Abstract
Humans are genetically unable to synthesize the common mammalian sialic acid N-glycolylneuraminic acid (Neu5Gc). However, Neu5Gc can be metabolically incorporated and covalently expressed on cultured human cell surfaces. Meanwhile, humans express varying and sometimes high titers of polyclonal anti-Neu5Gc antibodies. Here, a survey of human tissues by immunohistochemistry with both a monospecific chicken anti-Neu5Gc antibody and with affinity-purified human anti-Neu5Gc antibodies demonstrates endothelial expression of Neu5Gc, likely originating from Neu5Gc-rich foods like red meats. We hypothesized that the combination of Neu5Gc incorporation and anti-Neu5Gc antibodies can induce endothelial activation. Indeed, the incubation of high-titer human sera with Neu5Gc-fed endothelial cells led to Neu5Gc-dependent antibody binding, complement deposition, endothelial activation, selectin expression, increased cytokine secretion, and monocyte binding. The proinflammatory cytokine tumor necrosis factor-α also selectively enhanced human anti-Neu5Gc antibody reactivity. Anti-Neu5Gc antibodies affinity-purified from human serum also directed Neu5Gc-dependent complement deposition onto cultured endothelial cells. These data indicate a novel human-specific mechanism in which Neu5Gc-rich foods deliver immunogenic Neu5Gc to the endothelium, giving anti-Neu5Gc antibody- and complement-dependent activation, and potentially contributing to human vascular pathologies. In the case of atherosclerosis, Neu5Gc is present both in endothelium overlying plaques and in subendothelial regions, providing multiple pathways for accelerating inflammation in this disease.
Introduction
Sialic acids are monosaccharides typically found at the outer ends of glycan chains covering the surface of all vertebrate cells.1 The most commonly expressed sialic acid is N-acetylneuraminic acid (Neu5Ac), which is the precursor for N-glycolylneuraminic acid (Neu5Gc) synthesis, via action of the CMP-N-acetylneuraminic acid hydroxylase (CMAH) enzyme.1 Although both Neu5Ac and Neu5Gc are expressed in many mammals (including our closest evolutionary cousins, the chimpanzees), the single-copy human CMAH gene was pseudogenized approximately 2 to 3 million years ago.2 Because mice with a human-like Cmah defect have no detectable Neu5Gc,3,4 there is apparently no alternate mammalian pathway for Neu5Gc synthesis.
Although Neu5Gc is foreign to humans, our intracellular biochemical pathways cannot distinguish between Neu5Ac and Neu5Gc, which differ by one oxygen atom. Thus, exogenous Neu5Gc is taken up and metabolically incorporated into human cells, eventually being expressed on the cell surface as if it were made in the same cell.5–7 Furthermore, orally ingested Neu5Gc is taken up into the human body,8 and small amounts of Neu5Gc are present in normal human tissues.8 Interestingly, the Neu5Gc-rich foods identified to date are red meats, which have been associated with circulating inflammatory markers indicating endothelial dysfunction.9–12
Although Neu5Ac and Neu5Gc seem biochemically indistinguishable to human intracellular biosynthetic pathways, the latter is immunogenic in humans, leading to anti-Neu5Gc–specific antibody responses. Although such antibodies had been reported in some diseases,13–15 it was only recently recognized that all adult humans express them.6,7,16 Indeed, normal humans can express high titers of circulating anti-Neu5Gc immunoglobulin (Ig)G and IgM antibodies against a variety of Neu5Gc-containing epitopes commonly found on endothelial cells (see Figure 1B of Padler-Karavani et al17). Given this finding, and the presence of diet-derived Neu5Gc in human tissues, we here consider the effects of this combination on human endothelial cells.
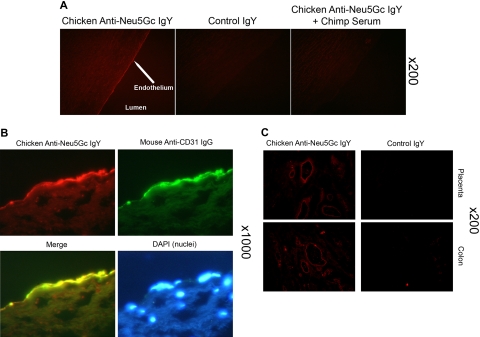
Figure 1.
Detection of Neu5Gc in aortic endothelium of human autopsy samples and microvasculature of colon and placenta. The chicken anti-Neu5Gc antibody (cGcAb) was used to detect the presence of Neu5Gc on the endothelium of autopsy samples of normal-appearing human aorta. Typical representatives of 8 autopsy samples studied are shown. The red Cy3 fluorescence represents labeling of endothelial cells of the aorta. (A) Specificity of the antibody was demonstrated by the lack of signal with the nonimmunized control chicken IgY (middle) and the abrogation of signal by adsorption with Neu5Gc-rich glycoproteins of chimpanzee serum (right). Magnification ×200. (B) Sections were double-stained with anti-CD31 for endothelial cells and counterstained with DAPI to visualize nuclei (magnification ×1000). (C) Sections of placenta (top) and colon (bottom) stain for Neu5Gc along microvasculature endothelial lining with the use of cGcAb. Control IgY (right) demonstrates specificity of signal (magnification ×200).
Endothelial activation is a common feature of many diseases, including those associated with inflammation and reperfusion injury.18,19 Antibody-mediated endothelial damage also has been described in many diseases, including primary autoimmune vasculitides,20,21 systemic autoimmune diseases with vascular involvement,22,23 as well as the early and late stages of atherosclerosis. Among primary autoimmune vasculitides such as Wegener granulamatosis, Kawasaki disease, and Henoch-Schonlein purpura, there is an association between antiendothelial cell antibodies (AECA) and disease status.22–27 Systemic lupus erythematosus also shows a relationship between AECA prevalence and disease status.28,29 Indeed, transfer of AECA into rabbits results in systemic lupus erythematosus–like nephritis.30 It is likely that such antibodies act in concert with other immunological factors to exacerbate vascular damage.20,24
A humoral immunological contribution to atherosclerosis is also recognized. Antibodies against oxidized low-density lipoprotein (LDL) epitopes are associated with coronary artery disease, are found in lesions, and are key players in endothelial damage and lesion progression.31,32 Infections also can generate a humoral response to bacterial HSP60, and the circulating anti-HSP60 antibodies can cross-react with stressed human aortic endothelial cells expressing modified autologous HSP60, causing an arteritis.33–36
The AECA epitopes thus far identified are proteins either constitutively expressed by endothelial cells or up-regulated by endothelial activation or inflammation.22,26,27,37,38 Here we hypothesize that metabolic incorporation of dietary Neu5Gc into human endothelial cell surface glycoproteins leads to an immunogenic endothelium that reacts with circulating human anti-Neu5Gc antibodies, causing complement deposition, endothelial activation, and increased leukocyte binding. In contrast to previous examples, the Neu5Gc-containing antigens are dependent on the endothelial cell's ability to metabolically incorporate Neu5Gc, which originates from food. To our knowledge, this is the first example of a “xeno-auto-antigen” that biochemically incorporates into human glycans and directs immunologic attack against “self.” Additional novel features are that it is diet induced and human specific, and it can potentially account for increases in circulating inflammatory markers associated with consumption of red meats.9–12
Methods
Human serum samples
Normal human sera were from apparently healthy adult volunteers at the University of California, San Diego, with approval from the Institutional Review Board and written informed consent in accordance with the Declaration of Helsinki. Samples were deidentified, assigned a code number, aliquoted, stored at −80°C, and thawed only once to preserve complement. For this study, we randomly used 14 of 37 previous donors17 solely on the basis of their availability to redonate blood.
Cell culture
Human umbilical vein endothelial cells (HUVECs, Lonza) were cultured in low-serum (2% fetal bovine serum [FBS]) media supplemented with growth/serum factors per manufacturer's instructions (EGM-2; Lonza). Cells were cultured in 24-well plates (BD Biosciences) in 5% CO2 at 37°C. To load with sialic acids, 3mM Neu5Gc or Neu5Ac (Inalco) was diluted in EGM-2 media and incubated with cells for 3 days. Although there were low levels of Neu5Gc-containing glycans in FBS, these can be competed out by incubating cells with 3mM Neu5Ac in the cell medium (data not shown). Cells were lifted with 10mM EDTA (ethylenediaminetetraacetic acid) solution without trypsin.
Detection of antibody binding and complement deposition on HUVECs by flow cytometry
HUVECs loaded with Neu5Ac or Neu5Gc were incubated with human sera diluted in media (endothelial basal medium without growth factors or FBS, EBM-2) at 1:5 (vol/vol) for IgG/IgM studies or 1:1 (vol/vol) for complement studies for 1 hour at 37°C. Cells were gently washed twice with cold phosphate-buffered saline (PBS) and incubated on ice for 30 minutes with either fluorescein isothiocyanate (FITC) mouse anti–human IgG, FITC mouse anti–human IgM (Zymed Labs), or FITC rabbit anti–human C3b antibody (Dako), diluted 1:100 in PBS containing 20mM EDTA. Cells were released by pipetting, fixed in 1% paraformaldehyde for 30 minutes on ice, and resuspended in PBS containing 10mM EDTA for flow cytometry with a FACSCalibur (BD Biosciences).
Detection of cell adhesion molecule expression on HUVECs by flow cytometry
Neu5Ac- or Neu5Gc-loaded cells were incubated in human sera diluted 1:1 in EBM-2 for 15 minutes (P-selectin) or 5 hours (E-selectin) at 37°C. As a control, complement activity in serum was heat-inactivated at 57°C for 30 minutes. After incubation, cells were released and incubated in either mouse anti–human CD62P or CD62E (BD Pharmingen) and diluted 1:100 in EBM-2 for 30 minutes on ice. Antibody binding was detected with Alexa-488 goat anti–mouse IgG (BD Pharmingen), diluted 1:100 in PBS containing 10mM EDTA, and quantified by the use of flow cytometry.
Peripheral blood mononuclear cell (PBMC) isolation
Whole blood was isolated into BD Vacutainers (+EDTA, BD Biosciences) by venipuncture from healthy donors, and PBMCs were isolated by the use of Ficoll-Paque PLUS (GE Healthcare). PBMCs were plated onto 10-cm tissue culture plates (BD Biosciences) and cultured in RPMI plus 10% FBS overnight.39,40
PBMC binding to HUVECs
Detailed methods are listed in the legend to Figure 4C.
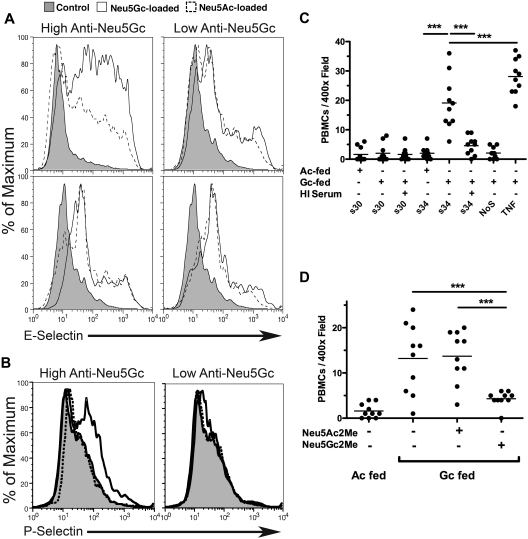
Figure 4.
Human serum enhances adhesion molecule expression and PBMC binding on Neu5Gc-loaded endothelium. (A) A representative example of surface E-selectin expression determined on Neu5Ac and Neu5Gc-loaded HUVECs after 5 hours of activation by low-titer serum (S30, right) or high-titer serum (S34) or heat-inactivated forms thereof (bottom). Control peaks (shaded curve) represent E-selectin expression on endothelial cells not activated by serum. (B) As described in panel A, except HUVECs were exposed to serum for 15 minutes only. Heat-inactivated serum controls are included as a negative control (shaded curve). Short serum incubations show a Neu5Gc-dependent expression of P-selectin that requires anti-Neu5Gc antibodies and is sensitive to heat inactivation of serum. This response was most evident in low passage HUVECs (passage # ≤ 3), consistent with the observed (data not shown) and reported passage-dependent loss of P-selectin expression in vitro.46 (C) HUVECs were cultured on 12-mm glass coverslips (Fisher) in 24-well plates and loaded with either Neu5Ac or Neu5Gc as described previously. Loaded cells were incubated with 50% human sera in EBM-2 for 4 hours at 37°C or with heat-inactivated serum as a control. A negative control was not exposed to human serum (NoS) and a positive control was exposed to TNF-α (10 ng/mL) for stimulation. At 4 hours, 105 PBMCs were added to each well and incubated at 37°C on an orbital shaker at 100 revolutions/minute for 1 hour. Cells were washed thrice in PBS and fixed in PBS containing 3% paraformadehyde. Bound PBMCs were counted in 10 randomly chosen fields at ×400 magnification. Data were averaged and expressed as cells/field. Data are presented as mean with the scatter. This panel is a representative example of 5 similar replicates. ***P < .001. (D) Same as panel C except that the α-methyl-glycosides (1mM) were incubated with the S34 before HUVEC stimulation. All groups were Neu5Gc loaded. We observed inhibition of PBMC binding in S34-stimulated HUVECs with Neu5Gc2Me but not Neu5Ac2Me. ***P < .001. Examples of fluorescent images from the experiment in panel D are shown in supplemental Figure 1. All results presented were performed at least 3 times.
Cytokine production by HUVECs
Detailed methods are in supplemental Table 1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Affinity purification of anti-Neu5Gc antibodies from human serum
Anti-Neu5Gc antibodies were purified from human sera on sequential affinity columns with immobilized human or chimpanzee sialoglycoproteins (rich with Neu5Ac or Neu5Gc, respectively) as described previously.4,17
Western blots
Biotinylated, affinity-purified human anti-Neu5Gc antibodies were studied by Western blotting against surface membrane glycoproteins of Neu5Ac (Ac)– or Neu5Gc (Gc)–loaded endothelial cells, and the banding pattern was compared with that generated with the cGcAb antibody. Details are in the legend to Figure 7A.
Figure 7.
Affinity-purified human anti-Neu5Gc antibodies bind specifically to Neu5Gc-loaded endothelial membrane glycoproteins and induce complement deposition. Affinity-purified human anti-Neu5Gc antibodies17 were used as a primary. (A) Biotinylated, affinity-purified human anti-Neu5Gc antibodies were studied by Western blotting against surface membrane glycoproteins of Neu5Ac (Ac)– or Neu5Gc (Gc)–loaded endothelial cells, and the banding pattern was compared with that generated with the cGcAb antibody. HUVECs loaded with either Neu5Ac or Neu5Gc were lifted with 100mM EDTA and sonicated. Membrane fractions were prepared, 3 μg denatured by boiling in SDS separated on 12.5% polyacrylamide mini gels (Bio-Rad), and electrotransferred to nitrocellulose membranes. Membranes were blocked overnight at 4°C with 0.5% Neu5Gc-free cold water fish gelatin (Sigma-Aldrich) in TBST. Primary antibodies were incubated for 5 hours at 4°C with either biotinylated anti-Neu5Gc antibodies purified from individual human sera, diluted 1:100 in TBST, or chicken anti-Neu5Gc antibody (cGcAb), diluted 1:50 000 in TBST. Purification of the cGcAb is described elsewhere.4 Membranes were washed 4 times for 5 minutes in TBST then incubated with Streptavidin-HRP (Bio-Rad), diluted 1:50 000, or HRP-anti-chicken-IgY (Jackson ImmunoResearch Laboratories), diluted 1:10 000, at room temperature for 45 minutes. Proteins were visualized by chemiluminescence detection (Pierce), followed by exposure to Kodak BioMax XAR film for 5 to 30 seconds. A representative example from 3 independently performed experiments is shown. (B) Anti-Neu5Gc IgG binding (x-axis) and complement deposition (y-axis) were simultaneously assessed on Neu5Ac- and Neu5Gc-loaded endothelium. Affinity-purified anti-Neu5Gc antibodies, low-titer human serum (S30), or both were incubated with Neu5Gc-loaded (top) or Neu5Ac-loaded (bottom) HUVECs. Although the low-titer human serum does not bind HUVECs in a Neu5Gc-dependent fashion (left), the affinity-purified anti-Neu5Gc antibodies are necessary and sufficient to bind Neu5Gc-loaded HUVECs in a Neu5Gc-dependent fashion (middle). Supplementing the low-titer serum with the purified anti-Neu5Gc antibodies allows complement deposition on HUVECs in a Neu5Gc-dependent manner (right). A representative result of 3 independent experiments is shown.
Immunohistochemistry
Anonymous autopsy tissue samples of 11 normal and diseased human aortas were obtained from the Co-operative Human Tissue Network, with approval from the Institutional Review Board of University of California, San Diego. Tissue was frozen in OCT (Sakura) by the use of isopentane-dry ice slurry and the tissue blocks kept at −80°C. Blocks were sectioned at 5 μm onto glass slides and allowed to dry overnight. Washing buffer was PBS containing 0.1% Tween (PBST; Sigma-Aldrich), which was used between each step of the procedure, and 0.5% fish gelatin/PBST was the diluting buffer. Frozen sections were blocked for endogenous biotin (Vector Laboratories), followed by fixation with 10% neutral buffered formalin (Fisher) for 20 minutes. Sections were then incubated at room temperature for 1 hour with the cGcAb at 1:500, then with biotinylated donkey anti–chicken IgY antibody (Jackson ImmunoResearch Laboratories) at 1:200, and then with Cy3-strepavidin (Jackson ImmunoResearch Laboratories) at 1:500. Then, if horseradish peroxidase (HRP) was the label, color was developed by the use of AEC (Vector Laboratories), nuclei counterstained, and the slides were mounted with an aqueous mounting media. Diseased aortic sections also were incubated with anti-CD68 antibodies (macrophage marker, BD Biosciences), anti-MDA antibody MDA2 (oxidized-LDL marker),41 or the endothelial markers anti–human CD31 (Dako) and VWF (Dako). Nuclei were sometimes revealed with DAPI (Vector Laboratories). Brightfield slides were viewed by the use of an Olympus BH2 microscope and photographs taken for panels by the use of the Olympus Magnafire software and Photoshop (Adobe).
Controls included pooled preimmune chicken IgY substituted for cGcAb; pretreatment of sections with 250 mU of Arthrobacter ureafaciens sialidase (EY Laboratories Inc) in 100mM sodium acetate, pH 5.5, for 2.5 hours at 37°C before fixation; or dilution of 1:500 cGcAb in blocking solution containing 10% chimpanzee serum (a source of Neu5Gc-containing glycoproteins42) and applied over tissue sections.
Frozen sections of aorta also were probed with biotinylated anti-Neu5Gc antibodies purified from human serum17 diluted 1:3 in blocking solution and detected with rabbit anti-biotin diluted 1:500 in blocking solution, followed by Cy3-anti-rabbit (Jackson ImmunoResearch Laboratories) diluted 1:500 in blocking solution, mounted with an aqueous mounting media containing DAPI (Vector Laboratories), and viewed at 400× and 1000× magnification as described previously.
Results
Immunohistochemical detection of Neu5Gc on endothelium of large and small human vessels
Immunohistochemistry was performed on human tissue sections by the use of a chicken polyclonal antibody (cGcAb) that is highly specific and sensitive for Neu5Gc-containing glycans. The specificity of this antibody for Neu5Gc was previously demonstrated by multiple methods4 and was further controlled by the use of a preimmune IgY preparation. Examples are shown of results from frozen sections of many tissues. The cGcAb detected Neu5Gc on aortic endothelial cells from autopsy samples (Figure 1A left panel). Specificity of staining is demonstrated by a lack of background signal with preimmunized-chicken IgY (Figure 1A middle panel), by a lack of signal when cGcAb was adsorbed out by Neu5Gc-rich chimpanzee serum (Figure 1A right panel), and by a lack of signal when tissue sections were sialidase pretreated (data not shown). Double-label staining with the endothelial marker CD31 (Figure 1B) confirmed that Neu5Gc accumulation occurs primarily on the endothelium. As discussed later (Figure 5), we also showed accumulation of Neu5Gc in endothelium overlying atherosclerotic plaques.
Figure 5.
Neu5Gc in the endothelium and subendothelium of atherosclerotic plaques. (A) Expression of Neu5Gc in endothelium overlying human aortic atherosclerotic plaques is shown by double labeling with the cGcAb anti-Neu5Gc antibody (left) the endothelial marker CD31 (center, see merge, right). White arrows indicate subendothelial Neu5Gc within lesions (left). (B) Samples of human aortic atherosclerotic plaques were identified by their characteristic appearance (hematoxylin and eosin, bottom right). Accumulation of macrophages (stained with anti-CD68, top middle) and oxidized-LDL (stained with malondialdehyde, top right) are shown. The cGcAb (top left) was used to detect the presence of Neu5Gc on the endothelium of the plaques. Control IgY and mouse IgG (bottom right and middle) demonstrates specificity of the staining. (C) Human aortic atherosclerotic plaque sections were double-stained with anti-CD68 (middle) for macrophages and cGcAb (left) for Neu5Gc. The merged fluorescent image (right) indicates that macrophages are recruited to the Neu5Gc-lined endothelium. (D) Endothelium was labeled with anti-VWF antibody (center; note that there is also some subendothelial green auto-fluorescence caused by macrophages and/or necrotic foci). In addition to typical endothelial-colocalized Neu5Gc staining, extensive subendothelial labeling with the cGcAb anti-Neu5Gc antibody (red channel, left) is seen in this lesion (see yellow in the merged image, right). All pictures in this figure are representative examples of multiple independent analyses on multiple samples from multiple individuals.
Neu5Gc is also readily detected in the microvasculature of many human tissues. Examples such as placenta and colon are shown in Figure 1C (left panels). Again, negligible background was demonstrated with nonimmunized chicken IgY (Figure 1C right panels). Thus, Neu5Gc is present in vivo on the endothelium of both human large and small vessels, in a position to interact with circulating anti-Neu5Gc antibodies.
Affinity-purified human anti-Neu5Gc antibodies bind to endothelium in human tissue sections
To show that the human anti-Neu5Gc antibodies also can bind these endothelial antigens, we used a recently devised novel affinity-purification of anti-Neu5Gc antibodies from human sera.17 The specificity of this enriched Ig preparation for Neu5Gc was previously demonstrated by showing its failure to bind tissues of Neu5Gc-deficient Cmah−− mice.17 As shown in Figure 2A (top panels), the purified and biotinylated human anti-Neu5Gc antibodies stained endothelial cells of the human aorta, similar to the pattern seen with cGcAb in Figure 1. Antibody binding was again Neu5Gc dependent because the staining was abrogated by preincubation with Neu5Gc-rich chimpanzee serum (Figure 2A middle panels) or when the tissue was pretreated with sialidase (Figure 2A bottom panels).
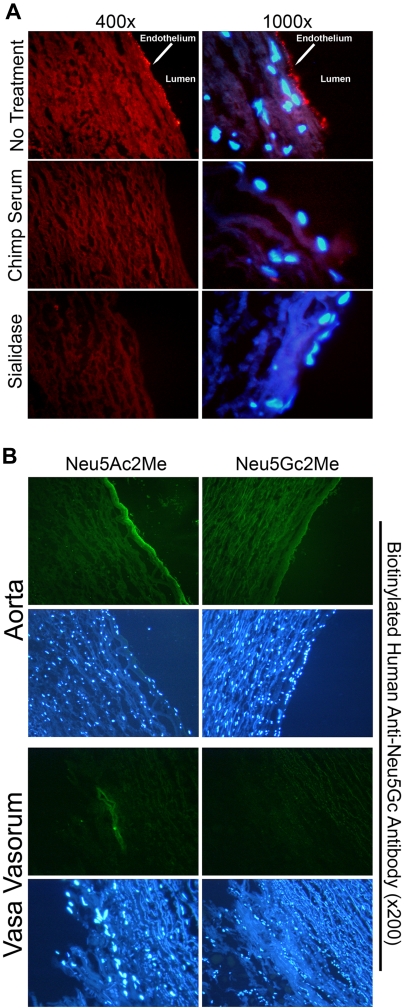
Figure 2.
Affinity-purified human anti-Neu5Gc antibodies bind specifically to Neu5Gc on endothelial cells of aorta sections and vasa vasorum. Affinity-purified human anti-Neu5Gc antibodies were used as a primary reagent on cryosections of autopsy samples of aorta. (A) Sections were incubated with the biotinylated, affinity-purified human anti-Neu5Gc antibodies followed by rabbit anti-biotin and then Cy3-anti-rabbit, with nuclear counterstaining using DAPI (top). Antibody staining was abrogated by adsorption with chimpanzee serum (middle) or by previous sialidase pretreatment (bottom). A representative example of 5 independent experiments is shown. (B) Sections were incubated with biotinylated, affinity-purified human anti-Neu5Gc antibodies followed by mouse anti-biotin and FITC antimouse antibody and nuclear counterstaining with DAPI. Primary antibody staining was abrogated by addition of Neu5Gc2Me but not by Neu5Ac2Me. The staining pattern is appreciated in both aorta and vasa vasorum. A representative example of 3 independent experiments on 8 individuals is shown (magnification ×200).
To further confirm Neu5Gc-specificity of the human antibodies, we studied the ability of methyl alpha-glycoside analogs of Neu5Gc (Neu5Gc2Me) or Neu5Ac (Neu5Ac2Me) to block the human Ig binding to endothelium. This synthetic mimic of native glycosidically linked Neu5Gc can effectively block the binding of human anti-Neu5Gc antibodies to Neu5Gc-containing glycans in enzyme-linked immunosorbent assays.17 Antibody binding to endothelium could be competed by Neu5Gc2Me but not by Neu5Ac2Me, confirming specificity for Neu5Gc-containing antigens on human aortic endothelium (Figure 2B). Interestingly, endothelial Neu5Gc expression also was detected in the vasa vasorum, the small vessels within the wall of the aorta. Again, Neu5Gc2Me but not Neu5Ac2Me was able to abolish this signal (Figure 2B). Thus, Neu5Gc present on endothelium lining large and small vessels was detected by affinity-purified human anti-Neu5Gc antibodies.
Anti-Neu5Gc antibodies from normal human sera bind to Neu5Gc-loaded human endothelial cells in vitro
To explore the pathogenic role of these human antibodies under more controlled conditions, we recapitulated the in vivo situation with an in vitro system. We previously demonstrated that human cells in culture can take up free Neu5Gc and metabolically incorporate it onto self-glycans.5,6 Human endothelial cells also exhibited this ability in a dose- and time-dependent manner, with up to one-third of the cell-surface Neu5Ac being replaced by Neu5Gc after a 3-day exposure to Neu5Gc in culture medium (data not shown, confirmed by the use of cGcAb as well as high-performance liquid chromatography analysis). With this in vitro system to model accumulation of Neu5Gc into human vasculature in vivo, we tested the reactivity of Neu5Gc-loaded endothelial cells against a panel of human sera collected from random healthy humans. Cells loaded with Neu5Ac were included to provide the optimal control for background reactivity, allowing Neu5Gc-dependent phenomena to be specifically determined.
The level of antibody binding assessed by flow cytometry ranged from no binding to levels far above the background with Neu5Ac feeding (see representative profiles with 4 human sera in Figure 3A; a more quantitative analysis of anti-Neu5Gc titers in the same healthy humans has been reported elsewhere17). We next observed variable Neu5Gc-specific reactivity in 14 random human sera for both IgG and IgM binding to intact cells, with human serum 34 (S34) giving the highest IgG binding and S35 giving the highest IgM binding. Arbitrarily normalizing IgG and IgM signals as a percent of the signal given by S34 in the same experiment (Figure 3B), we find that most persons (12 of 14) give relatively low IgG signals (defined as < 33% of the IgG signal given by S34), whereas S34 and S37 give the highest IgG signal. For IgM, we found that more persons (4 of 12) gave high anti-Neu5Gc IgM signals (at least 70% of the signal given by S34; Figure 3B).
Figure 3.
Human serum antibodies react against Neu5Gc-loaded endothelium. Human sera (S#), diluted 1:5, were assayed for their ability to bind HUVECs in a Neu5Gc-dependent manner. (A) Binding of human serum IgG (top) or IgM (bottom) to loaded HUVECs was assayed by flow cytometry. Neu5Gc-loaded cells (solid line), Neu5Ac-loaded cells (dotted line), and Neu5Gc-loaded cells stained with secondary antibody only (shaded curve). Representative individual human sera are shown. (B) IgG and IgM binding were normalized as a percentage value of the signal obtained with S34. The data are presented as mean ± SEM (n = 3). (C) Binding of anti-Neu5Gc IgG antibodies from S37 to Neu5Gc-loaded HUVEC was inhibited with the alpha-methyl glycoside, Neu5Gc2Me, in a dose-dependent manner and not by Neu5Ac2Me. (D) Classical complement, C3 convertase (C3b), was deposited onto Neu5Gc-loaded HUVEC (solid lines) only when incubated with high-titer anti-Neu5Gc serum (S34, right) and not low-titer anti-Neu5Gc serum (S30, left). Nonspecific complement was deposited onto Neu5Ac-loaded HUVEC (dotted lines) in a variable manner dependent on which sera was used, presumably attributable to unrelated serum factors. As a negative control, heat-inactivated serum was incubated with Neu5Gc-loaded HUVECs as a negative control (shaded curve). (E) Heat-inactivated S34 alone did not deposit complement in Neu5Gc-loaded HUVECs (shaded curve). Supplementation of S30 with heat-inactivated S34 shows Neu5Gc-dependent complement deposition in Neu5Gc-loaded (solid line) and not Neu5Ac-loaded HUVECs (dashed line). This finding indicates that anti-Neu5Gc antibodies are necessary and sufficient to mediate Neu5Gc-dependent complement deposition. All results presented in this figure were performed at least 3 times.
As an additional control, we assessed the ability of Neu5Gc2Me to block anti-Neu5Gc antibody binding. Using S37 as an example, we showed that Neu5Gc2Me inhibited anti-Neu5Gc IgG (Figure 3C) and IgM (not shown) binding to Neu5Gc-loaded endothelial cells in a dose-dependent manner. In contrast, we observed little inhibition by Neu5Ac2Me. Thus, antibodies from normal human sera bind specifically to Neu5Gc-fed endothelial cells, and this interaction is Neu5Gc dependent.
Human sera containing anti-Neu5Gc antibodies deposit complement onto Neu5Gc-loaded human endothelial cells
For further studies, we selected sera defined as “low-titer” or “high-titer” on the basis of reactivity against Neu5Gc-loaded endothelium (S30 and S34 from Figure 3B, respectively). Incubation of Neu5Gc-loaded endothelial cells with low-titer serum resulted in no complement deposition above levels observed with Neu5Ac-loaded endothelial cells (Figure 3D left panel). In contrast, high-titer serum gave a high level of Neu5Gc-specific complement deposition (Figure 3D right panel). Thus, the observed complement deposition is dependent on the anti-Neu5Gc antibody-antigen interaction. To confirm this, the high-titer serum S34 was heat-inactivated to deplete complement yet retains immunoglobulin activity.6 Heat-inactivated, high-titer sera failed to fix complement on endothelial cells (Figure 3D right panel, shaded curve). However, when heat-inactivated, high-titer serum was supplemented with fresh low-titer serum containing active complement, we again observed complement deposition in a Neu5Gc-dependent manner (Figure 3E).
Anti-Neu5Gc antibody-mediated complement deposition results in endothelial activation and increased leukocyte adhesion to Neu5Gc-loaded endothelial cells
Sublytic levels of complement deposition onto endothelial cells can induce cellular activation and up-regulation of surface adhesion molecules.43–45 We therefore examined Neu5Gc-loaded endothelial cells exposed to human serum containing anti-Neu5Gc antibodies. Five-hour incubations with 50% high-titer serum gave an increase in surface E-selectin expression in Neu5Gc-loaded endothelium over that in Neu5Ac-loaded cells (Figure 4A top left panel). As with antibody and complement deposition, there was some background with Neu5Ac-loaded cells, presumably because of other unrelated activating factors in the sera. Previous complement depletion by heat inactivation abrogated the difference in E-selectin expression between Neu5Gc- and Neu5Ac-loaded endothelium (Figure 4A bottom left panel). No differences in E-selectin expression were observed when cells were incubated with 50% low-titer serum (Figure 4A right panels).
In contrast to the transcriptionally regulated E-selectin surface expression upon activation, P-selectin surface expression occurs by mobilizing Weibel-Palade bodies to the plasma membrane.47 Shorter (15-minute) incubations of HUVECs with high-titer serum elicited cell-surface P-selectin expression that was Neu5Gc dependent and also sensitive to serum heat inactivation (Figure 4B). Thus, antibody-antigen binding with complement deposition is sufficient to induce cell-surface P-selectin presentation. Background P-selectin presentation was not evident in Neu5Ac-loaded cells nor in Neu5Gc-loaded cells exposed to low-titer or heat-inactivated serum.
We also studied cell lysates from such activated endothelial cells, looking for changes in cytokine production. Many cytokines were up-regulated in cells loaded with Neu5Gc and stimulated with fresh high titer of anti-Neu5Gc serum, in comparison with cells fed Neu5Ac and stimulated with the same fresh serum, or with cells loaded with Neu5Gc but exposed to heat-inactivated serum (supplemental Table 1). Many of the up-regulated cytokines were involved in monocyte recruitment (eg, CCL1/I-309, CCL8/MCP-2, CCL3/MIP-1α, CCL4/MIP-1β, CCL15/MIP-1δ, ICAM-1), leukocyte recruitment or differentiation (eg, macrophage colony-stimulating factor, granulocyte macrophage colony-stimulating factor, granulocyte colony-stimulating factor), or in the generation of a proinflammatory state (eg, interleukin-12 [IL-12] p70, IL-12 p40, IL-1α, interferon-γ [IFNγ]).
The aforementioned data confirm that binding of anti-Neu5Gc antibodies to Neu5Gc-containing glycans on endothelial cell surface and subsequent complement fixation causes endothelial activation. Recruitment of leukocytes to inflamed endothelium is a common pathway in endothelial damage, regardless of etiology. We therefore asked whether the high-titer anti-Neu5Gc antisera could specifically enhance binding of monocytes to Neu5Gc-loaded endothelial cells. Human PBMCs (105 per well) were added to Neu5Ac- or Neu5Gc-loaded endothelial cells, which had been exposed to high-titer (S34), low-titer (S30), or heat-inactivated serum for 4 hours. The cell layer was stained with a Cy3-anti-CD14 antibody to detect monocytes, and binding events were quantified by the counting of cells in 10 random 400× power fields. Results are summarized in Figure 4C.
As a positive control, tumor necrosis factor (TNF)-α–stimulated (10 ng/mL) endothelial cells demonstrated enhanced PBMC binding compared with cells not incubated with serum (28.1 ± 2.0 vs 2.1 ± 0.7 cells/field, respectively, P < .001). Incubation with low-titer serum did not significantly increase PBMC binding to Neu5Ac (1.8 ± 0.8 cells/field) or Neu5Gc-loaded endothelial cells (2.0 ± 1.0 cells/field). However, high-titer serum significantly enhanced PBMC binding to Neu5Gc-loaded cells over that seen with Neu5Ac-loaded cells (19.1 ± 3.1 vs 2.0 ± 0.7 cells/field, respectively, P < .001). Similar to the selectin and cytokine production data, enhanced PBMC binding to Neu5Gc-loaded endothelium induced by S34 was sensitive to heat inactivation (4.6 ± 1.0 cells/field, P < .001 vs fresh S34, n.s. vs no serum control). Thus, we observed a significant increase in PBMC binding only to Neu5Gc-loaded endothelium that was exposed to fresh serum with high anti-Neu5Gc antibodies.
Furthermore, PBMC binding to Neu5Gc-loaded endothelial cells, activated by high-titer serum, was sensitive to blocking by 1mM Neu5Gc2Me but not 1mM Neu5Ac2Me (Figure 4D). Representative immunofluorescence microscopy images of CD14-positive monocyte binding are also shown in supplemental Figure 1. We observed leukocyte binding and CD14 staining when endothelial cells are loaded with Neu5Gc and incubated with S34. There is substantially less leukocyte binding and CD14-positive binding on Neu5Gc-loaded endothelium when high-titered serum was preincubated with Neu5Gc2Me (supplemental Figure 1 bottom right panel) but not with Neu5Ac2Me (supplemental Figure 1 bottom left panel). These results further confirm that Neu5Gc antigen-antibody interaction is necessary for inducing subsequent leukocyte adhesion and that the human anti-Neu5Gc antibodies are exquisitely specific for the single oxygen atom difference between Neu5Gc versus Neu5Ac.
Endothelium overlying human atherosclerotic plaques contains Neu5Gc
Of the many human diseases associated with endothelial inflammation, the most prevalent is atherosclerosis, involving intimal accumulation of LDL cholesterol and oxidized LDL epitopes, exacerbated by many additional factors (see “Introduction” and “Discussion”). We therefore asked whether Neu5Gc accumulation occurs in atherosclerotic plaques. As shown in Figure 5A, Neu5Gc is expressed in the endothelium overlying atherosclerotic plaques, as evidenced by colocalization with the endothelial marker CD31. Plaques are detected not only by their characteristic appearance, but also by their accumulation of CD68+ macrophages and the lipid peroxidation epitope malondialdehyde (Figure 5B). This is shown more explicitly in Figure 5C, where fluorescent images are double-stained for Neu5Gc and CD68.
Subendothelial expression of Neu5Gc within atheromas
Whereas Neu5Gc expression in healthy aortic sections was localized to the endothelium of micro- and macrovasculature, areas of atherosclerotic human aorta exhibited novel subendothelial staining in addition to the typical endothelial staining (note the punctate, subendothelial Neu5Gc within the plaque in Figure 5A). In another example (Figure 5D), extensive and specific subendothelial Neu5Gc can be detected in the plaques. Thus, unlike the case in other blood vessels, Neu5Gc accumulation within atheromas provides an additional mechanism for circulating anti-Neu5Gc antibodies to accelerate inflammation.
An inflammatory cytokine enhances human anti-Neu5Gc antibody binding to Neu5Gc-loaded endothelium
Many immune processes involving the vasculature manifest flares of exacerbation, which is accompanied by a pathological production of inflammatory cytokines, notably TNF-α. This cytokine can also significantly alter the display patterns of surface sialic acids on human endothelial cells by up-regulating expression of the sialyltransferase ST6Gal-I.48 Thus, we hypothesized that local TNF-α production might enhance the anti-Neu5Gc-antibody binding profile to endothelial surfaces by altering the quantity and/or quality of Neu5Gc expression.
Endothelial cells also were treated with or without TNF-α during 18 hours of Neu5Gc or Neu5Ac feeding, and human serum antibody binding was then assessed by flow cytometry. As shown in Figure 6, TNF-α treatment increased binding of human Neu5Gc-dependent antibodies. In contrast, no difference was observed between treated and nontreated Neu5Ac-loaded endothelium, confirming that augmented antibody binding is Neu5Gc specific. Interestingly, TNF-α treatment did not actually cause an obvious increase in total Neu5Gc content as detected high-performance liquid chromatography analysis (data not shown). Thus, the TNF-α–induced increase in antibody binding represents a qualitative change in Neu5Gc antigen expression. We also studied IL-1β, IL-4, IL-6, IL-13, and IFNγ. Unlike TNF-α, none of these cytokines induced an increase in antibody binding to Neu5Gc-loaded endothelial cells, compared with mock-stimulated controls (data not shown). Thus, the TNF-α effect involves additional unknown factors, perhaps perpetuating a vicious cycle in the endothelium overlying atherosclerotic lesions.
Figure 6.
TNF-α augments human antibody reactivity against Neu5Gc-loaded endothelium. Endothelial cells were treated with TNF-α (10 ng/mL) during the last 18 hours of Neu5Gc loading. Antibody binding was assessed for both treated and nontreated cells after incubating with high-titer human serum (S34). Control peaks represent staining with FITC-conjugated secondary antibody alone. The experiment was performed multiple times and representative examples are shown. Several other cytokines studied (IL-1β, IL-4, IL-6, IL-13, and IFNγ) did not show this enhancing effect (not shown).
Affinity-purified human anti-Neu5Gc antibodies from human sera bind to specific glycoproteins in Neu5Gc-loaded endothelial cells and direct complement deposition
We further characterized the interplay among purified human anti-Neu5Gc antibodies, Neu5Gc-expressing endothelium, and complement. By using the purified human anti-Neu5Gc antibodies, we first investigated the nature of the endothelial glycoproteins presenting the Neu5Gc. Membrane proteins were isolated from Neu5Ac- and Neu5Gc-loaded endothelium and subjected to Western blotting. As shown in Figure 7A, the purified human anti-Neu5Gc antibodies recognized only membrane glycoproteins isolated from Neu5Gc-loaded endothelium. Interestingly, they bound only to a subset of the Neu5Gc-expressing glycoproteins detected by the broad-spectrum chicken cGcAb. This result further indicates that specific linkages and/or conformational presentations of Neu5Gc on endothelial cell glycans are selective targets for circulating human antibodies.
Finally, we asked whether purified human anti-Neu5Gc antibodies could also initiate complement deposition. Neu5Gc-loaded endothelial cells were incubated with low-titer serum, affinity-purified anti-Neu5Gc antibodies, or both, and then double-labeled for complement deposition and IgG binding (Figure 7B). As expected, incubation with a low-titer serum on Neu5Gc- or Neu5Ac-loaded endothelial cells resulted in a majority of cells being double negative (Figure 7B left column). In contrast, incubation of the purified anti-Neu5Gc antibodies on Neu5Gc-loaded endothelium resulted in 79% of cells becoming positive for IgG binding, whereas 86% of Neu5Ac-loaded cells remained double negative (Figure 7B middle column). Moreover, incubation of Neu5Gc-loaded endothelium with a low-titer serum in the presence of the purified anti-Neu5Gc antibodies resulted in 71% of cells becoming positive for both IgG and complement binding (Figure 7B right column, top panel). These data confirm that human anti-Neu5Gc antibody binding to Neu5Gc-expressing endothelium directs complement deposition and thus also likely drives Neu5Gc-dependent leukocyte adhesion in vivo.
Discussion
Taken together, these findings suggest a mechanism whereby anti-Neu5Gc antibodies can initiate, perpetuate, and/or exacerbate an inflammatory response at the endothelium, potentially playing a role in disease states such as atherosclerosis, wherein vascular inflammation is involved. Neu5Gc is a novel dietary and human-specific “xeno-auto-antigen” that may exacerbate a variety of vascular pathologies. Diets recommended by the US Department of Agriculture lead one to ingest approximately 10 mg of Neu5Gc per day.8 The primary dietary sources appear to be red meat (lamb, pork, and beef) and milk products, with Neu5Gc being conspicuously low in poultry and fish and absent from plants. Thus, long-term Neu5Gc intake and metabolic incorporation can explain our immunohistochemical finding of a strong Neu5Gc signal in the endothelial lining of blood vessels of human tissues. This scenario is confirmed in vitro by demonstrating that Neu5Gc in cell media is sufficient for its incorporation into cell-surface glycans in a time- and concentration-dependent manner.
We and others6,7,16,17 have previously shown that Neu5Gc is immunogenic in humans. Although it was previously thought that high antibody titers were found only in human diseases, we have recently found that the anti-Neu5Gc antibody response is quite prevalent17 and have confirmed here that these antibodies can bind Neu5Gc-loaded human endothelial cells (Figure 2A-B). Moreover we show that the purified anti-Neu5Gc human antibodies bound to Neu5Gc in human endothelial cells in tissue sections (Figure 2), similar to that seen when using the Neu5Gc-specific chicken cGcAb (Figure 1).
We hypothesized that the combination of Neu5Gc-containing glycans on endothelial cells and anti-Neu5Gc antibodies in circulation leads to antibody deposition on endothelial surfaces and subsequent complement deposition. This would promote a proinflammatory state in the vasculature and cell-surface expression of adhesion molecules (Figure 4). The nearly immediate up-regulation of P-selectin and longer-term de novo presentation of E-selectin provide a means for selectin-initiated rolling adhesion of leukocytes on the vessel wall. Moreover, up-regulation of cytokines by activated endothelial cells (see supplemental Table 1) that are involved in monocyte recruitment (CCL1, granulocyte macrophage colony stimulating factor, macrophage colony-stimulating factor) and generation of a proinflammatory state (IL-12 p70, IL-12 p40, IL-1α, IFNγ) suggest mutually reinforcing mechanisms for facilitating monocyte extravasation at sites of endothelial Neu5Gc incorporation.
Indeed, in vitro evidence confirmed that PBMCs adhered to endothelial cells in a Neu5Gc-dependent fashion only in the presence of anti-Neu5Gc antibodies (Figure 4C). Because leukocyte recruitment via endothelial interactions is central to many inflammatory vascular diseases, this human-specific immune response could significantly contribute to endothelial damage. Furthermore, the presence of TNF-α, a proinflammatory cytokine known to be up-regulated during flares of vascular damage, can enhance the impact of the human anti-Neu5Gc antibodies (Figure 6). In addition, complement binding can have direct toxic effects on the endothelium, further contributing to endothelial damage. Such processes could be involved in exacerbating both the early stages of atherosclerosis and the late ulcerating phases, as both are facilitated by endothelial inflammation.
Of note, the Neu5Gc is covalently bound to and expressed on surface glycoproteins of the endothelial cells. Thus, any shedding of Neu5Gc on glycoproteins would be minor, and generalized immune complex formation is unlikely to be significant. However, this possibility needs to be considered. Also, we have previously reported that circulating blood cells do not express Neu5Gc, even after human volunteer ingestion of a large amount of Neu5Gc.8 The reason for the difference between endothelial cells and blood cells is unknown, but it may have to do with differences in intracellular handling of exogenous Neu5Gc. Regardless, it is clear that the primary target of anti-Neu5Gc antibodies in circulation are endothelial cells.
In contrast to many other examples of AECAs, the humoral response to Neu5Gc is unique to humans, as compared with standard mammalian models. Interestingly, the Neu5Gc content of human tissues is likely to be chiefly dependent on dietary consumption of red meats, a food associated with increases in circulating markers of chronic inflammation.9–12 In this regard, we recently demonstrated that incorporation of dietary Neu5Gc into malignant cells can help explain the exacerbating effects of red meat consumption on carcinoma progression, a process also associated with chronic inflammation.49
Of course, more information is needed about how dietary Neu5Gc is absorbed and distributed and what regulates its incorporation into tissues. It is also necessary to learn which endothelial glycan epitopes are the primary carriers of metabolically incorporated Neu5Gc and which specific anti-Neu5Gc antibodies are likely to be implicated in various pathological conditions involving vascular inflammation. The broad spectrum and variable prevalence of the anti-Neu5Gc antibodies in humans raises the possibility that not all subpopulations of antibodies are equally pathogenic. For example, as detailed recently,17 the anti-Neu5Gc antibody response is more pronounced for certain presentations of Neu5Gc, such as Neu5Gcα2–6Galβ1–4Glc-, a sequence found in endothelium in vivo, and up-regulated by inflammatory cytokines such as TNF-α. Moreover, persons with similar anti-Neu5Gc IgG levels did not give similar levels of complement deposition or cell activation, potentially reflecting differences in IgG subclass composition. Understanding the presentation(s) of Neu5Gc (both the linkage type and underlying glycoprotein structure) that are targeted by anti-Neu5Gc antibodies in vivo would also define relevant antigens that could be used to define pathogenic anti-Neu5Gc antibodies.
Finally, other investigators50 have shown that conjugating a protein antigen to a foreign sugar can lead to enhanced production of antibodies against the protein itself, without the need for adjuvant. This response was dependent on the presence of antibodies against the foreign glycan, with the resulting immune complexes apparently acting as an adjuvant. Thus, antibodies that bind Neu5Gc on endothelial proteins could even prime a secondary immune response against these self-protein antigens themselves.
In conclusion, our studies describe a novel human-specific xeno-auto-antigen–mediated immunologic mechanism that could contribute significantly to human vascular pathologies, such as atherosclerosis. It is interesting that subendothelial Neu5Gc deposition also appears within atherosclerotic lesions, perhaps as the result of permeability changes in chronically inflamed endothelium. Regardless of the reason, the appearance of Neu5Gc within the lesions provides another potential mechanism to exacerbate inflammation via anti-Neu5Gc antibodies. Although much work is required to substantiate such examples of relevance to human disease, this hypothesis would support novel therapeutic approaches to reducing or dampening flares of immunologic responses against the endothelium. Such interventions could include reduction of dietary Neu5Gc intake and accumulation through simple diet-based interventions, enhanced elimination of Neu5Gc from humans, and/or reduction of anti-Neu5Gc antibodies titers.
Acknowledgments
This work was supported by NIH grants R01-GM32373 and R01-CA38701 to A.V. T.P. was a Medical Student fellow of the Howard Hughes Medical Institute (HHMI).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.P., C.J.G., and A.V. designed the studies; T.P., C.J.G., R.C., F.K., V.P.-K., and N.M.V. performed the research; H.C. and X.C. synthesized a critical reagent; and T.P., C.J.G., V.P.-K., J.L.W., N.M.V., and A.V. wrote the paper.
Conflict-of-interest disclosure: A.V. and N.M.V. are cofounders of Gc-Free Inc, a startup biotech company interested in the pathological consequences of Neu5Gc and Neu5Gc antibody interactions in humans. The remaining authors declare no competing financial interests.
Correspondence: Ajit Varki, 9500 Gilman Dr, University of California, San Diego, La Jolla, CA 92093-0687; e-mail: a1varki@ucsd.edu.
References
- 1.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446(7139):1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 2.Hayakawa T, Satta Y, Gagneux P, Varki A, Takahata N. Alu-mediated inactivation of the human CMP-N-acetylneuraminic acid hydroxylase gene. Proc Natl Acad Sci U S A. 2001;98(18):11399–11404. doi: 10.1073/pnas.191268198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hedlund M, Tangvoranuntakul P, Takematsu H, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27(12):4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diaz SL, Padler-Karavani V, Ghaderi D, et al. Sensitive and specific detection of the non-human sialic acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE. 2009;4(1):e4241. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280(6):4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175(1):228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- 7.Yin J, Hashimoto A, Izawa M, et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66(6):2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 8.Tangvoranuntakul P, Gagneux P, Diaz S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100(21):12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol. 2004;79(1):3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Garcia E, Schulze MB, Fung TT, et al. Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2004;80(4):1029–1035. doi: 10.1093/ajcn/80.4.1029. [DOI] [PubMed] [Google Scholar]
- 11.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675–684. doi: 10.1093/ajcn.82.3.675. quiz 714–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137(4):992–998. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 13.Malykh YN, Schauer R, Shaw L. N-glycolylneuraminic acid in human tumours. Biochimie. 2001;83(7):623–634. doi: 10.1016/s0300-9084(01)01303-7. [DOI] [PubMed] [Google Scholar]
- 14.Beer P. The heterophile antibodies in infectious mononucleosis and after injection of serum. J Clin Invest. 1936;15(6):591–599. doi: 10.1172/JCI100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morito T, Kano K, Milgrom F. Hanganutziu-Deicher antibodies in infectious mononucleosis and other diseases. J Immunol. 1982;129(6):2524–2528. [PubMed] [Google Scholar]
- 16.Zhu A, Hurst R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9(6):376–381. doi: 10.1034/j.1399-3089.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- 17.Padler-Karavani V, Yu H, Cao H, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18(10):818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinsey GR, Li L, Okusa MD. Inflammation in acute kidney injury. Nephron Exp Nephrol. 2008;109(4):e102–e107. doi: 10.1159/000142934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 20.Pall AA, Savage CO. Mechanisms of endothelial cell injury in vasculitis. Springer Semin Immunopathol. 1994;16(1):23–37. doi: 10.1007/BF00196711. [DOI] [PubMed] [Google Scholar]
- 21.Harper L, Savage CO. Pathogenesis of ANCA-associated systemic vasculitis. J Pathol. 2000;190(3):349–359. doi: 10.1002/(SICI)1096-9896(200002)190:3<349::AID-PATH524>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 22.Guilpain P, Mouthon L. Antiendothelial cells autoantibodies in vasculitis-associated systemic diseases. Clin Rev Allergy Immunol. 2008;35(1–2):59–65. doi: 10.1007/s12016-007-8069-3. [DOI] [PubMed] [Google Scholar]
- 23.Navarro M, Cervera R, Font J, et al. Anti-endothelial cell antibodies in systemic autoimmune diseases: prevalence and clinical significance. Lupus. 1997;6(6):521–526. doi: 10.1177/096120339700600608. [DOI] [PubMed] [Google Scholar]
- 24.Meroni P, Ronda N, Raschi E, Borghi MO. Humoral autoimmunity against endothelium: theory or reality? Trends Immunol. 2005;26(5):275–281. doi: 10.1016/j.it.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Gobel U, Eichhorn J, Kettritz R, et al. Disease activity and autoantibodies to endothelial cells in patients with Wegener's granulomatosis. Am J Kidney Dis. 1996;28(2):186–194. doi: 10.1016/s0272-6386(96)90300-5. [DOI] [PubMed] [Google Scholar]
- 26.Jamin C, Dugue C, Alard JE, et al. Induction of endothelial cell apoptosis by the binding of anti-endothelial cell antibodies to Hsp60 in vasculitis-associated systemic autoimmune diseases. Arthritis Rheum. 2005;52(12):4028–4038. doi: 10.1002/art.21401. [DOI] [PubMed] [Google Scholar]
- 27.Yang YH, Wang SJ, Chuang YH, Lin YT, Chiang BL. The level of IgA antibodies to human umbilical vein endothelial cells can be enhanced by TNF-alpha treatment in children with Henoch-Schonlein purpura. Clin Exp Immunol. 2002;130(2):352–357. doi: 10.1046/j.1365-2249.2002.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry GJ, Elston T, Khouri NA, Chan TM, Cameron JS, Frampton G. Antiendothelial cell antibodies in lupus: correlations with renal injury and circulating markers of endothelial damage. Q J Med. 1993;86(11):727–734. [PubMed] [Google Scholar]
- 29.Song J, Park YB, Lee WK, Lee KH, Lee SK. Clinical associations of anti-endothelial cell antibodies in patients with systemic lupus erythematosus. Rheumatol Int. 2000;20(1):1–7. doi: 10.1007/s002960000060. [DOI] [PubMed] [Google Scholar]
- 30.Matsuo S, Fukatsu A, Taub ML, Caldwell PR, Brentjens JR, Andres G. Glomerulonephritis induced in the rabbit by antiendothelial antibodies. J Clin Invest. 1987;79(6):1798–1811. doi: 10.1172/JCI113021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Binder CJ, Shaw PX, Chang MK, et al. The role of natural antibodies in atherogenesis. J Lipid Res. 2005;46(7):1353–1363. doi: 10.1194/jlr.R500005-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Palinski W, Tangirala RK, Miller E, Young SG, Witztum JL. Increased autoantibody titers against epitopes of oxidized LDL in LDL receptor-deficient mice with increased atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15(10):1569–1576. doi: 10.1161/01.atv.15.10.1569. [DOI] [PubMed] [Google Scholar]
- 33.Xu Q, Willeit J, Marosi M, et al. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341(8840):255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Katz RJ, Quyyumi AA, et al. Association of serum antibodies to heat-shock protein 65 with coronary calcification levels: suggestion of pathogen-triggered autoimmunity in early atherosclerosis. Circulation. 2004;109(1):36–41. doi: 10.1161/01.CIR.0000105513.37677.B3. [DOI] [PubMed] [Google Scholar]
- 35.Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- 36.Perschinka H, Wellenzohn B, Parson W, et al. Identification of atherosclerosis-associated conformational heat shock protein 60 epitopes by phage display and structural alignment. Atherosclerosis. 2007;194(1):79–87. doi: 10.1016/j.atherosclerosis.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Praprotnik S, Blank M, Meroni PL, Rozman B, Eldor A, Shoenfeld Y. Classification of anti-endothelial cell antibodies into antibodies against microvascular and macrovascular endothelial cells: the pathogenic and diagnostic implications. Arthritis Rheum. 2001;44(7):1484–1494. doi: 10.1002/1529-0131(200107)44:7<1484::AID-ART269>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 38.Del Papa N, Conforti G, Gambini D, et al. Characterization of the endothelial surface proteins recognized by anti-endothelial antibodies in primary and secondary autoimmune vasculitis. Clin Immunol Immunopathol. 1994;70(3):211–216. doi: 10.1006/clin.1994.1031. [DOI] [PubMed] [Google Scholar]
- 39.Brock TG, McNish RW, Coffey MJ, Ojo TC, Phare SM, Peters-Golden M. Effects of granulocyte-macrophage colony-stimulating factor on eicosanoid production by mononuclear phagocytes. J Immunol. 1996;156(7):2522–2527. [PubMed] [Google Scholar]
- 40.Delneste Y, Charbonnier P, Herbault N, et al. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101(1):143–150. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 41.Palinski W, Yla-Herttuala S, Rosenfeld ME, et al. Antisera and monoclonal antibodies specific for epitopes generated during oxidative modification of low density lipoprotein. Arteriosclerosis. 1990;10(3):325–335. doi: 10.1161/01.atv.10.3.325. [DOI] [PubMed] [Google Scholar]
- 42.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci U S A. 2006;103(20):7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zimmerman GA, McIntyre TM, Prescott SM. Adhesion and signaling in vascular cell-cell interactions. J Clin Invest. 1996;98(8):1699–1702. doi: 10.1172/JCI118967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foreman KE, Glovsky MM, Warner RL, Horvath SJ, Ward PA. Comparative effect of C3a and C5a on adhesion molecule expression on neutrophils and endothelial cells. Inflammation. 1996;20(1):1–9. doi: 10.1007/BF01487740. [DOI] [PubMed] [Google Scholar]
- 45.Foreman KE, Vaporciyan AA, Bonish BK, et al. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994;94(3):1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takano M, Meneshian A, Sheikh E, et al. Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am J Physiol Heart Circ Physiol. 2002;283(5):H2054–H2061. doi: 10.1152/ajpheart.01001.2001. [DOI] [PubMed] [Google Scholar]
- 47.Geng JG, Bevilacqua MP, Moore KL, et al. Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature. 1990;343(6260):757–760. doi: 10.1038/343757a0. [DOI] [PubMed] [Google Scholar]
- 48.Hanasaki K, Varki A, Stamenkovic I, Bevilacqua MP. Cytokine-induced beta-galactoside alpha-2,6-sialyltransferase in human endothelial cells mediates alpha2,6-sialylation of adhesion molecules and CD22 ligands. J Biol Chem. 1994;269(14):10637–10643. [PubMed] [Google Scholar]
- 49.Hedlund M, Padler-Karavani V, Varki NM, Varki A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A. 2008;105(48):18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benatuil L, Kaye J, Rich RF, Fishman JA, Green WR, Iacomini J. The influence of natural antibody specificity on antigen immunogenicity. Eur J Immunol. 2005;35(9):2638–2647. doi: 10.1002/eji.200526146. [DOI] [PubMed] [Google Scholar]