Abstract.
The GTP-binding proteins RhoA, Cdc42 and Rac1 regulate the organization and turnover of the cytoskeleton and cell-matrix adhesions, structures bridging cells to their support, and translating forces, external or generated within the cell. To investigate the specific requirements of Rho GTPases for biomechanical activities of clonal cell populations, we compared side-by-side stable lines of human fibroblasts expressing constitutively active (CA) RhoA, Cdc42 or Rac1. There was no marked effect of any CA GTPase on cell adhesion to different extracellular matrix proteins. Cell spreading was CA Rho GTPase specific and independent of the extracellular matrix proteins allowing adhesion. Mechanical properties were dramatically restricted by CA RhoA on bi- and in tri-dimensional surroundings, were boosted by CA Rac1 on bi-dimensional surroundings only, and were not or marginally affected by CA Cdc42. In conclusion, the action of Rho GTPases appears to depend on the task cells are performing.
Key words. RhoA, Rac1, Cdc42, QL mutants, fibroblast, adhesion, contraction, migration, cytoskeleton
The Rho subfamily of small GTPases, in particular the archetypal trio RhoA, Rac1 and Cdc42, governs the dynamics of the actin cytoskeleton [1]. These proteins thereby, regulate multiple cellular functions including cell migration, polarization, survival and proliferation as well as activation of transacting factors, their translocation to the nucleus and trafficking and positioning of organelles [2]. Like other small GTPases, Rho GTPases operate as binary molecular switches by cycling between active, GTP-bound and inactive, GDP-bound conformations [1, 3]. When GTP-bound, GTPases are targeted to the cell membrane where they activate defined sets of effectors specific for each GTPase [4, 5]. Rho GTPases are activated in response to different stimuli including soluble factors such as growth factors and cytokines, and integrin-mediated interactions with extracellular matrix proteins [1, 5]. Integrins are cell surface receptors integrating the biological and mechanical information from the extracellular matrix at specific sites of the cell membrane, the cell-matrix adhesions. There, the transmembrane integrins provide a physical link between extracellular matrix proteins and the intracellular cytoskeleton, allowing the transmission of mechanical forces necessary for cell adhesion and movement and for assembly and remodeling of the extracellular matrix [6, 7]. By controlling the organization of the cytoskeleton and cell-matrix adhesions, the trio Cdc42, Rac1 and RhoA regulates these integrin-linked mechanical functions [1, 8].
The functions of Rho GTPases and their signaling pathways have been mainly identified in experiments using specific dominant negative (displaying a higher affinity for GDP) or constitutively active (CA) (loss of the intrinsic GTPase activity) mutants generated by amino acid substitution [9–14]. Microinjection of CA RhoA in quiescent Swiss 3T3 fibroblasts induced the assembly of actin filaments into stress fibers, while CA Cdc42 and Rac1 triggered de novo actin polymerization and the formation of filopodia and lamellipodia, respectively. By using cell migration as a model of integrated mechanical functions, although each of the three Rho GTPases has a distinct function, their cooperation was shown to be critical in determining the final pattern of cytoskeleton organization. Cell migration is a multi-step process which has been extensively studied at a single-cell level. It includes the extension of a leading edge protrusion or lamellipodium, the establishment of new adhesion sites at the front, cell body contraction and detachment of adhesive structures at the cell rear. Achievement of each step requires proper spatiotemporal regulation and defined cooperation of Rho GTPases [15]. However, the action of Rho GTPases may depend on the task required of the cell and may vary according to fundamental biomechanical activities such as adhesion, migration and matrix remodeling. Moreover, biomechanical activities, in particular matrix remodeling, involve the coordinated action of an entire cell population. To test the role of Rho GTPases in the biomechanical activity of a cell population, we generated lines of fibroblasts stably transfected with CA forms (Cdc42-Q61L, Rac1-Q61L and RhoA-Q63L) of RhoA, Rac1 and Cdc42 and selected the most pertinent clones [16]. Using these clonal cell populations, we here compare side-by-side the role of RhoA, Rac1 and Cdc42 during cell adhesion, migration and matrix remodeling.
Materials and Methods
Plasmids and generation of stable transfectants. Wi-26 cells (SV40-transformed human lung fibroblasts) transfected with the cDNA coding for CA forms of RhoA (RhoA-Q63L), Rac1 (Rac1-Q61L) and Cdc42 (Cdc42-Q61L) in pIRESpuro vector (Clontech, Palo Alto, Calif.) were subcloned by limited dilution and amplified as reported elsewhere [16]. The cloned cells (hereafter referred to as RhoA-QL, Rac1-QL and Cdc42-QL) and the parental line transfected with the empty vector (hereafter referred to as control) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum (FCS), 200 mM glutamine and a mixture of antibiotics (streptomycin, penicillin, puromycin) at 37°C under 5% CO2 except where otherwise stated. All products were from Seromed/Biochrom (Berlin, Germany).
GTPase pull-down assays. Confluent cells were serumstarved for 24 h and lysed as previously described [16]. Active GTPases were pulled-down from the lysates (500 µl) using either the GST-PBD fusion protein with the Cdc42- and Rac1-binding region of PAK-1B, or the GST-RBD fusion protein with the RhoA-binding region of rhotekin [17, 18]. The total lysate (40 µl) and pull-down fractions were separated by SDS-PAGE on 15% acrylamide gels under reducing conditions. Proteins were transferred to nitrocellulose membranes and immunodetected with mouse monoclonal primary antibodies against either RhoA (Sc-418; Santa Cruz, Calif.), Rac1 (clone 05-389; Upstate Biotechnology, Lake Placid, N. Y.) or Cdc42 (clone 610928; Transduction Laboratories, San Diego, Calif.), followed by secondary horseradish peroxidase-conjugated antibodies (DAKO, Glostrup, Denmark). The signals were visualized using ECL (Amersham Biosciences Europe, Freiburg, Germany). Band intensities were quantified with NIH Scion image software and the relative amount of active, GTP-bound GTPase was normalized to the total content of GTPase in the lysate.
Immunofluorescence staining. Cells grown on glass coverslips for 24 h in complete medium were fixed with freshly prepared 2% paraformaldehyde in phosphate-buffered saline, pH 7.4 (PBS) for 15 min, permeabilized with ice-cold 0.2% Triton X-100 in PBS for 1 min and incubated with 3% bovine serum albumin (BSA, fraction V; Serva, Heidelberg, Germany) for 1 h. The cells were labeled with either affinity-purified rabbit antiserum against FHL2 [19] or mouse monoclonal antibody F-VII against vinculin (a gift from Dr. M. Glukhova, Institut Curie, Paris, France), followed by Cy3-conjugated secondary antibodies against rabbit or mouse immunoglobulins (Jackson, distributed through Dianova). Fibrillar actin was visualized with FITC-conjugated phalloidin (Sigma-Aldrich, Deisenhofen, Germany). The coverslips were mounted on histoslides and the stainings were observed by laser scanning confocal microscopy (Leica, Heidelberg, Germany) with single-channel excitation. Confocal images were acquired and stored using the Leica confocal software and mounted using Adobe Photoshop.
Cell adhesion and spreading assays. Multiwell tissue culture plates (96 wells; Costar, Bodenheim, Germany) were coated with collagen I (20 µg/ml; Seromed-Biochrom), laminin 1 (20 µg/ml; kindly provided by Dr. R. Timpl, Max-Planck Institut für Biochemie, Martinsried, Germany), fibronectin (40 µg/ml; Chemicon) or laminin 5 (5 µg/ml [20]). After saturation of the wells with 1% BSA, equal numbers of control, Cdc42-QL, Rac1-QL and RhoA-QL cells were seeded in triplicate for 15, 30 and 60 min in FCS-free DMEM. At the end of the experiments, non-adherent cells were removed by washing with PBS and adherent cells were fixed, stained with crystal violet and the extent of adhesion was quantified by colorimetry as previously reported [21]. To monitor cell spreading, photographs of adherent cells were taken from triplicate wells with a phase contrast microscope (Axiovert S100TV; Zeiss) equipped with a monochromatic digital camera (PowerShot G5; Canon, Tokyo, Japan). Spreading was quantified by visual counting of round (no visible cytoplasm) and spread (visible cytoplasm) cells in a population of at least 100 adherent cells on each photograph. Data were expressed as average of triplicate measurements ± SD.
Cell migration assays. Confluent cells were suspended by 0.05% trypsin and 0.02% EDTA in PBS, centrifuged and resuspended in FCS-containing DMEM at high cell density (106 cells/ml). Aliquots of the cell suspension (10 µl) were deposited as colonies in the center of duplicate wells (one colony/well, 24-well tissue culture plates; Costar) and the cells were allowed to attach at 37 °C in a humidified incubator. After 1 h, the colonies were washed with PBS and the wells were filled with 400 µl of FCS-free medium. At this time point (T0), a first photograph of each colony was taken with an inverted phase contrast microscope (Zeiss Axiovert S100TV, Leipzig, Germany) equipped with a CCD camera (Xillix MicroImager, Richmond, Canada) and further photographs of the colonies were captured automatically every 10 min for the next 800 min. Images were stored and processed using Openlab software (Improvision, Heidelberg, Germany). The sequences of images were converted to Quick Time movies to analyze cell migration tracks using Dynamic Image Analysis System software (Solltech, Oakdale, Iowa). Twenty tracks were analyzed under each condition and extracted migration parameters included cell velocity (speed in µm/min) and directed migration index defined by the ratio between the linear and the absolute distances covered by a cell during the time of recording. An index of 1 indicates that a cell moves following a straight path while a value of 0 indicates random movement. Differences between clones were analyzed by Student’s t test and were considered to be significant at p < 0.05.
Collagen gel contraction. Cells were seeded in triplicate at a density of 1.5×105 cells/ml into 32-mm bacteriological dishes (2 ml/dish; Renner, Dannstatt, Germany) in DMEM supplemented with 10% FCS, Na-ascorbate (50 µg/ml), antibiotics and 0.3 mg/ml of newborn calf skin, acid-extracted collagen I (IBFB-Pharma, Leipzig, Germany) as previously described [22]. The cultures were placed at 37°C to allow collagen polymerization (an intrinsic and specific property of collagen I) and gradual lattice contraction was monitored by measuring the gel diameter of triplicate set ups at successive time points up to 48 h. Data were expressed as average of triplicate measurements ± SD. Phase contrast photographs of gelembedded fibroblasts were taken using an Olympus IX-81 microscope equipped with a monochromatic digital camera.
Results
Basal Rho GTPase activities in RhoA-QL, Rac1-QL and Cdc42-QL fibroblasts. Clonal stable lines of Wi-26 fibroblasts that express the CA forms of RhoA, Rac1 and Cdc42 (RhoA-QL, Rac1-QL, Cdc42-QL) generated by glutamine to leucine mutation were established in our laboratories [16]. The mutation abolishes intrinsic GTPase activity and the protein should be locked in its GTP-bound, active conformation [3]. This was confirmed by pull-down assays demonstrating increased active forms of RhoA, Rac1 and Cdc42 in the selected clones [16] (fig. 1A, B). Because cooperation between the three Rho GTPases may result in an activation cascade [5, 23], such as Cdc42 activating Rac1, which in turn activates RhoA [10, 24], we measured the basal level of activity of each Rho GTPase in serum-starved mock-transfected parental cells (control) and selected QL clones (fig. 1A, B). The basal level of GTP-bound RhoA was equally very low in the control, Cdc42-QL and Rac1-QL cells and, as expected, high in RhoA-QL cells. GTP-bound Cdc42 was also low in the parental, RhoA-QL and Rac1-QL cells, and significantly higher in the Cdc42-QL clone. The level of GTP-bound Rac1 in the RhoA-QL and Cdc42-QL clones was as low as in the parental mock-transfected parental cells and increased in the Rac1-QL clone. Thus, there was no marked influence of any of the CA GTPases on the expression level and activation of the other two.
Figure 1.
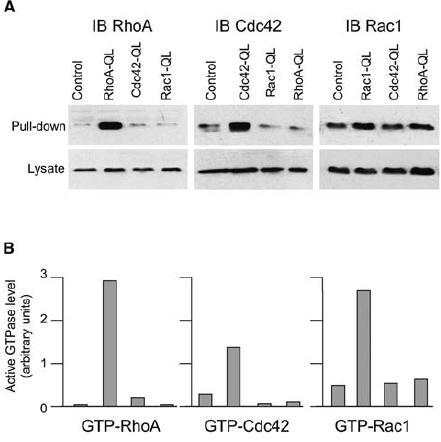
Basal levels of active GTPases in RhoA-QL, Rac1-QL and Cdc42-QL fibroblasts. Cells were serum-starved for 24 h and the activity of RhoA, Rac1 and Cdc42 was measured by pull-down assays in parental mock-transfected cells (control) and in the RhoA-QL, Rac1-QL and Cdc42-QL clones as indicated in A. Signals obtained for the pull-down samples correspond to 800 µl of lysate for the blots with anti-RhoA and anti-Cdc42 and 500 µl of lysate for the blot with anti-Rac1. Aliquots of the respective lysates (40 µl) were used to analyze the total amount of each GTPase. (B) Band intensity in the blots shown in A was quantified by densitometry and the relative amounts of active GTPase were normalized to the total content of GTPase in the lysate.
Distribution of fibrillar actin and cellular adhesions in RhoA-QL, Rac1-QL and Cdc42-QL fibroblasts. The effects of Rho GTPases on the organization of the actin cytoskeleton and cellular adhesions have been extensively documented after transient transfection or micro-injection of active forms of the GTPases. Namely, active Cdc42 and Rac1 induce de novo actin polymerization and cellular protrusions, i.e. filopodia and lamellipodia, respectively, while bundling of actin filaments into stress fibers occurs upon RhoA activation [9, 10, 14, 24]. To determine whether these features are reproduced in the stable QL mutants we have generated, we stained fibrillar actin and markers for nascent and mature adhesion complexes, FHL2 and vinculin, respectively. Observation of the staining by laser scanning confocal microscopy showed thin actin filaments in filopodia developed by Cdc42-QL cells as well as nascent and mature adhesion complexes at the cell periphery (fig. 2). Cells expressing Rac1-QL had lamellipodia containing a layer of cortical actin and thin actin filaments while the distribution of FHL2 and vinculin was similar to that observed in Cdc42-QL cells (fig. 2). In contrast, the RhoA-QL cells exhibited robust actin stress fibers and thick, strong vinculin-containing adhesion plaques, not only at the cell periphery but also over the entire ventral surface of the cells (fig. 2). Together, these results demonstrate that each different QL clone displayed specific cytoskeletal and cellular adhesion patterns as initially reported for Swiss 3T3 fibroblasts micro-injected with CA forms of RhoA, Rac1 and Cdc42 [9, 10, 24].
Figure 2.
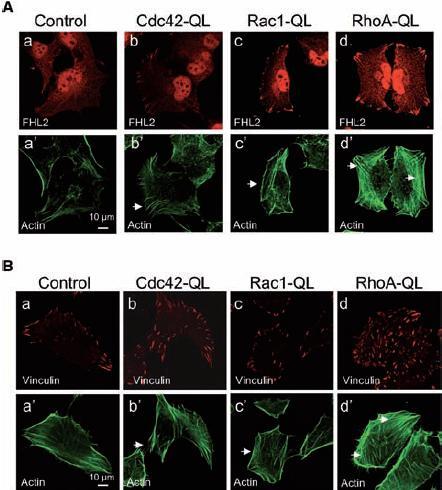
Fibrillar actin and focal adhesions in RhoA-QL, Rac1-QL, Cdc42-QL and control fibroblasts. Mock-transfected parental cells (control), RhoA-QL, Rac1-QL and Cdc42-QL cells were seeded on glass coverslips and grown for 24 h in complete medium. Nascent and mature adhesion complexes were stained with antibodies against FHL2 (A, a–d, red) and vinculin (B, a–d, red), respectively. Fibrillar actin was visualized with phalloidin-FITC (A, B, green, a′–d′). Arrows indicate filopodia (b′, Cdc42-QL), lamellipodia (c′, Rac1-QL) and actin stress fibers (d′, RhoA-QL).
Adhesion and spreading of Cdc42-QL, Rac1-QL and RhoA-QL fibroblasts on extracellular matrix proteins. To examine whether the stable QL mutations affect integrin-mediated adhesion, attachment of the different QL clones to collagen I, fibronectin, laminin 1 and laminin 5 was measured after different time points. These substrates were chosen for their property to mediate cell adhesion by different integrins [25]. Furthermore, although there are many reports on the role of Rho GTPases in cellular interactions with fibronectin [26–28], much less information is available regarding cell adhesion to collagens and laminins. The Cdc42-QL cells showed higher adhesion efficiency to collagen I, fibronectin, laminin 1 and laminin 5 than the control, Rac1-QL and RhoA-QL cells (fig. 3). This effect was observed already after 15 min of incubation on collagen I and fibronectin, and somewhat later on laminin 1 (30 min) and laminin 5 (60 min). The Rac1-QL cells had a lower adhesion efficiency to fibronectin, which was seen best after 30 and 60 min, and there was only little or no difference for their adhesion to the other three substrates when compared to control cells (fig. 3). The adhesion profiles for RhoA-QL and control cells to the four tested extracellular matrix proteins were similar, except for a reduction in adhesion to laminin 5 after 60 min (fig. 3).
Figure 3.
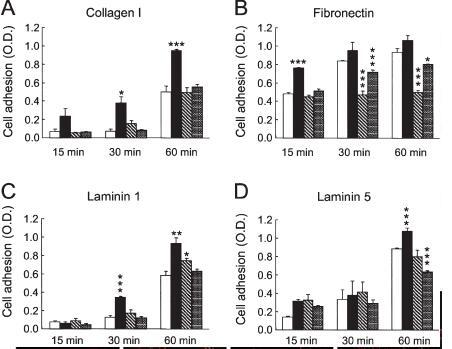
Adhesion of RhoA-QL, Rac1-QL, Cdc42-QL and control fibroblasts to extracellular matrix proteins. Equal numbers of control (white columns), Cdc42-QL (black columns), Rac1-QL (hatched columns), and RhoA-QL (gray columns) cells were seeded in triplicate wells coated with optimal concentrations of collagen I (A; 20 µg/ml), fibronectin (B; 40 µg/ml), laminin 1 (C; 20 µg/ml) and laminin 5 (D; 5 µg/ml). The extent of cell adhesion to the different substrates was measured after 15, 30 and 60 min by crystal violet staining and color readings. The mean absorbance of triplicate wells and SDs are shown. Except for late time points on fibronectin, the numbers of Cdc42-QL mutant cells adhering on the different substrates were significantly higher than either the control or the other two QL mutants. *p<0.01, **p<0.001, ***p<0.0001.
Next we examined cell spreading on different extracellular matrix proteins. A specific cell morphology started to show after 30 min and was seen best after 60 min (fig. 4A–D). A distinct characteristic of Cdc42-QL cells was that they formed clusters of aggregated cells on all substrates tested (fig. 4A–D). Digitally zoomed images showed the presence of filopodia connecting adjacent Cdc42-QL cells (fig. 4E, arrows). The Rac1-QL cells were polarized with a fan-shaped morphology and lamellipodia on all four substrates (fig. 4E). Under all conditions tested, RhoA-QL cells showed a well-spread, flat, unpolarized morphology, with the nucleus totally circumscribed by cytoplasm (fig. 4E). Thus the spreading morphology of RhoA-QL, Rac1-QL and Cdc42-QL fibroblasts appeared to be specific for each CA Rho GTPase and independent of the extracellular matrix proteins to which cells adhere. Quantification of cell spreading by counting the number of round (no visible cytoplasm) and spread (visible cytoplasm) cells in the population of adherent cells showed that spreading of Cdc42-QL cells was clearly reduced compared to Rac1-QL, RhoA-QL and control fibroblasts (fig. 4F–I).
Figure 4.
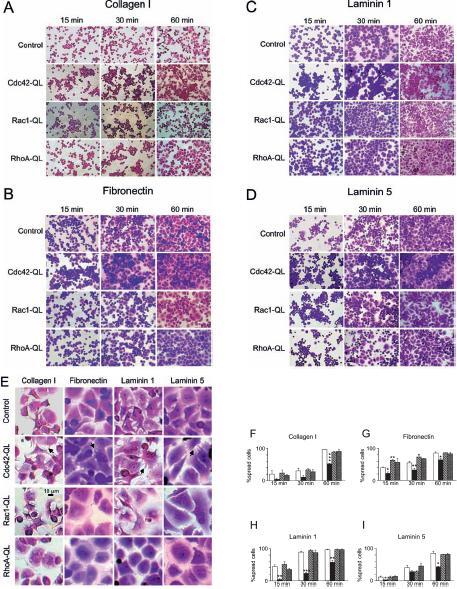
Spreading of RhoA-QL, Rac1-QL, Cdc42-QL and control fibroblasts on extracellular matrix proteins. After 15, 30 and 60 min of adhesion to collagen I (A), fibronectin (B), laminin 1 (C) and laminin 5 (D), Cdc42-QL, Rac1-QL, RhoA-QL and control cells were stained with crystal violet and photographed under phase contrast microscopy at an original magnification of ×200. Details of the specific morphology of cells are shown at higher magnification in E (arrows indicate filopodia in Cdc42-QL cells). Spreading of control (white columns), Cdc42-QL (black columns), Rac1-QL (hatched columns), and RhoA-QL (gray columns) on collagen I (F), fibronectin (G), laminin 1 (H) and laminin 5 (I), was quantified by counting the number of round and spread cells in a population of at least 100 adherent cells on each photograph. Each column represents the mean average of counts determined on three separate photographs for each condition. Bars indicate SD. Statistically significant differences are indicated: *p<0.01, **p<0.001, ***p<0.0001.
Migration of Cdc42-QL, Rac1-QL and RhoA-QL fibroblasts. Cell migration is critical during embryogenesis and development, and it continues to play an essential role in the adult organism, ranging from normal physiological activities, such as wound healing, to pathological situations such as tumor invasion. Rho GTPases play a pivotal role in regulating the mechanical pathways most relevant to cell migration, with RhoA and Rac1 often exerting antagonistic effects by segregating actin filaments in different subcompartments [15, 29]. To investigate the influence of CA RhoA, Rac1 and Cdc42 on migration, the different clones were monitored by time-lapse video microscopy (supplementary videos)1. Rac1-QL fibroblasts extended lamellipodia (supplementary video Rac1) and had significantly moved away from their original position with a speed of 0.27 ± 0.06 µm/min (fig. 5A) and a directed migration index of 0.7 ± 0.1 (fig. 5B), indicating persistent migration. The migratory behavior of Cdc42-QL cells was more complex, with single cells extending several filopodia quickly in different directions and cell clusters migrating by extending protrusions at their leading edges (supplementary video Cdc42). The migration parameters of Cdc42-QL cells were however not different from that of mock-transfected cells (supplementary video control), with a velocity of 0.15 ± 0.06 versus 0.15 ± 0.05 µm/min (fig. 5A) and a directed migration index of 0.4 ± 0.2 versus 0.5 ± 0.2 (fig. 5B). In contrast, RhoA-QL fibroblasts were still very close to the original position at the end of the experiment (supplementary video RhoA), displaying a velocity of 0.06 ± 0.02 µm/min (fig. 5A) and a directed migration index of 0.2 ± 0.1 (fig. 5B). There was, however, very intense cell membrane movement with successive rapid extension and retraction of protrusions for every single RhoA-QL cell (supplementary video RhoA).
Figure 5.
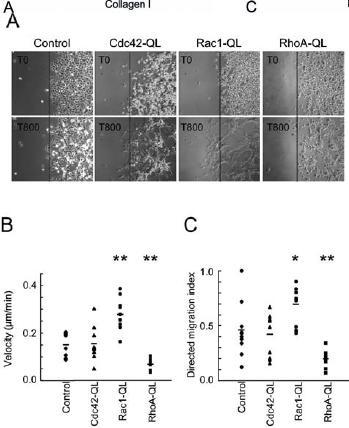
Migration of RhoA-QL, Rac1-QL, Cdc42-QL and control fibroblasts. Small colonies (10 µl) of Cdc42-QL, Rac1-QL, RhoA-QL and control cells were seeded in serum-containing medium in the center of tissue culture wells and allowed to attach at 37 °C. After 1 h, the cells were washed several times with serum-free medium and the wells were filled with serum-free medium. At this point, cell migration was recorded at the border of the colonies by time-lapse video microscopy for 800 min, with one picture every 10 min (supplemental movies). Pictures in A show the colony margin of control and QL cells at the onset (T0) and end of recording (T800). Single-cell tracking was used to determine the velocity (B) and directed migration index (C) of the different clones as described in Materials and methods. *p<0.02 and **p<0.005 versus mock-transfected (control) cells (Student’s t test; n = 20 for each group).
Contraction of collagen lattices by Cdc42-QL, Rac1-QL and RhoA-QL cells. To investigate the mechanical properties of the QL cells within a tri-dimensional network, the transfected fibroblasts were cultivated in gels of collagen I, because it has the intrinsic and specific property to polymerize into an organized fibrillar network [30]. Cultivating fibroblasts within floating gels of polymerized collagen results in contraction of the collagen matrix by the cells over time [30] and this model is therefore best suited to test the integrity of integrin-mediated interactions between the extracellular matrix and the cytoskeletal system in a tissue-like environment [7]. Compared to control cells, collagen gel contraction was delayed for all three QL mutants (fig. 6A). A delay of about 5 h was observed before the Cdc42-QL and Rac1-QL mutants started to contract the gels. Thereafter, contraction kinetics were similar for the two lines and, despite the delay, contraction nevertheless reached control values after 48 h (fig. 6A). In contrast, the RhoA-QL cells displayed negligible contraction for 24 h and had not achieved at 48 h the same extent of gel contraction as the controls and the two other QL cell lines (fig. 6A). Within the contracting gels, the morphology of Cdc42-QL and Rac1-QL fibroblasts (fig. 6C, D) was similar to that of the mock-transfected parental cells (fig. 6B), with multiple dendritic, inter-connected cellular extensions. In contrast, RhoA-QL fibroblasts embedded within the collagen lattices differed significantly from the other cells as they failed to develop dendritic cellular extensions and remained round and isolated (fig. 6E).
Figure 6.

Contraction of collagen gels by RhoA-QL, Rac1-QL, Cdc42-QL and control fibroblasts. Equal numbers of control (empty squares), Cdc42-QL (empty circles), Rac1-QL (black squares), and RhoA-QL (black circles) cells were seeded within triplicate gels of collagen I. Collagen gel contraction was monitored by photographing the gels at successive time intervals and the gel diameters were measured and plotted as a function of time (A). Each point represents the average of three independent experiments. Please note that the SDs were so low that the error bars are not visible. Gel contraction was delayed for the three QL clones, the largest delay being observed for the RhoA-QL cells. Photographs show the morphology of mock-transfected parental (B), Cdc42-QL (C), Rac1-QL (D) and RhoA-QL (E) cells embedded within the gel of collagen I.
Discussion
The role of Cdc42, Rac1 and RhoA in the organization of the actin cytoskeleton has been extensively investigated at a single-cell level by transient overexpression or microinjection of recombinant dominant negative or CA forms in 3T3 fibroblasts [1]. In this report we used lines of human fibroblasts stably transformed with CA forms of the three Rho GTPases to investigate by a panel of functional tests the behavior of clonal cell populations. This strategy has several advantages compared to transient overexpression of genes encoding the GTPases or to microinjection of the recombinant proteins. The activity of the GTPase is similar to that observed in parental cells upon induction with the cognate agonists. Furthermore, beside the fact that all cells express the transgene, which is rarely the case in transient expression experiments, a nearly unlimited number of identical cells can be obtained.
Locking Rho GTPases in the GTP-bound, active state can be achieved by mutating glycine in position 12 (Rac1, Cdc42) or 14 (RhoA) to valine (G12V or G14V) or, alternatively, by substituting glutamine 61 (Rac1, Cdc42) or 63 (RhoA) to leucine (Q61L or Q63L). These two mutated variants have mostly been used interchangeably, but distinct differences in cellular behavior have been described for the two forms of CA RhoA [31, 32]. Both mutations, G14V and Q63L, are located in nucleotide-binding pockets and interfere with hydrolysis of the γ-phosphate of GTP, rendering the protein constitutively active. Although the two mutants have an overall high degree of structural similarity, the affinity for the inhibitory accessory protein RhoGDI is lower for the G14V than Q63L mutant, suggesting that RhoA-Q63L functions as a more active constitutive mutant than RhoA-G14V [33]. Consistent with previous observations, CA RhoA-Q63L induced actin assembly into stress fibers and focal contacts while CA Cdc42 and Rac1 triggered the formation of filopodia and lamellipodia, respectively. The cytoskeletal modifications induced by stable expression of CA Rho-GTPases are compatible with fibroblast survival and proliferation [16]. Furthermore, as shown in figure 1, locking one of the three GTPases into its active GTP-bound conformation did not affect the basal level of activity of the other two. Together these observations demonstrate that the Cdc42-QL, Rac1-QL and RhoA-QL cell lines are suitable and stable models with the requisite cytoskeletal features to compare side-by-side the role of the three GTPases in functional assays involving their mechanical properties.
Rho GTPases were shown to be important for cell adhesion; in particular, Rac1 and RhoA enhance integrin clustering, thereby increasing adhesive strength, without however, changing integrin affinity for extracellular matrix ligands [5, 34]. In fibroblasts, distinct sets of integrins initiate adhesion to different extracellular matrix proteins, for example α1β1, α2β1 and α11β1 for collagen I, α2β1 and α6β1 for laminin 1, α3β1 for laminin 5 and α5β1 for fibronectin. Overexpression of CA RhoAQL and Rac1-QL had no marked effect on the kinetics and overall fibroblast adhesion to these extracellular matrix proteins. In contrast, the number of Cdc42-QL adherent cells was distinctly increased on all substrates. Enhanced cell adhesion to extracellular matrix proteins has been proposed to result from increased receptor affinity or post-receptor events involving cell spreading and/or integrin clustering [35, 36]. However, microscopic monitoring of the morphology of adherent cells revealed that a large proportion of Cdc42-QL fibroblasts were round and associated into clusters, which was not the case for RhoA-QL and Rac1-QL cells. As also observed by video microscopy, many live Cdc42-QL fibroblasts had a rather round body and formed compact groups of cells. This strongly suggests that the enhanced number of adherent Cdc42-QL cells results from cell-cell interaction rather than increased adhesion to the substrates. This is consistent with the role of Cdc42 in promoting intercellular adhesion [37, 38] by regulation of E-cadherin-mediated cell-cell adhesion [39]. Also, the overall shape of Rac1-QL and RhoA-QL fibroblasts appeared to be specific for the active GTPase. Together, these results indicate that spreading of the QL cells is dictated by the CA GTPase, regardless of the nature of the extracellular matrix proteins and integrins permitting cell adhesion.
Beside allowing cells to attach to the extracellular matrix support, cell-matrix adhesions allow the transmission of forces needed for cell movement and matrix remodeling which are key processes in many physiological and pathological conditions [6, 7]. As these processes are thought to be controlled by Rho GTPases, we examined how the CA proteins would impact on the migration and matrix remodeling of the QL fibroblasts. Our study shows that RhoA-QL fibroblasts are not able to locomote, while CA Rac1 increases migration and CA Cdc42 has no effect on the process. The inhibitory effect observed with CA RhoA is consistent with increased migration observed after RhoA inhibition [14, 26]. In other reports, cell motility was reduced with variable amplitudes after transient transfection with a different CA mutant (G14V) of RhoA [14, 40–42]. Also, and in contrast to the observation that transient overexpression of RhoA inhibits membrane protrusions in Chinese hamster ovary fibroblast-like cells [43], RhoA-QL fibroblasts were capable of rapidly extending and retracting cell membrane protrusions in different directions, although these were inefficient to initiate locomotion. Similarly variable effects were reported for cells transiently expressing CA Rac1 and Cdc42, ranging from no effect of CA Rac1 and CA Cdc42 [14] to enhanced migration with CA Cdc42 [44] or decreased invasion by CA Rac1 [45]. Variability in these observations may represent cell type-specific features or differences in transient transfection efficiency.
Further interesting features of the QL cells, in particular for CA RhoA, were revealed when we tested their ability to contract tri-dimensional collagen lattices. Compared to the mock-transfected cells, gel contraction was delayed for all three mutants. A likely explanation for the observed delay in contraction by the CA Rac1 and Cdc42 is that cellular extensions needed for cell locomotion along collagen fibers and transmission of mechanical forces [7] may be established at a slower rate. Nevertheless, the two mutants eventually develop an overall contraction equal to the controls. In contrast, the overall contractile capacity of the RhoA-QL mutant was decreased and it failed to develop extensions inter-connecting adjacent cells. Apparently, our results are in contrast to the report by Chrzanowska-Wodnicka and Burridge [12], who described increased contractility following RhoA activation by introducing the G14V mutation into 3T3 fibroblasts. However, the models used here, a tri-dimensional collagen network, and in their study, wrinkling of silicone rubber membranes are different. In our model, the tri-dimensional collagen network lacks mechanical loading, while the other is a gold-coated, planar geometry, which confers different mechanical cues to the cells. This interpretation is supported by findings that contraction of floating collagen lattices in the presence of serum does not require Rho kinase activity, while that of cells in a more constrained mechanical environment does [46]. Moreover, recent studies showed that Rho activity is regulated in a feedback manner by mechanical forces with contraction of floating lattices being paralleled by a decrease in active Rho [47]. This suggests that RhoA activity during collagen lattice contraction is not an on/off response, but rather requires tight temporal and spatial regulation. We think that in our RhoA-QL fibroblasts, the critical balance between inactive and active RhoA and regulation of the activity of the associated factors is no longer maintained, resulting in particular in a non-dendritic shape, a locomotive defect and the inability to compact and remodel collagen fibers proximal to the cell surface. This is underscored by RhoA-QL fibroblasts displaying focal adhesions all over the ventral surface. We therefore anticipate that CA RhoA inhibits the turnover of focal adhesions, at least those located at the ventral surface of the cells, preventing detachment of the cell body to enable locomotion in both bi- and tri-dimensional substrates. Alternatively, the increased number of focal adhesions observed in RhoA-QL cells may not transmit adequate traction for cell movement. Indeed, small adhesions have been shown to transmit strong propulsive traction forces, whereas mature focal adhesions exert weaker forces [48]. Taken together, side-by-side comparison of the QL mutants of RhoA, Rac1 and Cdc42 revealed alterations with respect to spreading morphology, migration behavior and collagen lattice contraction specific for each GTPase, with the most severe effects seen in the RhoA-QL mutants. Most of the experimental demonstrations of the role of the Rho GTPases in cytoskeleton-mediated biomechanical functions (adhesion, spreading, migration and traction) of various types of cell have been obtained by microinjection or transient transfection of mutated forms of the Rho GTPases or by interfering with their activity using biological or pharmacological mediators, procedures that do not offer the required specificity. The selection and amplification of clonal lines of cells expressing a mutated form of each Rho GTPase, as here for the CA forms, provides a uniform population of cells most suitable for comparative investigation of the mechanical behaviour not only of individual cells but also of large groups of cells. Such features are most evident for Cdc42-QL cells and their cell-cell interactions on various extracellular matrix proteins and for the generation of traction forces on bi-dimensional (migration) or within tri-dimensional (collagen gels) substrates as demonstrated for RhoA-QL cells. Worth noting is that these transfected human fibroblasts have undergone a large number of population doublings without losing the altered signalling activity of each of the Rho GTPases on the cytoskeleton [16, this report]. This indicates that hyperactive Rho GTPases allow survival and multiplication of cells even though the precise modulation of cell architecture required for progression in the cell cycle is disturbed and that the action of Rho GTPases appears to depend on the task cells are performing.
Acknowledgment
We are grateful to Dr. M. Glukhova for the gift of antibodies and M. Pesch and G. Scherr for excellent technical assistance. This work was supported by the Deutsche Zentrum für Luft- und Raumfahrt (50WB0321), the Deutsche Forschungsgemeinschaft (AU 86/5-3 and KR 558/13), the Center for Molecular Medicine (TV10, TV80), the Medical Faculty of the University of Cologne (Köln Fortune), the Prodex Agency of ESA (PEA 90095 and 90099) and the Belgian Fonds National de la Recherche Scientifique. M. A. is a researcher from the Centre National de la Recherche Scientifique.
Footnotes
Sequential images were stored using Openlab software and used to create Quick Time movies.
Received 12 September 2005; received after revision 5 October 2005; accepted 1 November 2005
References
- 1.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S., Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 3.Krengel U., Schlichting L., Scherer A., Schumann R., Frech M., John J., et al. Three-dimensional structures of H-ras p21 mutants: molecular basis for their inability to function as signal switch molecules. Cell. 1990;62:539–548. doi: 10.1016/0092-8674(90)90018-A. [DOI] [PubMed] [Google Scholar]
- 4.Bishop A. L., Hall A. Rho GTPases and their effector proteins. Biochem. J. 2000;348:241–255. doi: 10.1042/0264-6021:3480241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burridge K., Wennerberg K. Rho and Rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/S0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 6.Ingber D. E. Tensegrity: the architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997;59:575–599. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 7.Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/S0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 8.Humphries M. J., Travis M. A., Clark K., Mould A. P. Mechanisms of integration of cells and extracellular matrices by integrins. Biochem. Soc. Trans. 2004;32:822–825. doi: 10.1042/BST0320407. [DOI] [PubMed] [Google Scholar]
- 9.Ridley A. J., Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 10.Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 11.Hotchin N. A., Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J. Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrzanowska-Wodnicka M., Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J. Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Leeuwen F. N., van Delft S., Kain H. E., van der Kammen R. A., Collard J. G. Rac regulates phosphorylation of the myosin-II heavy chain, actinomyosin disassembly and cell spreading. Nat. Cell Biol. 1999;4:242–248. doi: 10.1038/12068. [DOI] [PubMed] [Google Scholar]
- 14.Nobes C. D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raftopoulou M., Hall A. Cell migration: Rho GTPases lead the way. Dev. Biol. 2004;265:23–32. doi: 10.1016/j.ydbio.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Servotte S., Zhang Z.-G., Lambert C. A., Ho T. T. G., Chometon G., Eckes B. et al. (2005) Cytoskeletal modifications induced by stable expression of constitutively active Rho-GTPases are compatible with fibroblast survival and proliferation. Protoplasma. in press
- 17.Ren X. D., Kiosses W. B. and Schwartz M. A. (1999) Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton EMBO J. 18: 578–585 [DOI] [PMC free article] [PubMed]
- 18.Sander E. E., ten Klooster J. P., van Delft S., van der Kammen R. A., Collard J.G. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J. Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Mourabit H., Müller S., Tunggal L., Paulsson M., Aumailley M. Analysis of the adaptor function of the LIM domain-containing protein FHL2 using an affinity chromatography approach. J. Cell. Biochem. 2004;92:612–625. doi: 10.1002/jcb.20096. [DOI] [PubMed] [Google Scholar]
- 20.Tasanen K., Tunggal L., Chometon G., Bruckner-Tuderman L., Aumailley M. Keratinocytes from patients lacking collagen XVII display a migratory phenotype. Am. J. Pathol. 2004;164:2027–2038. doi: 10.1016/S0002-9440(10)63762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousselle P., Aumailley M. Kalinin is more efficient than laminin in promoting adhesion of primary keratinocytes and some other epithelial cells and has a different requirement for integrin receptors. J. Cell Biol. 1994;125:205–214. doi: 10.1083/jcb.125.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kessler D., Dethlefsen D., Haase I., Plomann M. F., Hirche F., Krieg T., et al. Fibroblasts in mechanically stressed collagen lattices assume a ’synthetic’ phenotype. J. Biol. Chem. 2001;276:36575–36585. doi: 10.1074/jbc.M101602200. [DOI] [PubMed] [Google Scholar]
- 23.Van Aelst L., D’souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 24.Nobes C. D., Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 25.van der Flier A., Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305:285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- 26.Arthur W. T., Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol. Biol. Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danen E. H., Sonneveld P., Brakebusch C., Fässler R., Sonnenberg A. The fibronectin-binding integrins α5β1 and αvβ3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moyano J. V., Maqueda A., Casanova B., Garcia-Pardo A. α4β1 integrin/ligand interaction inhibits α5β1-induced stress fibers and focal adhesions via down-regulation of RhoA and induces melanoma cell migration. Mol. Biol. Cell. 2003;14:3699–3715. doi: 10.1091/mbc.E02-10-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malliri A., Collard J. G. Role of Rho-family proteins in cell adhesion and cancer. Curr. Opin. Cell Biol. 2003;15:583–589. doi: 10.1016/S0955-0674(03)00098-X. [DOI] [PubMed] [Google Scholar]
- 30.Bell E., Ivarsson B., Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. USA. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mayer T., Meyer M., Janning A., Schiedel A. C., Barnekow A. A mutant form of the rho protein can restore stress fibers and adhesion plaques in v-src transformed fibroblasts. Oncogene. 1999;18:2117–2128. doi: 10.1038/sj.onc.1202537. [DOI] [PubMed] [Google Scholar]
- 32.Michaelson D., Silletti J., Murphy G., D’Eustachio P., Rush M., Philips M. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longenecker K., Read P., Lin S. K., Nakamoto R. K., Derewenda Z. S. Structure of a constitutively activated RhoA mutant (Q63L) at 1.55 Angstrom resolution. Acta Cryst. 2003;D59:876–880. doi: 10.1107/s0907444903005390. [DOI] [PubMed] [Google Scholar]
- 34.Kaibuchi K., Kuroda S., Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu. Rev. Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 35.Stewart M., Hogg N. Regulation of leukocyte integrin function: affinity vs. avidity. J. Cell. Biochem. 1996;61:554–561. doi: 10.1002/(SICI)1097-4644(19960616)61:4<554::AID-JCB8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Bazzoni G., Hemler M. E. Are changes in integrin affinity and conformation overemphasized? Trends Biochem. Sci. 1998;23:30–34. doi: 10.1016/S0968-0004(97)01141-9. [DOI] [PubMed] [Google Scholar]
- 37.Fukata M., Nakagawa M., Kuroda S., Kaibuchi K. Cell adhesion and Rho small GTPases. J. Cell Sci. 1999;112:4491–4500. doi: 10.1242/jcs.112.24.4491. [DOI] [PubMed] [Google Scholar]
- 38.Vasioukhin V., Bauer C., Yin M., Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 39.Kuroda S., Fukata M., Nakagawa M., Fujii K., Nakamura T., Ookubo T., et al. Role of IQGAP1, a target of the small GTPases Cdc42 and Rac1, in regulation of E-cadherin-mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 40.Tkach V., Bock E., Berezin V. The role of RhoA in the regulation of cell morphology and motility. Cell Motil. Cytoskeleton. 2005;61:21–33. doi: 10.1002/cm.20062. [DOI] [PubMed] [Google Scholar]
- 41.Gutjahr M.C., Rossy J., Niggli V. Role of Rho, Rac, and Rho-kinase in phosphorylation of myosin light chain, development of polarity, and spontaneous migration of Walker 256 carcinosarcoma cells. Exp. Cell Res. 2005;308:422–438. doi: 10.1016/j.yexcr.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Zhou H., Kramer R.H. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 2005;280:10624–10635. doi: 10.1074/jbc.M411900200. [DOI] [PubMed] [Google Scholar]
- 43.Cox E.A., Sastry S.K., Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol. Biol Cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olivo C., Vanni C., Mancini P., Silengo L., Torrisi M.R., Tarone G., et al. Distinct involvement of cdc42 and RhoA GTPases in actin organization and cell shape in untransformed and Dbl oncogene transformed NIH3T3 cells. Oncogene. 2000;19:1428–1436. doi: 10.1038/sj.onc.1203440. [DOI] [PubMed] [Google Scholar]
- 45.Banyard J., Anand-Apte B., Symons M., Zetter B.R. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. doi: 10.1038/sj.onc.1203338. [DOI] [PubMed] [Google Scholar]
- 46.Lee D. J., Ho C. H., Grinnell F. LPA-stimulated fibroblast contraction of floating collagen matrices does not require Rho kinase activity or retraction of fibroblast extensions. Exp. Cell Res. 2003;289:86–94. doi: 10.1016/S0014-4827(03)00254-4. [DOI] [PubMed] [Google Scholar]
- 47.Olson M. F. Contraction reaction: mechanical regulation of Rho GTPase. Trends Cell Biol. 2004;14:111–114. doi: 10.1016/j.tcb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 48.Beningo K. A., Dembo M., Kaverina I., Small J. V., Wang Y. L. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]


