Summary
Molecular mechanisms underlying the reciprocal regulation of the two major surface lipoproteins and virulence factors of Borrelia burgdorferi, OspA and OspC, are not fully understood. Herein, we report that inactivation of the ospAB operon resulted in overproduction of OspC and many other lipoproteins via the constitutive activation of the Rrp2-RpoN-RpoS pathway. Complementing the ospAB mutant with a wild-type copy of ospA, but not an ospA variant that lacks the lipoprotein signal sequence, restored normal regulation of the Rrp2-RpoN-RpoS pathway; these results indicate that the phenotype was not caused by spurious mutations. Interestingly, while most of the ospAB mutant clones displayed a constitutive ospC expression phenotype, some ospAB mutant clones showed little or no ospC expression. Further analyses revealed that this OspC-negative phenotype was independent of abrogation of ospAB. While activation of the Rrp2-RpoN-RpoS pathway was recently shown to downregulate ospA, our findings suggest that reduction of OspA can also activate this pathway. We postulate that the activation of the Rrp2-RpoN-RpoS pathway and downregulation of OspA form a positive feedback loop that allows spirochaetes to produce and maintain a constant high level of OspC and other lipoproteins during tick feeding, a strategy that is critical for spirochaetal transmission and mammalian infection.
Introduction
Dramatic alterations in surface lipoprotein profiles is a key strategy that the Lyme disease pathogen, Borrelia burgdorferi, has evolved in order to adapt to the two contrasting host environments in arthropods and vertebrates (Philipp, 1998; Haake, 2000; Anguita et al., 2003; Schwan, 2003; Singh and Girschick, 2004). The importance of differential gene expression in the enzootic cycle of B. burgdorferi is underscored by the findings that two major virulence factors, outer surface lipoproteins OspA and OspC, undergo a reciprocal alteration of expression during tick feeding (Schwan et al., 1995; Montgomery et al., 1996; de Silva et al., 1996; Akins et al., 1998; Schwan and Piesman, 2000; Ohnishi et al., 2001). OspA, an adhesin essential for spirochaetal colonization in tick midguts (Pal et al., 2000; 2004a; Yang et al., 2004), is mainly expressed in unfed ticks. During tick engorgement, ospA is concomitantly downregulated with upregulation of ospC, a virulence gene that is indispensable for B. burgdorferi to establish initial infection in mammals, and although controversial, for the migration of the bacteria from the midgut to tick salivary glands (Grimm et al., 2004; Pal et al., 2004b; Ramamoorthi et al., 2005; Stewart et al., 2006; Tilly et al., 2006). Spirochaetes also display a high level of OspC and low level of OspA during the early phase of replication in the mammalian host (Hodzic et al., 2003; Schwan, 2003). Furthermore, B. burgdorferi variants that either do not express ospC or constitutively express ospA were found to be incapable of establishing infection in mammals (Grimm et al., 2004; Strother and de Silva, 2005). Although spirochaetes exhibiting all combinations of OspA and OspC expression have also been observed in tick midguts during feeding (Ohnishi et al., 2001), the inverse regulation of OspA and OspC is thought to be important for spirochaetal migration between ticks and mammals and the initial colonization within both hosts.
Differential expression of ospA and ospC has served as a paradigm for studying the molecular mechanisms underlying temporal gene regulation in B. burgdorferi. Such investigations have led to the discovery of a central regulatory pathway, the Rrp2-RpoN-RpoS regulatory network (also referred as the σ54-σS sigma factor cascade), which controls production of OspC and numerous other differentially expressed genes in B. burgdorferi (Hübner et al., 2001; Yang et al., 2003a; Fisher et al., 2005; Burtnick et al., 2007; Caimano et al., 2007; Gilbert et al., 2007; Lybecker and Samuels, 2007; Smith et al., 2007). In this pathway, an NtrC-like bacterial two-component response regulator and σ54-dependent transcriptional activator, Rrp2, in concert with RpoN (σ54), controls the production of the second alternative sigma factor RpoS (σS). RpoS, a global regulator, then upregulates many B. burgdorferi genes including ospC (Hübner et al., 2001; Yang et al., 2003a; 2005; Fisher et al., 2005; Burtnick et al., 2007; Caimano et al.., 2007; Gilbert et al.., 2007; Lybecker and Samuels, 2007; Smith et al., 2007; Boardman et al., 2008; Ouyang et al., 2008). Recent data indicate that this pathway is not operative in flat ticks but becomes activated during tick feeding (Caimano et al., 2007). Taken together, there is a growing body of evidence supporting the notion that the Rrp2-RpoN-RpoS pathway functions as a master regulatory network that modulates differential gene expression to ensure spirochaete’s transmission in ticks and establishment of infection in mammals.
In addition to controlling ospC expression, the Rrp2-RpoN-RpoS pathway was recently shown to be required for the downregulation of ospA. By employing a model for cultivating bacteria in a host-adapted state (spirochaetes cultivated in a dialysis membrane chamber implanted into rat peritoneal cavities, also referred as the ‘DMC model’) (Caimano et al., 2005; 2007). Caimano et al. (2005) demonstrated that whereas wild-type B. burgdorferi displayed a OspA−/OspC+ phenotype, the rpoS mutant that was incapable of expressing ospC could no longer downregulate ospA and displayed a OspA+/OspC− phenotype. This finding provided compelling evidence that the reciprocal regulation ospA and ospC expression is interconnected at the molecular level. The notion that the Rrp2-RpoN-RpoS pathway controls reciprocal regulation of ospA and ospC was further supported by a recent study of gene expression at a single-cell level using the flow cytometry method for in vitro cultivated spirochaetes (Srivastava and de Silva, 2008).
The mechanism by which the Rrp2-RpoN-RpoS pathway becomes activated remains unclear. During in vitro cultivation, several signals including elevated temperature, high cell density, low pH, increased CO2 concentrations and mammalian host chemicals have been implicated in inducing the expression of ospC and other lipoproteins via the activation of the Rrp2-RpoN-RpoS pathway (Schwan et al., 1995; Stevenson et al., 1995; Indest et al., 1997; Akins et al., 1998; Carroll et al., 1999; Obonyo et al., 1999; Tokarz et al., 2004; Hyde et al., 2007). It remains unclear how these signals activate the Rrp2-RpoN-RpoS pathway, and whether B. burgdorferi senses the same signals during replication in ticks and mice to activate this pathway. Although it has been postulated that the response regulator Rrp2 is activated by a putative cognate sensor kinase Hk2 (Yang et al., 2003a), a recent report showed that inactivation of hk2 does not affect activation of the Rrp2-RpoN-RpoS pathway, at least during in vitro cultivation (Burtnick et al., 2007).
In this report, we demonstrate that inactivation of ospA and its cotranscribed homologue, ospB, results in constitutive activation of the Rrp2-RpoN-RpoS pathway. This finding suggests that a decrease in the production of OspA can be one of the mechanisms that activate the Rrp2-RpoN-RpoS pathway, which contributes to the reciprocal regulation of ospA and ospC. Therefore, whereas activation of the Rrp2-RpoN-RpoS pathway serves to downregulate ospA expression, reduction of OspA can, in turn, further activate the Rrp2-RpoN-RpoS pathway. Such a positive feedback loop likely ensures that B. burgdorferi maintains its overall outer membrane architecture as well as a constant high level of OspC and other lipoproteins for the successful transmission and infection in mammals.
Results
Inactivation of ospAB results in constitutive production of OspC and many other antigens
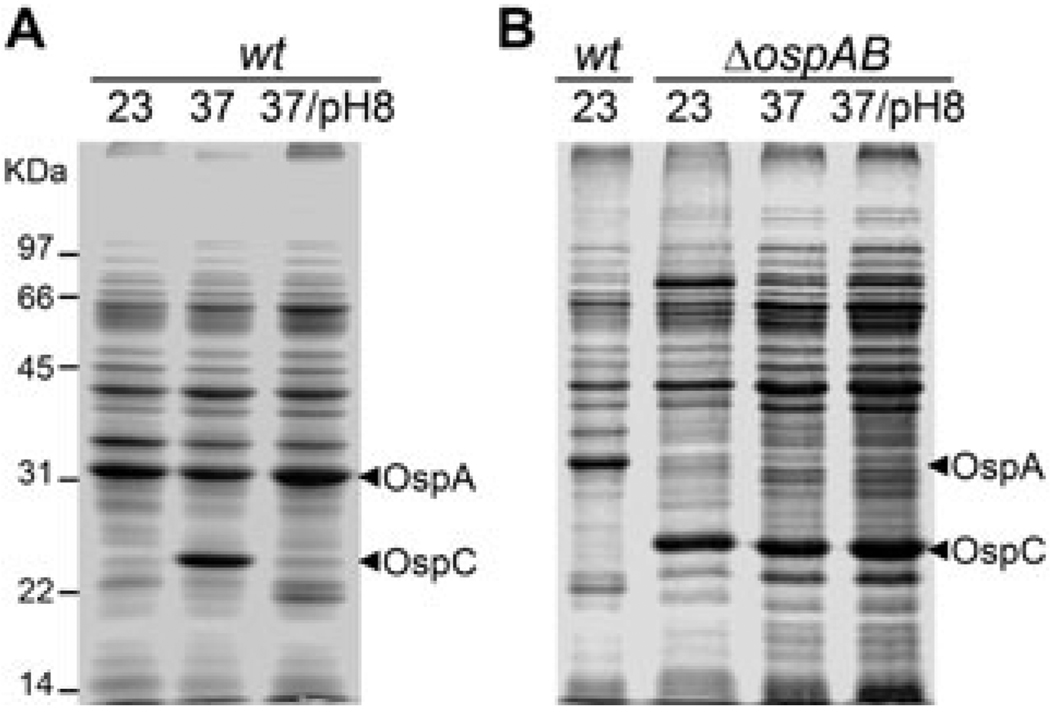
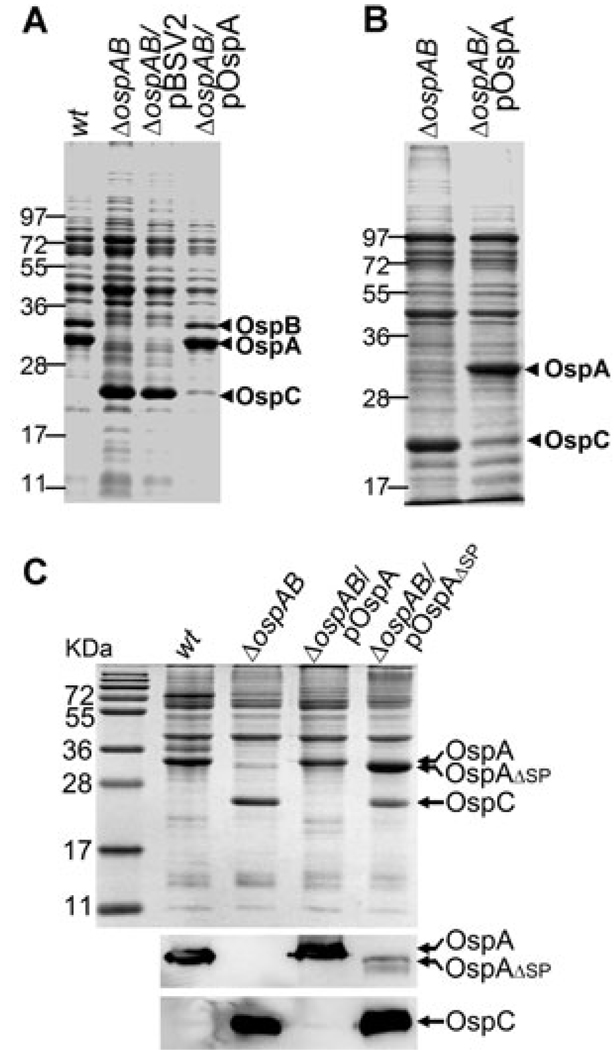
We previously reported on the disruption of the ospAB operon that encodes OspA and its homologue OspB. The ospAB mutant remained infectious in mice, but was unable to colonize and replicate in ticks (Yang et al., 2004). Because OspA and OspB are abundant surface lipoproteins, we sought to assess the influence that abrogation of ospAB might have on overall B. burgdorferi gene expression. The ospAB mutant and the parental strain BbAH130, an infectious clone of B. burgdorferi strain 297, were cultivated under varying conditions of temperature and pH. While the wild-type strain could not produce OspC at lower temperature or increased pH, the ospAB mutant showed constitutive production of OspC, regardless of culture temperature and pH (Fig. 1).
Fig. 1.
Abrogation of ospAB results in constitutive activation of ospC. Wild-type infectious clone BbAH130 of B. burgdorferi strain 297 (wt) and the isogenic ospAB mutant (ΔospAB) were cultivated in BSK-H medium (pH 7.5) at 23°C (23), 37°C (37) or 37°C with media adjusted to pH 8.0 (37/pH 8). Spirochaetes were harvested at a cell density of 5 × 107 ml−1 and cell lysates were subjected to SDS-PAGE and proteins stained with Coomassie blue. Each gel lane was loaded with lysates of approximately 5 × 107 spirochaetes. Numbers at the left of the figure denote molecular mass markers in kilodaltons. The bands corresponding OspA and OspC were labelled on the right.
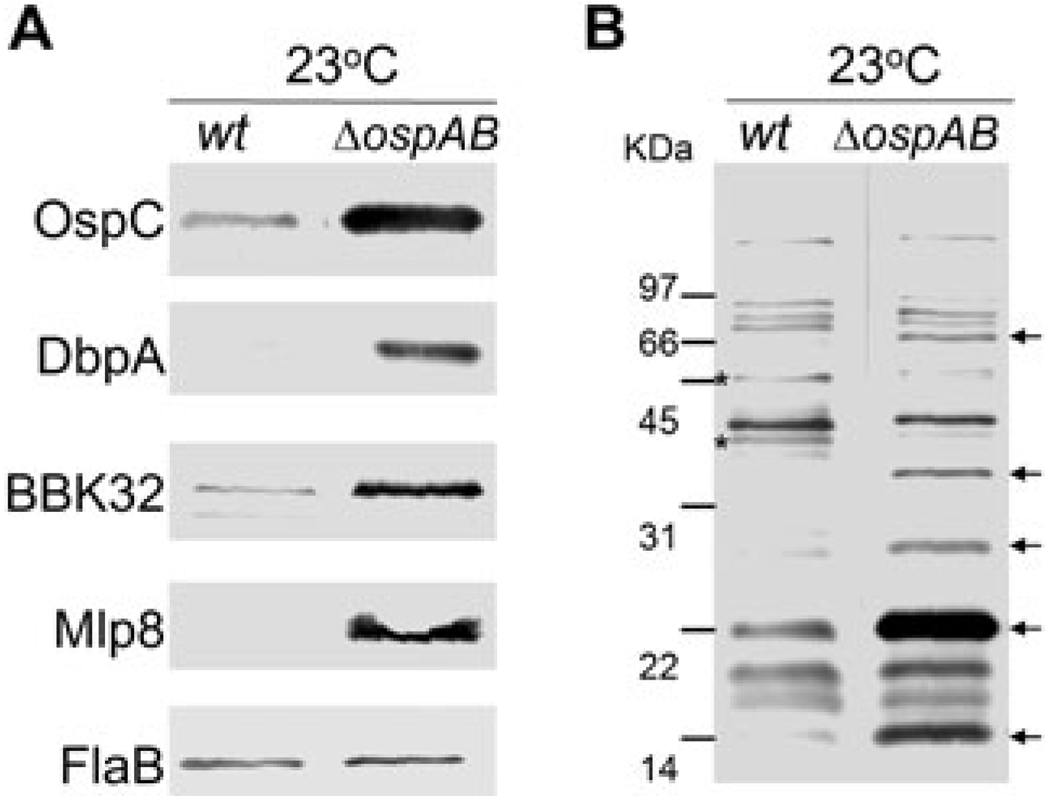
To determine whether abrogation of ospAB affected expression of other lipoproteins known to be differentially expressed, immunoblot analyses were performed with specific antibodies against three temperature-induced lipoproteins including DbpA, BBK32 and Mlp8 (Hübner et al., 2001; Yang et al., 2003b; He et al., 2007). As shown in Fig. 2A, all three lipoproteins were produced in the ospAB mutant at 23°C, suggesting that they are constitutively expressed in the ospAB mutant. To further assess the overall influence of deleting ospAB on the B. burgdorferi antigen profile, an immunoblot analysis was performed using pooled sera from mice infected via tick bite with B. burgdorferi strain 297. While at least two antigens appeared to be downregulated in the ospAB mutant, deletion of ospAB resulted in the overexpression of several additional antigens by the spirochaetes grown at the lower temperature (Fig. 2B).
Fig. 2.
Influences of production of selected lipoproteins and overall antigen profile by inactivation of ospAB. Wild-type (wt) and the isogenic ospAB mutant (ΔospAB) were cultivated in BSK-H medium (pH 7.5, 23°C) and harvested at a cell density of 5 × 107 ml−1.
A. Whole-cell lysates were probed with antibodies directed against the respective lipoproteins (indicated on the left). FlaB serves to confirm equal loading of lysates in each lane.
B. Whole-cell lysates were probed with pooled antisera (2 week to 16 week post infection) from mice infected with B. burgdorferi strain 297 by tick bites. Arrowheads denote antigenic proteins not expressed in wild-type spirochaetes and asterisks indicate antigenic proteins not expressed in the ospAB mutant.
Overproduction of OspC in the ospAB mutant is due to constitutive activation of the Rrp2-RpoN-RpoS pathway
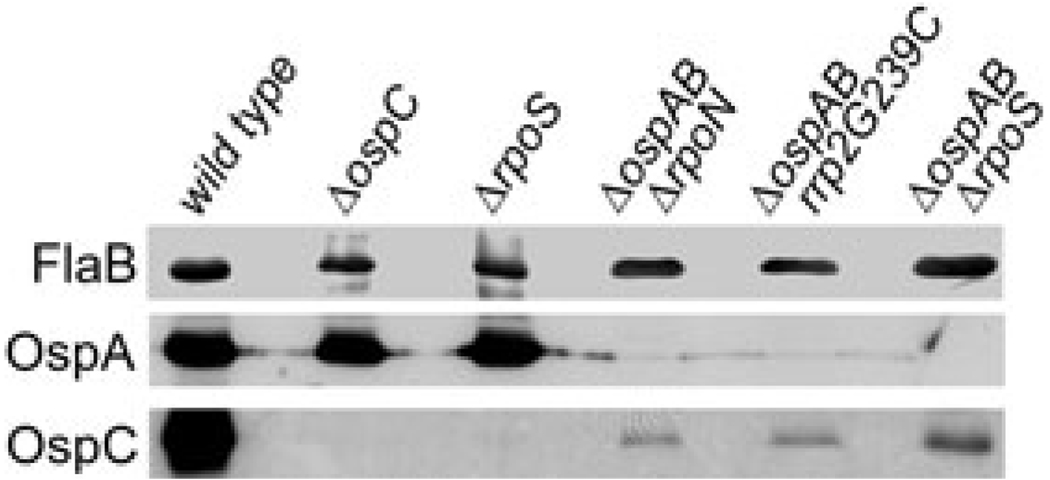
Temperature-induced expression of ospC, dbpA and bbk32 is mediated by the Rrp2-RpoN-RpoS pathway (Hübner et al., 2001; Yang et al., 2003a; Eggers et al., 2004; Gilbert et al., 2007; He et al., 2007). To determine whether the constitutive expression of ospC in the ospAB mutant is also dependent on this pathway, the rrp2, rpoN or rpoS gene was mutated in the ospAB mutant background. The strategies employed to generate these mutants have been described in prior studies (Hübner et al., 2001; Yang et al., 2003a). As shown in Fig. 3, a point mutation (G239C) in Rrp2 or inactivation of rpoN or rpoS in the ospAB mutant greatly diminished the constitutive production of OspC in the ospAB mutant. These data indicate that overexpression of ospC in the ospAB mutant is due to constitutive activation of the Rrp2-RpoN-RpoS pathway.
Fig. 3.
Mutation or inactivation of rpoS, rpoN or rrp2 abolishes constitutive expression of ospC in the ospAB mutant. The ospAB mutant (ΔospAB, lane 2), the ospAB and rpoS double mutant (ΔospAB ΔrpoS, lane 3), the ospAB and rpoN double mutant (ΔospAB ΔrpoN, lane 4) and the ospAB and rrp2 double mutant (ΔospAB rrp2G239C, lane 5) were cultivated at 35°C and harvested at 5 × 107 spirochaetes ml−1. Whole-cell lysates were subjected to SDS-PAGE (Coomassie blue stain) (left) and immunoblot (right) analyses. For immunoblotting, monoclonal antibody directly against OspC was used.
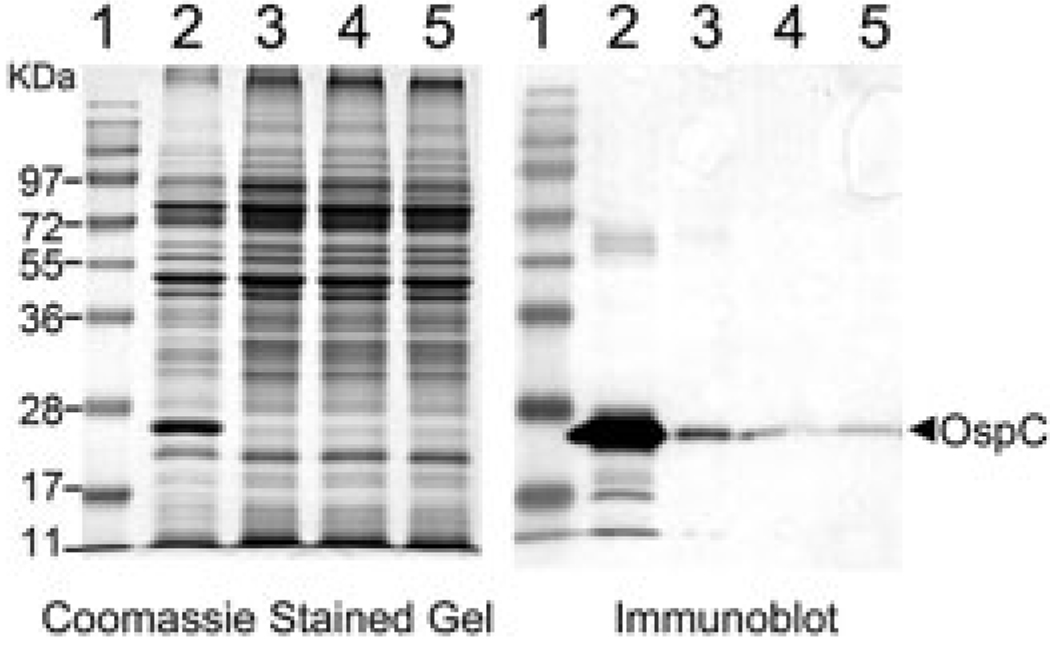
Expression of ospC has been previously shown to be exclusively controlled by RpoS (Hübner et al., 2001; Eggers et al., 2004; Yang et al., 2005; Gilbert et al., 2007). However, we observed a low level of OspC in the ospAB mutant even when rpoS, rpoN or rrp2 was mutated (in the ospAB rpoS double mutant, Fig. 3, lanes 3–5). Therefore, we reasoned that inactivation of ospAB might result in both RpoS-dependent and RpoS-independent ospC expression. To confirm this observation, we compared levels of OspC among various B. burgdorferi strains including the wild-type strain, the ospC mutant, the rpoS mutant and the three double mutants. Spirochaetes were cultivated at elevated temperature (35°C) and lysates were subjected to immunoblot assays. As shown in Fig. 4, OspC was indeed readily detectable in all three double mutants. Thus, abrogation of ospAB constitutively activates ospC by two means: constitutive activation of the Rrp2-RpoN-RpoS pathway (which contributes to the major portion of high levels of OspC), and activation of a novel form of ospC expression that is RpoS-independent.
Fig. 4.
Detection of RpoS-independent OspC production in the ospAB mutant. Wild-type strain, the ospC mutant (ΔospC), the rpoS mutant (ΔrpoS) and ΔospAB ΔrpoN, ΔospAB rrp2G239C ΔospAB ΔrpoS double mutants were cultivated at 35°C and whole-cell lysates subjected to immunoblot analysis with monoclonal antibody against either OspC or FlaB (indicated on the left). Note that the samples were loaded in every other lane to avoid possible contamination from the wild-type sample.
Complementation of the ospAB mutant with ospA alone restores repression of the Rrp2-RpoN-RpoS pathway at 23°C
Constitutive activation of the Rrp2-RpoN-RpoS pathway by the ospAB mutant could be due to spurious mutations that locked the pathway in the active state. To rule out this possibility and determine whether the phenotype observed in the ospAB mutant was due solely to the loss of ospAB expression, the ospAB mutant was complemented with a shuttle vector carrying a wild-type copy of ospAB (pOspAB). Unlike the ospAB mutant, the complemented strain was no longer able to produce OspC at 23°C (Fig. 5A). Moreover, complementation of the ospAB mutant with ospA alone restored the temperature-dependent repression of ospC expression (Fig. 5B). These data indicate that constitutive activation of the Rrp2-RpoN-RpoS pathway was due to abrogation of ospA, and not by spurious mutants.
Fig. 5.
Complementation of the ospAB mutant with a wild-type copy of ospAB or ospA alone restores repression of ospC at 23°C. Wild-type strain (wt), the ospAB mutant (ΔospAB), the ospAB transformed with either the shuttle vector pBSV2 alone (ΔospAB/pBSV2), the shuttle vector carrying a wild copy of ospAB (ΔospAB/pOspAB) (A), the shuttle vector carrying ospA only (ΔospAB/pOspA) (B) or the shuttle vector carrying an ospA variant lacking the region encoding the lipoprotein signal sequence (ΔospAB/pOspAΔSP) (C, top) were cultivated at 23°C. Whole-cell lysates were subjected to SDS-PAGE and stained with Coomassie blue, and to immunoblot using antibodies against OspA or OspC (C, bottom). The bands corresponding to OspA, OspB and OspC were labelled on the right.
Restoring repression of the Rrp2-RpoN-RpoS pathway requires lipidated OspA
How does the loss of OspA, a surface-associated lipoprotein, activate the Rrp2-RpoN-RpoS pathway? The lack of knowledge regarding the upstream events that activate Rrp2 has hampered efforts to further delineate how the upstream factors of the pathway are affected by deletion of ospAB. As an alternative approach to gain further insight into the nature of ospAB deletion influencing the Rrp2-RpoN-RpoS pathway, we sought to investigate whether surface localization of OspA is required for restoring the repression of the pathway at 23°C. The rationale was that because both Rrp2 and its putative sensor kinase Hk2 are predicted to be cytoplasmic proteins, it is possible that the OspA precursor present in the cytoplasm may directly influence the activation of Rrp2. To test this, the ospAB mutant was complemented with a shuttle vector harbouring a variant OspA that lacked the N-terminal lipoprotein signal sequence. Unlike wild-type OspA, the OspA variant lacking the lipoprotein signal sequence (ospA-ΔSP) failed to restore the repression of ospC at 23°C (Fig. 5C). These data indicate that surface localization of a lipidated OspAis required for restoring repression of the Rrp2-RpoN-RpoS pathway in the ospAB mutant at 23°C.
OspC expression in antibody escape ospAB mutants
Previously, several OspAB-lacking mutants, referred to as OspA antibody escape mutants, were isolated (Sadziene et al., 1992; 1995). These variants were selected as antibody-resistant mutants by in vitro incubation of wild-type B. burgdorferi strain B31 with monoclonal antibody against OspA.
The genetic background of these mutants was less defined, with most of the clones having lost the entire lp54 plasmid (harbouring ospAB) along with many other endogenous plasmids (Sadziene et al., 1993b; 1995). Nevertheless, studies with these mutants have contributed to our understanding of function and regulation of ospAB (Sadziene et al., 1992; 1993a; 1995). To further validate the ospAB phenotype observed herein, we sought to examine whether antibody escape ospAB mutants also exhibit constitutive activation of the Rrp2-RpoN-RpoS pathway.
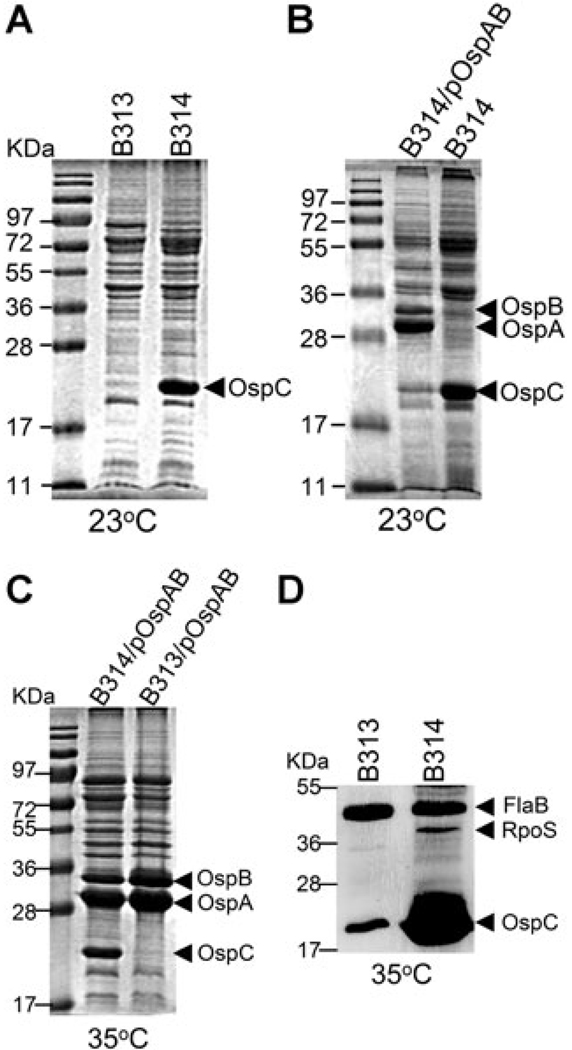
It has been reported previously by Sadziene et al. (1995) that antibody escape mutant strains show heterogeneous expression of ospC. Whereas one of the antibody escape mutant clone, B314, expressed high levels of OspC at 35°C, the other clone B313 had low or undetectable levels of OspC (Sadziene et al., 1995). Therefore, we first examined whether B314 displays a constitutive ospC expression phenotype. Indeed, similar to the isogenic ospAB mutant described above, lower temperature failed to downregulate OspC in B314, indicating that B314 constitutively expressed ospC regardless of culture temperature (Fig. 6A). B313 did not express ospC at 23°C, which was expected as it did not express ospC even at elevated temperature (Fig. 6A) (Sadziene et al., 1995). Thus, the antibody escape ospAB mutants have at least two distinct phenotypes: constitutive ospC expression at both 35°C and 23°C (in B314) and diminished ospC expression regardless of culture temperature (in B313).
Fig. 6.
Analyses of OspC and RpoS production in the antibody escape ospAB mutants. Strains B313, B314 and B313 and B314 complemented with a wild-type copy of ospAB that are designated B313/pOspBAB and B314/pOspAB, respectively (labelled on top), were cultivated at either 23°C or 35°C. Whole-cell lysates were subjected to SDS-PAGE (Coomassie blue stain) (A–C) and immunoblot (D) analyses. For immunoblotting, a mixture of monoclonal antibodies directly against FlaB, RpoS and OspC was used. The bands corresponding to OspA, OspB, OspC, FlaB and RpoS were labelled on the right.
B313, which does not demonstrate constitutive expression of ospC, is defective in activation of the Rrp2-RpoN-RpoS pathway
To determine which ospC expression phenotype observed in the antibody escape mutants was due to the loss of ospAB, and to gain insight into the heterogeneity of ospC expression among these mutants, we complemented both B313 and B314 with pOspAB. Unlike B314, the complemented strain B314/pOspAB no longer expressed ospC at 23°C. Yet, similar to wild-type spirochaetes, B314/pOspAB responded to culture temperature shift and produced OspC at 35°C (Fig. 6B and C). These data indicate that ospAB alone could restore the normal temperature-dependent regulation of ospC in B314, and that the lack of ospAB is responsible for the constitutive ospC expression phenotype in B314, despite its lacking lp54 and many other endogenous plasmids (Sadziene et al., 1993b; 1995).
Contrary to the results obtained with B314, B313 transformed with pOspAB remained incapable of expressing ospC, even under the elevated temperature condition (Fig. 6C). This finding indicates that the defect in ospC expression observed with B313 was not related to the loss of ospAB. To determine whether B313 has a defect in the Rrp2-RpoN-RpoS pathway, levels of RpoS were assessed in both B313 and B314 by immunoblotting. As shown in Fig. 6D, while RpoS was readily detected in B314, RpoS was not observed in B313 when cultivated at 35°C. Thus, the low level of ospC expression of B313 is due to an inability to fully induce the Rrp2-RpoN-RpoS pathway by elevated temperature. Note that growing cultures to a high cell density or at a reduced pH, environmental factors that readily activate the Rrp2-RpoN-RpoS pathway, also failed to induce high level of ospC expression in B313 (data not shown).
Heterogeneity of ospC expression among allelic exchange ospAB mutants
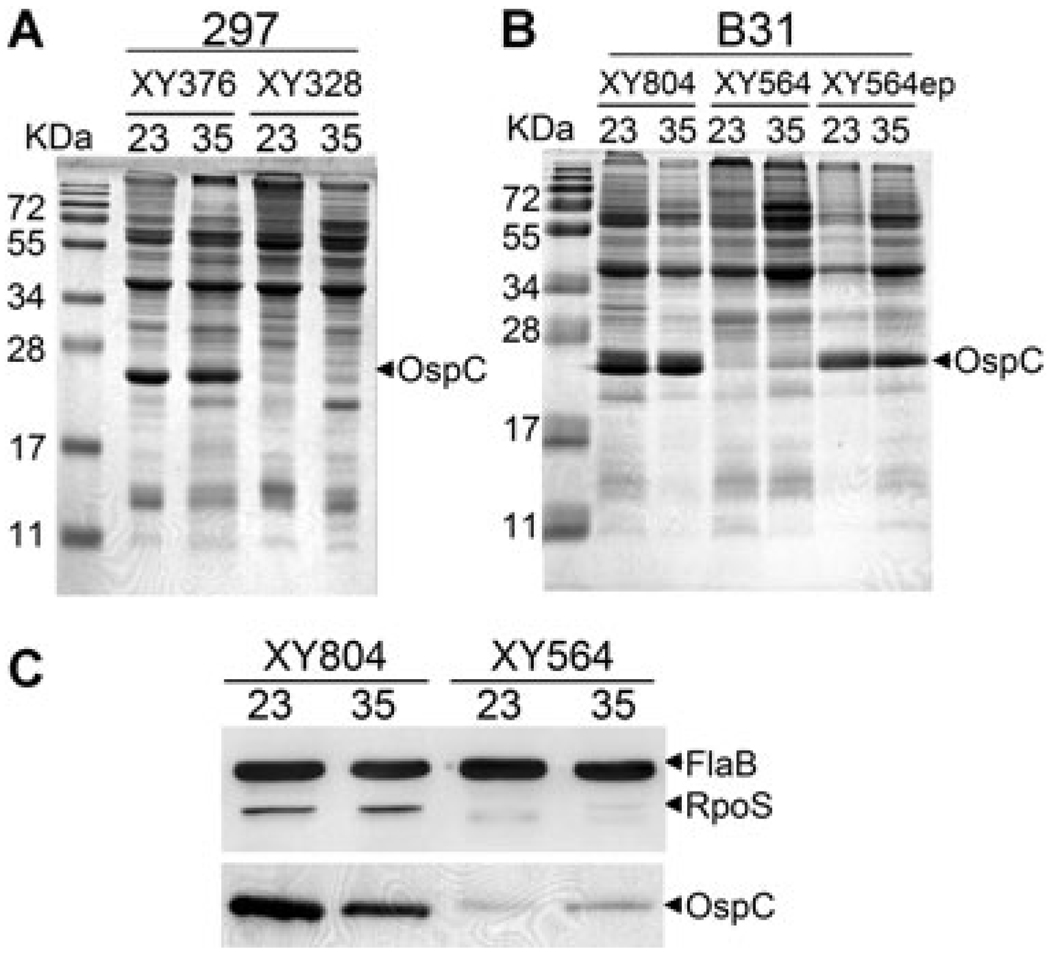
Variation in ospC expression among the antibody escape ospAB mutants prompted us to examine whether the isogenic ospAB mutants also displayed a heterogeneous ospC expression phenotype. Multiple ospAB mutant clones were subsequently generated in strain 297 and their protein profiles were examined. Although the majority of the ospAB mutant clones constitutively expressed ospC, one out of five clones of the ospAB mutant clones showed no or low OspC at 23°C (represented by clone XY328, Fig. 7A). Further characterization revealed that XY328 was unable to express ospC regardless of culture temperature conditions (Fig. 7A). These data suggest that similar to the antibody escape mutant B313, this non-ospC-expressing isogenic ospAB mutant (XY328) has a defect in temperature-induced activation of the Rrp2-RpoN-RpoS pathway.
Fig. 7.
Heterogeneity of OspC production among isogenic ospAB mutants. Representative ospAB mutant clones in the backgrounds of B. burgdorferi strains 297 (A) and B31 (B). Strains were cultivated at either 23°C or 35°C. Whole-cell lysates were subjected to SDS-PAGE (Coomassie blue stain). The band corresponding to OspC was labelled on the right.
C. Immunoblotting of samples in 7B with a mixture of monoclonal antibodies directly against FlaB and RpoS.
As the genome sequence of strain 297 is currently not available, we were unable to use a comparative genomic analysis of the previously described ospAB mutants to further decipher the molecular mechanism underlying this heterogenic ospC regulation. Therefore, we generated ospAB mutants in 5A4NP1, an infectious clone of a sequenced B. burgdorferi strain B31(Kawabata et al., 2004). Similar to the ospAB mutants generated in the strain 297 background, heterogeneity of ospC expression was also observed among B31-derived ospAB mutant clones; clone XY804 displayed constitutive production of OspC and RpoS, whereas clone XY564 expressed low ospC at either temperature conditions (Fig. 7B and C). Thus, the influence on ospC expression by abrogation of ospAB occurs in both strains 297 and B31.
In an effort to define the nature of defect in non-ospC-expressing ospAB mutant clones, the plasmid profile of XY564 was analysed. XY564 had an identical endogenous plasmid profile as the parental strain 5A4NP1 (data not shown), suggesting that the defect in ospC expression was not due to loss of one or more endogenous plasmids. We then examined the infectivity of XY564 in mammalian hosts; the rationale being that although ospAB is dispensable for mammalian infection, a defect in ospC expression could impair the ability of XY564 to establish infection in mice. Interestingly, spirochaetes were readily cultured from ear punch biopsies harvested at 10–14 days post infection from mice that were needle-inoculated with XY564. Furthermore, the recovered spirochaetes, which were then designated as strain XY564ep, began to constitutively produce OspC at both 23°C and 35°C (Fig. 7B). These data suggest that the ospC expression defect in XY564 is reversible upon mammalian host adaptation.
Discussion
Borrelia burgdorferi has a unique outer membrane structure in which it lacks lipopolysaccharide (Takayama et al., 1987), a major component of the outer membrane of Gram-negative bacteria and that contributes greatly to the structural integrity of the bacteria. The absence of lipopolysaccharide appears to be partially compensated by the presence of abundant outer surface lipoproteins in B. burgdorferi. Given that OspA and OspC are two major outer surface lipoproteins, it is thus reasonable to postulate that a reduction in the quantity of one may have significant impact on the overall spirochaetal outer membrane architecture, unless there is a compensatory increase in the other. The results presented in this study provide direct experimental evidence supporting the compensatory effect between OspA and OspC: permanent removal of OspA resulted in constitutive expression of ospC. The compensatory structural role of OspA and OspC is further supported by the observation that restoration of the production of OspA, but not a non-lipidated OspA, restored the repression of ospC at 23°C. Therefore, reciprocal regulation of ospA and ospC serves two purposes: in addition to their distinct and vital functions at different stages of the enzootic cycle (Grimm et al., 2004; Pal et al., 2004a; Yang et al., 2004), OspA and OspC compensate each other in maintaining the overall membrane architecture. This shared structural function by surface lipoproteins was also recently shown to be important in protecting spirochaetes against the host’s innate immune response (Radolf and Caimano, 2008; Xu et al., 2008a).
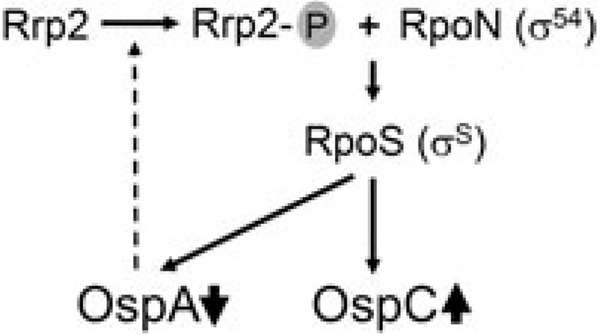
This study also identified one of the molecular mechanisms responsible for governing the inverse pattern of expression of ospA and ospC. Caimano et al. (2005) recently showed that under the host-adapted DMC condition, both upregulation of OspC and downregulation of OspA require the Rrp2-RpoN-RpoS pathway. We now show that the inverse also is true, in that reduction of OspA can activate the Rrp2-RpoN-RpoS pathway. These findings suggest that activation of the Rrp2-RpoN-RpoS pathway and downregulation of OspA form a positive feedback circuit (Fig. 8).We hypothesize that such a feedback loop is important to B. burgdorferi ’s transmission and mammalian infection: during tick feeding, an unknown signal serves to activate the Rrp2-RpoN-RpoS pathway that in turn, leads to repression of ospA expression presumably via an unknown transcriptional repressor. The reduction in surface-associated OspA further activates the Rrp2-RpoN-RpoS pathway. Such a feedback loop likely allows the Rrp2-RpoN-RpoS pathway to achieve and sustain a maximal level of activation, to ensure B. burgdorferi’s successful migration in ticks and for establishing infection in mammals.
Fig. 8.
Proposed model for the positive feedback circuit between the activation of Rrp2-RpoN-RpoS regulatory pathway and the reduction of OspA (see text).
Elevated temperature is one of the most important environmental cues that induce OspC production under in vitro cultivation conditions. Although decrease in the level of DNA supercoiling has been shown to be one of the mechanisms for temperature-independent regulation of ospC (Alverson et al., 2003), the recent report by Gilbert et al. (2007) using an inducible system of controlling rpoS expression demonstrated that increasing level of RpoS is sufficient to increase ospC expression at 23°C. Therefore, the temperature-induced expression of ospC is predominantly due to an increase of RpoS expression. Our finding that OspC synthesis in the ospAB mutant is independent of temperature shift by a constitutive expression of rpoS is consistent with this notion. In addition, we observed an RpoS-independent ospC expression in this study (Fig. 4). Although this form of ospC expression appeared less abundant, to our knowledge, this is the first demonstration that ospC can be expressed independent of RpoS. Although the physiological significance of this mechanism for ospC expression remains unclear, our finding suggests that under certain conditions, ospC can be transcribed either from a different sigma70-dependent promoter, or from the identified RpoS-dependent promoter sequence by the sigma70-polymerase holoenzyme.
The result that abrogation of ospAB constitutively activates ospC via the Rrp2-RpoN-RpoS pathway seems contradictory to several well-documented observations. First, while in vitro cultivation of B. burgdorferi at elevated temperature and cell density or reduced pH induces ospC expression, ospA is still expressed in these cells when levels of total mRNA and protein are assessed. Therefore, it was thought that the aforementioned in vitro conditions do not induce reciprocal regulation of ospA and ospC. However, a recent study demonstrated that this notion is incorrect. Using flow cytometry, Srivastava and de Silva (2008) identified two subpopulations of spirochaetes in cultures cultivated at 35°C: whereas the majority of spirochaetes remained OspA+/OspC− (62.3%), the other subpopulation showed upregulation of ospC concomitantly with the downregulation of ospA (OspA−/OspC+, 23.7%). These data indicate that although the overall level of OspA might be high in the total population of spirochaetes cultivated at 35°C, reciprocal regulation of OspA and OspC is evident when expression is examined at the single-cell level (Srivastava and de Silva, 2008).
Another previous observation that seems to be contradictory with reciprocal regulation of OspA and OspC is that B. burgdorferi displays a OspA−/OspC− phenotype during persistent infection in mice (Liang et al., 2002a). In the early phase of mammalian infection, spirochaetes display an OspA−/OspC+ phenotype. OspC, a potent immunogen, then undergoes downregulation, which is one of the strategies for B. burgdorferi to evade the host adaptive immune response (Liang et al., 2002a,b; Xu et al., 2006). Although OspA may be expressed transiently at a certain point during mammalian infection (Kalish et al., 1993), OspA is often absent during persistent infection. Thus, these spirochaetes display an OspA−/OspC− phenotype rather than a reciprocal pattern. Several recent findings could explain this phenomenon. First, although ospC is downregulated during persistent infection, the Rrp2-RpoN-RpoS pathway appears to remain ‘ON’; Xu et al. (2007; 2008b) showed that the immune-mediated ospC repression is ospC-specific and not due to the repression of the whole Rrp2-RpoN-RpoS pathway. Rather, repression of ospC is mediated via the inverted repeat cis-elements upstream of the ospC promoter, and these inverted repeats did not affect RpoS-dependent activation of ospC in vitro (Eggers et al., 2004; Yang et al., 2005). In fact, while ospC is downregulated, the expression of other RpoS-dependent genes such as dbpA and bbk32 remains high during persistent infection (Liang et al., 2002a,b). Thus, although ospA and ospC are not expressed in an inverse pattern during persistent infection, OspA and the Rrp2-RpoN-RpoS pathway remain reciprocally regulated. Then how do these spirochaetes that lack both OspA and OspC maintain their membrane architecture? A logical explanation would be that B. burgdorferi upregulates other surface lipoproteins to compensate for the loss of both OspA and OspC, while at the same time preventing the induction of the host antibody response. One such candidate is VlsE, a homologue of OspC that undergoes antigenic variation during mammalian infection (Zhang et al., 1997). Indeed, several studies indicate that vlsE expression is significantly upregulated during persistent infection (Liang et al., 2002a,b; 2004; Crother et al., 2008).
The notion of reciprocal regulation of OspA and OspC is further complicated by the heterogeneous expression of ospC observed among ospAB mutants. It was well documented that antibody escape ospAB mutants vary in ospC expression (Sadziene et al., 1995). We found that the isogenic ospAB mutants also displayed a heterogeneous ospC expression phenotype. We further showed that the heterogeneity of ospC expression did not affect the ospAB mutant’s ability to infect mice. It remains to be determined whether these ospAB mutant clones with different ospC expression phenotypes behave differently in the tick vector. Interestingly, a recent report by Battisti et al. (2008) showed that an ospA mutant in B31 is capable of survival in the tick vector, a phenotype that is different from the 297 ospAB mutant (Yang et al., 2004). Further study is warranted to determine whether the different phenotype in ticks is associated with a possible variation in ospC expression among the ospA mutants.
It is noteworthy that the ospAB mutant clones that did not express ospC were incapable of inducing high level of ospC even when cultivated at conditions conducive to OspC induction (e.g. elevated temperature). In fact, the loss of ospC expression in these clones is not due to abrogation of ospAB, but rather due to an inability to fully activate the Rrp2-RpoN-RpoS pathway for an unknown mechanism that is not related to ospAB. As a result, these clones lacked both major surface lipoproteins OspA and OspC. Therefore, these OspC-negative ospAB mutant clones (XY328 and XY564) aggregated and grew much slower in vitro than OspA−/OspC+ clones (XY376, XY804 or XY564ep, data not shown), the phenotypes that have been reported previously for the antibody escape ospAB mutant B313 (Sadziene et al., 1995). Thus, although the heterogeneity of ospC expression among ospAB mutants is an interesting observation, it does not challenge the conclusion that abrogation of ospAB cumulates in constitutive activation of the Rrp2-RpoN-RpoS pathway.
The mechanism of heterogeneous ospC expression among ospAB mutants remains unclear. One simple explanation would be that the ospAB mutants incapable of expressing ospC have acquired an intrinsic genetic defect to activate the Rrp2-RpoN-RpoS pathway. However, this appears not to be the case for at least one of the isogenic ospAB mutants, XY564: although XY564 initially expressed little ospC, it began to constitutively express ospC after recovery from mice. Therefore, the ospC phenotype in clone XY564 is reversible. Interestingly, although the antibody escape ospAB mutant strain B313 was shown low expression of ospC by Sadziene et al. (1992; 1995) and by this study, one previous report by Shang et al. (2000) showed that B313 appeared expressing a high level of ospC. Although the mechanisms of the phenotype reversion in XY564 and possibly in B313 are not clear, these observations support the reversible ‘ON’/‘OFF’ switch model for OspC production reported recently by Srivastava and de Silva (2008). The authors proposed that there is a stochastic effect at the cellular level in the absence of genetic variation that defines an individual cell’s fate in terms of the activation of ospC (via the Rrp2-RpoN-RpoS pathway). Building on this model, we postulate that the ospC expression phenotype in each individual ospAB mutant clone is predefined by the ‘ON’ or ‘OFF’ OspC status of that cell prior to the disruption of ospAB in that cell. In other words, the ospAB mutant clones XY376 and XY804 (with constitutive OspC production, Fig. 7) were derived from a subpopulation of wild-type spirochaetes with OspA−/OspC+ phenotype at 35°C, whereas XY328 and XY564 arose from the result of inactivation of ospAB in OspA+/OspC− cells. Irreversible removal of OspA from the outer membrane of OspA−/OspC+ cells locked the cells in OspC ‘ON’ state (clones XY376 and XY804). Permanent removal of OspA in OspA+/OspC− spirochaetes did not alter the OspC ‘OFF’ state in vitro, resulting in an OspA−/OspC− phenotype in these cells in vitro (clones XY328 and XY564).
In conclusion, the data presented here further support the reciprocal regulation of ospA and ospC, and uncovered one of the mechanisms underlying such regulation. That is, downregulation of OspA upregulates OspC via activation of the Rrp2-RpoN-RpoS pathway. We postulate that during tick feeding and in the early phase of mammalian infection, activation of the Rrp2-RpoN-RpoS pathway and reduction of OspA form a positive feedback loop to achieve a steady-state high level of OspC and other lipoproteins, to ensure a successful transmission to and colonization of the mammalian host. How abrogation of ospAB constitutively activates the Rrp2-RpoN-RpoS the pathway remains obscure, but it is tempting to speculate that the upstream factor for Rrp2 activation is capable of sensing the overall outer membrane architecture. Future analysis of the gene expression profiles of such ospAB mutants (e.g. XY376) will likely provide insight into factors/pathways that mediate such effects. In addition, our characterization of heterogeneous ospC expression in ospAB mutants supports the model of stochastic regulation of the Rrp2-RpoN-RpoS pathway proposed by Srivastava and de Silva (2008). Further genome-wide comparison of expression profiles between the constitutive OspC ‘ON’ and ‘OFF’ ospAB mutants (e.g. XY804 versus XY564) will provide further insight into the stochastic nature of the activation of the Rrp2-RpoN-RpoS pathway.
Experimental procedures
Bacterial strains and growth conditions
Borrelia burgdorferi strains BbAH130, an infectious clone of strain 297, and XY376, the original ospAB mutant, were reported previously (Yang et al., 2004). Strain 5A4NP1 (a gift from Dr Steve Norris, University of Texas Health Science Center at Houston) is an infectious clone of strain B31 harbouring a kanamycin-resistance marker inserted in the restriction modification gene bbe02 on plasmid lp25 (Kawabata et al., 2004). The antibody escape mutants B313 and B314 were generously provided by Dr Wolfram Zuckert (University of Kansas Medical Center) (Sadziene et al., 1992; 1995). Borreliae were cultivated in vitro in BSK-H medium (Sigma, St Louis, MO) supplemented with 6% normal rabbit serum (Pel Freez Biologicals, Rogers, AR) at either 23°C or 35°C. 1 M Sodium hydroxide was used for achieving the pH 8 BSK-H medium (Yang et al., 2000). Cultures were harvested at late logarithmic phase of growth (1 × 107 spirochaetes ml−1 for cultures at 23°C; 5 × 107 spirochaetes ml−1 for cultures at 35°C).
B. burgdorferi transformations
Electrocompetent B. burgdorferi cells were prepared and transformed as previously described (Samuels, 1995; Yang et al., 2004). Relevant antibiotic concentrations used for mutant selection were: 50 ng ml−1 for erythromycin, 100 µg ml−1 for streptomycin and 300 µg ml−1 for kanamycin. The strategy for creating the ospAB mutant in 5A4NP1 was described previously (Yang et al., 2004). To generate double mutants of ΔospAB ΔrpoS, ΔospA ΔrpoN and ΔospAB rrp2G269C, an ospAB mutant derived from BbAH130, XY376, was transformed with each corresponding suicide vector that was previously used for inactivating rpoS or rpoN or mutating rrp2 (Hübner et al., 2001; Yang et al., 2003a). The shuttle vector carrying the wild-type ospAB (pOspAB) used for complementation has been described previously (Yang et al., 2004). To create the shuttle vector pOspA that carries the wild-type ospA gene only, pOspAB was digested with AhdI and BstApI, treated with Large Klenow fragment, and re-ligated, resulting in the deletion of the majority of ospB (a small 5’ portion of the ospB gene that encodes the first 56 amino acids of OspB still remained). To create the shuttle vector pOspA-ΔSP that carries the ospA variant encoding a non-lipidated OspA, a portion of the 5’ ospA gene on pOspA was deleted using the QuikChange Site-Directed Mutagenesis kit (Strategene, La Jolla, CA), resulting in the replacement of first 17 amino acids (from Met to Cys) of the N-terminal signal sequence of OspA with Met-Arg-Ser.
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis and immunoblotting
SDS-PAGE and immunoblotting were carried out as previously described (Yang et al., 1999). Monoclonal antibodies against FlaB and OspC were described previously, and immunoblots were developed with 4-chloro-1-naphthol as the substrate (Yang et al., 2000; 2005). Pooled sera collected from C3H/HeJ mice infected with wild-type strain 297 via infestation with Ixodes scapularis nymphs (2–16 weeks post infestation) were previously described (Yang et al., 2003a).
Mouse infection
All animal experimentation was approved by the Institutional Animal Care and Use Committee at Indiana University. For mammalian infection studies, 3- to 4-week-old C3H/SCID mice (Harlan, Indianapolis, IN) were inoculated via intradermal/subcutaneous injection with 1 × 105 spirochaetes. At 14 days post inoculation, ear punch biopsies were collected and spirochaetes were cultured in BSK-H medium supplemented with 1× Borrelia antibiotic mixture (Sigma, Saint Louis, MO). After cultivation for 1–2 weeks, dark-field microscopy was used to examine cultures for the presence of motile spirochaetes.
Acknowledgements
We thank Drs. W. Zuckert, A. Barbour, S. Norris, P. Rosa, P. Stewart for generously supplying strains B313, B314, 5A4NP1 and the plasmid pBSV2. We thank Dr. Bauer for comments on the manuscript. The work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Disease (AR-54023; X.F.Y.) and the National Institute of Allergy and Infectious Diseases (AI-72144; X.F.Y.) (AI-59602; M.V.N.), American Heart Association Scientist Development Grant (X.F.Y.), and in part by the Indiana INGEN of Indiana University, funded by the Lilly Endowment (X.F.Y.). This investigation was conducted in part, in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR015481-01 from the National Center for Research Resources, National Institutes of Health.
References
- Akins DR, Bourell KW, Caimano MJ, Norgard MV, Radolf JD. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest. 1998;101:2240–2250. doi: 10.1172/JCI2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alverson J, Bundle SF, Sohaskey CD, Lybecker MC, Samuels DS. Transcriptional regulation of the ospAB and ospC promoters from Borrelia burgdorferi. Mol Microbiol. 2003;48:1665–1677. doi: 10.1046/j.1365-2958.2003.03537.x. [DOI] [PubMed] [Google Scholar]
- Anguita J, Hedrick MN, Fikrig E. Adaptation of Borrelia burgdorferi in the tick and the mammalian host. FEMS Microbiol Rev. 2003;27:493–504. doi: 10.1016/S0168-6445(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastroc PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76:5228–5237. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman BK, He M, Ouyang Z, Xu H, Pang X, Yang XF. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect Immun. 2008;76:3844–3853. doi: 10.1128/IAI.00467-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. Insights into the complex regulation of rpoS. Borrelia burgdorferi. Mol Microbiol. 2007;65:277–293. doi: 10.1111/j.1365-2958.2007.05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Eggers CH, Gonzalez CA, Radolf JD. Alternate sigma factor RpoS is required for the in vivo-specific repression of Borrelia burgdorferi plasmid lp54-borne ospA and lp6.6 genes. J Bacteriol. 2005;187:7845–7852. doi: 10.1128/JB.187.22.7845-7852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caimano MJ, Iyer R, Eggers CH, Gonzalez C, Morton EA, Gilbert MA, et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol Microbiol. 2007;65:1193–1217. doi: 10.1111/j.1365-2958.2007.05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JA, Garon CF, Schwan TG. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect Immun. 1999;67:3181–3187. doi: 10.1128/iai.67.7.3181-3187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crother TR, Champion CI, Whitelegge JP, Aguiler R, Wu XY, Blanco DR, et al. Temporal analysis of the antigenic composition of Borrelia burgdorferi during infection in rabbit skin. Infect Immun. 2008;72:5072. doi: 10.1128/IAI.72.9.5063-5072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers CH, Caimano MJ, Radolf JD. Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes. J Bacteriol. 2004;186:7390–7402. doi: 10.1128/JB.186.21.7390-7402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MA, Grimm D, Henion AK, Elias AF, Stewart PE, Rosa PA, Gherardini FC. Borrelia burgdorferi σ54 is required for mammalian infection and vector transmission but not for tick colonization. Proc Natl Acad Sci USA. 2005;102:5162–5167. doi: 10.1073/pnas.0408536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MA, Morton EA, Bundle SF, Samuels DS. Artificial regulation of ospC expression in Borrelia burgdorferi. Mol Microbiol. 2007;63:1259–1273. doi: 10.1111/j.1365-2958.2007.05593.x. [DOI] [PubMed] [Google Scholar]
- Grimm D, Tilly K, Byram R, Stewart PE, Krum JG, Bueschel DM, et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc Natl Acad Sci USA. 2004;101:3142–3147. doi: 10.1073/pnas.0306845101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haake DA. Spirochaetal lipoproteins and pathogenesis. Microbiology. 2000;146:1491–1504. doi: 10.1099/00221287-146-7-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Boardman BK, Yan D, Yang XF. Regulation of expression of the fibronectin-binding protein BBK32 in Borrelia burgdorferi. J Bacteriol. 2007;189:8377–8380. doi: 10.1128/JB.01199-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodzic E, Feng S, Freet KJ, Barthold SW. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect Immun. 2003;71:5042–5055. doi: 10.1128/IAI.71.9.5042-5055.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner A, Yang X, Nolen DM, Popova TG, Cabello FC, Norgard MV. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc Natl Acad Sci USA. 2001;98:12724–12729. doi: 10.1073/pnas.231442498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JA, Trzeciakowski JP, Skare JT. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J Bacteriol. 2007;189:437–445. doi: 10.1128/JB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indest KJ, Ramamoorthy R, Sole M, Gilmore RD, Johnson BJB, Philipp MT. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–2779. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata H, Norris SJ, Watanabe H. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect Immun. 2004;72:7147–7154. doi: 10.1128/IAI.72.12.7147-7154.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Jacobs MB, Bowers LC, Philipp MT. An immune evasion mechanism for spirochetal persistence in Lyme borreliosis. J Exp Med. 2002a;195:415–422. doi: 10.1084/jem.20011870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Nelson FK, Fikrig E. Molecular adaptation of Borrelia burgdorferi in the murine host. J Exp Med. 2002b;196:275–280. doi: 10.1084/jem.20020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FT, Yan J, Mbow ML, Sviat SL, Gilmore RD, Mamula M, Fikrig E. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect Immun. 2004;72:5759–5767. doi: 10.1128/IAI.72.10.5759-5767.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybecker MC, Samuels DS. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol Microbiol. 2007;64:1075–1089. doi: 10.1111/j.1365-2958.2007.05716.x. [DOI] [PubMed] [Google Scholar]
- Montgomery RR, Malawista SE, Feen KJM, Bockenstedt LK. Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med. 1996;183:261–269. doi: 10.1084/jem.183.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonyo M, Munderloh UG, Fingerle V, Wilske B, Kurtti TJ. Borrelia burgdorferi in tick cell culture modulates expression of outer surface proteins A and C in response to temperature. J Clin Microbiol. 1999;37:2137–2141. doi: 10.1128/jcm.37.7.2137-2141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi J, Piesman J, de Silva AM. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc Natl Acad Sci USA. 2001;98:670–675. doi: 10.1073/pnas.98.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang Z, Blevins JS, Norgard MV. Transcriptional interplay among the regulators Rrp2, RpoN, and RpoS in Borrelia burgdorferi. Microbiology. 2008;154:2641–2658. doi: 10.1099/mic.0.2008/019992-0. [DOI] [PubMed] [Google Scholar]
- Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Fikrig E. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest. 2000;106:561–569. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal U, Li X, Wang T, Montgomery RR, Ramamoorthi N, deSilva AM, et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell. 2004a;119:457–468. doi: 10.1016/j.cell.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, Flavell RA, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004b;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp MT. Studies on OspA: a source of new paradigms in Lyme disease research. Trends Microbiol. 1998;6:44–47. doi: 10.1016/S0966-842X(97)01201-8. [DOI] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ. The long strange trip of Borrelia burgdorferi outer-surface protein C. Mol Microbiol. 2008;69:1–4. doi: 10.1111/j.1365-2958.2008.06226.x. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, et al. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Rosa PA, Thompson PA, Hogan DM, Barbour AG. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992;176:799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Barbour AG, Rosa PA, Thomas DD. An OspB mutant of Borrelia burgdorferi has reduced invasiveness in vitro and reduced infectivity in vivo. Infect Immun. 1993a;61:3590–3596. doi: 10.1128/iai.61.9.3590-3596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Wilske B, Ferdows MS, Barbour AG. The cryptic ospC gene of Borrelia burgdorferi B31 is located on a circular plasmid. Infect Immun. 1993b;61:2192–2195. doi: 10.1128/iai.61.5.2192-2195.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadziene A, Thomas DD, Barbour AG. Borrelia burgdorferi mutant lacking Osp: biological and immunological characterization. Infect Immun. 1995;63:1573–1580. doi: 10.1128/iai.63.4.1573-1580.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels DS. Electrotransformation of the spirochaete Borrelia Burgdorferi. In: Nickoloff JA, editor. Methods in Molecular Biology. Totowa, NJ: Humana Press; 1995. pp. 253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans. 2003;31:108–112. doi: 10.1042/bst0310108. [DOI] [PubMed] [Google Scholar]
- Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–388. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, Golde WT, Dolan MC, Rosa PA. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang ES, Champion CI, Wu X-Y, Skare JT, Blanco DR, Miller JN, Lovett MA. Comparison of protection in rabbits against host-adapted and cultivated Borrelia burgdorferi following infection-derived immunity or immunization with outer membrane. Infect Immun. 2000;68:4189–4199. doi: 10.1128/iai.68.7.4189-4199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva AM, Telford SR, Brunet LR, Barthold SW, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Girschick HJ. Molecular survival strategies of the Lyme disease spirochete Borrelia burgdorferi. Lancet Infect Dis. 2004;4:575–583. doi: 10.1016/S1473-3099(04)01132-6. [DOI] [PubMed] [Google Scholar]
- Smith AH, Blevins JS, Bachlani GN, Yang XF, Norgard MV. Evidence that RpoS (σS) in Borrelia burgdorferi is controlled directly by RpoN (σ54/σN) J Bacteriol. 2007;189:2139–2144. doi: 10.1128/JB.01653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SY, de Silva AM. Reciprocal expression of ospA and ospC in single cells of Borrelia burgdorferi. J Bacteriol. 2008;190:3429–3433. doi: 10.1128/JB.00085-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Schwan TG, Rosa PA. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PE, Wang X, Bueschel DM, Clifton DR, Grimm D, Tilly K, et al. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect Immun. 2006;74:3547–3553. doi: 10.1128/IAI.00158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strother KO, de Silva A. Role of Borrelia burgdorferi linear plasmid 25 in Infection of Ixodes scapularis Ticks. J Bacteriol. 2005;187:5776–5781. doi: 10.1128/JB.187.16.5776-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama K, Rothenberg RJ, Barbour AG. Absence of lipopolysaccharide in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1987;55:2311–2313. doi: 10.1128/iai.55.9.2311-2313.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect Immun. 2006;74:3554–3564. doi: 10.1128/IAI.01950-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarz R, Anderton JM, Katona LI, Benach JL. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect Immun. 2004;72:5419–5432. doi: 10.1128/IAI.72.9.5419-5432.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Seemanapalli SV, McShan K, Liang FT. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect Immun. 2006;74:5177–5184. doi: 10.1128/IAI.00713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Identification of an ospC operator critical for immune evasion of Borrelia burgdorferi. Mol Microbiol. 2007;64:220–231. doi: 10.1111/j.1365-2958.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Essential protective role attributed to the surface lipoproteins of Borrelia burgdorferi against innate defences. Mol Microbiol. 2008a;69:15–26. doi: 10.1111/j.1365-2958.2008.06264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, McShan K, Liang FT. Verification and dissection of the ospC operator by using flaB promoter as a reporter in Borrelia burgdorferi. Microbial Pathogen. 2008b;45:70–78. doi: 10.1016/j.micpath.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Popova TG, Hagman KE, Wikel SK, Schoeler GB, Caimano MJ, et al. Identification, characterization, and expression of three new members of the Borrelia burgdorferi Mlp (2.9) lipoprotein gene family. Infect Immun. 1999;67:6008–6018. doi: 10.1128/iai.67.11.6008-6018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Goldberg MS, Popova TG, Schoeler GB, Wikel SK, Hagman KE, Norgard MV. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol Microbiol. 2000;37:1470–1479. doi: 10.1046/j.1365-2958.2000.02104.x. [DOI] [PubMed] [Google Scholar]
- Yang XF, Alani SM, Norgard MV. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc Natl Acad Sci USA. 2003a;100:11001–11006. doi: 10.1073/pnas.1834315100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Hubner A, Popova TG, Hagman KE, Norgard MV. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect Immun. 2003b;71:5012–5020. doi: 10.1128/IAI.71.9.5012-5020.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–648. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XF, Lybecker MC, Pal U, Alani SM, Blevins J, Revel AT, et al. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J Bacteriol. 2005;187:4822–4829. doi: 10.1128/JB.187.14.4822-4829.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-R, Hardham JM, Barbour AG, Norris SJ. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]