846001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Hussain MA, Lee J, Miller CP, Habener JF. POU domain transcription factor brain 4 confers pancreatic alpha-cell-specific expression of the proglucagon gene through interaction with a novel proximal promoter G1 element. Mol Cell Biol. 1997;17:7186–7194. doi: 10.1128/mcb.17.12.7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A. 2008 doi: 10.1073/pnas.0805803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J, Heller RS, Funder-Nielsen T, Pedersen EE, Lindsell C, Weinmaster G, Madsen OD, Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163–176. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- Johansson KA, Dursun U, Jordan N, Gu G, Beermann F, Gradwohl G, Grapin-Botton A. Temporal control of neurogenin3 activity in pancreas progenitors reveals competence windows for the generation of different endocrine cell types. Dev Cell. 2007;12:457–465. doi: 10.1016/j.devcel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- Petersson B, Hellman B. Effects of Long Term Administration of Glucagon on the Pancreatic Islet Tissue of Rats and Guinea-Pigs. Acta Endocrinol (Copenh) 1963;44:139–149. doi: 10.1530/acta.0.0440139. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Induction of islet cell neogenesis in the adult pancreas: the partial duct obstruction model. Microsc Res Tech. 1998;43:337–346. doi: 10.1002/(SICI)1097-0029(19981115)43:4<337::AID-JEMT8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sander M, Neubuser A, Kalamaras J, Ee HC, Martin GR, German MS. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- Sander M, Sussel L, Conners J, Scheel D, Kalamaras J, Dela Cruz F, Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- Theis M, Mas C, Doring B, Degen J, Brink C, Caille D, Charollais A, Kruger O, Plum A, Nepote V, et al. Replacement by a lacZ reporter gene assigns mouse connexin36, 45 and 43 to distinct cell types in pancreatic islets. Exp Cell Res. 2004;294:18–29. doi: 10.1016/j.yexcr.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Wang Q, Elghazi L, Martin S, Martins I, Srinivasan RS, Geng X, Sleeman M, Collombat P, Houghton J, Sosa-Pineda B. Ghrelin is a novel target of Pax4 in endocrine progenitors of the pancreas and duodenum. Dev Dyn. 2008;237:51–61. doi: 10.1002/dvdy.21379. [DOI] [PubMed] [Google Scholar]
- Webb GC, Akbar MS, Zhao C, Swift HH, Steiner DF. Glucagon replacement via micro-osmotic pump corrects hypoglycemia and alpha-cell hyperplasia in prohormone convertase 2 knockout mice. Diabetes. 2002;51:398–405. doi: 10.2337/diabetes.51.2.398. [DOI] [PubMed] [Google Scholar]
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van De Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.