Abstract
Multiple sclerosis is an inflammatory demyelinating disease of the central nervous system (CNS) that is thought to be caused by a combination of genetic and environmental factors. To date, considerable evidence has associated Epstein–Barr virus (EBV) infection with disease development. However, it remains controversial whether EBV infects multiple sclerosis brain and contributes directly to CNS immunopathology. To assess whether EBV infection is a characteristic feature of multiple sclerosis brain, a large cohort of multiple sclerosis specimens containing white matter lesions (nine adult and three paediatric cases) with a heterogeneous B cell infiltrate and a second cohort of multiple sclerosis specimens (12 cases) that included B cell infiltration within the meninges and parenchymal B cell aggregates, were examined for EBV infection using multiple methodologies including in situ hybridization, immunohistochemistry and two independent real-time polymerase chain reaction (PCR) methodologies that detect genomic EBV or the abundant EBV encoded RNA (EBER) 1, respectively. We report that EBV could not be detected in any of the multiple sclerosis specimens containing white matter lesions by any of the methods employed, yet EBV was readily detectable in multiple Epstein–Barr virus-positive control tissues including several CNS lymphomas. Furthermore, EBV was not detected in our second cohort of multiple sclerosis specimens by in situ hybridization. However, our real-time PCR methodologies, which were capable of detecting very few EBV infected cells, detected EBV at low levels in only 2 of the 12 multiple sclerosis meningeal specimens examined. Our finding that CNS EBV infection was rare in multiple sclerosis brain indicates that EBV infection is unlikely to contribute directly to multiple sclerosis brain pathology in the vast majority of cases.
Keywords: B cells, Epstein–Barr virus, multiple sclerosis brain
Introduction
Multiple sclerosis is an inflammatory demyelinating disease of the central nervous system (CNS). It is thought to be caused by autoimmune processes in response to a combination of both genetic and environmental factors (Hafler, 2004; Giovannoni et al., 2006). After decades of study, many causal factors have been implicated in multiple sclerosis development, including a large number of bacterial and viral pathogens (Hafler, 2004; Giovannoni et al., 2006). While inconsistent results have left the involvement of many of these pathogens unresolved, considerable evidence has linked the human herpes Epstein–Barr virus (EBV) to disease development (Ascherio and Munger, 2007; Pohl, 2009; Salvetti et al., 2009).
EBV is a human DNA herpes virus that predominantly infects B cells (Thorley-Lawson, 2001). It causes latent asymptomatic infection in most individuals and infectious mononucleosis in adolescents and young adults. EBV has five programmes of gene usage: one programme is used to produce the virus (lytic programme), while four other programmes are associated with latent infection in which no virus is produced (Thorley-Lawson and Gross, 2004). The latency programmes include; the growth programme (also referred to as Latency III), which involves the expression of up to nine distinct latent proteins; the default programme (also referred to as latency II) in which only three genes are expressed [EBV nuclear antigen 1 (EBNA1), latent membrane proteins (LMP) 1 and 2A]; the EBNA-1 programme (EBNA-1 expression only), which allows viral DNA in the latency programme to divide; and the latency programme, in which few or no genes are expressed. While the lytic, growth and default programmes are immunogenic as a result of EBV protein expression, the latency programme, in which few or no genes are expressed, allows the virus to evade immune detection and persist at low levels in >95% of all adults. Although immunohistochemistry for EBV protein expression can reliably detect EBV infection in three of the four latency programmes if performed correctly, definitive evidence for the presence of EBV is based on the detection of either genomic EBV or the small, abundant, nuclear EBV encoded RNAs (EBER1 and EBER2), which are expressed at high levels during all phases of latent infection.
EBV infection has been associated with the development of a number of cancers including Burkitt's lymphoma (Epstein et al., 1964; Leder, 1985; Callan et al., 1998), nasopharyngeal carcinoma (Muir, 1972; Gregory et al., 1990), Hodgkin lymphoma (Anagnostopoulos et al., 1995) and immunoblastic lymphomas (Niedobitek, 1999). However, several studies have shown an association between EBV infection and several autoimmune diseases such as systemic lupus erythematosus (James et al., 2006) and multiple sclerosis (Ascherio and Munger, 2007; De Jager et al., 2008).
EBV has a number of properties that make it an attractive candidate as a potential causal factor of multiple sclerosis development, including its ubiquitous expression, its ability to cause latent infection, and its ability to undergo periodic reactivation (Haahr and Hollsberg, 2006). After decades of research, considerable evidence supporting a role for EBV in multiple sclerosis development has emerged primarily from sero-epidemiological and immunological studies (Ascherio and Munger, 2007, 2008). This evidence includes; the statistically higher EBV seropositivity rate in adult multiple sclerosis patients (99%) relative to controls (90%–95%), despite the ubiquitous nature of EBV infection in adults (Bray et al., 1983; Sumaya et al., 1985; Wandinger et al., 2000; Haahr and Hollsberg, 2006; Ascherio and Munger, 2007); the dramatically increased EBV seropositivity rate in rare paediatric multiple sclerosis cases (83%–99%) relative to controls (42%–72%) (Alotaibi et al., 2004; Pohl et al., 2006); increased serum antibody titres to certain EBV antigens up to 20 years before clinical onset of multiple sclerosis (retrospective studies) (Levin et al., 2005; DeLorenze et al., 2006); and the presence of increased numbers of EBV reactive CD8 (Hollsberg et al., 2003; Cepok et al., 2005; Lunemann et al., 2006; Jilek et al., 2008) and CD4 T cells (Lunemann et al., 2006; Lunemann et al., 2008) in the periphery of multiple sclerosis patients. In addition, studies have reported an increased risk of developing multiple sclerosis following infectious mononucleosis (Operskalski et al., 1989; Lindberg et al., 1991; Thacker et al., 2006). Furthermore, several reports have shown reactivity of the immunoglobulin present in the cerebrospinal fluid (CSF) of multiple sclerosis patients to EBV antigens (Bray et al., 1992; Rand et al., 2000; Cepok et al., 2005). Finally, T cell clones isolated in our laboratory, recognizing an immunodominant epitope of myelin basic protein from patients with multiple sclerosis, cross-reacted with EBV-derived proteins (Ota et al., 1990; Wucherpfennig and Strominger, 1995) as did EBV reactive T cells isolated from multiple sclerosis patients in an independent study (Lunemann et al., 2008).
Despite these observations, it remains uncertain whether EBV infection is a causal factor in multiple sclerosis development or whether its disease association is a consequence of dysregulated immune function. With regard to the former, it has been speculated that EBV could contribute to multiple sclerosis pathogenesis; via an indirect effect on immune function; through molecular mimicry between EBV and CNS antigens; or alternatively by undergoing periodic re-activation within the CNS thus serving as a direct target of immune-mediated CNS demyelination. However, in certain respects EBV infection in multiple sclerosis patients does not appear perturbed as no substantial increases have been observed in viral DNA load in the blood of multiple sclerosis patients relative to controls (Wagner et al., 2004; Lunemann et al., 2006, 2007). Furthermore, within the CNS, it remains unclear whether EBV infection is a characteristic feature of multiple sclerosis brain with several studies generating contrasting results (Hilton et al., 1994; Opsahl and Kennedy, 2007; Serafini et al., 2007). While an early study using EBER in situ hybridization (ISH) reported an absence of EBV in a cohort of 10 multiple sclerosis cases (Hilton et al., 1994) and results from another study were inconclusive possibly due to poor tissue quality (Opsahl and Kennedy, 2007), a recent study reported that EBV infection was prominent in a large percentage of multiple sclerosis CNS B cells in >95% of multiple sclerosis cases (21 of 22 cases) examined using both ISH and immunohistochemistry (Serafini et al., 2007).
Given the conflicting results reported in these studies, we investigated whether EBV infection was a characteristic feature of multiple sclerosis brain by analysing a large cohort of multiple sclerosis specimens containing white matter lesions with a heterogeneous B cell infiltrate (adult and paediatric multiple sclerosis) and a second cohort of multiple sclerosis specimens containing B cell infiltration within the meninges and parenchymal B cell aggregates. EBV infection was examined in multiple sclerosis specimens and controls using multiple methodologies including in situ hybridization, immunohistochemistry and two independent real-time PCR methodologies that measured both genomic EBV and an abundant EBV-associated non-polyadenylated RNA called EBER1. Both real-time PCR methodologies were capable of detecting two or fewer EBV infected B cells. Despite an exhaustive search with these highly sensitive methodologies, we report that EBV was undetectable in all multiple sclerosis white matter lesions examined by all methodologies employed. Furthermore, EBV was not detected by ISH in our second cohort of multiple sclerosis specimens, despite the identification of parenchymal B cell aggregates and loose B cell infiltration within the meninges within a subset of the specimens examined. Our molecular analysis performed on matching frozen specimens yielded similar results with EBV detected at low levels in only 2 of the 12 cases examined. Collectively, these results led us to conclude EBV infection was not a characteristic feature of multiple sclerosis brain. While our results do not exclude the potential for an indirect role for EBV infection in multiple sclerosis immunopathology, our finding that EBV infection was rare in multiple sclerosis brain indicates that EBV is unlikely to contribute directly to multiple sclerosis brain pathology in the vast majority of cases.
Methods
Cell lines
The EBV-positive lymphoblastoid cell line IB4 (gift of David Thorley-Lawson, Tufts University) was used as a positive control for genomic (W repeat) DNA PCR and EBER1 RNA (cDNA) PCR. The EBV-negative human Jurkat T cell line was used as a negative control. Cell lines were cultured at 37°C in 5% CO2 in RPMI 1640 supplemented with 10% foetal calf serum, 2 mM sodium pyruvate, 2 mM glutamine and 100 IU each of penicillin and streptomycin.
Tissue specimens
Post-mortem formalin-fixed, paraffin-embedded multiple sclerosis tissue blocks (from autopsy and biopsy cases) and snap-frozen multiple sclerosis tissue specimens from autopsy cases were obtained from the Department of Pathology at Brigham and Women's Hospital, the Human Brain and Spinal Fluid Resource Centre at the University of California Los Angeles, the UK Multiple Sclerosis Tissue Bank and the Department of Neuropathology at the University Medical Centre Göttingen, Germany. A formalin-fixed EBV-positive Hodgkin lymphoma isolated from lymph node, a formalin-fixed EBV-positive lymphoma isolated from lung (autopsy) and snap-frozen post-transplant B cell lymphomas were obtained from the Department of Pathology at Brigham and Women's Hospital. Two formalin-fixed CNS lymphomas were obtained from the Massachusetts General Hospital. Each site collected samples using a protocol approved by the institutional review board for human subjects. A total of 23 formalin-fixed, paraffin-embedded tissue specimens from 12 multiple sclerosis cases (multiple sclerosis cohort 1) with confirmed B cell infiltrate and 12 paraformaldehyde fixed-frozen multiple sclerosis specimens containing meningeal tissue from 12 autopsy cases (multiple sclerosis cohort 2) were examined for EBV by ISH. A total of 17 snap-frozen multiple sclerosis lesions with confirmed B cell infiltrate from five autopsy cases (four cases had formalin-fixed, paraffin-embedded blocks) and 12 snap-frozen multiple sclerosis specimens containing meningeal tissue (all 12 cases had fixed-frozen blocks as well) from 12 autopsy cases were examined for EBV by real-time PCR (Hochberg et al., 2004). It must be noted that all post-mortem snap-frozen tissue specimens would have taken several hours to process in contrast to biopsied material.
Tissue processing for DNA and RNA isolation
For DNA and RNA isolation, snap-frozen multiple sclerosis specimens were sectioned using a cryostat. For multiple sclerosis specimens containing white matter lesions, eight alternating 28-μm sections (two 14-μm sections) were used to isolate DNA (four samples) or RNA (four samples). For multiple sclerosis specimens containing meningeal tissue, seven 10-μm sections were cut with minimal waste. Sections 1, 4 and 7 were used for histology, 2 and 5 for DNA and 3 and 6 for RNA. DNA and RNA were isolated using DNeasy and RNeasy kits (Qiagen), respectively, as per manufacturer's instructions.
EBV detection by real-time PCR
Real-time PCR for the detection of EBV genomic BamHI-W repeats and EBER1 RNA were performed as described (Hochberg et al., 2004). PCR was performed in a 20 µl volume on the ABI Prism 5700 RT Thermal Cycler (Perkin Elmer). Thermal cycling was initiated with a first denaturation step at 95°C for 20 s, and continued with 50 cycles of amplification at 95°C for 3 s and 60°C for 30 s. For each reaction, 5 µl of DNA or cDNA template was added to a master mix containing 10 µl of Universal TaqMan Master Mix (Applied Biosystems) and 1 µl each of the forward, reverse primers and fluorogenic probe (18 μM each). The primers used for the W repeats and EBER1 were:
W Forward AGTGGGCTTGTTTGTGACTTCA
W Reverse GGACTCCTGGCGCTCTGAT
W probe 6-FAM-TTACGTAAGCCAGACAGCAGCCAATTGTC- TAMRA
EBER1 Forward ACCGAAGACGGCAGAAAGC
EBER1 Reverse CCTACGCTGCCCTAGAGGTTT
EBER1 probe 6-FAM-ACAGACACCGTCCTCACCACCCG-TAMRA
Commercial probes to CD20 (Hs00544818_m1), β-2 microglobulin (Hs00187842_m1) and β-actin (401846), were used as per manufacturer's (Applied Biosystems) instructions using the same cycling conditions as indicated above.
Immunohistochemistry
Immunohistochemistry on formalin-fixed, paraffin-embedded specimens was performed using 4-μm thick tissue sections. Briefly, slides were soaked in xylene, passed through graded alcohols, and then rehydrated in distilled water. Slides were then pre-treated with ready-to-use Dako Target Retrieval Solution (DAKO) in a steam pressure cooker (Decloaking Chamber, Biocare Medical), per manufacturer's instructions, followed by washing in distilled water. All further steps were performed at room temperature in a hydrated chamber. Slides were pre-treated with Peroxidase Block (DAKO) for 5 min to quench endogenous peroxidase activity. Murine monoclonal anti-human CD20 (DAKO, clone L26, ready-to-use), murine monoclonal anti-LMP-1 (Menarini, clone EBV CS1-4) or murine anti-EBNA2 (DAKO, clone PE2) was applied for 1 h (diluted in DAKO Antibody Diluent). Slides were washed in 50 mM Tris–Cl, pH 7.4, and detected with anti-mouse Envision+ kit (DAKO) per manufacturer's instructions. After further washing, immunoperoxidase staining was developed using a DAB chromogen (DAKO) and counterstained with hematoxylin. LMP-1 and EBNA2 staining was detected with the DAKO LSAB®2 system K5001.
Immunohistochemistry on frozen specimens was performed using 10-μm thick acetone-fixed, optimal cutting temperature compound (O.C.T.)-embedded tissue sections. The slides were soaked in –20°C acetone for 5 min and then left to air dry for 30 min at room temperature. All further steps were performed at room temperature in a hydrated chamber. Slides were pre-treated with Peroxidase Block (DAKO) for 5 min to quench endogenous peroxidase activity. Murine monoclonal anti-human CD20 (DAKO, clone L26) was applied in a ready-to-use form for 1 h. Slides were washed in 50 mM Tris–C1, pH 7.4, and detected with DAKO Mouse Envision kit (DAKO) as per manufacturer's instructions. After further washing, immunoperoxidase staining was developed using a 3,3′-Diaminobenzidine (DAB) chromogen (DAKO) and counterstained with haematoxylin.
EBER ISH
EBER ISH was performed using 4-μm thick formalin-fixed, paraffin-embedded tissue sections as described previously (Kutok et al., 2001). Briefly, slides were initially baked at 60°C for 30 min. Using a Ventana Discovery machine, slides were deparaffinized using EZ-prep solution (Ventana Medical Systems Inc.), fixed with 10% paraformaldehyde (PFA) (37°C for 20 min), digested with proteinase K (20 μg/ml; 37°C for 10 min; Roche Diagnostics) and then denatured (60°C for 2 min). EBER probe was then added to the slides (100 μl/slide; diluted 1:50 in RiboHybe Reagent (Ventana) and allowed to hybridize at 37°C for 6 h. Slides were washed twice with 1× saline-sodium citrate (SSC) at 47°C for 6 min, then once with 50 mM Tris–Cl, pH 7.4 for 5 min. Rabbit anti-fluorescein isothiocyanate (DAKO) diluted 1:200 in Peroxidase Block (DAKO) was then applied to each slide for 30 min at room temperature. Slides were then washed with 50 mM Tris–Cl, pH 7.4. The EBER probe was detected with anti-rabbit Envision+ kit (DAKO) as per manufacturer's instructions. After further washing, immunoperoxidase staining was developed using a DAB chromogen (DAKO) and counterstained with haematoxylin. On a subset of multiple sclerosis tissue sections, ISH was performed manually using the DAKO EBER-ISH probe Y5200 or a GAPDH probe (DAKO) and detection was performed using the DAKO detection system Y5201 as per manufacturer's instructions. A negative probe (DAKO) composed of random PNA probes was also used as per manufacturer's instructions.
Epstein–Barr virus (EBER) ISH was performed on formalin fixed-frozen specimens using 10-μm thick sections. Slides were immersed in 4°C methanol for 1 min and allowed to air dry for 30 min prior to hybridization. Using a Ventana Discovery machine, slides were digested with proteinase K (20 μg/ml; 37°C for 10 min; Roche Diagnostics) and then denatured (60°C for 2 min). EBV probe (Novocastra) was then added to the slides (100 μl/slide; diluted 1:4 in RiboHybe Reagent (Ventana) and allowed to hybridize at 37°C for 6 h. Slides were washed twice with 1× SSC at 47°C for 6 min, then once with 50 mM Tris–Cl, pH 7.4 for 5 min. Rabbit anti-fluorescein isothiocyanate (DAKO) diluted 1:200 in Peroxidase Block (DAKO) was then applied to each slide for 30 min at room temperature. Slides were then washed with 50 mM Tris–Cl, pH 7.4. The probe was detected with anti-rabbit Envision+ kit (DAKO) according to manufacturer's instructions. After further washing, immunoperoxidase staining was developed using a DAB chromogen (DAKO) and counterstained with haematoxylin.
Results
EBV was not detectable in multiple sclerosis lesions by ISH
To address whether EBV infection is a characteristic feature of multiple sclerosis brain, a total of 63 formalin-fixed multiple sclerosis tissue specimens (each containing white matter lesions) from 12 multiple sclerosis cases were initially examined for a CD20-positive B cell infiltrate, which allowed us to identify a subset of cases that had the potential to harbour EBV infection for further analysis. From this screen, a total of 23 specimens (including two spinal cord lesions) from 12 multiple sclerosis cases (adult and paediatric) were selected for further characterization (Supplementary Table S1; multiple sclerosis cohort 1). Luxol Fast Blue (LFB) staining confirmed the presence of white matter lesions within the multiple sclerosis tissue specimens chosen (Fig. 1A, D and G) and a prominent (Fig. 1B and E) or sparse (Fig. 1H) B cell infiltrate that was mainly in the perivascular space. However, all 23 multiple sclerosis tissue specimens were negative for the EBV transcript EBER (Fig. 1C, F, I and L) by ISH (Kutok et al., 2001), which is expressed at high levels during all phases of latent EBV infection. In contrast to the absence of EBER expression in these multiple sclerosis tissue specimens, EBER was readily detected (brown nuclear staining) in several control tissues including multiple CNS lymphomas (Fig. 1J and K, Supplementary Fig. S2A), a Hodgkin lymphoma isolated from lymph node (Supplementary Fig. S1A) and a post-transplant lymphoma (autopsy) isolated from lung (Supplementary Fig. S1B). Furthermore, ISH detected the house-keeping gene GAPDH in the majority of cells in both an EBV-positive CNS lymphoma and several multiple sclerosis lesions, confirming that ISH worked robustly on all formalin-fixed tissue specimens analysed (Supplementary Fig. S2). In addition to ISH, a subset of the multiple sclerosis specimens was also examined for the expression of several EBV latent proteins using immunohistochemistry. Consistent with our ISH data, all lesions were negative for LMP1, which is expressed during the growth and default programmes and also EBNA2, which is expressed during the growth programme (Supplementary Fig. S3). Conversely, both LMP1 and EBNA2 were readily detectable in a Hodgkin lymphoma (Supplementary Fig. S3B inset) and a post-transplant lymphoma (Supplementary Fig. S3C inset), respectively.
Figure 1.
ISH revealed EBER+ cells were not present in multiple sclerosis brain. LFB staining identified regions of considerable demyelination (plaques) in representative multiple sclerosis tissue specimens obtained from patients multiple sclerosis_1 (A and D) and _2 (G); blue = positive staining for myelin; pink = negative for myelin. Immunohistochemistry identified a prominent CD20+ B cell infiltrate (B and E) in adjacent sections within demyelinated areas (brown-positive staining for antigen) in some specimens and a sparse CD20+ B cell infiltrate in others (H). ISH revealed no EBER+ cells within demyelinated regions (C, F and I). EBER was readily detected (brown nuclear staining) in two EBV-positive CNS lymphomas (J and K). Figures A, D and G (100×); B, C, E and F (200X); H and I (400×); J, K and insets (1000×). (L) ISH revealed no EBER+ in any of the 23 multiple sclerosis specimens examined obtained from 12 autopsy cases, yet all specimens had a detectable CD20+ B cell infiltrate. Each dot represents the total number of positive cells counted in five 200× fields. The bar represents mean cell number observed over the 23 multiple sclerosis specimens examined.
EBV was not detectable in multiple sclerosis lesions by quantitative real-time PCR
The absence of detectable EBV as assessed by ISH and immunohistochemistry in our cohort of multiple sclerosis tissue specimens suggested that EBV infection was probably absent from multiple sclerosis brain. However, it remained possible that EBV infection was present at low levels that may have been missed through the examination of individual tissue sections using the above methodologies. To complement the above methodologies and to widen our search for EBV, we employed two highly sensitive real-time PCR methodologies to detect genomic EBV (BamHI-W repeat region) or the abundant, EBV-encoded RNA called EBER1. Using the EBV-positive lymphoblastoid cell line IB4, which contains approximately four integrated copies of the EBV genome (Hurley et al., 1991), we first demonstrated that our real-time PCR methodologies could detect genomic EBV (Fig. 2A) and EBER1 (Fig. 2B) in a single EBV-positive cell but not in the EBV-negative Jurkat T cell line (Supplementary Fig. S4). Importantly, the B cell marker CD20 could also be detected at the single-cell level in the EBV-positive B cell line (Fig. 2B) but not in the EBV-negative T cell line (Supplementary Fig. S4B). This specificity was confirmed further using snap-frozen tissue specimens where EBV was readily detectable in DNA or RNA isolated from an EBV-positive but not an EBV-negative post-transplant B cell lymphoma (Fig. 2C and D).
Figure 2.
Quantitative real-time PCR could detect genomic EBV and EBER1 in a single EBV infected cell. The EBV-positive lymphoblastoid (IB4) cell line was sorted (100 cells to 1 cell) into a 96-well plate and real-time PCR performed (in triplicate) as described in Methods section. Genomic EBV W repeats (A), EBER1 (B) and CD20 (B) were detectable in a single EBV-positive cell. Actin (A) and B2M (B) served as controls. Genomic EBV (C) and EBER1 (D) were also readily detectable in an EBV-positive post-transplant B cell lymphoma and not in an EBV-negative B cell lymphoma. Real-time PCR was performed for 50 cycles, and values shown indicate 50-Ct.
Having confirmed the sensitivity and specificity of our EBV detection assays, we then serially sectioned 17 snap-frozen multiple sclerosis specimens from five multiple sclerosis cases, each with a B cell infiltrate confirmed by immunohistochemistry, then isolated DNA or RNA from alternating sections for analysis (Fig. 3A). As shown in Fig. 3C, all multiple sclerosis specimens had a strong signal for the house-keeping gene β-2 microglobulin and a robust signal for CD20, confirming the presence of a B cell infiltrate in all specimens examined. However, consistent with our ISH data, genomic EBV (Fig. 3B) or EBER1 (Fig. 3C) were not detected. To confirm the sensitivity of our assays on frozen tissue specimens, we demonstrated that genomic EBV was readily detected in DNA isolated from as few as two sorted EBV-positive cells (IB4 cell line) that were processed in the presence of multiple sclerosis brain tissue (Fig. 3D) as was EBER1 when RNA isolated from one sorted EBV-positive cell was processed in a similar fashion (Fig. 3E).
Figure 3.
EBV was not detectable by quantitative real-time PCR in multiple sclerosis specimens with a confirmed B cell infiltrate. Multiple sclerosis specimens containing white matter lesions were serially sectioned for alternate DNA or RNA isolation (A; processed under same conditions used for post-transplant B cell lymphomas). Genomic EBV (B) or EBER1 (C) was not detectable by real-time PCR (performed in triplicate) in all multiple sclerosis specimens examined. All multiple sclerosis specimens contained a detectable CD20+ B cell infiltrate (C). Few EBV-positive cells (IB4) could be detected when processed with a multiple sclerosis tissue specimen for DNA (two cell sensitivity; D) or RNA (one cell sensitivity; E). Actin (B and D) and B2M (C and E) served as controls for real-time PCR. Each data point represents the mean value (50-Ct) over the four samples processed for DNA or RNA.
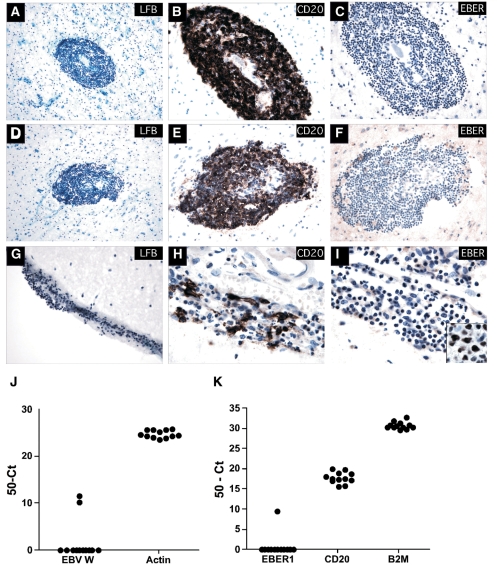
EBV was largely absent from a second cohort of multiple sclerosis specimens that included B cell infiltration within the meninges and parenchymal B cell aggregates
Although EBV appeared to be largely absent from multiple sclerosis specimens containing white matter lesions, it remained possible that EBV resides almost exclusively in the recently described ectopic B cell follicles, located near or in the meninges (Serafini et al., 2007) although this study reported that EBV was found in almost all multiple sclerosis specimens examined, regardless of whether follicles were present or not. To address this possibility, 12 multiple sclerosis specimens (one fixed-frozen and one matching snap-frozen block from each case) from the same tissue bank as those used in the Serafini study were examined for EBV infection (Supplementary Table S1; multiple sclerosis cohort 2). LFB staining (Fig. 4A, D and G), together with immunohistochemistry identified B cell aggregates in a subset of cases (3 of 12) within the brain parenchyma (Fig. 4B and E), with several cases (4 of 12) possessing a loose B cell infiltrate within the meninges (Fig. 4H). However, many specimens (7 of 12) contained a sparse parenchymal B cell infiltrate (not shown), with no B cell infiltration detectable within the meninges. Despite the variable B cell infiltrate observed in these specimens, EBV was not detectable in any of the cases examined by ISH (Fig. 4C, F and I), yet was readily detectable in multiple control tissues including a CNS lymphoma (Fig. 4I inset). Next, snap-frozen multiple sclerosis specimens from the same cases were sectioned (10 μm sections) and examined for EBV using our real-time PCR methodologies. Largely consistent with our ISH data, EBV was rare in these samples, being detected in only 2 of the 12 cases examined (Fig. 4J and K), suggesting that while EBV infection could occasionally be detected, it was not a characteristic feature of multiple sclerosis brain. It should be stressed, however, that while the identity of the EBV-derived products was confirmed by directly sequencing the real-time PCR products (not shown); the level of EBV infection in these samples was minimal. This was reflected by the low strength of EBER1 signal relative to CD20 and our observation that genomic EBV and EBER1 were detected in only one specimen, with genomic EBV detected in a single section of another case. Furthermore, our inability to detect EBV in these same cases by ISH, led us to conclude that it was likely that very few EBV-positive cells were present in these samples.
Figure 4.
EBV was detected in very few multiple sclerosis specimens containing B cell infiltration within the meninges and parenchymal B cell aggregates. Fixed-frozen tissue specimens were sectioned with minimal waste and stained with LFB, CD20 and EBER. LFB staining identified B cell aggregates in regions of considerable demyelination (A and D—Multiple sclerosis_UK10) (blue = positive staining for myelin; pink = negative for myelin). Dense CD20+ B cell aggregates are shown in the brain parenchyma in one case (B and E—Multiple sclerosis_UK10), with loose B cell infiltrates identified within the meninges (G) of several others (H and not shown), while most cases had a diffuse B cell infiltrate. ISH revealed no EBER+ in all 12 multiple sclerosis specimens examined (C, F and I and not shown). An EBV-positive CNS lymphoma (I inset) served as a positive control for EBER ISH. Snap-frozen multiple sclerosis tissue specimens from the same cases were serially sectioned (10 μm sections; see Methods section) for alternate DNA or RNA isolation. Genomic EBV (J) was detected in 2 of 12 multiple sclerosis samples examined while EBER1 (K) was detected in one sample with detectable genomic EBV. All samples had a detectable CD20-positive B cell infiltrate (K). Note the sample with detectable genomic EBV but not EBER1 only contained EBV within one of two tissue sections examined (value reported is the average seen within the positive section only). Figures A and D (200×); B, C, E, F and G (400×); H, I and inset (1000×).
Discussion
After decades of research, a large body of evidence has emerged to support a role for EBV infection in multiple sclerosis disease development. However, precisely how EBV may contribute to multiple sclerosis pathology remains unknown. Early studies using ISH to detect EBV infection in multiple sclerosis brain yielded negative (Hilton et al., 1994) or inconclusive (Opsahl and Kennedy, 2007) results. However, recent work has challenged these findings (Serafini et al., 2007), citing that a failure to detect EBV in multiple sclerosis brain may have resulted from poor tissue preservation, analysis of multiple sclerosis material without relevant inflammatory infiltrates containing B cells or methodological differences. In their study, Serafini and colleagues (2007) reported that the EBV-associated RNA EBER and EBV protein products were, in fact, present in a large percentage of infiltrating B lymphocytes (40%–90%) in >95% of multiple sclerosis brain cases examined, yet were absent from other cases of inflammatory neurological disease. Furthermore, the authors suggested that EBV infection may be a prerequisite for multiple sclerosis and that the dysregulated EBV infection observed in multiple sclerosis patients may result in a chronic inflammatory state that triggers disease. If true, these results would have significant therapeutic implications for multiple sclerosis as they suggest that vaccination against EBV or regulation of the existing infection may prevent or eliminate disease.
Given the potential therapeutic importance of EBV infection in the CNS and the conflicting reports over its presence in multiple sclerosis brain, we investigated whether EBV infection was a characteristic feature of multiple sclerosis brain using multiple methodologies including ISH, immunohistochemistry and two highly sensitive PCR methodologies that can detect a very low number of EBV-infected B cells. Initially, we characterized the immune cell infiltrate in a large number of multiple sclerosis specimens in order to select a cohort of multiple sclerosis lesions that had a heterogeneous B cell infiltrate and thus had the potential to harbour EBV. From this analysis, a total of 23 multiple sclerosis tissue specimens from 12 autopsy cases were selected from both adult and paediatric multiple sclerosis cases for further analysis. However, while all multiple sclerosis specimens containing white matter lesions examined possessed a heterogeneous B cell infiltrate (Fig. 1B, E, H and L), EBV was not detectable by ISH (Fig. 1). These results were supported by the absence of EBV protein expression in a subset of specimens examined by immunohistochemistry (LMP1 and EBNA2; Supplementary Fig. S3). In addition, it should be stressed that the absence of EBV in the multiple sclerosis tissue specimens examined is unlikely to reflect sample size as Serafini et al. (2007) reported that EBV infection was present in >95% of multiple sclerosis cases examined.
Our inability to detect EBV by ISH or immunohistochemistry indicated that EBV infection in these samples was most likely absent or alternatively, present at low levels that may have been missed using the above technologies on single sections of multiple sclerosis lesions. Consequently, we employed two highly sensitive real-time PCR methodologies for the detection of EBV in multiple sclerosis specimens from multiple cases that were shown to have a B cell infiltrate. Two independent quantitative real-time PCR protocols were used for the detection of genomic EBV or EBER1, with the latter expressed at high levels in cells latently infected with EBV. Together with EBER ISH, these approaches are the ‘gold standard’ for EBV detection. However, these real-time PCR methodologies have the added advantage that the PCR products can be sequenced to provide definitive evidence for EBV infection. Seventeen multiple sclerosis lesions from five autopsy cases were extensively sectioned for DNA or RNA isolation (Fig. 3). However, consistent with our ISH results, no EBV was detected, despite the fact that all lesions harboured a B cell infiltrate (Fig. 3) and that our assays were extremely sensitive, in that as few as two EBV-positive cells provided a positive signal (Figs 2 and 3).
In the recent study by Serafini and colleagues (2007), it was reported that ectopic B cell follicles within the meninges were the main site of EBV persistence, although EBV was found within almost all multiple sclerosis specimens with relevant inflammatory material, whether follicles were present or not. Our observation that EBV was not detectable in our cohort of multiple sclerosis white matter lesions that harboured a B cell infiltrate led us to assess whether EBV infection was restricted to multiple sclerosis tissue specimens containing B cell infiltration within the meninges and parenchyma (Fig. 4). To address this, we examined 12 multiple sclerosis specimens (12 fixed-frozen and 12 snap-frozen blocks), which were obtained from the same tissue bank as those used in the Serafini study where the EBV positivity rate was reported to be >95%. These specimens represented adjacent tissue blocks from some (not all) of the same cases. Consistent with this previous study, large B cell aggregates were identified in a subset of cases predominantly within the brain parenchyma, with loose B cell infiltrates found in the meninges in a subset of cases (Fig. 4). In contrast to the prior study (Serafini et al., 2007), EBV was not detectable by ISH in all cases examined while EBV was detectable in our positive control (Fig. 4I inset). Consistent with our ISH data, EBV was absent in 10 of the 12 matching frozen cases examined by real-time PCR. Genomic EBV and EBER1 were detected in only one case. In the other case, only genomic EBV was detected in one section, despite the fact that B cells were detected in both sections examined. These combined data demonstrate that while we could, as expected, occasionally detect the low levels of EBV signals in human tissue, EBV was largely absent in multiple sclerosis brain.
Based on the seroepidemiological evidence obtained over several decades, a growing number of studies have shown an association between EBV and multiple sclerosis. These findings include but are not limited to; the higher incidence of EBV infection in both adult and paediatric multiple sclerosis cases relative to controls (Bray et al., 1983; Sumaya et al., 1985; Wandinger et al., 2000; Alotaibi et al., 2004; Haahr and Hollsberg, 2006; Pohl et al., 2006; Ascherio and Munger, 2007); and the increased numbers of EBV reactive CD8 (Hollsberg et al., 2003; Cepok et al., 2005; Lunemann et al., 2006; Jilek et al., 2008) and CD4 T cells (Lunemann et al., 2006, 2008) in the periphery of multiple sclerosis patients. However, in the absence of increased viral levels in the serum of multiple sclerosis patients (Wagner et al., 2004; Lunemann et al., 2006, 2007), or significant infection levels within the CSF of multiple sclerosis patients (Alvarez-Lafuente et al., 2008), and our finding that EBV was largely absent from multiple sclerosis brain, the contribution EBV may make to multiple sclerosis development remains unknown.
While the seroepidemiology studies have demonstrated a clear association between EBV infection and multiple sclerosis, it must be stressed that caution should be taken in interpreting these results in terms of a causal relationship. This is because it remains possible that the observed association is a consequence of host factors that predispose individuals to both multiple sclerosis and infection with certain viruses such as EBV (Niller et al., 2008). Furthermore, many studies have reported increased antibody titres to a range of pathogens in multiple sclerosis patients including EBV, measles virus (Panelius et al., 1971; Shirodaria et al., 1987), rubella virus (Shirodaria et al., 1987) and Chlamydia pneumoniae (Sriram et al., 1999) as evidence for potential involvement in disease development. However, the involvement of many of these pathogens has been called into question, as vaccination against measles, mumps and rubella has not altered the incidence of multiple sclerosis.
In addition to its association with multiple sclerosis, epidemiological studies also support an association between EBV infection and systemic lupus erythematosus (James et al., 2006). Furthermore, EBV infection has also been implicated in a number of other autoimmune conditions including, but not limited to, rheumatoid arthritis (RA), Sjogrens syndrome and autoimmune thyroiditis (reviewed in Niller et al., 2008). Not surprisingly, the implicated diseases are thought to include a B cell-mediated role in their immunopathology and like the situation in multiple sclerosis, the per cent of these patients that have been infected by EBV exceeds that of the general population. However, no direct role for EBV infection in the immunopathology in any autoimmune disease has been demonstrated. The suspected causal role of autoimmunity by EBV presumably began when antibodies directed toward the virus were shown to be elevated in patients with systemic lupus erythematosus (Evans et al., 1971). Since then a number of autoantibodies reactive with both auto-antigens and EBV components, arising presumably through molecular mimicry, have been found in RA and systemic lupus erythematosus and multiple sclerosis (Niller et al., 2008). These and other findings have continued to support the notion of an EBV-associated causative role in autoimmunity. An example of direct evidence for such an autoimmune disease-propagating event would show that pre-existing auto-reactive B cells become immortalized through EBV infection and are protected against mechanisms of peripheral tolerance such as anergy and thus go on to produce disease-associated autoantibodies. It is this mechanism that has been proposed to play a role in the immunopathology of RA (Pender, 2003). Investigation into the presence of EBV in the RA synovium, the site in which immune-mediated pathogenesis is observed, indicated EBER RNA was detectable in 8 of 34 specimens examined (Takei et al., 1997). Subsequent studies failed to confirm these findings (Alspaugh et al., 1983; Fox et al., 1986), indicating that a causal role for EBV infection in the pathology of RA is indirect if present at all. Our investigation into the role EBV plays at the site of injury in multiple sclerosis, the CNS, has led us to the same conclusion.
In summary, despite an exhaustive search using multiple methodologies we have shown that EBV appears largely absent from multiple sclerosis brain. While our findings do not exclude the notion that EBV may contribute to multiple sclerosis via an indirect effect on immune function or through molecular mimicry between EBV and CNS antigens, our results lead us to conclude that EBV infection is unlikely to contribute directly to multiple sclerosis immunopathology in the vast majority of cases.
Supplementary material
Supplementary material is available at Brain online.
Funding
US National Institutes of Health (U01DK6192601, R01NS024247, P01AI39671 and P01NS38037 to D.A.H.); National Multiple Sclerosis Society (RG2172C9 and RG3308A10 to D.A.H.); a Career Transition Fellowship from the National Multiple Sclerosis Society (TA 3000A to K.C.O.); National Health and Medical Research Council of Australia CJ Martin Biomedical Research Fellowship (to S.N.W); and the 6th Framework of the European Union, NeuroproMiSe (LSHM-CT-2005-018637 to W.B. and C.S.).
Supplementary Material
Acknowledgements
We sincerely thank Professor David Thorley-Lawson (Department of Pathology, Sackler School of Biomedical Sciences, Tufts University, Boston, MA 02111, USA) for discussions regarding EBV detection and for kindly providing the EBV-positive lymphoblastoid cell line (IB4) for assay optimization. We thank the C.S. Kubik Laboratory for Neuropathology at Massachusetts General Hospital for provision of tissue samples. We also sincerely thank Professor Frederick Wang (Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA) for helpful discussions.
Glossary
Abbreviations
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EBER
EBV encoded RNA
- EBNA1
EBV nuclear antigen 1
- EBV
Epstein–Barr virus
- ISH
in situ hybridization
- LMP
latent membrane protein
References
- Alotaibi S, Kennedy J, Tellier R, Stephens D, Banwell B. Epstein-Barr virus in pediatric multiple sclerosis. JAMA. 2004;291:1875–9. doi: 10.1001/jama.291.15.1875. [DOI] [PubMed] [Google Scholar]
- Alspaugh MA, Shoji H, Nonoyama M. A search for rheumatoid arthritis-associated nuclear antigen and Epstein-Barr virus specific antigens or genomes in tissues and cells from patients with rheumatoid arthritis. Arthritis Rheum. 1983;26:712–20. doi: 10.1002/art.1780260603. [DOI] [PubMed] [Google Scholar]
- Alvarez-Lafuente R, Garcia-Montojo M, De Las Heras V, Dominguez-Mozo M, Bartolome M, Benito-Martin M, et al. Herpesviruses and human endogenous retroviral sequences in the cerebrospinal fluid of multiple sclerosis patients. Mult Scler. 2008;14:595–601. doi: 10.1177/1352458507086425. [DOI] [PubMed] [Google Scholar]
- Anagnostopoulos I, Hummel M, Stein H. Frequent presence of latent Epstein-Barr virus infection in peripheral T cell lymphomas. A review. Leuk Lymphoma. 1995;19:1–12. doi: 10.3109/10428199509059657. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–99. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- Ascherio A, Munger KL. Epidemiology of multiple sclerosis: from risk factors to prevention. Semin Neurol. 2008;28:17–28. doi: 10.1055/s-2007-1019126. [DOI] [PubMed] [Google Scholar]
- Bray PF, Bloomer LC, Salmon VC, Bagley MH, Larsen PD. Epstein-Barr virus infection and antibody synthesis in patients with multiple sclerosis. Arch Neurol. 1983;40:406–8. doi: 10.1001/archneur.1983.04050070036006. [DOI] [PubMed] [Google Scholar]
- Bray PF, Luka J, Culp KW, Schlight JP. Antibodies against Epstein-Barr nuclear antigen (EBNA) in multiple sclerosis CSF, and two pentapeptide sequence identities between EBNA and myelin basic protein. Neurology. 1992;42:1798–804. doi: 10.1212/wnl.42.9.1798. [DOI] [PubMed] [Google Scholar]
- Callan MF, Tan L, Annels N, Ogg GS, Wilson JD, O'Callaghan CA, et al. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J Exp Med. 1998;187:1395–402. doi: 10.1084/jem.187.9.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepok S, Zhou D, Srivastava R, Nessler S, Stei S, Bussow K, et al. Identification of Epstein-Barr virus proteins as putative targets of the immune response in multiple sclerosis. J Clin Invest. 2005;115:1352–60. doi: 10.1172/JCI23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jager PL, Simon KC, Munger KL, Rioux JD, Hafler DA, Ascherio A. Integrating risk factors: HLA-DRB1*1501 and Epstein-Barr virus in multiple sclerosis. Neurology. 2008;70:1113–8. doi: 10.1212/01.wnl.0000294325.63006.f8. [DOI] [PubMed] [Google Scholar]
- DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Arch Neurol. 2006;63:839–44. doi: 10.1001/archneur.63.6.noc50328. [DOI] [PubMed] [Google Scholar]
- Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–3. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- Evans AS, Rothfield NF, Niederman JC. Raised antibody titres to E.B. virus in systemic lupus erythematosus. Lancet. 1971;1:167–8. doi: 10.1016/s0140-6736(71)91937-4. [DOI] [PubMed] [Google Scholar]
- Fox RI, Chilton T, Rhodes G, Vaughan JH. Lack of reactivity of rheumatoid arthritis synovial membrane DNA with cloned Epstein Barr virus DNA probes. J Immunol. 1986;137:498–501. [PubMed] [Google Scholar]
- Giovannoni G, Cutter GR, Lunemann J, Martin R, Munz C, Sriram S, et al. Infectious causes of multiple sclerosis. Lancet Neurol. 2006;5:887–94. doi: 10.1016/S1474-4422(06)70577-4. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Rowe M, Rickinson AB. Different Epstein-Barr virus-B cell interactions in phenotypically distinct clones of a Burkitt's lymphoma cell line. J Gen Virol. 1990;71(Pt 7):1481–95. doi: 10.1099/0022-1317-71-7-1481. [DOI] [PubMed] [Google Scholar]
- Haahr S, Hollsberg P. Multiple sclerosis is linked to Epstein-Barr virus infection. Rev Med Virol. 2006;16:297–310. doi: 10.1002/rmv.503. [DOI] [PubMed] [Google Scholar]
- Hafler DA. Multiple sclerosis. J Clin Invest. 2004;113:788–94. doi: 10.1172/JCI21357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton DA, Love S, Fletcher A, Pringle JH. Absence of Epstein-Barr virus RNA in multiple sclerosis as assessed by in situ hybridisation. J Neurol Neurosurg Psychiatry. 1994;57:975–6. doi: 10.1136/jnnp.57.8.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg D, Souza T, Catalina M, Sullivan JL, Luzuriaga K, Thorley-Lawson DA. Acute infection with Epstein-Barr virus targets and overwhelms the peripheral memory B-cell compartment with resting, latently infected cells. J Virol. 2004;78:5194–204. doi: 10.1128/JVI.78.10.5194-5204.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollsberg P, Hansen HJ, Haahr S. Altered CD8+ T cell responses to selected Epstein-Barr virus immunodominant epitopes in patients with multiple sclerosis. Clin Exp Immunol. 2003;132:137–43. doi: 10.1046/j.1365-2249.2003.02114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley EA, Klaman LD, Agger S, Lawrence JB, Thorley-Lawson DA. The prototypical Epstein-Barr virus-transformed lymphoblastoid cell line IB4 is an unusual variant containing integrated but no episomal viral DNA. J Virol. 1991;65:3958–63. doi: 10.1128/jvi.65.7.3958-3963.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JA, Harley JB, Scofield RH. Epstein-Barr virus and systemic lupus erythematosus. Curr Opin Rheumatol. 2006;18:462–7. doi: 10.1097/01.bor.0000240355.37927.94. [DOI] [PubMed] [Google Scholar]
- Jilek S, Schluep M, Meylan P, Vingerhoets F, Guignard L, Monney A, et al. Strong EBV-specific CD8+ T-cell response in patients with early multiple sclerosis. Brain. 2008;131:1712–21. doi: 10.1093/brain/awn108. [DOI] [PubMed] [Google Scholar]
- Kutok JL, Pinkus GS, Dorfman DM, Fletcher CD. Inflammatory pseudotumor of lymph node and spleen: an entity biologically distinct from inflammatory myofibroblastic tumor. Hum Pathol. 2001;32:1382–7. doi: 10.1053/hupa.2001.29679. [DOI] [PubMed] [Google Scholar]
- Leder P. The state of and prospects for molecular genetics in Burkitt's lymphoma. IARC Sci Publ. 1985;60:475–6. [PubMed] [Google Scholar]
- Levin LI, Munger KL, Rubertone MV, Peck CA, Lennette ET, Spiegelman D, et al. Temporal relationship between elevation of Epstein-Barr virus antibody titers and initial onset of neurological symptoms in multiple sclerosis. JAMA. 2005;293:2496–500. doi: 10.1001/jama.293.20.2496. [DOI] [PubMed] [Google Scholar]
- Lindberg C, Andersen O, Vahlne A, Dalton M, Runmarker B. Epidemiological investigation of the association between infectious mononucleosis and multiple sclerosis. Neuroepidemiology. 1991;10:62–5. doi: 10.1159/000110248. [DOI] [PubMed] [Google Scholar]
- Lunemann JD, Edwards N, Muraro PA, Hayashi S, Cohen JI, Munz C, et al. Increased frequency and broadened specificity of latent EBV nuclear antigen-1-specific T cells in multiple sclerosis. Brain. 2006;129:1493–506. doi: 10.1093/brain/awl067. [DOI] [PubMed] [Google Scholar]
- Lunemann JD, Jelcic I, Roberts S, Lutterotti A, Tackenberg B, Martin R, et al. EBNA1-specific T cells from patients with multiple sclerosis cross react with myelin antigens and co-produce IFN-gamma and IL-2. J Exp Med. 2008;205:1763–73. doi: 10.1084/jem.20072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunemann JD, Kamradt T, Martin R, Munz C. Epstein-Barr virus: environmental trigger of multiple sclerosis? J Virol. 2007;81:6777–84. doi: 10.1128/JVI.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir CS. Cancer of the head, & neck. Nasopharyngeal cancer. Epidemiology and etiology. JAMA. 1972;220:393–4. doi: 10.1001/jama.220.3.393. [DOI] [PubMed] [Google Scholar]
- Niedobitek G. The Epstein-Barr virus: a group 1 carcinogen? Virchows Arch. 1999;435:79–86. doi: 10.1007/s004280050402. [DOI] [PubMed] [Google Scholar]
- Niller HH, Wolf H, Minarovits J. Regulation and dysregulation of Epstein-Barr virus latency: implications for the development of autoimmune diseases. Autoimmunity. 2008;41:298–328. doi: 10.1080/08916930802024772. [DOI] [PubMed] [Google Scholar]
- Operskalski EA, Visscher BR, Malmgren RM, Detels R. A case-control study of multiple sclerosis. Neurology. 1989;39:825–9. doi: 10.1212/wnl.39.6.825. [DOI] [PubMed] [Google Scholar]
- Opsahl ML, Kennedy PG. An attempt to investigate the presence of Epstein Barr virus in multiple sclerosis and normal control brain tissue. J Neurol. 2007;254:425–30. doi: 10.1007/s00415-006-0316-7. [DOI] [PubMed] [Google Scholar]
- Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- Panelius M, Salmi AA, Halonen P, Penttinen K. Measles antibodies detected with various techniques in sera of patients with multiple sclerosis. Acta Neurol Scand. 1971;47:315–30. doi: 10.1111/j.1600-0404.1971.tb07486.x. [DOI] [PubMed] [Google Scholar]
- Pender MP. Infection of autoreactive B lymphocytes with EBV, causing chronic autoimmune diseases. Trends Immunol. 2003;24:584–8. doi: 10.1016/j.it.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Pohl D. Epstein-Barr virus and multiple sclerosis. J Neurol Sci. doi: 10.1016/j.jns.2009.03.028. (in press) [DOI] [PubMed] [Google Scholar]
- Pohl D, Krone B, Rostasy K, Kahler E, Brunner E, Lehnert M, et al. High seroprevalence of Epstein-Barr virus in children with multiple sclerosis. Neurology. 2006;67:2063–5. doi: 10.1212/01.wnl.0000247665.94088.8d. [DOI] [PubMed] [Google Scholar]
- Rand KH, Houck H, Denslow ND, Heilman KM. Epstein-Barr virus nuclear antigen-1 (EBNA-1) associated oligoclonal bands in patients with multiple sclerosis. J Neurol Sci. 2000;173:32–9. doi: 10.1016/s0022-510x(99)00298-1. [DOI] [PubMed] [Google Scholar]
- Salvetti M, Giovannoni G, Aloisi F. Epstein-Barr virus and multiple sclerosis. Curr Opin Neurol. 2009;22:201–6. doi: 10.1097/WCO.0b013e32832b4c8d. [DOI] [PubMed] [Google Scholar]
- Serafini B, Rosicarelli B, Franciotta D, Magliozzi R, Reynolds R, Cinque P, et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J Exp Med. 2007;204:2899–912. doi: 10.1084/jem.20071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirodaria PV, Haire M, Fleming E, Merrett JD, Hawkins SA, Roberts SD. Viral antibody titers. Comparison in patients with multiple sclerosis and rheumatoid arthritis. Arch Neurol. 1987;44:1237–41. doi: 10.1001/archneur.1987.00520240019006. [DOI] [PubMed] [Google Scholar]
- Sriram S, Stratton CW, Yao S, Tharp A, Ding L, Bannan JD, et al. Chlamydia pneumoniae infection of the central nervous system in multiple sclerosis. Ann Neurol. 1999;46:6–14. [PubMed] [Google Scholar]
- Sumaya CV, Myers LW, Ellison GW, Ench Y. Increased prevalence and titer of Epstein-Barr virus antibodies in patients with multiple sclerosis. Ann Neurol. 1985;17:371–7. doi: 10.1002/ana.410170412. [DOI] [PubMed] [Google Scholar]
- Takei M, Mitamura K, Fujiwara S, Horie T, Ryu J, Osaka S, et al. Detection of Epstein-Barr virus-encoded small RNA 1 and latent membrane protein 1 in synovial lining cells from rheumatoid arthritis patients. Int Immunol. 1997;9:739–43. doi: 10.1093/intimm/9.5.739. [DOI] [PubMed] [Google Scholar]
- Thacker EL, Mirzaei F, Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol. 2006;59:499–503. doi: 10.1002/ana.20820. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol. 2001;1:75–82. doi: 10.1038/35095584. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–37. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- Wagner HJ, Munger KL, Ascherio A. Plasma viral load of Epstein-Barr virus and risk of multiple sclerosis. Eur J Neurol. 2004;11:833–4. doi: 10.1111/j.1468-1331.2004.00871.x. [DOI] [PubMed] [Google Scholar]
- Wandinger K, Jabs W, Siekhaus A, Bubel S, Trillenberg P, Wagner H, et al. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology. 2000;55:178–84. doi: 10.1212/wnl.55.2.178. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig KW, Strominger JL. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell. 1995;80:695–705. doi: 10.1016/0092-8674(95)90348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.