Abstract
White matter tracts, which play a crucial role in the coordination of information flow between different regions of grey matter, are particularly vulnerable to multiple sclerosis. Many studies have shown that the white matter lesions in multiple sclerosis are associated with focal abnormalities of grey matter, but little is known about the alterations in the coordinated patterns of cortical morphology among regions in the disease. Here, we used cortical thickness measurements from structural magnetic resonance imaging to investigate the relationship between the white matter lesion load and the topological efficiency of structural cortical networks in multiple sclerosis. Network efficiency was defined using a ‘small-world’ network model that quantifies the effectiveness of information transfer within brain networks. In this study, we first classified patients (n = 330) into six subgroups according to their total white matter lesion loads, and identified structural brain networks for each multiple sclerosis group by thresholding the corresponding inter-regional cortical thickness correlation matrix, followed by a network efficiency analysis with graph theoretical approaches. The structural cortical networks in multiple sclerosis demonstrated efficient small-world architecture regardless of the lesion load, an organization that maximizes the information processing at a relatively low wiring cost. However, we found that the overall small-world network efficiency in multiple sclerosis was significantly disrupted in a manner proportional to the extent of total white matter lesions. Moreover, regional efficiency was also significantly decreased in specific brain regions, including the insula and precentral gyrus as well as regions of prefrontal and temporal association cortices. Finally, we showed that the lesions also altered many cortical thickness correlations in the frontal, temporal and parietal lobes. Our results suggest that the white matter lesions in multiple sclerosis might be associated with aberrant neuronal connectivity among widely distributed brain regions, and provide structural (morphological) evidence for the notion of multiple sclerosis as a disconnection syndrome.
Keywords: cortical thickness, connectivity, MRI, multiple sclerosis, small-world networks
Introduction
Multiple sclerosis is a chronic, progressive and degenerative disease of the central nervous system that is usually accompanied by an impairment of cognitive functions such as memory, attention and speed of information processing, in addition to sensory and motor deficits. The impairment arises predominantly from impaired neuronal conduction due to white matter lesions (Ormerod et al., 1987; Newcombe et al., 1991; Evangelou et al., 2000; Filippi et al., 2001; Griffin et al., 2001; Ge et al., 2004; Poonawalla et al., 2008; for reviews, see Rovaris et al., 2005), which can disrupt functional integrity between widely distributed brain regions (Lowe et al., 2002; Au Duong et al., 2005a, b; Audoin et al., 2006a; Cader et al., 2006; Lowe et al., 2008; Tecchio et al., 2008). There is also increasing evidence that multiple sclerosis is associated with global (Chard et al., 2002; Dalton et al., 2004; Sastre-Garriga et al., 2005; Rovaris et al., 2006; Fisniku et al., 2008) and regional (Miller et al., 2002; Charil et al., 2003; Sailer et al., 2003; Chen et al., 2004; Audoin et al., 2006b; Calabrese et al., 2007; Charil et al., 2007; Jasperse et al., 2007) morphological abnormalities in the grey matter. Here, we report on multiple sclerosis-related alterations in the coordinated patterns of cortical morphology derived from structural magnetic resonance imaging (MRI).
Recent advances in structural MRI analysis have demonstrated that the morphological features of human cerebral cortex carry important brain connectivity information. For instance, several researchers have observed correlations in grey matter morphology (e.g. volume, density or thickness) between various anatomically or functionally linked brain areas such as the frontotemporal (Bullmore et al., 1998; Lerch et al., 2006), frontoparietal (Wright et al., 1999) and contralateral interhemispheric regions (Mechelli et al., 2005). We previously demonstrated that the coordinated variations in human regional cortical thickness, as derived from structural MRI (MacDonald et al., 2000; Kim et al., 2005), follow a ‘small-world’ topology at a macroscale level (He et al., 2007), characterized by a high degree of local clustering and short path-lengths linking individual network nodes (Watts and Strogatz, 1998). This small-world property suggests that the human cerebral cortex is optimally organized to support both modularized and distributed information processing (Bassett and Bullmore, 2006; Stam and Reijneveld, 2007). We have also recently utilized the small-world cortical thickness network model to investigate the topological organization of structural brain networks in Alzheimer's disease, in which abnormal patterns of cortical thickness correlation were found (He et al., 2008). Given that, in multiple sclerosis, white matter lesions interrupt neuronal pathways connecting cortical regions, we hypothesized that patients would also show white matter lesion related alterations in the pattern of cortical thickness correlation, and that these alterations would depend on the white matter lesion load. Here, we utilized the ‘network efficiency metrics’, first defined by Latora and Marchiori (2001), to quantify small-world cortical thickness correlation patterns. The efficiency measure provides many technical and conceptual advantages for the characterization of complex systems (see Materials and methods section for details), and it has been recently applied to both primate and human brain network analysis (Latora and Marchiori, 2001; Achard and Bullmore, 2007; Iturria-Medina et al., 2008; Liu et al., 2008; Wang et al., 2009). However, few studies utilized the efficiency measures to characterize the topological organization of human cortical morphology.
To test our hypothesis, we analysed the cortical thickness data from a group of 330 relapsing–remitting multiple sclerosis patients (Charil et al., 2007). We defined six subgroups using their total white matter lesion loads (TWMLL) as a measure of disease severity. After parcellating the entire cerebral cortex into 54 areas, we calculated the correlation matrix of regional cortical thickness across subjects within each subgroup. The resulting correlation matrix for each subgroup was then thresholded to construct an undirected graph representing the underlying structural cortical network. Finally, we applied graph theoretical approaches to investigate how the network efficiency in multiple sclerosis changes with TWMLL across subgroups.
Materials and methods
Subjects
The study included 330 patients with clinically definite relapsing–remitting multiple sclerosis, characterized by a minimum of two or more attacks of multiple sclerosis within the preceding 2 years, as defined by the Poser criteria (Poser et al., 1983). The mean age of the patients (female/male: 153/177) was 38.35 years (SD: 5.93; range: 22.2–48.1). All patients had undergone yearly MRI scans and clinical evaluations for 2 years as part of a phase III clinical trial. The study, designed to explore the effects of an oral formulation of bovine myelin (MyloralTM, Autoimmune Inc., Lexington MA, USA) (Weiner, 1997; Charil et al., 2003), revealed no differences in the frequency of relapses or progression of disability between the treated and placebo groups. All patients had been relapse-free for at least 28 days prior to entering the study. The exclusion criteria for the study involved treatment with corticosteroids within the preceding 28 days, treatment with any experimental immunomodulating drug or interferon within the preceding 60 days, and treatment with cyclophosphamide, azathioprine, methotrexate or cyclosporine within the preceding 2 years. Patients had tested negative for the presence of human immunodeficiency virus or human T-lymphotropic virus and did not suffer from other serious neurological diseases. Written informed consent was obtained from each patient and this study was approved by the Institutional Review Boards at the 14 participating sites (four in Canada and 10 in the US, listed in the acknowledgements). More detailed clinical and demographic characteristics of the patients have been described previously (Charil et al., 2003, 2007).
Image acquisition
The MRI scans were performed on one of three 1.5 T cameras at the 14 participating sites (GE Signa, Philips ACS-II, or Siemens SP 4000). T1, T2 and proton density (PD) images were acquired for each patient with the following sequences: T1-weighted [3D gradient echo scan, time repetition (TR)/time echo (TE) = 35/11 ms, flip angle = 45°, slices = 60, slice thickness = 3 mm, orientation = transverse, resolution = 256 × 192 matrix, field of view (FOV) = 240 × 240 mm2, NEX = 1] and T2/PD-weighted (2D multislice fast spin-echo scan, TR/TE1/TE2 = 3000/30/80 ms, slices = 54, slice thickness = 3 mm, orientation = transverse, resolution = 256 × 192 matrix, FOV =240 × 240 mm2, NEX = 0.5). Prior to the study, the data coordinating centre (Montreal Neurological Institute, MNI) of this study ensured standardization of all acquisition procedures. All data analyses were also conducted at the coordinating centre.
Measurements of white matter lesion volume and cortical thickness
The procedures of the measurements of white matter lesion volume and cortical thickness in the multiple sclerosis patients have been previously described (Charil et al., 2007). Briefly, the native MRI were first registered into the stereotaxic space using a nine-parameter linear transformation (Collins et al., 1994). Simultaneously, the images were corrected for non-uniformity artifacts using the N3 algorithm (Sled et al., 1998). The registered and corrected images were segmented into grey matter, white matter, cerebro-spinal fluid, multiple sclerosis lesions and background using an advanced neural net classifier (Zijdenbos et al., 2002). Some data that failed the automated segmentation of lesions were manually corrected. As a result, all data passed quality control. This procedure yields the relative white matter lesion volume for each patient since the head size had been normalized by the linear transformation. Since the MRI-based structural brain networks were constructed on the basis of cortical thickness data of a group of subjects (He et al., 2007) (for details, see Network construction section), we therefore separated patients into six groups according to their TWMLL [(0–2), (2–4), (4–8), (8–16), (16–32) and (32+) cm3], with fifty-five subjects in each group (Table 1). In this assignment, TWMLL was simply modelled by an increasing exponential function. It should be noted that there is currently no definitive way to perform such an assignment; our arbitrary approach enabled us to explore changes in network properties with increasing TWMLL in multiple sclerosis.
Table 1.
Demographics and clinical characteristics of patients
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 | |
|---|---|---|---|---|---|---|
| TWMLL (cm3) | 0–2 | 2–4 | 4–8 | 8–16 | 16–32 | 32+ |
| n | 55/62 | 55/61 | 55/73 | 55/87 | 55/85 | 55/57 |
| Age (year) | 40.02 (5.08) | 38.06 (6.03) | 36.30 (5.78) | 39.57 (5.99) | 37.72 (5.80) | 38.44 (6.27) |
| Gender (M/F) | 30/25 | 26/29 | 33/22 | 36/19 | 30/25 | 22/33 |
| Average TWMLL (cm3) | 1.004 | 2.920 | 5.817 | 12.023 | 21.99 | 43.378 |
Multiple sclerosis patients were classified into six groups according to their TWMLL values. Note that the number of subjects retained from each group was different from the initial classification (see the denominator of the second row of the table, n). We excluded the subjects whose regional cortical thickness fell 3 SDs from the mean of their group. After this, Group 6 only included 55 subjects, which was less than other groups. Given that the multiple sclerosis brain networks were constructed by calculating the regional cortical thickness correlations across subjects, we thus selected the same number of subjects (55) for each group. Subjects were included if their TWMLL values showed minimum differences with the mean TWMLL of their group. The average TWMLL value of the included subjects for each group was shown in the bottom row of the table.
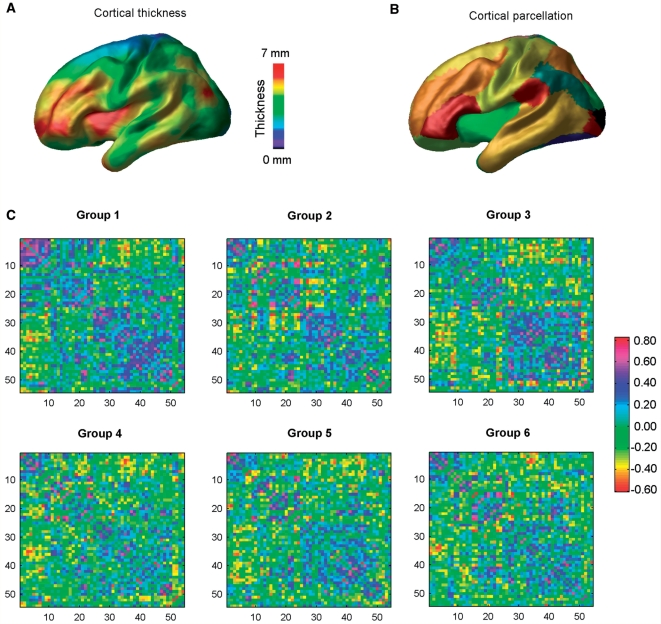
The inner and outer grey matter surfaces of each brain (40 962 vertices per surface and a total of 81 920 polygons) were automatically extracted from the classified images using the Constrained Laplacian-based Automated Segmentation with Proximities (CLASP) algorithm (MacDonald et al., 2000; Kim et al., 2005). Cortical thickness was defined as the distance between the linked vertices on the inner and outer surfaces (Lerch and Evans, 2005). Each cortical thickness map was then blurred by using a 20 mm surface-based blurring kernel to improve sensitivity (Chung et al., 2003). The cortical thickness measurement algorithm has been validated using both manual measurements (Kabani et al., 2001) and simulation approaches (Lerch and Evans, 2005; Lee et al., 2006). A representative average cortical thickness map is shown in Fig. 1A.
Figure 1.
A flowchart for the construction of structural cortical networks in multiple sclerosis. (A) A representative cortical thickness map obtained from anatomical MRI (MacDonald et al., 2000; Kim et al., 2005). The colour bar indicates thickness. (B) The entire cerebral cortex was segmented into 54 areas according to a prior surface parcellation from the ICBM152 dataset (He et al., 2007). The cortical areas are displayed on the average surface, each colour representing a single region. (C) The inter-regional correlation matrix for each multiple sclerosis group was obtained by calculating Pearson's correlations between regional cortical thicknesses across subjects within the group. The colour bar indicates the correlation coefficient between regions. The correlation matrices were further thresholded into a set of binarized matrices to construct the multiple sclerosis structural cortical networks. For details, see Materials and methods section.
Network construction
Structural cortical networks were constructed from the regional cortical thickness measurements from MRI (He et al., 2007). The entire cerebral cortex was first parcellated into 54 cortical regions according to a prior surface segmentation (He et al., 2007) (Fig. 1B) from the ICBM152 data (Mazziotta et al., 2001). The surface segmentation was obtained using Automated Non-linear Image Matching and Anatomical Labelling package (Collins et al., 1995). For each subject, regional cortical thickness was then computed as the average thickness of all vertices belonging to that region. A linear regression analysis was further performed at every cortical region to remove the effects of multiple confounding variables (age, gender, age-gender interaction, MRI acquisition site, lesion volume and overall mean cortical thickness). The residuals of this regression were then used to substitute for the raw cortical thickness values of each region. We then obtained the inter-regional correlation matrix Rij (i, j = 1, 2 … N, here N = 54) for each of the six patients groups by calculating Pearson's correlation coefficients across subjects between the residual cortical thicknesses of every pair of regions. Finally, these correlation matrices were thresholded into a set of binarized matrices that describe the topological organization of the structural cortical networks. In this study, we adopted two different thresholding approaches. First, the same correlation thresholding values (0 < R < 1) were applied to all the group correlation matrices to construct the structural brain networks. This allow us to examine the absolute network efficiency in each group. For the second approach, we applied a cost threshold value (0 < C < 1, see below) to all the group correlation matrices. Here, the cost was computed as the ratio of the number of actual connections divided by the maximum possible number of connections in the network. This step normalizes the six subgroup networks to have the same number of nodes and edges and allows an examination of the relative network efficiency in each group. The absolute and relative network efficiency measurements quantify distinct aspects of topological network organization. The absolute efficiency metrics, based upon correlation thresholds, capture the network efficiency but cannot completely detect the alterations in the topological organization of each multiple sclerosis group because there is a different number of edges in each network. The relative efficiency metrics based upon cost thresholds captures the changes in network organization of each group by imposing on each network the same number of edges or wiring cost for compensatory adaptations. Thus, the absolute and relative efficiency measurements provide a way to characterize fully the organizational changes in the structural cortical network in multiple sclerosis.
Network analysis
Small-world efficiency
Small-world network parameters (clustering coefficient, Cp and characteristic path length, Lp) were originally proposed by Wattz and Strogatz (1998). In this study, we employed a single network efficiency measure to quantify the small-world behaviour of structural cortical networks in multiple sclerosis (Latora and Marchiori, 2001). It not only deals with disconnected graphs and/or non-sparse graphs, but also provides a clear physical meaning for topological characterization of the networks. For a graph (network) G with N nodes and K edges, the global efficiency of G can be computed as (Latora and Marchiori, 2001):
| (1) |
where dij is the shortest path length between node i and node j in G. The local efficiency of G is measured as (Latora and Marchiori, 2001):
| (2) |
where Eglob(Gi) is the global efficiency of Gi, the subgraph of the neighbors of node i. The graph G is considered to be a small-world network if it meets the following criteria: Eglob(Gregular) <Eglob(G) < Eglob(Grandom) and Eloc(Grandom) < Eloc(G) < Eloc(Gregular), where Eglob(Gregular), Eglob(Grandom), Eloc(Gregular) and Eloc(Grandom) are the global and local efficiency values of node- and degree-matched regular and random networks. It is noted that there is currently no definitive way to select a single threshold to construct brain structural networks. Thus, we computed the integrated absolute global and local efficiencies as  and
and  , where Eglobal(r) and Elocal(r) are the global and local efficiency functions of the variable, correlation. We repeated this process for the relative efficiencies (
, where Eglobal(r) and Elocal(r) are the global and local efficiency functions of the variable, correlation. We repeated this process for the relative efficiencies (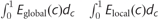 and), but with respect to cost, c, rather than correlation, r, which allows us to explore the changes in topological organization with TWMLL after correcting low-level correlation differences among groups, as described previously (Achard and Bullmore, 2007; He et al., 2008).
and), but with respect to cost, c, rather than correlation, r, which allows us to explore the changes in topological organization with TWMLL after correcting low-level correlation differences among groups, as described previously (Achard and Bullmore, 2007; He et al., 2008).
Regional nodal characteristics
To determine the nodal (regional) characteristics of structural cortical networks in multiple sclerosis, we computed three measures: the nodal correlation strength and its regional absolute and relative efficiency. The correlation strength of node i was defined as (Jiang et al., 2004; Lerch et al., 2006):
| (3) |
where Rij is the correlation coefficient between node i and node j in G. Snodal(i) measures the average correlation extent by which a given node, i, is connected to the rest of the network. The regional nodal efficiency of node i was measured as (Achard and Bullmore, 2007)
| (4) |
where dij is the shortest path length between node i and node j in G. Enodal(i) measures the average shortest path length between a given node, i, and other nodes in the network. Similar to the integrated overall network efficiency measures above, we defined the integrated absolute and relative nodal efficiency as  and
and 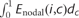 , where Enodal(i, r) and Enodal(i, c) are the regional nodal efficiency functions of variables, correlation and cost, respectively. The integrated nodal efficiency allows us to characterize nodal properties in the brain networks without the selection of a specific network threshold. A given region was considered to be affected by the white matter lesion load if its values for one or more of the above measures correlated with TWMLL.
, where Enodal(i, r) and Enodal(i, c) are the regional nodal efficiency functions of variables, correlation and cost, respectively. The integrated nodal efficiency allows us to characterize nodal properties in the brain networks without the selection of a specific network threshold. A given region was considered to be affected by the white matter lesion load if its values for one or more of the above measures correlated with TWMLL.
Statistical analysis
To determine whether the network topology in multiple sclerosis were correlated with white matter lesion load, we performed a linear regression analysis of each network parameter [ ,
,  ,
,  ,
, 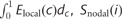 ,
,  and
and 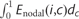 ] against the log volume of white matter lesions [log2(TWMLL)]. In addition, the relation between the cortical thickness correlations and lesion loads was also assessed by a regression analysis.
] against the log volume of white matter lesions [log2(TWMLL)]. In addition, the relation between the cortical thickness correlations and lesion loads was also assessed by a regression analysis.
Results
Small-world efficiency in the structural cortical networks in multiple sclerosis
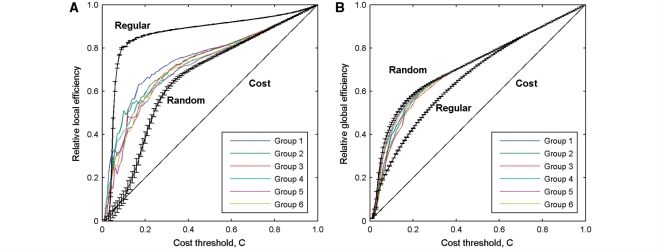
Figure 1 shows the cortical thickness correlation matrices of the six multiple sclerosis groups of increasing lesion load. Visual examination indicated that there were similar correlation patterns among the groups at all lesion loads. The correlation patterns were also approximately consistent with those in young healthy subjects (He et al., 2007). Graph theoretical analysis revealed that the efficiency curves of structural cortical networks were intermediate compared with those of the matched regular and random networks over a wide range of network costs (Fig. 2). In other words, the multiple sclerosis structural brain networks had a higher local and global efficiency than comparable random and regular networks, respectively. This is indicative of high topological efficiency, which is a typical feature of small-world networks (Latora and Marchiori, 2001). In addition, the structural cortical networks in multiple sclerosis exhibited an economical behaviour since both local and global efficiency rose much faster than the required wiring cost (Fig. 2). For example, the multiple sclerosis brain networks reached an efficiency of ∼50% when only ∼20% wiring cost was required. These findings are in accordance with recent human brain structural networks studies based upon cortical thickness measurements (He et al., 2007, 2008) and functional networks studies using resting functional MRI (Achard and Bullmore, 2007).
Figure 2.
The local and global efficiency of the random and regular, and actual brain networks of each multiple sclerosis group as a function of cost. The multiple sclerosis brain networks showed higher local efficiency than the matched random networks (A), and higher global efficiency than the matched regular networks (B) at a wide range of cost thresholds. Thus, the multiple sclerosis brain networks for each group exhibited small-world properties regardless of disease severity. The brain networks were also found to be economical since both the local and global efficiency were much higher than the required cost. Note that the regular and random networks in the plots had the same number of nodes and edges as the real networks.
Small-world efficiency versus TWMLL
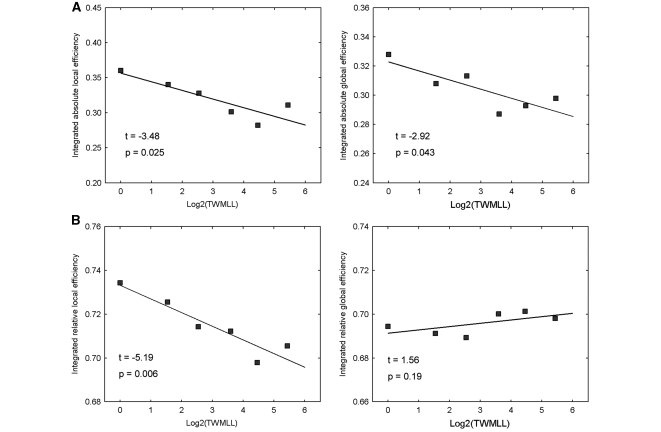
We found that the white matter lesion loads in multiple sclerosis significantly impaired the efficiency of structural cortical networks. The multiple sclerosis patients exhibited significantly decreased integrated absolute local [t(4) = –3.48, P < 0.025] and global efficiency [t(4) = –2.92, P < 0.043] in the structural cortical networks with increasing TWMLL (Fig. 3A). In addition, there were also significant decreases [t(4) = –5.19, P < 0.006] in the integrated relative local efficiency with the TWMLL (Fig. 3B). However, the integrated relative global efficiency only exhibited a slight non-significant increase.
Figure 3.
Changes in integrated absolute and relative network efficiency with lesion load. (A) Plots showing the significant decreases of integrated absolute local and global efficiency with TWMLL. (B) Plots showing the significant decreases of integrated relative local efficiency and slight (non-significant) increases of integrated relative global efficiency with TWMLL.
Regional nodal characteristics versus TWMLL
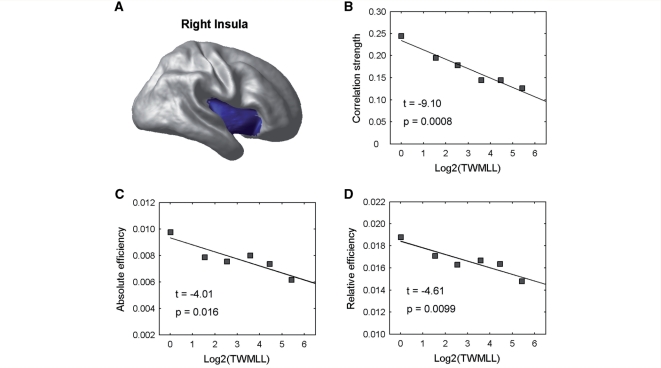
The white matter lesion load was also found to have a deleterious effect on the nodal characteristics of the structural brain networks (Table 3). First, several regional correlation strengths in the cortical network, involving the insula, precentral gyrus, prefrontal and temporal association cortices, showed significant decreases with the TWMLL. On the other hand, we also found decreased integrated absolute regional network efficiency in these same regions, and increased in the parahippocampal gyrus and angular gyrus with the TWMLL. Finally, we found that the relative regional network efficiency also decreased with the TWMLL in the insula, precentral gyrus and prefrontal association cortex, and increased in the parahippocampal gyrus and angular gyrus. Interestingly, the insula displayed the most significant decreases with the TWMLL for all three measures (Fig. 4).
Table 2.
Cortical surface regions of interest
| Index | Anatomical regions | Abbreviation | Index | Anatomical regions | Abbreviation |
|---|---|---|---|---|---|
| (1,2) | Superior frontal gyrus | SFG | (29,30) | Inferior temporal gyrus | ITG |
| (3,4) | Middle frontal gyrus | MFG | (31,32) | Uncus | UNC |
| (5,6) | Inferior frontal gyrus | IFG | (33,34) | Medial occipitotemporal gyrus | MOTG |
| (7,8) | Medial frontal gyrus | MdFG | (35,36) | Lateral occipitotemporal gyrus | LOTG |
| (9,10) | Precentral gyrus | PrCG | (37,38) | Parahippocampal gyrus | PHG |
| (11,12) | Lateral fronto-orbital gyrus | LOFG | (39,40) | Occipital pole | OP |
| (13,14) | Medial front-orbital gyrus | MOFG | (41,42) | Superior occipital gyrus | SOG |
| (15,16) | Superior parietal lobule | SPL | (43,44) | Middle occipital gyrus | MOG |
| (17,18) | Supramarginal gyrus | SMG | (45,46) | Inferior occipital gyrus | IOG |
| (19,20) | Angular gyrus | ANG | (47,48) | Cuneus | CUN |
| (21,22) | Precuneus | PCU | (49,50) | Lingual gyrus | LING |
| (23,24) | Postcentral gyrus | PoCG | (51,52) | Cingulate region | CING |
| (25,26) | Superior temporal gyrus | STG | (53,54) | Insula | INS |
| (27,28) | Middle temporal gyrus | MTG |
The regions are listed according to an existing atlas (Kabani et al., 1998).
Table 3.
Regional nodal characteristics versus TWMLL
| Anatomical regions |
t-score (P-value) |
||
|---|---|---|---|
| Correlation strength | Absolute efficiency | Relative efficiency | |
| Right insula | –9.10 (0.0008) | –4.01 (0.016) | –4.61 (0.0099) |
| Right inferior frontal gyrus | –5.91 (0.004) | –2.52 (0.065) | –4.35 (0.012) |
| Right precentral gyrus | –4.52 (0.010) | NS | –3.66 (0.021) |
| Right middle frontal gyrus | –3.93 (0.017) | –2.64 (0.057) | –3.57 (0.023) |
| Left middle temporal gyrus | –3.23 (0.032) | NS | NS |
| Left insular | –2.60 (0.060) | NS | NS |
| Right middle temporal gyrus | NS | –2.78 (0.050) | NS |
| Right superior middle gyrus | NS | –2.64 (0.057) | NS |
| Left parahippocampal gyrus | NS | 2.85 (0.047) | NS |
| Right parahippocampal gyrus | NS | NS | 2.97 (0.041) |
| Left angular gyrus | NS | 2.59 (0.061) | 2.79 (0.049) |
The t-values indicate significant decreases in nodal characteristics with increasing white matter lesion load, and vice versa. NS = non-significant.
Figure 4.
Changes in regional nodal characteristics of the insula with lesion load. (A) Insular region used in the analysis mapped onto a cortical surface. (B) Plot showing the decrease of the correlation strength in the insula with TWMLL. (C) Plot showing the decrease of the integrated absolute regional efficiency in the insula with TWMLL. (D) Plot showing the decrease of the integrated relative regional efficiency in the insula with TWMLL.
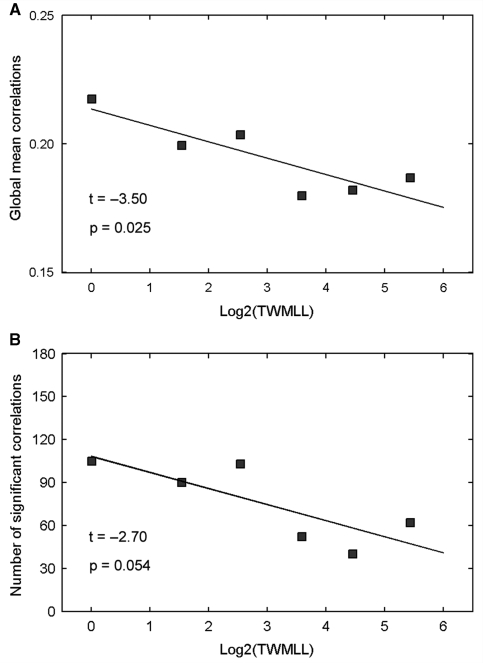
Regional cortical thickness correlations versus TWMLL
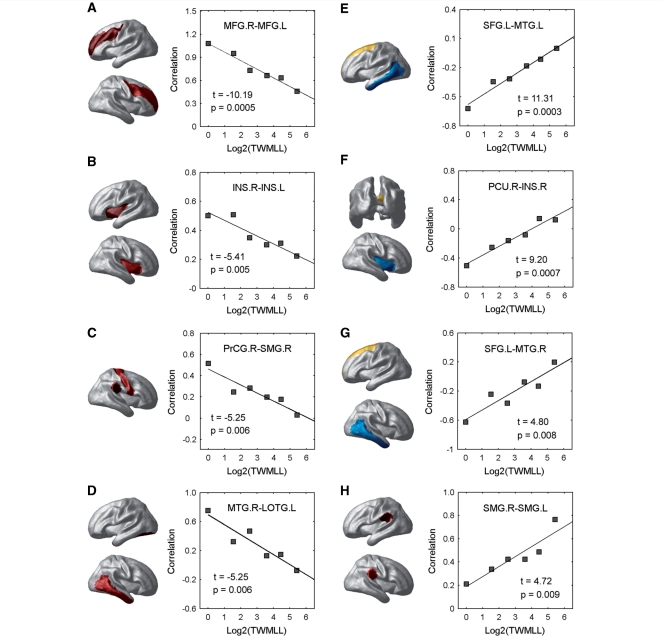
We also examined the relation between the regional cortical thickness correlations and the TWMLL. The global mean correlation decreased significantly [t(4) = –3.50, P = 0.025] with the TWMLL (Fig. 5A). The number of significant cortical correlations [false discovery rate procedure (Genovese et al., 2002), q < 0.05] in each group also decreased with the TWMLL [t(4) = –2.70, P = 0.054] (Fig. 5B). Further statistical analysis revealed significant cortical thickness correlation changes (P < 0.01, uncorrected) in various cortico-cortical pairs (Fig. 6). For instance, decreased positive correlations were found between the bilateral middle frontal gyrus, bilateral insula, precentral/supramarginal gyrus and middle temporal/lateral occipitotemporal gyrus (Fig. 6A–D), and decreased negative correlations were found between the superior frontal/middle temporal gyrus and precuneus/insula (Fig. 6E–G). Additionally, we also noted increased positive inter-hemispheric correlation between the supramarginal gyri (Fig. 6H).
Figure 5.
Changes in global cortical thickness correlations with lesion load. (A) Plot showing the decrease of the average correlations in multiple sclerosis with TWMLL. The average correlations were obtained by calculating the average values of inter-regional correlation matrices (Fig. 1). (B) Plot showing the decrease in the number of significant correlations with TWMLL.
Figure 6.
Changes in regional cortical thickness correlations with lesion load. (A–D) Plots showing the decrease of the inter-regional positive correlations in multiple sclerosis with TWMLL. (E–G) Plots showing the decrease of the inter-regional negative correlations in multiple sclerosis with TWMLL. (H) Plot showing the increase of the inter-regional positive correlations in multiple sclerosis with TWMLL. Note that pairs of regions were only listed if (i) they were significantly non-zero in at least one group (q < 0.05, FDR-corrected); and (ii) they showed significant decrease with lesion load (P < 0.01, uncorrected). All regions involved were mapped onto a cortical surface.
Discussion
We used cortical thickness measurements from structural MRI to investigate alterations in brain structural networks in multiple sclerosis. Previously, we showed that the thickness of human cerebral cortex was organized in a small-world architecture as characterized by a short characteristic path length and high clustering coefficient (corresponding to high global and local efficiency) (He et al., 2007). We now show that increasing white matter lesion volume in multiple sclerosis was significantly correlated with the impairment of both the global and local topological organization. These results provide new insights into the understanding of the organizational principles of human cortical morphology and white matter lesion related alterations in multiple sclerosis.
Small-world structural brain networks in multiple sclerosis
The human brain is a complex interconnected dynamical system. Recent studies have explored the intrinsic topological properties underlying the network organization of the human brain. For instance, neurophysiological and neuroimaging techniques have shown that brain functional activity is organized with a small-world topology at macroscale (Achard et al., 2006; Bassett and Bullmore, 2006; Stam et al., 2007, 2009; Liu et al., 2008; Wang et al., 2009; Rubinov et al., 2009), characterized by a high clustering coefficient and small characteristic path lengths linking individual network nodes (Watts and Strogatz, 1998). Specifically, Achard and Bullmore (2007) demonstrated that brain functional networks had economical small-world properties in terms of global and local efficiency of parallel information processing at a low wiring cost. At the same time, several MRI studies have also demonstrated such an architecture in the structural cortical networks in humans (He et al., 2007, 2008; Bassett et al., 2008). Thus, our findings of high global and local efficiency in the cortical networks in our group of multiple sclerosis patients are compatible with previous brain networks studies. Global efficiency is mainly associated with long-range connections ensuring effective interactions or rapid transfers of information between and across remote cortical regions that are believed to form the basis of many cognitive processes. Local efficiency is predominantly associated with short-range connections between nearby regions that mediate modularized information processing or fault-tolerance of a network (Latora and Marchiori, 2001). Computational modelling simulation (Sporns et al., 2000) and experimental studies (Chen et al., 2008) have suggested the emergence of such a small-world topology when networks are evolved for the high complexity of dynamic behaviour defined as an optimal balance between local specialization and global integration. Our findings provide empirical evidence to support the theory that human cortical morphology has evolved into a complex but efficient neuronal architecture to maximize the power of parallel information processing at a low cost.
Impaired network efficiency of the whole brain in multiple sclerosis
Although the brain networks of all multiple sclerosis groups retained a small-world topology regardless of lesion load, both absolute global and local efficiency of the structural brain networks nevertheless deteriorated as white matter lesion load increased. As discussed previously, the global and local efficiency of brain networks are predominantly associated with long- and short-range connections, respectively. Thus, our finding regarding the loss of absolute cortical network efficiency indicates a disrupted coordination of cortical thickness among widely distributed brain regions in the multiple sclerosis patients. The hypothesis can be further supported by the reduced number of significant regional cortical thickness correlations as lesion load increases. In multiple sclerosis, the increased lesion volume usually occurs in the periventricular regions with rich fibre connections to multiple cortical regions and has been shown to cause cortical neuronal damage due in part to the transection of axons and subsequent retrograde neuronal loss (Sailer et al., 2003). One could thus speculate that the white matter lesions in multiple sclerosis cause aberrant neuronal integrity between spatially distributed brain regions, which is likely responsible for the impairment of cognitive functions (Au Duong et al., 2005a, b; Morgen et al., 2006) and information processing speed (Reicker et al., 2007) commonly observed in the disease. We also observed that, when keeping the brain networks of each multiple sclerosis group at the same wiring cost, the relative local efficiency was significantly reduced as lesion load increased, whereas the relative global efficiency was slightly (non-significantly) increased (Fig. 3B). Since the global network efficiency is mainly associated with long-range connections, the slight increase in the relative global efficiency might reflect fewer long-range connections in the real state in the cortical networks of multiple sclerosis patients with higher TWMLLs that were compensated for adaptation in the relative state. Additionally, the results also indicated that white matter lesions in multiple sclerosis were associated with more impairment of the short-range connections (mainly associated with local efficiency) than the long-range connections. Together, our findings of decreased relative local efficiency and slightly increased relative global efficiency suggest that the structural brain networks in the multiple sclerosis patients tend to have a more randomized configuration as the lesion load increases. Random networks have less modularized information processing or fault-tolerance as compared to small-world networks (Latora and Marchiori, 2001). Therefore, the loss of small-world topological efficiency reported here represents a less optimal network organization in the multiple sclerosis patients as the lesion volume increases.
Impaired regional efficiency in multiple sclerosis
White matter lesions were also found to selectively and significantly impair the nodal characteristics of several cortical regions in the structural brain networks in the multiple sclerosis patients, including the insula, precentral gyrus and prefrontal and temporal association cortices. Many previous studies in monkeys and humans have demonstrated that these regions tend to be highly interconnected with each other and with many other cortical regions (Augustine, 1996; Mesulam, 1998; Luppino and Rizzolatti, 2000). Moreover, structural neuroimaging and pathological studies have also demonstrated that these regions displayed the most pronounced cortical thinning (Sailer et al., 2003; Charil et al., 2007), volume loss (Audoin et al., 2006b; Morgen et al., 2006), and cortical plaques (Kutzelnigg and Lassmann, 2006) in multiple sclerosis. Other studies have also indicated impaired functional connectivity of these regions in multiple sclerosis. For instance, by studying spontaneous low-frequency blood oxygen level-dependent fluctuations with functional MRI, Lowe et al. (2002) found that multiple sclerosis patients exhibited reduced functional connectivity in the precentral gyrus. Using a working memory task, Au Duong and colleagues (2005a, b) demonstrated reduced functional connectivity in the prefrontal cortex in multiple sclerosis. In this study, we also observed increased regional efficiency in the parahippocampal gyrus and angular gyrus as the lesions increased. Although previous studies have indicated that patients have enhanced functional reserve or compensatory recruitment of cognitive resources (Cader et al., 2006), it is still unclear what the increased efficiency in the two regions reflects. Further studies of structural and functional brain connectivity in multiple sclerosis will be helpful to address this issue. One could speculate that the cortical regions showing abnormalities in local efficiency might be more vulnerable to cortical or adjacent white matter multiple sclerosis lesions.
Impaired cortical thickness correlations in multiple sclerosis
White matter lesions also affected various regional cortical thickness correlations in the multiple sclerosis patients. We observed several decreased inter-hemispheric correlations, most notably between the prefrontal cortices and between the insular cortices. Corpus callosum connects homologous cortical regions and plays a vital role in information transfer between the two hemispheres. It is prone to multiple sclerosis lesions and atrophy, as shown in several autopsy and MRI studies (Barnard and Triggs, 1974; Simon et al., 1987; Barkhof et al., 1998; Manson et al., 2008). Moreover, callosal atrophy in multiple sclerosis also correlates with impaired cognitive function (Paolillo et al., 2000; Pelletier et al., 2001). Thus, the decreased inter-hemispheric cortical thickness correlations between homologous regions could be related to the disruptions of the corpus callosum. Other decreased positive correlations were also found between the precentral and supramarginal gyrus, and between the middle temporal and lateral occipitotemporal gyrus. The results are compatible with many previous studies showing that these regions are especially vulnerable to grey matter loss (Sailer et al., 2003; Morgen et al., 2006; Ceccarelli et al., 2008) or decreased functional connectivity (Lowe et al., 2002, 2008) in multiple sclerosis. We also observed decreased negative cortical thickness correlations in the patients as the lesion load increased. The phenomenon of negative correlations in cortical morphology has been demonstrated in several previous studies in normal subjects (Mechelli et al., 2005; He et al., 2007), and patients with schizophrenia (Mitelman et al., 2005) and Alzheimer's disease (He et al., 2008); however, the underlying mechanism remains unclear. One possible explanation for the reduced negative correlations in multiple sclerosis is that they arise from weakened inter-regional inhibitory relationships caused by white matter pathway disruptions, leading to a reorganization of inter-regional associations.
Structural disruptions likely reflect functional deficits in multiple sclerosis
In the present study, we showed that the white matter lesions in multiple sclerosis are associated with the changes in structural correlations among cortical regions. Previously, we demonstrated that the human brain structural networks constructed by structural correlations follow a small-word organization and the network hubs tend to be the highly connected association cortex regions (He et al., 2007). These features of the structural networks are in accordance with the findings of spontaneous brain functional networks derived from functional MRI in a no-task or resting state (Achard et al., 2006), suggesting that the structural brain networks are activity dependent. Recently, Skudlarski and colleagues (2008) showed that the human brain structural connectivity (white matter pathways) was broadly consistent with resting-state spontaneous brain functional connectivity. There is also increasing evidence suggesting that the damage to structural connectivity in multiple sclerosis correlates with the altered functional connectivity among regions (Rocca et al., 2007). Thus, the structural disruptions in multiple sclerosis shown here are likely to reflect functional disruptions. We speculate that the disrupted white matter pathways likely lead to functional disconnections, which then lead to structural disconnections (disruptive cortical thickness correlations).
Structural brain networks of multiple sclerosis versus Alzheimer's disease
Recently, we investigated the structural brain networks of Alzheimer's disease using cortical thickness measurements, and demonstrated small-world alterations (He et al., 2008). In the present study, we also showed multiple sclerosis-related changes in the structural brain networks. Although the loss of small-world properties was observed in both studies, there were distinct patterns (Table 4). In Alzheimer's disease, the structural brain networks tended to take on a more regular configuration (increased local clustering and decreased path length) compared with controls. However, in multiple sclerosis, the structural brain networks tended toward a more random configuration (decreased local clustering and slightly increased paths) as the white matter lesion loads increase (note however that we could not compare our multiple sclerosis patients to matched controls). These differences between Alzheimer's disease and multiple sclerosis could be related to specific regional abnormalities in the brain networks. For example, patients with Alzheimer's disease showed abnormalities at network hubs (regions with high topological centrality) predominately in the temporal and parietal regions, whereas multiple sclerosis patients had abnormalities mainly in the insula, precentral gyrus and prefrontal and temporal association cortices (Table 4). These regional differences likely reflect disease-specific pathophysiologies. One could thus expect that the approach of structural brain networks analysis applied here provides a promising tool to elucidate the mechanisms responsible for different clinical manifestations of brain disease or damage. Nonetheless, it should be noted that we only correlated the changes of topological organization of structural brain networks with the white matter lesion load in multiple sclerosis patients. It is still unknown how the brain network organization of multiple sclerosis differs from that of healthy subjects and whether this approach can be used as a novel method to diagnose disease and monitor progression. Further graph analysis of structural and functional data as well as longitudinal studies will be helpful to address these issues.
Table 4.
Comparisons of topological properties in structural brain networks of Alzheimer's disease versus multiple sclerosis
| Study | Small-world network efficiency | Regional nodal efficiency |
|---|---|---|
| Alzheimer's disease (He et al., 2008) | An increase in local network efficiency but a decrease in global efficiency as compared to controls, suggesting a more regular configuration. | A decrease in regional efficiency of temporal and parietal association cortex areas with an increase for more primary occipital areas as compared to controls. |
| Multiple sclerosis (the current study) | A decrease in local network efficiency but a non-significant change in global efficiency with increasing white matter lesion load, suggesting a more random configuration. | A decrease in regional efficiency of insula and precentral gyrus as well as prefrontal and temporal association cortex areas, and an increase in the parahippocampal gyrus and angular gyrus with increasing white matter lesion load. |
Note that, in the previous Alzheimer's disease study, we characterized the small-world properties and regional nodal characteristics of the structural brain networks by using clustering coefficient/shortest path length, and nodal betweenness centrality, respectively (He et al., 2008). For comparative purpose, here the Alzheimer's disease results are described from an efficiency perspective.
Methodological issues
Several methodological issues need to be addressed. First, the structural brain networks for each multiple sclerosis group were obtained by calculating the correlations of regional cortical thickness across subjects. Thus, we could not explore the relation between the network parameters and brain lesions in individual subjects. However, recent studies have demonstrated that the graph theoretical analysis of brain networks can also be implemented by using resting-state functional MRI (Salvador et al., 2005; Achard et al., 2006; Liu et al., 2008; Wang et al., 2009) and diffusion MRI (Hagmann et al., 2007; Iturria-Medina et al., 2008; Gong et al., 2009) data that allow measurements of spontaneous brain functional connectivity and white matter integrity at the individual level, respectively. Specifically, combinations of such multi-modal neuroimaging approaches should yield a more comprehensive understanding of how structural disruptions in the brain network architecture are associated with functional deficits, in multiple sclerosis and in other brain diseases. Second, we showed that the white matter lesion load had significant correlations with the disruption of network efficiency in multiple sclerosis. However, we cannot assert a direct and unique causal relationship between the white matter lesions and the organizational alterations of the brain networks. In fact, previous studies using magnetization transfer imaging, spectroscopy and diffusion MRI have demonstrated that multiple sclerosis is associated with diffuse white matter injury as well as diffuse grey matter injury and white matter elapsed inflammation (for reviews, see Filippi et al., 2001; Rovaris et al., 2005). Thus, pathological processes affecting both the white matter and grey matter are likely to contribute to the network disorganization in multiple sclerosis. Third, we measured changes in the multiple sclerosis cortical structural networks as a function of the TWMLL. As different white matter structures are associated with distinct neuronal pathways, one could speculate that lesion location should distinctly influence cortical morphology (Charil et al., 2007). The relationship between lesion location and brain network topology would be an interesting topic for future research. Fourth, it remains unclear whether the topological changes described here in relapsing-remitting multiple sclerosis can be generalized to other clinical phenotypes. Finally, it would be interesting in future work to correlate disruptions in network topology with different clinical manifestations of the disease.
Conclusion
In this study, we used regional cortical thickness measurements to demonstrate that human brain structural networks exhibit economical small-world properties as characterized by high local and global efficiency at a relatively low wiring cost. More importantly, we showed that the network efficiency had significant decrements with increased white matter lesion load in multiple sclerosis, with the most prominent changes in specific brain regions, including the insula, precentral gyrus and prefrontal and temporal association areas. The decreased network efficiency in multiple sclerosis might reflect disrupted functional neuronal organization of spatially distributed cortical regions. Our findings are compatible with the notion that multiple sclerosis is associated with disruptions in the integrity of large-scale interconnected brain systems. The present study has implications for understanding how the topological alterations in large-scale brain networks underlie functional deficits in multiple sclerosis patients. The topology-based cortical network analysis also provides a new way to understand the pathophysiology of specific functional deficits (in cognitive function, mood, coordination or gait) and, possibly, to evaluate disease progression.
Funding
National Institutes of Health Human Brain Project (Grant #NIH 9P01 EB001955-11); Fonds de Recherche en Santé du Quebec salary award (to A.D.); National Natural Science Foundation of China (Grant #30870667, Y.H.).
Acknowledgements
We wish to thank the participating sites in the imaging component of the Myloral study: St Michael's Hospital (Toronto, ON), Montreal Neurological Hospital (Montreal, PQ), University of British Colombia (Vancouver, BC), Ottawa General Hospital (Ottawa, ON), University of Maryland (Baltimore, MD), Lehigh Magnetic Imaging Center (Allentown, PA), Maimonides Medical Center Brooklyn, NY), St John Medical Center (Tulsa, OK), University of Minnesota (Minneapolis, MN), Washington University (St Louis, MO), Cornell Medical Center (New York, NY), University of Southern California (Los Angeles, CA), and Carolinas Medical Center (Charlotte, NC).
References
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au Duong MV, Audoin B, Boulanouar K, Ibarrola D, Malikova I, Confort-Gouny S, et al. Altered functional connectivity related to white matter changes inside the working memory network at the very early stage of MS. J Cereb Blood Flow Metab. 2005a;25:1245–53. doi: 10.1038/sj.jcbfm.9600122. [DOI] [PubMed] [Google Scholar]
- Au Duong MV, Boulanouar K, Audoin B, Treseras S, Ibarrola D, Malikova I, et al. Modulation of effective connectivity inside the working memory network in patients at the earliest stage of multiple sclerosis. Neuroimage. 2005b;24:533–8. doi: 10.1016/j.neuroimage.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Audoin B, Au Duong MV, Malikova I, Confort-Gouny S, Ibarrola D, Cozzone PJ, et al. Functional magnetic resonance imaging and cognition at the very early stage of MS. J Neurol Sci. 2006a;245:87–91. doi: 10.1016/j.jns.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Audoin B, Davies GR, Finisku L, Chard DT, Thompson AJ, Miller DH. Localization of grey matter atrophy in early RRMS: A longitudinal study. J Neurol. 2006b;253:1495–501. doi: 10.1007/s00415-006-0264-2. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev. 1996;22:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Barkhof FJ, Elton M, Lindeboom J, Tas MW, Schmidt WF, Hommes OR, et al. Functional correlates of callosal atrophy in relapsing-remitting multiple sclerosis patients. A preliminary MRI study. J Neurol. 1998;245:153–8. doi: 10.1007/s004150050196. [DOI] [PubMed] [Google Scholar]
- Barnard RO, Triggs M. Corpus callosum in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1974;37:1259–64. doi: 10.1136/jnnp.37.11.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–23. doi: 10.1177/1073858406293182. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–48. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore ET, Woodruff PW, Wright IC, Rabe-Hesketh S, Howard RJ, Shuriquie N, et al. Does dysplasia cause anatomical dysconnectivity in schizophrenia? Schizophr Res. 1998;30:127–35. doi: 10.1016/s0920-9964(97)00141-2. [DOI] [PubMed] [Google Scholar]
- Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129:527–37. doi: 10.1093/brain/awh670. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Atzori M, Bernardi V, Morra A, Romualdi C, Rinaldi L, et al. Cortical atrophy is relevant in multiple sclerosis at clinical onset. J Neurol. 2007;254:1212–20. doi: 10.1007/s00415-006-0503-6. [DOI] [PubMed] [Google Scholar]
- Ceccarelli A, Rocca MA, Pagani E, Colombo B, Martinelli V, Comi G, et al. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage. 2008;42:315–22. doi: 10.1016/j.neuroimage.2008.04.173. [DOI] [PubMed] [Google Scholar]
- Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–37. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- Charil A, Dagher A, Lerch JP, Zijdenbos AP, Worsley KJ, Evans AC. Focal cortical atrophy in multiple sclerosis: relation to lesion load and disability. Neuroimage. 2007;34:509–17. doi: 10.1016/j.neuroimage.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Charil A, Zijdenbos AP, Taylor J, Boelman C, Worsley KJ, Evans AC, et al. Statistical mapping analysis of lesion location and neurological disability in multiple sclerosis: application to 452 patient data sets. Neuroimage. 2003;19:532–44. doi: 10.1016/s1053-8119(03)00117-4. [DOI] [PubMed] [Google Scholar]
- Chen ZJ, He Y, Rosa-Neto P, Germann J, Evans AC. Revealing modular architecture of human brain structural networks by using cortical thickness from MRI. Cereb Cortex. 2008;18:2374–81. doi: 10.1093/cercor/bhn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JT, Narayanan S, Collins DL, Smith SM, Matthews PM, Arnold DL. Relating neocortical pathology to disability progression in multiple sclerosis using MRI. Neuroimage. 2004;23:1168–75. doi: 10.1016/j.neuroimage.2004.07.046. [DOI] [PubMed] [Google Scholar]
- Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, et al. Deformation-based surface morphometry applied to grey matter deformation. Neuroimage. 2003;18:198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- Collins DL, Holmes CJ, Peters TM, Evans AC. Automatic 3-D modelbased neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, et al. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–7. doi: 10.1093/brain/awh126. [DOI] [PubMed] [Google Scholar]
- Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM. Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain. 2000;123(Pt 9):1845–9. doi: 10.1093/brain/123.9.1845. [DOI] [PubMed] [Google Scholar]
- Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–11. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- Fisniku LK, Chard DT, Jackson JS, Anderson VM, Altmann DR, Miszkiel KA, et al. Grey matter atrophy is related to long-term disability in multiple sclerosis. Ann Neurol. 2008;64:247–54. doi: 10.1002/ana.21423. [DOI] [PubMed] [Google Scholar]
- Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, et al. Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging. 2004;20:1–7. doi: 10.1002/jmri.20083. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19:524–36. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin CM, Chard DT, Ciccarelli O, Kapoor B, Barker GJ, Thompson AI, et al. Diffusion tensor imaging in early relapsing-remitting multiple sclerosis. Mult Scler. 2001;7:290–7. doi: 10.1177/135245850100700504. [DOI] [PubMed] [Google Scholar]
- Hagmann P, Kurant M, Gigandet X, Thiran P, Wedeen VJ, Meuli R, et al. Mapping human whole-brain structural networks with diffusion MRI. PLoS ONE. 2007;2:e597. doi: 10.1371/journal.pone.0000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen ZJ, Evans AC. Small-world anatomical networks in the human brain revealed by cortical thickness from MRI. Cereb Cortex. 2007;17:2407–19. doi: 10.1093/cercor/bhl149. [DOI] [PubMed] [Google Scholar]
- He Y, Chen Z, Evans A. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer's disease. J Neurosci. 2008;28:4756–66. doi: 10.1523/JNEUROSCI.0141-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturria-Medina Y, Sotero RC, Canales-Rodriguez EJ, Aleman-Gomez Y, Melie-Garcia L. Studying the human brain anatomical network via diffusion-weighted MRI and Graph Theory. Neuroimage. 2008;40:1064–76. doi: 10.1016/j.neuroimage.2007.10.060. [DOI] [PubMed] [Google Scholar]
- Jasperse B, Vrenken H, Sanz-Arigita E, de Groot V, Smith SM, Polman CH, et al. Regional brain atrophy development is related to specific aspects of clinical dysfunction in multiple sclerosis. Neuroimage. 2007;38:529–37. doi: 10.1016/j.neuroimage.2007.07.056. [DOI] [PubMed] [Google Scholar]
- Jiang T, He Y, Zang Y, Weng X. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004;22:63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabani N, Le Goualher G, MacDonald D, Evans AC. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage. 2001;13:375–80. doi: 10.1006/nimg.2000.0652. [DOI] [PubMed] [Google Scholar]
- Kabani N, MacDonald D, Holmes C, Evans A. Fourth International Conference on Functional Mapping of the Human Brain Mapping Conference Montreal-PQ Canada. 1998. 3D atlas of the human brain. [Google Scholar]
- Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. Neuroimage. 2005;27:210–21. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lassmann H. Cortical demyelination in multiple sclerosis: a substrate for cognitive deficits? J Neurol Sci. 2006;245:123–6. doi: 10.1016/j.jns.2005.09.021. [DOI] [PubMed] [Google Scholar]
- Latora V, Marchiori M. Efficient behaviour of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Lee JK, Lee JM, Kim JS, Kim IY, Evans AC, Kim SI. A novel quantitative cross-validation of different cortical surface reconstruction algorithms using MRI phantom. Neuroimage. 2006;31:572–84. doi: 10.1016/j.neuroimage.2005.12.044. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage. 2005;24:163–73. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Worsley K, Shaw WP, Greenstein DK, Lenroot RK, Giedd J, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31:993–1003. doi: 10.1016/j.neuroimage.2006.01.042. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Beall EB, Sakaie KE, Koenig KA, Stone L, Marrie RA, et al. Resting state sensorimotor functional connectivity in multiple sclerosis inversely correlates with transcallosal motor pathway transverse diffusivity. Hum Brain Mapp. 2008;29:818–27. doi: 10.1002/hbm.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–92. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Luppino G, Rizzolatti G. The organization of the frontal motor cortex. News Physiol Sci. 2000;15:219–24. doi: 10.1152/physiologyonline.2000.15.5.219. [DOI] [PubMed] [Google Scholar]
- MacDonald D, Kabani N, Avis D, Evans AC. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage. 2000;12:340–56. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- Manson SC, Wegner C, Filippi M, Barkhof F, Beckmann C, Ciccarelli O, et al. Impairment of movement-associated brain deactivation in multiple sclerosis: further evidence for a functional pathology of interhemispheric neuronal inhibition. Exp Brain Res. 2008;187:25–31. doi: 10.1007/s00221-008-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Friston KJ, Frackowiak RS, Price CJ. Structural covariance in the human cortex. J Neurosci. 2005;25:8303–10. doi: 10.1523/JNEUROSCI.0357-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Miller DH, Barkhof F, Frank JA, Parker GJ, Thompson AJ. Measurement of atrophy in multiple sclerosis: pathological basis, methodological aspects and clinical relevance. Brain. 2002;125:1676–95. doi: 10.1093/brain/awf177. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Buchsbaum MS, Brickman AM, Shihabuddin L. Cortical intercorrelations of frontal area volumes in schizophrenia. Neuroimage. 2005;27:753–70. doi: 10.1016/j.neuroimage.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Morgen K, Sammer G, Courtney SM, Wolters T, Melchior H, Blecker CR, et al. Evidence for a direct association between cortical atrophy and cognitive impairment in relapsing-remitting MS. Neuroimage. 2006;30:891–8. doi: 10.1016/j.neuroimage.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Newcombe J, Hawkins CP, Henderson CL, Patel HA, Woodroofe MN, Hayes GM, et al. Histopathology of multiple sclerosis lesions detected by magnetic resonance imaging in unfixed postmortem central nervous system tissue. Brain. 1991;114(Pt 2):1013–23. doi: 10.1093/brain/114.2.1013. [DOI] [PubMed] [Google Scholar]
- Ormerod IE, Miller DH, McDonald WI, du Boulay EP, Rudge P, Kendall BE, et al. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain. 1987;110(Pt 6):1579–616. doi: 10.1093/brain/110.6.1579. [DOI] [PubMed] [Google Scholar]
- Paolillo A, Pozzilli C, Gasperini C, Giugni E, Mainero C, Giuliani S, et al. Brain atrophy in relapsing-remitting multiple sclerosis: relationship with 'black holes', disease duration and clinical disability. J Neurol Sci. 2000;174:85–91. doi: 10.1016/s0022-510x(00)00259-8. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Suchet L, Witjas T, Habib M, Guttmann CR, Salamon G, et al. A longitudinal study of callosal atrophy and interhemispheric dysfunction in relapsing-remitting multiple sclerosis. Arch Neurol. 2001;58:105–11. doi: 10.1001/archneur.58.1.105. [DOI] [PubMed] [Google Scholar]
- Poonawalla AH, Hasan KM, Gupta RK, Ahn CW, Nelson F, Wolinsky JS, et al. Diffusion-tensor MR imaging of cortical lesions in multiple sclerosis: initial findings. Radiology. 2008;246:880–6. doi: 10.1148/radiol.2463070486. [DOI] [PubMed] [Google Scholar]
- Poser CM, Paty DW, Scheinberg L, McDonald WI, Davis FA, Ebers GC, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–31. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Reicker LI, Tombaugh TN, Walker L, Freedman MS. Reaction time: An alternative method for assessing the effects of multiple sclerosis on information processing speed. Arch Clin Neuropsychol. 2007;22:655–64. doi: 10.1016/j.acn.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Pagani E, Absinta M, Valsasina P, Falini A, Scotti G, et al. Altered functional and structural connectivities in patients with MS: a 3-T study. Neurology. 2007;69:2136–45. doi: 10.1212/01.wnl.0000295504.92020.ca. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Gass A, Bammer R, Hickman SJ, Ciccarelli O, Miller DH, et al. Diffusion MRI in multiple sclerosis. Neurology. 2005;65:1526–32. doi: 10.1212/01.wnl.0000184471.83948.e0. [DOI] [PubMed] [Google Scholar]
- Rovaris M, Judica E, Gallo A, Benedetti B, Sormani MP, Caputo D, et al. Grey matter damage predicts the evolution of primary progressive multiple sclerosis at 5 years. Brain. 2006;129:2628–34. doi: 10.1093/brain/awl222. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, et al. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30:403–16. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer M, Fischl B, Salat D, Tempelmann C, Schonfeld MA, Busa E, et al. Focal thinning of the cerebral cortex in multiple sclerosis. Brain. 2003;126:1734–44. doi: 10.1093/brain/awg175. [DOI] [PubMed] [Google Scholar]
- Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15:1332–42. doi: 10.1093/cercor/bhi016. [DOI] [PubMed] [Google Scholar]
- Sastre-Garriga J, Ingle GT, Chard DT, Cercignani M, Ramio-Torrenta L, Miller DH, et al. Grey and white matter volume changes in early primary progressive multiple sclerosis: a longitudinal study. Brain. 2005;128:1454–60. doi: 10.1093/brain/awh498. [DOI] [PubMed] [Google Scholar]
- Simon JH, Schiffer RB, Rudick RA, Herndon RM. Quantitative determination of MS-induced corpus callosum atrophy in vivo using MR imaging. AJNR Am J Neuroradiol. 1987;8:599–604. [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Calhoun VD, Hampson M, Skudlarska BA, Pearlson G. Measuring brain connectivity: diffusion tensor imaging validates resting state temporal correlations. Neuroimage. 2008;43:554–61. doi: 10.1016/j.neuroimage.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Sporns O, Tononi G, Edelman GM. Theoretical neuroanatomy: relating anatomical and functional connectivity in graphs and cortical connection matrices. Cereb Cortex. 2000;10:127–41. doi: 10.1093/cercor/10.2.127. [DOI] [PubMed] [Google Scholar]
- Stam CJ, de Haan W, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer's disease. Brain. 2009;132:213–24. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Small-world networks and functional connectivity in Alzheimer's disease. Cereb Cortex. 2007;17:92–9. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1:3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio F, Zito G, Zappasodi F, Dell' Acqua ML, Landi D, Nardo D, et al. Intra-cortical connectivity in multiple sclerosis: a neurophysiological approach. Brain. 2008;131:1783–92. doi: 10.1093/brain/awn087. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang L, Zang Y, Yang H, Tang H, Gong Q, et al. Parcellation-dependent small-world brain functional networks: a resting-state fMRI study. Hum Brain Mapp. 2009;30:1511–23. doi: 10.1002/hbm.20623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature. 1998;393:440–2. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–51. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sharma T, Ellison ZR, McGuire PK, Friston KJ, Brammer MJ, et al. Supra-regional brain systems and the neuropathology of schizophrenia. Cereb Cortex. 1999;9:366–78. doi: 10.1093/cercor/9.4.366. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic ‘pipeline’ analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]