Abstract
Background
Emotional interference tasks may be useful in probing anterior cingulate cortex (ACC) function to understand abnormal attentional control in individuals with specific phobia.
Methods
In a 3T functional MRI scanner, individuals with specific phobias of the animal subtype (SAP, n=12) and healthy comparison adults (HC, n=12) completed an event-related emotional counting Stroop (ecStroop) task. Individuals were presented phobia-related, negative, and neutral words and were instructed to report via button-press the number of words displayed on each trial.
Results
Compared to the HC group, the SAP group exhibited greater rostral ACC activation (i.e., greater response to phobia-related words than neutral words). In this same contrast, HCs exhibited greater right amygdala and posterior insula activations as well as greater thalamic deactivations than the SAP group. Both groups exhibited anterior cingulate, dorsomedial prefrontal cortex (dmPFC), inferior frontal gyrus/insula, and amygdala activations as well as thalamic deactivations. Psychophysiological interaction (PPI) analysis highlighted a network of activation in these regions in response to phobia-related words in the SAP group.
Conclusions
Taken together, these findings implicate a circuit of dysfunction that is linked to attention abnormalities in individuals with SAP.
Keywords: anxiety, attention, fMRI, cingulate, amygdala, psychophysiological interaction
1. Introduction
Individuals with specific phobias are characterized by exaggerated anxiety responses to feared objects (e.g., animals, places). When an individual with specific phobia is exposed to the feared object, real or imagined, anxiety symptoms are experienced often leading to avoidance behaviors that interfere with normal daily activities [1]. Because the fears are circumscribed to specific objects (e.g., spiders, snakes, rodents) and animal phobias are common, symptom provocation studies have been most widely used to identify brain regions implicated in the pathophysiology of specific animal phobia (SAP). Exaggerated anterior cingulate cortex (ACC), dorsomedial prefrontal cortex (dmPFC), insular cortex, and amygdala activations in response to phobia-related videos [2], pictures [3, 4], and words [5] compared to neutral stimuli have been reported in SAP. Although provocation studies have identified the regions involved in the evoked fear response, the psychological processes that are presumably dysfunctional (e.g. attention) are not isolated using these paradigms.
Attention biases towards threat have been demonstrated in individuals with specific phobias [6–8]. One functional MRI (fMRI) study that manipulated attention while individuals with spider phobias viewed pictures of spiders showed that cognitive labeling elicited similar regions of activation as symptom provocation tasks [3]. Our research group has experience administering the emotional counting Stroop (ecStroop) task, an emotional interference task where individuals are presented emotional or neutral words and asked to report via button-press the number of words displayed, as a means to probe ACC function [9]. The ecStroop task measures response (i.e., reaction time) as an index of attention control to maintain cognitive set (i.e., word counting), while participants are presented emotional vs. neutral distracters. In healthy subjects, rostral ACC (rACC) activation is greater in response to negative words relative to neutral words [10]. Likewise, in a study of post-traumatic stress disorder (PTSD), trauma-exposed non-PTSD subjects activated rACC in response to combat-related relative to neutral words, whereas PTSD patients failed to activate this region [11]. We wished to extend this investigation to individuals with SAP to examine ACC function in response to the ecStroop emotional interference task.
Previous fMRI studies using the ecStroop task have employed block designs. However, an event-related design may overcome limitations inherent to fMRI block designs and provide added utility in future studies. Rostral ACC responses, a main region of interest for ecStroop tasks, may habituate over time [10–12]. Unlike previous block-design ecStroop experiments, rapid event-related designs may be more resistant to habituation effects, due to the intermixing of conditions. Additionally, event-related designs would allow individual trials to be examined, rather than being constrained to examine averaged effects over entire blocks of trials. Studying multiple anxiety disorders (e.g., SAP, PTSD) to determine specificity of ACC response would require symptom-specific trials for each disorder, resulting in long periods of non-disorder specific word blocks. An event-related design allows trial types to be interspersed accordingly and would provide added utility to determine disorder specificity.
In this fMRI study, an event-related ecStroop task, designed to be tested with multiple anxiety disorders, was administered to individuals with SAP and healthy comparison (HC) subjects. It was hypothesized that the circuitry highlighted in symptom provocation studies of SAP (i.e., ACC, dmPFC, insula, and amygdala) would be activated in response to phobia-related relative to neutral words to a greater extent in the SAP vs. HC group. Further, the interregional connectivity between these specific nodes may be altered; therefore, we used functional connectivity analysis to test whether such between-group differences existed.
2. Methods
2.1 Subjects
Prior to enrollment, written informed consent was obtained from all individuals in accordance with the Partners Human Research Committee.
Individuals were recruited from local advertisements to participate in this neuroimaging experiment as paid volunteers. All participants were between 18 and 40 years old, right-handed, English speaking, and had normal or corrected-to-normal vision. Participants denied current or past history of head injury, learning disability, major medical illness, or substance abuse/dependence (>6 months). All subjects were free of any psychotropic medication. Group characteristics are outlined in Table 1.
Table 1.
Group Demographics
| SAP (n=12) | Healthy Control (n=12) | |
|---|---|---|
| Gender (males) | 5 | 4 |
| Age (years) | 25.2±4.5 | 26.7±5.5 |
| Education (years) | 15.8±2.4 | 16.2±1.3 |
| ASI | 16.8±9.0* | 8.8±4.9 |
| BAI | 4.3±3.2* | 1.5±1.8 |
| BDI | 3.5±3.6* | 1.0±1.5 |
| STAI-Trait | 37.2±6.5* | 31.6±5.1 |
| STAI-State | 32.0±7.9 | 29.0±9.0 |
Group means ± standard deviations are presented for specific animal phobia (SAP) and healthy control groups. ASI=Anxiety Sensitivity Index, BAI=Beck Anxiety Inventory, BDI=Beck Depression Inventory, Spielberger STAI= State-Trait Anxiety Inventory.
Significant group difference at P<0.05 (two-tailed) indicates SAP reported higher levels of anxiety and depression than healthy control group.
The Structured Clinical Interview for DSM-IV (SCID) [13] was administered to all participants. Individuals meeting DSM-IV criteria for SAP, with phobias of snakes, spiders, and/or rodents were included. In our final sample, 6 individuals had snake phobias, 8 individuals had spider phobias, and 7 individuals had rodent phobias. Six individuals with SAP had combinations of these animal phobia types. All individuals were free from any past or current Axis I psychiatric disorder, other than SAP for the SAP group. For all participants, indices of anxiety and depressive symptom severity were measured using the Anxiety Sensitivity Index, Spielberger State-Trait Anxiety Inventory, Beck Anxiety Inventory, and Beck Depression Inventory [14–17].
The final study sample included 12 individuals with SAP and 12 HC subjects, matched for age, gender, and years of education. This final sample was obtained after 1 SAP and 1 HC were excluded for excessive head movement and 2 HCs were excluded for incomplete behavioral or imaging data.
2.2 Procedures
2.2.1 The emotional counting Stroop task
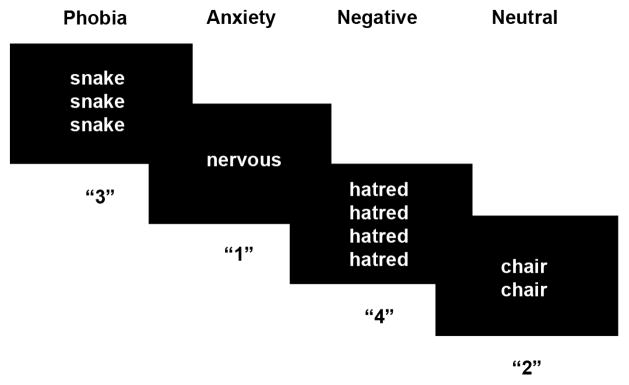
Participants completed an event-related version of the ecStroop task (Figure 1) [9]. In this task, participants viewed 1–4 identical words on each trial. Participants were instructed to respond (via button press) the number of words presented. The keys mapped 1–4 from left to right most positions. Participants were instructed to respond with the left middle finger for “1”, left index finger for “2”, right index finger for “3” and right middle finger for “4”.
Figure 1.
Event-Related Emotional Counting Stroop (ecStroop) Task. For each trial, subjects reported the number of words on the screen via button press. Phobia-related, anxiety-related, negative and neutral words were presented.
Prior to performing the ecStroop task, each subject participated in a separate paradigm involving masked presentations of fear, happy and neutral faces [18].
2.2.2 Stimuli
Eighty-four unique words were each presented for four separate trials during the course of the experiment. Phobia-related words (e.g., snakes, slithering, bite), anxiety-related words (e.g., distress, nervous, worry), general negative (e.g., hatred, frustrated, punish), and neutral words (e.g., chair, mild, describe) were presented. The phobia-related words were selected to represent words associated with snake, spider, and rodent phobias. General anxiety-related, PTSD-related and panic disorder-related (PD) words were also presented. All sets of words were matched in terms of word length, part of speech, and frequency of usage in the English language [19].
Four functional runs of this task were completed. Across the entire experiment, the four trials of a particular word included all possible response permutations (i.e., 1, 2, 3, and 4 word presentations). All 84 words were presented once per run, along with 20 fixation trials. Each trial consisted of a 1450 ms word display followed by 50 ms fixation (i.e., a fixation point centered on the screen). The word order was determined using an optimization tool for event-related fMRI designs [20], which introduces “jitter” by interspersing 1–5 consecutive fixation trials within each run to maximize the ability to deconvolve the blood oxygenation level dependent (BOLD) signal according to trial type.
2.2.3 Apparatus
Words were displayed in white text and centered on a black background via standardized software (E-Prime, Inc, 1.1) using a Dell Inspiron 6000 laptop computer with Intel® Pentium® M Processor 750 (1.86GHz/2MB Cache/533MHz FSB) and projected via a Sharp XG-2000V color LCD projector (Osaka, Japan) onto a rear-projection screen.
Magnetic resonance images were collected with a Siemens Trio 3.0T whole-body high-speed imaging device equipped for echo planar imaging (EPI) (Siemens Medical Systems, Iselin NJ) and a 12-channel gradient head coil.
2.2.4 Functional MRI Data Acquisition
An automated scout image was acquired and localized shimming procedures were performed to optimize field homogeneity. A high resolution 3D MPRAGE sequences (TR/TE/flip angle=2530ms/3.45ms/7°, 1.3mm in-plane resolution, and 1.3 mm slice thickness) were collected for spatial normalization. Then, a T1-EPI (TR/TE =10sec/34ms) and T2-weighted (TR/TE/flip angle/FOV=5210sec/103ms/90°/200 mm) sequences were gathered to monitor scanner function and to assist in registration of the functional data to the high-resolution anatomical scan. Functional MRI images reflecting BOLD signal were acquired using a gradient echo T2*-weighted sequence (TR/TE/flip angle/FOV=1.5sec/30ms/90°/200 mm). Four functional runs were completed after reaching longitudinal magnetization equilibrium, yielding 104 acquisition volumes per run. The T1-EPI, T2, and gradient-echo functional images were collected in the same plane (25 axial slices angled approximating the Anterior Commissure-Posterior Commissure line) with the same slice thickness (5mm; voxel size 3.125 × 3.125 × 5mm, no interslice skip), excitation order (interleaved) and phase encoding (anterior-to-posterior, right-to-left for T2).
2.2.5 Post-scan ratings
Immediately after scanning, all subjects rated the word stimuli according to relevance (0=low to 6=high), arousal (0=low to 6=high) and valence (−3=negative to +3=positive). Relevance reflected how appropriate the words were to the individual’s personal concerns and fears. Arousal reflected the emotional intensity of the word. Valence reflected the extent to which the word was unpleasant or pleasant.
2.2.6 Data Analysis
Behavioral
Online (reaction time and accuracy) and post-scan (relevance, arousal, and valence ratings) behavioral data were analyzed using a 2 (group: SAP, HC) × 4 (word type: phobia, anxiety, negative and neutral) repeated measures ANOVA in SPSS 15.0. Statistical significance was determined using an alpha-level of 0.05. Where appropriate, a multiple comparison correction was applied. Post-hoc analyses using two-tailed t-tests were performed where indicated.
BOLD Activation Patterns
Preprocessing and image analysis was completed in SPM5 [21]. The parameters to motion-correct the functional images to the mean image were calculated using 6-parameter rigid body spatial registration. In addition, any EPI motion-related susceptibility was removed via unwarping. Each anatomical MPRAGE image was co-registered to each individual’s mean functional image. Segmentation parameters were used to normalize the functional images to the SPM5’s MNI T1 2×2×2 template. Finally, the functional images were spatially smoothed with a 6mm full-width-half-maximum (FWHM) Gaussian filter.
A general linear model was created for each individual. The data were convolved with the canonical hemodynamic response function (HRF) and modeled using the different word types as conditions: phobia-related, general anxiety-related, PTSD-related, panic-related, negative, and neutral words as well as fixation. A high pass filter with 128 second cut-off was used to eliminate low-frequency drift and AR1 correction was used to remove any temporal autocorrelation. To isolate within-brain voxels, the SPM masking threshold was reduced and an explicit mask representing the combined gray and white matter volume was included. A series of estimated betas, one for each regressor, was generated to minimize the error term within the model. Contrasts were generated by comparing the beta weights associated with BOLD activation in response to phobia-related relative to neutral words (i.e., phobia>neutral) across all runs. In addition, deactivations (i.e., neutral>phobia) were also examined.
A whole-brain voxel-wise analysis was conducted using random effects analysis. Contrast images from individual analyses were entered into a 2nd-level model. One-sample and two-sample t-tests were conducted to determine significant activation and group interaction effects. We examined the ACC, dmPFC, insula and amygdala, a priori regions of interest (ROI). Significant activation threshold was set at P<0.005 uncorrected, with a cluster criterion of at least 5 contiguous voxels. Brain regions were identified by visual inspection and cross-referenced with the Talairach atlas [22]. Montreal Neurological Institute (MNI) coordinates are reported throughout.
Percent signal change values were extracted using functionally-defined regions based on the whole-brain voxelwise analysis group × condition interaction, using the same activation threshold to determine the activation and deactivation patterns in response to phobia and neutral conditions. MarsBar [23] was used to define each ROI and to extract BOLD data, in terms of the mean BOLD signal within the ROI. The percent signal change value relative to fixation was calculated. Where applicable, paired t-tests determined significant condition effects in each group. Significance was determined using an alpha-level of 0.05.
Functional Connectivity
Psychophysiological interaction (PPI) analysis was conducted to identify significant differences in functional connectivity based on task [24, 25]. In other words, regions may be more functionally connected (i.e., covary together more strongly) during specific tasks and this analysis tests the differences in regional covariance based on task by testing differences in regression slopes. PPI analysis uses a design matrix that incorporates a psychological variable (e.g., task), the time course of the seed region (i.e., physiological variable), and the interaction between the psychological variable and the physiological variable. Of note, PPI analysis does not provide information regarding direction of regional influence.
In the current study, we were interested in the connectivity between the right rACC, a region showing a significant group difference in activation to phobia relative to neutral words, and the amygdala, dmPFC, insula, and thalamus. A 6mm radius sphere around the peak activation voxel [(12,46,24)] in the omnibus test of phobia vs. neutral words was used to extract activation from this seed ROI. A model including the psychological condition, time course of rACC, and the interaction term was created for each individual to determine whether region-region interactions varied by task (i.e., phobia-related words vs. neutral words). The interaction effect was entered into a 2nd-level random effects analysis to determine whether task-dependent functional connectivity differed by group (i.e., individuals with SAP vs. HCs). Within-group and between-group analyses were investigated to determine differences in inter-regional interactions. Significant differences in functional connectivity depending on task were detected using a P<0.005 uncorrected threshold and spatial extent of at least 5 contiguous voxels.
3. Results
3.1 Behavioral Results
In terms of reaction time and accuracy, no group effects or group × condition interactions were statistically significant [all P>0.1]. Reaction times were slower in response to phobia-related words than negative words [t(23)=1.9, P<0.04, one-tailed] and neutral words [t(23)=1.8, P<0.04, one-tailed], indicating an emotional interference effect. Differences in accuracy rates were not statistically significant across word types [condition effect: F(3,66)=0.06, P>0.9]. Table 2.
Table 2.
On-line Behavioral Responses
| SAP (n=12) | Healthy Control (n=12) | |
|---|---|---|
| Accuracy (% Correct) | ||
| Phobia-Related Words | 96.4±2.8 | 98.1±2.6 |
| Anxiety-Related Words | 97.4±3.2 | 97.4±2.6 |
| Negative Words | 97.8±3.0 | 96.6±2.8 |
| Neutral Words | 97.1±1.9 | 96.6±3.4 |
| Reaction Time (ms) | ||
| Phobia-Related Words | 747.4±54.4 | 728.6±81.1 |
| Anxiety-Related Words | 740.9±59.2 | 732.8±86.4 |
| Negative Words | 741.6±61.2 | 715.7±75.4 |
| Neutral Words | 741.4±50.7 | 722.6±78.5 |
Group means ± standard deviations are presented for specific animal phobia (SAP) and healthy control groups. No significant between-group differences were found.
Ratings showed a significant group × condition interaction [all F(3,66)>4.1, P<0.02]. Compared to HCs, SAP individuals rated the phobia-related words as more relevant (SAP: 3.0±1.8, HC: 1.5±1.5), more arousing (SAP: 3.3±1.4, HC: 1.5±1.4), and more negative (SAP: −1.8±0.6, HC: −0.9±0.8) [all t(22)>2.2, P<0.04]. In addition, consistent with the condition manipulations, main effects of condition were evident across groups. Phobia-related words, anxiety-related words and negative words were rated as more relevant, more arousing and more negative than neutral words [condition effect: all F(3,66)>10.6, P<0.001].
3.2 Imaging Results
Between-group patterns
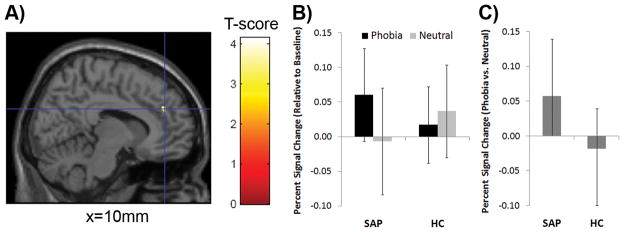
Group differences in neural response to phobia-related vs. neutral words during the ecStroop task were detected in a priori regions, including ACC, insula, amygdale, and thalamus. Individuals with SAP exhibited greater right rACC activation than HCs. Individuals with SAP exhibited greater right rACC activation in response to phobia-related words relative to neutral words [t(11)=−2.7, P<0.02], whereas the response between the two conditions did not differ in HCs [t(11)=1.1, P>0.3]. Figure 2
Figure 2.
Group difference detected in rostral anterior cingulate (rACC). A) Individuals with specific animal phobia (SAP) had greater right rostral anterior cingulate (rACC) activation in response to phobia-related compared to neutral words [(10, 42, 28)] compared to healthy controls (HC) in the emotional Counting Stroop task. Activation is displayed in sagittal view of the single subject overlay within SPM using a P<0.005 uncorrected display threshold. MNI coordinates are reported. B) Percent signal change values fixation were extracted from this functionally defined region of interest (ROI), rACC, showing SAP group exhibited greater response to phobia-related than neutral words C) Group differences in rACC activation in response to phobia-related vs. neutral words also confirm group difference in activation. Mean ± Standard deviations are displayed.
For the phobia related vs. neutral words contrast, in comparison to SAP, HCs exhibited greater activations in lateral amygdala and posterior insula, as well as a greater thalamic deactivation. Table 3.
Table 3.
Between-Group Comparisons of BOLD Activation/Deactivation in response to Phobia-related relative to Neutral words
| MNI Coordinates | |||||
|---|---|---|---|---|---|
| A priori Regions of Interest | x | y | z | Z-score | k |
| ACTIVATION (Phobia-Related > Neutral Words) | |||||
| Specific Animal Phobics>Healthy Controls | |||||
| Rostral Anterior Cingulate/Dorsomedial prefrontal Cortex BA32/BA9 | 10 | 42 | 28 | 3.08 | 13 |
| Healthy Controls>Specific Animal Phobics | |||||
| R Amygdala | 30 | −2 | −22 | 2.95 | 6 |
| Posterior insula | 38 | −22 | 6 | 3.09 | 32 |
| DEACTIVATION (Neutral > Phobia-Related Words) | |||||
| Healthy Controls>Specific Animal Phobics | |||||
| Thalamus | −14 | −8 | −4 | 3.12 | 7 |
| −2 | −20 | 6 | 2.65 | ||
Within-group patterns
In response to phobia-related compared to neutral words, both SAP and HC groups exhibited activations in the rACC/dmPFC (BA 32/8), inferior frontal gyrus, insula and lateral amygdala. Additionally, thalamic deactivations (i.e., greater response to neutral words compared to phobia-related words) were detected in both groups. Table 4.
Table 4.
Within-Group Comparisons of BOLD Activation/Deactivation in response to Phobia-related relative to Neutral Words
| MNI Coordinates | |||||
|---|---|---|---|---|---|
| A priori Regions of Interest | x | y | z | Z-score | k |
| ACTIVATION (Phobia-Related > Neutral Words) | |||||
| Specific Animal Phobics | |||||
| A priori regions | |||||
| R rACC/dMPFC (BA32/BA9) | 12 | 46 | 30 | 3.83 | 211 |
| 12 | 54 | 16 | 4.03 | ||
| 6 | 52 | 36 | 2.91 | ||
| L rACC/dMPFC (BA32/BA9) | −10 | 44 | 36 | 3.32 | 22 |
| −6 | 38 | 32 | 2.73 | ||
| L rACC (BA32) | −12 | 44 | 16 | 3.61 | 42 |
| R dACC (BA24) | 8 | 24 | 20 | 3.67 | 18 |
| L dACC/dMPFC (BA32/BA8) | 12 | 18 | 46 | 3.12 | 6 |
| L dMPFC (BA9) | −10 | 58 | 28 | 4.34 | 83 |
| L dMPFC (BA8) | −2 | 48 | 40 | 2.71 | 5 |
| L Rostral dMPFC (BA9/10) | −14 | 62 | 16 | 3.4 | 33 |
| L Amygdala | −30 | 2 | −22 | 4.64 | 66 |
| −22 | −10 | −30 | 3.71 | ||
| −20 | −8 | −18 | 3.01 | ||
| −30 | 10 | −20 | 2.92 | 7 | |
| L Insula/Inferior Frontal Gyrus BA44/45 | −50 | 22 | 6 | 3.8 | 404 |
| −52 | 36 | 6 | 3.7 | ||
| −40 | 24 | 2 | 3.52 | ||
| L Insula | −30 | 30 | −8 | 3.2 | 20 |
| Healthy Controls | |||||
| Apriori Regions | |||||
| L rACC (BA32) | −4 | 28 | 42 | 3.22 | 10 |
| L dMPFC (BA8) | −8 | 54 | 40 | 3.6 | 77 |
| −14 | 44 | 44 | 3.1 | ||
| R Amygdala | 32 | 10 | −20 | 3.06 | 10 |
| L Inferior Frontal Gyrus BA46 | −46 | 42 | 6 | 3.49 | 109 |
| −40 | 48 | −2 | 3.26 | ||
| L Inferior Frontal Gyrus BA46/Insula | −48 | 18 | 16 | 2.82 | 7 |
| R Posterior Insula | 40 | −18 | 8 | 3.37 | 35 |
| DEACTIVATION (Neutral > Phobia-Related Words) | |||||
| Specific Animal Phobics | |||||
| Thalamus | −6 | −22 | −4 | 2.98 | 7 |
| Healthy Controls | |||||
| Thalamus | 6 | −20 | 10 | 4.3 | 24 |
| −10 | −6 | −2 | 3.01 | 7 | |
| −12 | −22 | 16 | 2.93 | 5 | |
3.3 PPI and Functional Connectivity
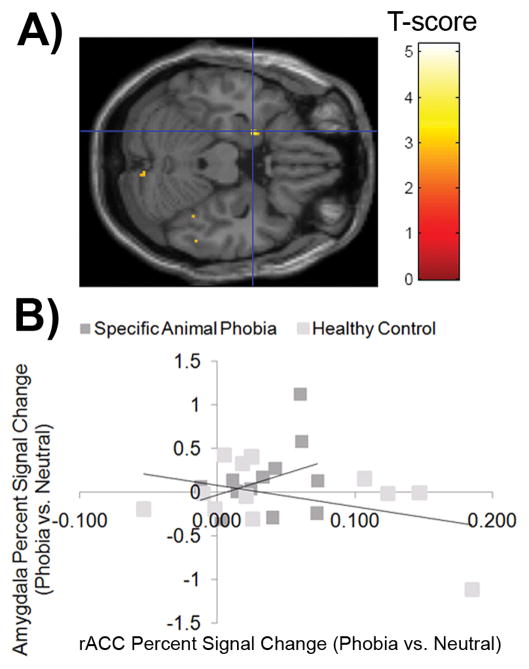
Group differences in connectivity between these regions activated in response to phobia vs. neutral words, the task of interest, were examined using PPI. The rACC-left amygdala connectivity in response to phobia-related relative to neutral words was significantly greater in the SAP group compared to the HC group. Table 5. After extracting signal from both regions (i.e. rACC and left amygdala), the pattern indicated a positive relationship between rACC and left amygdala activation in individuals with SAP; whereas, the activation in the rACC showed a negative relationship with left amygdala activation in the HC group. Of note, both correlations independently were not significant. Figure 3.
Table 5.
Functional Connectivity with rACC seed region dependent on task (phobia-related words vs. neutral words)
| MNI Coordinates | |||||
|---|---|---|---|---|---|
| A priori Regions of Interest | x | y | z | Z-score | k |
| Specific Animal Phobics, Greater connectivity during phobia-related words | |||||
| L Amygdala | −20 | 2 | −22 | 2.85 | 12 |
| Insula | 32 | 28 | −12 | 2.83 | 10 |
| dMPFC | 8 | 66 | 26 | 3.18 | 14 |
| Thalamus | −20 | −6 | 2 | 4.01 | 24 |
| Healthy Controls, Greater connectivity during neutral words | |||||
| Thalamus | 16 | −16 | 8 | 3.45 | 45 |
| Specific Animal Phobics>Healthy Controls, Greater connectivity during phobia-related words | |||||
| L Amygdala | −18 | 0 | −16 | 3.05 | 11 |
Figure 3.
Task-dependent Functional Connectivity Patterns with Rostral Anterior Cingulate. A) Task-dependent functional connectivity was examined between rACC, the seed region defined by omnibus test of significance between phobia-related and neutral words [(12,46,24)], and a priori regions of interest. The SPM-map displays greater rACC-amygdala covariance in response to phobia-related words vs. neutral words. Activation is displayed in axial view of the single subject overlay within SPM using a P<0.005 uncorrected display threshold. B) Based on significant task-dependent functional connectivity at P<0.005 uncorrected in an omnibus test of significance, the left amygdala was identified at (−22, 0, 22). Percent signal change values in response to phobia relative to neutral words relative to baseline fixation were extracted from this functionally defined region of interest (ROI) responses and correlated with the extracted values of the rACC seed region.
In individuals with SAP, region-to-region covariance between rACC and left amygdala, dmPFC, insula, and thalamus was greater in response to phobia-related words compared to neutral words. On the other hand, in HCs, region-to-region covariance between rACC and the thalamus was greater in response to neutral words compared to phobia-related words.
4. Discussion
In summary, the event-related ecStroop task was used to examine the neural activation patterns of response to distracting threat-related words during a cognitive task (i.e., counting words) in SAP and HC groups. Across all participants, reaction time data showed a significant interference effect between phobia-related and neutral words. With regard to brain activation patterns, a significant group × condition interaction was noted in the right rACC, where the SAP group showed greater activation than the HC group for the phobia-related vs. neutral word contrast. HCs exhibited greater right amygdala and posterior insula activations as well as greater thalamic deactivation than the SAP group. The SAP group exhibited an activation pattern similar to that previously observed in symptom provocation studies and the current functional connectivity analysis complemented these results. Using PPI analysis, the rACC-left amygdala functional connectivity in response to phobia-related words relative to neutral words was significantly greater in the SAP group compared to the HC group. Taken together, these findings implicate a neural circuit of dysfunction in individuals with SAP, reflecting attention abnormalities exhibited as either enhanced salience of phobia-related words or deficient emotion regulation.
A group difference was detected in the ACC, a heterogeneous region involved in monitoring, evaluation, and attentional control [26–28]. Regulation via attentional control is needed as limited resources may be available to complete the cognitive task when emotional distracters are present. Attention biases have been demonstrated using emotional Stroop tasks, implying that attention is engaged by phobia-related stimuli [6–8]; yet, it is unclear from such demonstrations whether engagement and/or disengagement of attention is affected. In a transcranial magnetic stimulation study, inactivation of the anterior and mid-cingulate regions abolished reaction time differences in a counting Stroop task [29], providing evidence that the ACC plays a role in mediating attention control during emotional interference. In this study, exaggerated rACC activation may signify increased salience, heightened sensitivity or a lower perceptual threshold for detecting fear, which draws attention towards threat cues more readily. Alternatively, rACC response could indicate emotion regulation needed to shift attention to the cognitive task. In HCs, voluntary suppression of affect in response to negative emotional pictures engages the ACC, the mPFC and the insula [30, 31], the same network detected in this study. When voluntarily controlled, decreased negative emotion has been observed to correlate with ACC and insula activation [31], providing support for the concept that this network may reflect emotion regulation. Of note, as anxiety increases, the rACC may become inefficient at controlling attention [32]. In a study that combined an n-back task and pain anticipation, the rACC activation was reduced in the more demanding cognitive task [33]. Given the lack of group differences in reaction time although finding a group difference in rACC activation, it is unclear whether individuals with SAP are able to perform the ecStroop task similarly to controls because of rACC activation or in spite of differences in rACC recruitment. No significant correlations between magnitude of rACC activation and reaction times during the task were detected, which would have provided support for either of these possibilities. However, we can speculate that while the difficulty level does not appear to reach ceiling, the data suggest that the task may require additional attentional control reflected by exaggerated rACC activation.
Given that our previous work detected rACC activation in HC groups, at first glance, it may seem surprising that rACC activation was found in the SAP group only. However, prior studies of the ecStroop, showing rACC activation in healthy volunteers, did so in response to negative relative to neutral words [10, 11]. In the current study, for the HC group, the phobia-words, like the neutral words, may not interfere with the cognitive task at hand because the phobia-related words may not evoke an emotional response. In this study, group differences in arousal and relevance ratings between the phobia-related and neutral words were only detected in the SAP group; therefore, arousal and relevance differences may be necessary to elicit rACC activation.
In whole-brain voxelwise analysis, HCs exhibited a greater activation in response to phobia-related words relative to neutral words in the right amygdala, mapped to the most lateral aspect of the central amygdala. This result is unexpected as several imaging studies have reported exaggerated amygdala responses to phobia-related pictures in SAP individuals relative to HCs [3, 4, 34]. While some studies have reported exaggerated amygdala activation in response to phobia-related stimuli, other studies did not [35–37]. Several studies have shown that the attention task demands (e.g., cognitive rating) modulate amygdala activation to emotional stimuli [38, 39]. Amygdala activation in the SAP may be reduced because the ecStroop involves implicit, rather than explicit, processing of emotional stimuli. Alternatively, the individuals with SAP, unlike HCs, may have sustained amygdala responses throughout the paradigm due to heightened anticipation of a phobia-related word. In other words, the overriding effect of sustained anxiety may minimize the ability to detect the differential between event-related trials. In addition, a meta-analysis reports the amygdala is preferentially activated by visual emotional stimuli (e.g. spider pictures) [28], suggesting that there may be a fundamental difference in amygdala activation in response to phobia-related images and words. In this study, phobia-related words may engage the amygdala through top-down mechanism, contrasted with bottom-up processing of phobia-related pictures. Thus, the rACC, in a top-down manner, may mitigate emotional interference and accompanied exaggerated amygdala response to phobia-related words in SAPs.
Of note, the analysis of rACC- left amygdala functional connectivity showed a direct correlation in SAP but an inverse correlation in HC groups. In this study, the significant rACC-amygdala functional connectivity in response to phobia-related words in the SAP group is consistent with finding a stronger dmPFC/ACC-amygdala connection during voluntary reappraisal [40] and may provide evidence that rACC activation is adaptive. The laterality of these amygdala findings is noteworthy. Previous work has postulated that the left amygdala may reflect sustained activity in response to threat and the right amygdala may be more involved in initial orienting [41]; therefore, these two groups may be recruiting the amygdala in different ways for different reasons. One possibility is that the heightened danger value of the phobia-words in SAP has been transferred to rACC while the minimal threat signal conveyed by phobia-related words in HCs continues to reside within the amygdala.
In addition to the rACC and amygdala, the thalamus is also highlighted as a region involved in this network in the context of this paradigm. The HC group exhibited greater thalamic deactivation and the functional connectivity between the rACC and thalamus was stronger for neutral words compared to phobia-related words. One potential explanation may be that the sensory input for phobia-related words relayed via the thalamus to the rACC and amygdala may be more salient (i.e., personally significant) to SAP individuals. Alternatively, the cognitive demands of the ecStroop task may alter the prefrontal modulation of the sensory signals through the thalamus [42]. Of note, this interpretation is mere speculation.
Two positions concerning the relationship between task performance and neural activation differences exist in the neuroimaging literature. Matched task performance and confirmation that both groups are able to successfully complete the task allows researchers to interpret that the differences in neural activation are not confounded by group differences in behavioral response. On the other hand, finding group differences in behavior and neural activation may suggest that the physiology is directly linked to the behavioral deficits manifested in the disorder. In this study, significant group differences were found in neural activation in the absence of group differences in the interference effect. Neural activation may be more sensitive to group differences than behavioral differences. An increased sample size may be required to yield behavioral differences in interference effect between these groups. In addition, the absence of group differences may be explained by the use of word stimuli, which are not as potent as a visual picture or live object.
Future studies are needed to elucidate the underlying psychological processes involved in the anxiety response in individuals with SAP. This paradigm alone could not distinguish between fear response and its associated regulation. However, psychological experiments like the dot-probe task may be combined with magnetoencephalography, a neuroimaging modality with superior temporal resolution, to clearly differentiate these possibilities. In addition, separate paradigms examining symptom provocation and emotion regulation in the same subjects may provide answers. In this study, individuals with specific phobias were limited to the animal subtype, and future studies are needed to determine whether these effects will generalize across different types of phobias (e.g., blood, heights). This event-related ecStroop task was shown to elicit activation across the brain regions of a predicted network in SAP individuals. Future studies using this paradigm can be used across multiple anxiety disorders (e.g. PTSD, panic disorder, and SAP) to determine the specificity of ACC response during attentional control when exposed to emotional distracters. Taken together, the results from the previous and current ecStroop tasks suggest that PTSD and SAP may be quite different in rACC response; however, without using the same paradigm definitive conclusions cannot be made regarding disorder-specificity. In the future, this event-related ecStroop paradigm may be used to answer this question of disorder specificity.
Acknowledgments
We thank Lauren M. Price for her assistance with preprocessing data, Jan Beuke for his assistance in scanning, and Mary Foley for her technical assistance.
This work was supported by NIMH grant MH070730 (SLR) and in part by the Intramural Research Program of the NIH, NIMH (JCB).
Footnotes
A previous version of this work was presented at the Anxiety Disorders Association of America annual conference in March 2007 and at the American College of Neuropsychopharmacology annual conference in December 2007.
References
- 1.First MB, et al. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision, DSM-IV-TR. [Google Scholar]
- 2.Straube T, et al. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006;29(1):125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Straube T, Mentzel HJ, Miltner WH. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biol Psychiatry. 2006;59(2):162–70. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Dilger S, et al. Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neurosci Lett. 2003;348(1):29–32. doi: 10.1016/s0304-3940(03)00647-5. [DOI] [PubMed] [Google Scholar]
- 5.Straube T, et al. Brain activation to phobia-related words in phobic subjects. Neurosci Lett. 2004;372(3):204–8. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 6.Kindt M, Brosschot JF. Phobia-related cognitive bias for pictorial and linguistic stimuli. J Abnorm Psychol. 1997;106(4):644–8. doi: 10.1037//0021-843x.106.4.644. [DOI] [PubMed] [Google Scholar]
- 7.Thorpe SJ, Salkovskis PM. Information processing in spider phobics: the Stroop colour naming task may indicate strategic but not automatic attentional bias. Behav Res Ther. 1997;35(2):131–44. doi: 10.1016/s0005-7967(96)00093-9. [DOI] [PubMed] [Google Scholar]
- 8.van den Hout M, et al. Preconscious processing bias in specific phobia. Behav Res Ther. 1997;35(1):29–34. doi: 10.1016/s0005-7967(96)00080-0. [DOI] [PubMed] [Google Scholar]
- 9.Whalen PJ, et al. The emotional counting Stroop: a task for assessing emotional interference during brain imaging. Nat Protoc. 2006;1(1):293–6. doi: 10.1038/nprot.2006.45. [DOI] [PubMed] [Google Scholar]
- 10.Whalen PJ, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44(12):1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 11.Shin LM, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50(12):932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- 12.Phan KL, et al. Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology. 2003;28(7):1344–50. doi: 10.1038/sj.npp.1300186. [DOI] [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders - Patient Edition (SCID-I/P, 11/2002 revsion) New York: Biometrics Research Department, New York State Psychiatric Institute; 2002. [Google Scholar]
- 14.Beck A, Steer R. Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 15.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 16.Spielberger CD. Manual for the state trait anxiety inventory (Form Y) (“Self-evaluation questionnaire”) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 17.Taylor S, et al. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess. 2007;19(2):176–88. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- 18.Rauch SL, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47(9):769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 19.Kucera HaWNF. Computational analysis of present-day American English. Providence: Brown University Press; 1967. [Google Scholar]
- 20.Greeve D. Freesurfer optseq program. [cited; Available from: https://surfer.nmr.mgh.harvard.edu/optseq/
- 21.Friston K. Statistical Parametric Mapping. [cited; Available from: http://www.fil.ion.ucl.ac.uk/spm.
- 22.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Thieme; Stuttgart: 1988. [Google Scholar]
- 23.Matthew Brett J-LA, Valabregue Romain, Poline Jean-Baptiste. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 24.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 25.Gitelman DR, et al. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19(1):200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- 26.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 27.Carter CS, et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc Natl Acad Sci U S A. 2000;97(4):1944–8. doi: 10.1073/pnas.97.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phan KL, et al. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 29.Hayward G, Goodwin GM, Harmer CJ. The role of the anterior cingulate cortex in the counting Stroop task. Exp Brain Res. 2004;154(3):355–8. doi: 10.1007/s00221-003-1665-4. [DOI] [PubMed] [Google Scholar]
- 30.Eippert F, et al. Regulation of emotional responses elicited by threat-related stimuli. Hum Brain Mapp. 2007;28(5):409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phan KL, et al. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 32.Bishop S, et al. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7(2):184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 33.Kalisch R, et al. Levels of appraisal: a medial prefrontal role in high-level appraisal of emotional material. Neuroimage. 2006;30(4):1458–66. doi: 10.1016/j.neuroimage.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Schienle A, et al. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neurosci Lett. 2005;388(1):1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 35.Rauch SL, et al. A positron emission tomographic study of simple phobic symptom provocation. Arch Gen Psychiatry. 1995;52(1):20–8. doi: 10.1001/archpsyc.1995.03950130020003. [DOI] [PubMed] [Google Scholar]
- 36.Wright CI, et al. Amygdala and insular responses to emotionally valenced human faces in small animal specific phobia. Biol Psychiatry. 2003;54(10):1067–76. doi: 10.1016/s0006-3223(03)00548-1. [DOI] [PubMed] [Google Scholar]
- 37.Wik G, et al. A functional cerebral response to frightening visual stimulation. Psychiatry Res. 1993;50(1):15–24. doi: 10.1016/0925-4927(93)90020-i. [DOI] [PubMed] [Google Scholar]
- 38.Taylor SF, et al. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 39.McClure EB, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 40.Banks SJ, et al. Amygdala frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wright CI, et al. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18(3):660–9. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 42.Das P, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26(1):141–8. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]