Abstract
Spinal and bulbar muscular atrophy is an X-linked motor neuron disease caused by a CAG repeat expansion in the androgen receptor gene. To characterize the natural history and define outcome measures for clinical trials, we assessed the clinical history, laboratory findings and muscle strength and function in 57 patients with genetically confirmed disease. We also administered self-assessment questionnaires for activities of daily living, quality of life and erectile function. We found an average delay of over 5 years from onset of weakness to diagnosis. Muscle strength and function correlated directly with serum testosterone levels and inversely with CAG repeat length, age and duration of weakness. Motor unit number estimation was decreased by about half compared to healthy controls. Sensory nerve action potentials were reduced in nearly all subjects. Quantitative muscle assessment and timed 2 min walk may be useful as meaningful indicators of disease status. The direct correlation of testosterone levels with muscle strength indicates that androgens may have a positive effect on muscle function in spinal and bulbar muscular atrophy patients, in addition to the toxic effects described in animal models.
Keywords: Kennedy disease, spinal and bulbar muscular atrophy, motor neuron disease, androgens
Introduction
Spinal and bulbar muscular atrophy (SBMA, Kennedy's disease) is an X-linked, adult onset motor neuron disease characterized by slowly progressive weakness of the bulbar and extremity muscles. SBMA patients may become wheelchair dependent 20–30 years after onset (Tsukagoshi et al., 1965; Kennedy et al., 1968). Involvement of bulbar muscles may lead to dysarthria and dysphagia (Ferrante et al., 1997). Fasciculations often occur, particularly around the mouth and in the tongue (Ferrante et al., 1997). Affected individuals frequently have muscle cramps and tremor. Other common neurological features include decreased or absent deep tendon reflexes (Antonini et al., 2000) and sensory loss (Ferrante et al., 1997; Antonini et al., 2000). SBMA patients also often have signs of androgen insensitivity, such as gynecomastia and reduced fertility (Dejager et al., 2002).
SBMA is a member of the family of CAG-polyglutamine expansion diseases that includes Huntington's disease and seven spinocerebellar ataxias (Lieberman et al., 2000). Previous studies of SBMA and the other polyglutamine diseases have shown that the length of the CAG expansion correlates inversely with age of disease onset, i.e. the longer the expansion the earlier the onset (La Spada et al., 1992). In SBMA, CAG repeat length has also been reported to correlate with motor and sensory nerve conduction abnormalities (Suzuki et al., 2008).
The SBMA repeat expansion is in the first exon of the androgen receptor gene (La Spada et al., 1991). The androgen receptor is a nuclear receptor that normally regulates gene expression after ligand binding. The primary androgen receptor ligands are testosterone and dihydrotestosterone. Studies in animal models and patients indicate that these ligands are important for the development of the disease. This evidence includes the following observations: (i) male transgenic mice expressing the mutant androgen receptor have a neuromuscular deficit resembling SBMA and females are much less affected; (ii) when the male mice are castrated (Chevalier-Larsen et al., 2004) or treated with the anti-androgen leuprorelin (Katsuno et al., 2002, 2003), the phenotype is improved, and when female transgenic mice are given testosterone, the SBMA phenotype becomes fully manifested; (iii) in humans, heterozygous female carriers of the disease gene are generally asymptomatic, and even homozygous females in one reported family had only mild symptoms (Sobue et al., 1993; Schmidt et al., 2002). Together, this evidence supports the hypothesis that SBMA disease manifestations are primarily due to a ligand-dependent toxic gain of function in the mutant androgen receptor.
While the clinical features of SBMA are fairly well known, little natural history data are available to inform the selection of sample size, outcome measures, and biomarkers for SBMA intervention trials (Katsuno et al., 2006). Additional retrospective data would help to define the natural history of SBMA and guide the design of future clinical trials. Here we present an analysis of retrospective and cross-sectional data collected from 57 patients with SBMA.
Patients and methods
Patients and clinical evaluations
Subjects were recruited to the National Institutes of Health (NIH) Clinical Research Center in Bethesda, MD for the purpose of participating in a trial to assess the efficacy and safety of the 5-alpha reductase inhibitor dutasteride in SBMA. An NIH Institutional Review Board approved the protocol, and the study was conducted, and informed consent obtained, in accordance with the principles of the Declaration of Helsinki. Subject demographics are summarized in Table 1. Fifty-four subjects were self-identified as European-American, two as Asian-American and one as African-American. The relative lack of ethnic minorities may be due in part to founder effects in SBMA (Tanaka et al., 1996; Lund et al., 2000) and in part to recruitment bias. All subjects were clinically and genetically diagnosed with SBMA before entrance into the study, and genetic confirmation with repeat length was obtained for each. At the initial screening visit the subjects underwent a series of tests to characterize their disease status. Additional natural history information was obtained by a mailed questionnaire.
Table 1.
Demographics of SBMA patients
| Mean ± SD (range) | |
|---|---|
| Disease milestones (years) | |
| Age at first muscle weakness | 41 ± 10 (18–64) |
| Age at first presentation for medical care | 44 ± 9 (22–64) |
| Age at clinical diagnosis | 47 ± 9 (29–66) |
| Age at genetic diagnosis | 47 ± 10 (29–75) |
| Age at evaluation for study | 53 ± 10 (37–79) |
| Mean intervals between milestones (years) | |
| First muscle weakness to first medical attention | 2.2 |
| First muscle weakness to clinical diagnosis | 5.5 |
| First muscle weakness to genetic diagnosis | 5.9 |
| First muscle weakness to evaluation for study | 11.8 |
| First medical attention to clinical diagnosis | 3.1 |
| First medical attention to genetic diagnosis | 3.5 |
| Self-assessed activity level | |
| Activity level before disease onset (1–5, 5 high) | 4.6 ± 0.6 (3–5) |
| Current activity level (1–5, 5 high) | 2.5 ± 1.1 (1–5) |
| Genetic | |
| CAG repeat length (number) | 46.7 ± 2.5 (41–53) |
| Family history | |
| Positive | 34 |
| Negative | 16 |
Fifty-seven patients were genotyped, and 45–50 provided information for each of the other demographic variables.
Strength and functional testing
Quantitative muscle assessment (QMA), a measure of maximal voluntary isometric muscle contraction, was done with a fixed frame dynamometer (strain gauge tensiometer) and computer-aided data acquisition (The Computer Source, Gainesville, GA). The mean of three efforts for each muscle group was recorded (Andres et al., 1996; Mathieu et al., 2003). Normative values were obtained from a group of 10 healthy men matched with the SBMA subjects for age and body mass index. In the healthy volunteers, we found inter- and intra-rater reliability of the QMA testing protocol used in this study to be very good, with interclass and intraclass correction coefficients of 0.95 and 0.93, respectively.
For the timed walk, we used the 2 min walk test, since it is more feasible than longer tests (Brooks et al., 2007), particularly for repetitive testing in subjects with ambulatory difficulty (Light et al., 1997), and it can be performed in patients with and without assistive devices (Rossier et al., 2001). As previously reported, the timed 2 min walk test has high intra- and inter-rater reliability (interclass and intraclass correction coefficients of 0.97 and 0.98, respectively) (Rossier et al., 2001; Miller et al., 2002). As described by Light et al. (1997), subjects walked back and forth a distance of 50 ft for 2 min. The test was repeated three times with an opportunity for the subjects to rest between trials. The mean distance of the three trials and use of optional assistive devices were recorded (Table 4).
Table 4.
SBMA patient muscle strength and function
| SBMA mean ± SD (range) | SBMA percent of healthy control (%) | |
|---|---|---|
| QMA (kg) | ||
| Shoulder abduction | 8 ± 3 (0–16) | 41 |
| Elbow extension | 7 ± 4 (0–19) | 38 |
| Elbow flexion | 12 ± 6 (1–32) | 48 |
| Wrist flexion | 12 ± 5 (3–26) | 54 |
| Hand grip | 22 ± 10 (6–52) | 53 |
| Pinch | 5 ± 2 (2–12) | 48 |
| Upper extremity composite [137 ± 26 (95–186)a]** | 66 ± 25 (18–140) | 48 |
| Total upper extremity weight scaled (kg/kg) [1.7 ± 0.3 (1.2–2.2)a]** | 0.8 ± 0.3 (0.1–1.5) | 47 |
| Hip abduction | 16 ± 6 (3–30) | 57 |
| Hip extension | 38 ± 16 (9–93) | 61 |
| Hip flexion | 14 ± 6 (4-33) | 70 |
| Knee extension | 17 ± 11 (3–50) | 40 |
| Ankle dorsiflexion | 13 ± 7 (0–35) | 63 |
| Lower extremity composite [176 ± 46 (108–259)a]** | 98 ± 41 (28–231) | 56 |
| Total lower extremity weight scaled (kg/kg) [2.1 ± 0.5 (1.2–2.9)a]** | 1.1 ± 0.5 (0.3–2.4) | 52 |
| Total force [313 ± 70 (217–445)a]** | 164 ± 63 (63–372) | 52 |
| Total force weight scaled (kg/kg) [3.8 ± 0.8 (2.4–5.0)a]** | 1.9 ± 0.8 (0.7–3.9) | 50 |
| Timed 2 min walk (m) | ||
| Ambulatory with an assistive device | 66 ± 23 (30–104) | 38 |
| Ambulatory without assistive device | 136 ± 44 (15–208) | 77 |
| All timed walks [176 (130–237)b] | 109 ± 50 (15–208) | 62 |
The 56 SBMA patients assessed by QMA and timed 2 min walk had age and body mass index means of 53 ± 10 years and 28 ± 5 kg/m2, respectively. QMA values shown for the right side only. For the timed 2 min walk, use of an assistive device was optional, and 22 of the 56 subjects chose to do so. The timed walk values are the average of three trials for each subject. Composite and total scores are shown in bold.
Mean ± SD (range) for 10 healthy male volunteers with a mean age of 51 ± 6 years and body mass index of 26 ± 4 kg/m2.
Normative data reported for a 12 person cohort of men and women with a mean age of 68 ± 10 years (Light et al., 1997).
**P < 0.0001 for comparison of SBMA and healthy controls.
Neurophysiological studies
Fifty-four SBMA subjects underwent nerve conduction studies and motor unit number estimation (MUNE). Nerve conduction studies were done using standard methodology and reported according to lab normative values (Liveson and Ma, 1992). Four sensory nerves (median, ulnar, radial and sural nerves) and two motor nerves (median and peroneal nerves) were studied. Only the right median and peroneal compound motor action potential (CMAP) values and right average sensory nerve action potential (SNAP) values were used for the correlation and regression analyses.
MUNE was performed on a Nicolet Viking Select electromyography machine using the statistical MUNE program. The MUNE was modified by excluding small motor unit potentials under 40 µV (Shefner et al., 2004) to account for greater motor unit instability noted in SBMA (Lehky et al., 2009). For the SBMA subjects, the MUNE was evaluated in the right abductor pollicis brevis (n = 48); if the right abductor pollicis brevis was very atrophic and had a very low CMAP, the left abductor pollicis brevis was also evaluated (n = 4). Fourteen healthy male volunteers for the MUNE were recruited through the Patient Recruitment and Public Liaison Office at the NIH, with informed consent, and had a similar age distribution to the SBMA subjects (55 ± 8 years). In these healthy subjects, the right and left abductor pollicis brevis MUNEs were evaluated, although abnormal median CMAPs, defined as CMAP <4.5 mV or distal latency >4.5 ms were excluded.
Self-assessment testing
Subjects completed a nine question assessment of ability to perform specific activities of daily living (ADL). The ADL assessment was modified from the ADL subscale in the Friedreich's Ataxia Rating Scale (Subramony et al., 2005) by replacing the question about bladder function with one about handwriting (0 = normal; 1 = slightly slow or small, all words are legible; 2 = moderately slow or small, all words are legible; 3 = severely affected, not all words are legible; 4 = the majority of words are not legible). Subjects rated each item from 0 (normal) to 4 (fully impaired) in 0.5 increments. For the data analysis, the scores were inverted to give a total potential score of 36 (normal).
The subjects also completed the Medical Outcomes Study 36-item Short Form Version 2 questionnaire (SF-36v2), a 4 week-recall quality of life assessment that has been widely used previously (Riazi et al., 2003; Finas et al., 2006). Using the statistical analysis system analysis code provided by QualityMetric, Inc. (Lincoln, RI), raw scores were converted into norm-based scores with a mean of 50 and a standard deviation (SD) of 10 (Ware et al., 2007). The SF-36v2 can be condensed into two summary measures: the physical component summary (PCS) and the mental component summary (MCS). These summary scores were used in the statistical analysis (Table 7 and Supplementary Tables 1 and 2), and national norm-based scores from Ware et al. (2007) were used for comparison with the SBMA population (Table 6).
Table 7.
Regression analysis of disease measures relative to repeat length, age and testosterone level
| CAG repeat length | Age | Total testosterone | |
|---|---|---|---|
| Muscle function | |||
| QMA weight scaled | −3.50, P = 0.001 | −3.27, P = 0.002 | 2.88, P = 0.006 |
| Timed 2 min walk | −3.16, P = 0.003 | −3.50, P = 0.001 | 2.30, P = 0.03 |
| Neurophysiological | |||
| Peroneal CMAP | −2.72, P = 0.009 | ||
| Average SNAP | −4.96, P < 0.0001 | ||
| MUNE | −2.36, P = 0.02 | ||
| Self-assessment | |||
| Total ADL | −2.33, P = 0.03 | −2.85, P = 0.007 | 3.93, P = 0.0004 |
| SF-36v2 PCS | 3.98, P = 0.0002 | ||
| Total IIEF | −2.48, P = 0.02 | −3.10, P = 0.003 |
Results of multiple linear regression analysis, where the significance of associations of each variable (CAG repeat length, age, and total testosterone) with the respective disease measures (muscle strength and function, neurophysiological measures, and self-assessment questionnaires) are shown, as adjusted for the other two variables. Each cell contains t-test statistics and the corresponding P-value. Only associations that were selected by the stepwise analysis and found to be significant (P < 0.05) are shown.
Table 6.
Self-assessment in SBMA (ranked most to least affected)
| Raw SBMA mean ± SD (range) | SBMA% maximum score | Healthy control% maximum score | SBMA percent of healthy control (%) | |
|---|---|---|---|---|
| ADLa (unable to unaffected) | ||||
| Walking (0–4.0) | 2.1 ± 1.1 (1.0–4.0) | 53 | ||
| Handwriting (0–4.0) | 2.4 ± 1.2 (0–4.0) | 60 | ||
| Falling (0–4.0) | 2.6 ± 1.0 (1.0–4.0) | 65 | ||
| Swallowing (0–4.0) | 2.7 ± 0.9 (1.0–4.0) | 68 | ||
| Speech (0–4.0) | 2.8 ± 0.9 (1.0–4.0) | 70 | ||
| Dressing (0–4.0) | 3.1 ± 0.8 (1.0–4.0) | 78 | ||
| Personal hygiene (0–4.0) | 3.1 ± 0.9 (1.0–4.0) | 78 | ||
| Cutting food and handling utensils (0–4.0) | 3.5 ± 0.7 (1.0–4.0) | 88 | ||
| Quality of sitting position (0–4.0) | 3.8 ± 0.4 (2.5–4.0) | 95 | ||
| Total score (0–36.0) | 25.9 ± 5.0 (15.0–35.5)** | 72 | ||
| Quality of life (SF-36v2)b (low to high) | ||||
| Physical functioning (10–30) | 16.5 ± 5.8 (10–30) | 29 | 51 | 57 |
| Role physical (4–20) | 11.4 ± 5.3 (4–20) | 36 | 50 | 71 |
| General health (5–25) | 15.6 ± 5.0 (3–25) | 43 | 50 | 86 |
| Social functioning (2–10) | 7.6 ± 2.4 (2–10) | 44 | 51 | 86 |
| Vitality (4–20) | 11.7 ± 3.5 (4–19) | 45 | 52 | 87 |
| Role emotional (3–15) | 12.6 ± 2.9 (3–15) | 46 | 51 | 90 |
| Bodily pain (2–12) | 6.4 ± 0.8 (4–7) | 48 | 50 | 96 |
| Mental health (5–25) | 19.9 ± 3.6 (8-25) | 49 | 51 | 96 |
| Physical component summary (0–100) | 34.3 ± 11.0 (16–58)** | 34 | 50 | 68 |
| Mental component summary (0–100) | 52.2 ± 11.6 (14–67) | 52 | 51 | 102 |
| Erectile function (IIEF)c (low to high) | ||||
| Intercourse satisfaction (0–15) | 5.5 ± 5.7 (0–15) | 37 | 70 | 53 |
| Overall satisfaction (2–10) | 5.5 ± 3.0 (2–10) | 44 | 83 | 53 |
| Orgasmic function (0–10) | 5.6 ± 4.4 (0–10) | 56 | 88 | 64 |
| Erectile function (1–30) | 15.2 ± 12.5 (1–30) | 47 | 71 | 66 |
| Sexual desire (2–10) | 5.9 ± 2.4 (2–10) | 49 | 63 | 78 |
| Total score (5–75) | 37.8 ± 26.2 (5–75) | 47 | 80 | 59 |
a SBMA percentages for ADL (n = 53) are based on the maximum possible score for each component. **P < 0.0001 for comparison between SBMA and an unaffected total score of 36.
b The component scores reported for the SF-36v2 (based on a 4 week-recall period, n = 53) are as defined by Ware et al. (2007) and were normalized using the statistical analysis system coding protocol provided by QualityMetric, Inc. (Lincoln, RI). National normalized scores were obtained from a 1998 survey of U.S. subjects, and the reported averages were calculated for men 35–74 years of age, weighted according to age and sample number (n = 2053–2071) (Ware et al., 2007). **P < 0.0001 for comparison between SBMA and age-matched controls.
c The percent max scores for the IIEF (n = 53) were adjusted for changes in range, i.e. [(raw mean SBMA score – minimum possible score)/(raw score range) x 100%]. Values for healthy controls with a mean age of 55 years (31–86) asymptomatic for erectile dysfunction were obtained from Dinsmore et al. (1999; n = 109).
**P < 0.0001 for comparison between SBMA and an unaffected total score of 36. Composite and total scores are shown in bold.
The 15 question International Index of Erectile Function (IIEF) survey was used to assess sexual satisfaction and function. The IIEF has been shown to have good test–retest reliability and discriminant validity (Rosen et al., 1997, 2002).
Data analysis
Statistical analyses were performed with statistical analysis system 9.1.3 (SAS Institute, Inc., Cary, NC) or SUDAAN 9.0 (Research Triangle Institute, Inc., Cary, NC). In this study, descriptive statistics such as mean, SD, standard error (SE), range, and 95% confidence interval (CI) of mean were used to summarize the quantitative measures for the SBMA subjects. The national estimates presented in Table 3 were generated from the 2005–2006 National Health and Nutrition Examination Survey using weights recommended by the National Center for Health Statistics (http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm) and statistical software capable of accommodating the complex sampling design of the national survey (descriptive methods available on request). Either a one sample or a two sample t-test was used to compare SBMA patients with published control data, where available. Satterthwaite's method was used if necessary. P-values were two-sided, and a level of significance of 0.05 was used.
Table 3.
Serum markers at initial evaluation with percent out of range
| SBMA mean ± SE (range) | Reference | Out of reference range (%) |
National estimate for | ||
|---|---|---|---|---|---|
| [95% mean CI] | range | Low | High | healthy control mean ± SE | |
| Biochemical profile | |||||
| Fasting glucose (mg/dl) | 105 ± 2 (79–189) [101, 109] | 70–115 | 0 | 12 | 109 ± 2 |
| Creatine kinase (U/l) | 1159 ± 114 (118–4434) [935, 1382] | 52–386 | 0 | 88 | |
| Total cholesterol (mg/dl) | 204 ± 5 (125–283) [195, 213] | 100–200 | 0 | 53 | 201 ± 1 |
| Triglycerides (mg/dl) | 180 ± 19 (37–850) [143, 217] | 160 ± 5 | |||
| High-density lipoprotein (mg/dl) | 51 ± 2 (34–82) [48, 54] | 49 ± 0.4 | |||
| Low-density lipoprotein (mg/dl) | 141 ± 4 (66–224) [133, 150] | 65–129 | 0 | 67 | 120 ± 1 |
| Aspartate aminotransferases (U/l) | 46 ± 2 (24–109) [41, 50] | 6–41 | 0 | 72 | 28 ± 1 |
| Alanine aminotransferases (U/l) | 54 ± 3 (24–111) [49, 60] | 9–34 | 0 | 60 | 30 ± 1 |
| Lactate dehydrogenase (U/l) | 216 ± 8 (125–373) [201, 231] | 113–226 | 0 | 35 | 129 ± 1 |
| Gamma glutamyl transferase (U/l) | 22 ± 2 (7–86) [19, 26] | 11–52 | 11 | 4 | 40 ± 3 |
| Hormonal profile | |||||
| Total testosterone (ng/dl)** | 621 ± 35 (187–1570) [553, 689] | 240–950 | 4 | 9 | 520 ± 4 |
| Free testosterone (ng/dl)** | 14 ± 1 (4–26.7) [12, 15] | 9–30 | 14 | 0 | 10 ± 0.1 |
| Dihydrotestosterone (ng/dl)** | 45 ± 3 (4–157) [38, 51] | 30–85 | 28 | 4 | 26 ± 0.4 |
| Androstenedione (ng/dl)* | 100 ± 9 (41–518) [82, 118] | 40–150 | 0 | 5 | 121 ± 3 |
| Oestradiol (pg/ml)** | 60 ± 4 (25–163) [52, 68] | <20–56 | 0 | 39 | 38 ± 1 |
Fasting glucose and creatine kinase reference ranges were set by the NIH Department of Laboratory Medicine (Bethesda, MD); cholesterol and low-density lipoprotein reference ranges represent desirable and optimal/near optimal ranges, respectively. Serum dihydrotestosterone reference levels are as reported by Esoterix, Inc. (Austin, TX), and serum oestradiol reference ranges are from the NIH Department of Laboratory Medicine (Bethesda, MD). The remaining serum hormonal ranges are as set by the Mayo Medical Laboratories (Rochester, MN). National estimates of means and SEs for the biochemical profile were generated from the 2005–2006 National Health and Nutrition Examination Survey using a nationally representative sample of 1480 men with a mean age of 53.2 years (range 37–79). For the hormonal profile, healthy control mean values and SEs were obtained from Wu et al. (1995; n = 1102) and Litman et al. (2007; n = 1612–1880).
*P < 0.05, **P < 0.0001 for comparison of SBMA and healthy controls.
Spearman's correlation coefficients were used to assess correlations between the various measures. For multivariate analysis, stepwise multiple linear regressions were first done to select the best subset of covariates among age, CAG repeat length, total testosterone, free testosterone and dihydrotestosterone. As a result, age, CAG repeat length and total testosterone (not dihydrotestosterone or free testosterone) were selected. Depending on the selected subset of covariates, a simple or multiple linear regression analysis was then done to assess the influence of the chosen covariates for each measure. The analysis was repeated with duration of weakness in place of age.
Results
Clinical and genetic background
The average age at evaluation for this study was 53 years, and the average age reported for onset of muscle weakness was 41 years (Table 1). The average age of diagnosis was 47 years, 5.5 years after the onset of weakness. There was an average delay of 3.5 years from first medical attention to genetic diagnosis. Fifteen (32%) of 47 respondents reported being misdiagnosed before the final SBMA diagnosis; six of these were misdiagnosed with amyotrophic lateral sclerosis. As indicated in Table 2, the most common presenting symptom was muscle cramps (n = 22), followed by tremors and leg weakness (n = 16). Half the patients reported that the weakness was first noticeable in the legs, followed by bulbar symptoms in 33%. The mean CAG repeat length in the androgen receptor gene was 47 (range 41–53). Thirty-four (68%) of the subjects had a known positive family history.
Table 2.
SBMA onset distribution
| Number (%) | |
|---|---|
| Presenting symptoms | |
| Bulbar weakness | 1 (1) |
| Arm weakness | 5 (7) |
| Leg weakness | 16 (23) |
| Breast enlargement | 5 (7) |
| Cramps | 22 (32) |
| Tremor | 16 (23) |
| Other | 4 (6) |
| Area of first muscle weakness | |
| Bulbar | 20 (33) |
| Arm | 10 (17) |
| Leg | 30 (50) |
Presenting symptoms were assessed retrospectively for 57 patients at the time of evaluation. Some patients reported more than one symptom at onset. Numbers in parentheses indicate percent of total symptoms reported. Presenting symptoms noted as “other” include choking, muscle twitching (fasciculations) and musculoskeletal pain.
Biochemical and hormonal profiles
Biochemical and hormonal profiles for 57 subjects are listed in Table 3. Creatine kinase was elevated in 88% of the patients. Aspartate and alanine aminotransferases and lactate dehydrogenase were also elevated above the reference range in 72, 60 and 35% of patients, respectively. However, this may not be due to liver damage, since mean gamma glutamyl transferase levels were not increased compared to national estimates.
Twelve of the 57 SBMA subjects were taking medication to correct hyperlipidemia. Many subjects had total cholesterol (53%) and low-density lipoprotein (67%) levels elevated above the recommended levels, with mean levels of 204 and 141 mg/dl, respectively. However, total cholesterol, high-density lipoprotein (HDL), triglyceride and fasting glucose were all similar to control levels reported by the 2005–2006 National Health and Nutrition Examination Survey. Only low-density lipoprotein showed significant divergence. The same result was observed when subjects taking hyperlipidaemia-correcting medication were excluded.
Serum hormone values were generally within the reference ranges (Table 3). However, sizeable minorities of subjects had abnormally low levels of free testosterone or dihydrotestosterone. The mean SBMA oestradiol levels were elevated compared to the reference range and to healthy controls. Moreover, when SBMA androgen levels were compared with age-matched healthy controls (Wu et al., 1995; Litman et al., 2007), a divergence was seen between SBMA subjects and healthy controls in total testosterone, free testosterone, dihydrotestosterone, androstenedione and oestradiol levels.
Strength and functional measures
Table 4 summarizes the subjects' performance on QMA and timed 2 min walk (n = 56). Compared with healthy controls the subjects had decreased total (50%), lower extremity (52%) and upper extremity (47%) composite peak isometric forces as scaled for body weight. The strength in 11 muscle groups ranged from 38 to 70% of healthy control values. The weakness was asymmetric (>5% left–right difference) in 62% of the subjects, and interestingly the dominant side was weaker in over two thirds of these (69%).
The average distance covered in the timed 2 min walk test was 109 ± 50 m (range 15–208 m). Twenty-two of the 56 subjects used assistive devices such as canes, walkers, or ankle-foot orthoses; these covered a mean distance of 66 ± 23 m, with an average velocity of 0.55 m/s. The mean for 34 subjects who did not use gait aids was 136 ± 44 m, with an average velocity of 1.13 m/s. According to Light et al. (1997) a cohort of 12 healthy control men and women with a mean age of 68 ± 10 years had an average ambulation of 176 m in the timed 2 min walk (range 130–237 m).
Neurophysiological studies
Nerve conduction study results for 54 SBMA subjects are summarized in Table 5. Ninety-four to 100% of SBMA subjects had low SNAP amplitude depending on the nerve, and 28 and 52% had low CMAP amplitudes in the median and peroneal nerves, respectively.
Table 5.
Neurophysiological studies
| Mean ± SD (range) | Reference range | Abnormally low (%) | |
|---|---|---|---|
| Motor nerve conduction (mV) | |||
| Median CMAP | 6.3 ± 3.1 (0.3–13.9) | ≥4.5 mV | 28 |
| Peroneal CMAP | 2.7 ± 2.0 (0–6.7) | ≥2.5 mV | 52 |
| Sensory nerve conduction (µV) | |||
| Median SNAP | 5.4 ± 3.8 (0–23) | ≥15 µV, ≥10 µV over 60 years | 98 |
| Ulnar SNAP | 3.9 ± 3.2 (0–16) | ≥15 µV, ≥10 µV over 60 years | 96 |
| Radial SNAP | 5.1 ± 3.3 (0–13) | ≥15 µV, ≥10 µV over 60 years | 100 |
| Sural SNAP | 1.9 ± 2.1 (0–8) | ≥6 µV, may be absent over 60 years | 94 |
CMAP and SNAP were performed on both sides (n = 54). Only right-sided values were used in the correlation and regression analyses (Tables 7 and 8). For the sural nerve the percent abnormal is for subjects < 60 years of age.
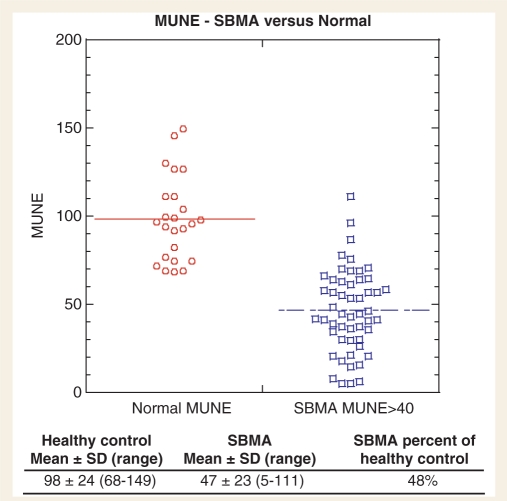
Fifty-two SBMA subjects had MUNEs done in the abductor pollicis brevis muscle (Fig. 1). Twenty-four MUNEs were performed in the 14 healthy volunteers (mean age 55 ± 8 years). There was a significant difference in MUNE between the SBMA subjects (47 ± 23) and the healthy controls (98 ± 24) (P < 0.0001). Only three SBMA subjects had a MUNE within one standard deviation of the mean control value.
Figure 1.
The statistical MUNE study was performed on the abductor pollicis brevis of healthy controls (n = 24) and SBMA patients (n = 52). The SBMA subjects tested had a mean median CMAP of 6.3 ± 3.1 mV. The MUNE data from the SBMA patients were adjusted to exclude single motor unit potentials less than 40 µV (Lehky et al., 2009). Control subjects had a mean age of 55 ± 8 years (42–69 years) and a mean median CMAP of 9.7 ± 2.8 mV. The control subjects did not have any small motor unit potential values less than 40 µV, therefore no further adjustment of the MUNE data were needed.
Self-assessment testing
The results of the ADL questionnaire are summarized in Table 6. The total ADL score is the sum of all nine responses, with a lower score indicating more severe impairment (n = 56). The mean total ADL score was 25.9 ± 5.0, 72% of the best possible score. The most affected ADL areas were walking, handwriting, falling, swallowing and speech (53-70% of the maximal scores). Only 9 of 56 subjects reported no difficulty with falls.
In assessing the quality of life in the SBMA population by the SF-36v2, we compared the SBMA norm-based scores to those from a national age-matched male cohort of over 2000 respondents (Ware et al., 2007). The SBMA population reported the following SF-36v2 components as less than 90% of national norms: physical functioning (56%), role physical (71%), general health (85%), social functioning (86%) and vitality (87%). The PCS and MCS were divergent, with scores of 69% and 102% of the national age-matched norm for men 35–74 years of age, respectively.
Five components of erectile function were assessed using the IIEF (n = 53). Intercourse and overall satisfaction were most affected (37 and 46% of maximal). Erectile function was rated at 47% of maximal and 66% that of healthy controls. Four of 53 respondents were taking prescription medication for erectile dysfunction, and there were anecdotal reports of benefit. The mean total IIEF score was 47% of maximal and 59% that of healthy control individuals who were not symptomatic for erectile dysfuction (mean age of 55, n = 108–109) (Dinsmore et al., 1999).
Correlations and multivariate analysis
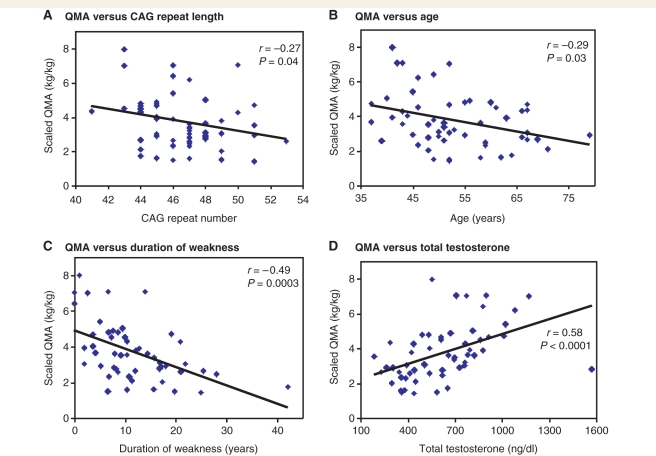
In patients with SBMA, possible phenotype-modifying variables other than CAG repeat length have yet to be identified. Therefore, we evaluated our phenotypic measures for correlation with age, disease duration and androgen levels as well as CAG repeat length (Supplementary Table 1). There was an inverse correlation between CAG repeat length and weight-scaled QMA (P = 0.04, Fig. 2A). Age at evaluation correlated inversely with measures of muscle strength and function and neurophysiology: QMA (P = 0.03, Fig. 2B), timed 2 min walk (P = 0.005, Supplementary Fig. 1B), peroneal CMAP (P = 0.005), and average SNAP (P < 0.0001). Age also correlated inversely with the total ADL score (P = 0.002, Supplementary Fig. 2B) and the total IIEF score (P = 0.02). Disease duration also correlated inversely with muscle strength and function, neurophysiological measures (median and peroneal CMAP), ADL and physical quality of life (Fig. 2C, Supplementary Figs 1C and 2C, Supplementary Table 1).
Figure 2.
(A–D) Correlation of QMA scores with CAG repeat length, age, duration of weakness and total testosterone levels. The values shown are for total weight-scaled QMA (right plus left).
In contrast, total testosterone correlated directly with muscle strength and function, as indicated by QMA and timed 2 min walk (Fig. 2D, Supplementary Fig. 1D). Free testosterone and dihydrotestosterone showed similar correlations (Supplementary Table 1). Free testosterone also correlated positively with a nerve conduction measure, the average SNAP (P = 0.006). All androgens correlated positively with the total ADL score and the physical functioning component of the SF-36v2 (Supplementary Table 1, Supplementary Fig. 2D). Androgens did not correlate significantly with total IIEF scores, with or without the inclusion of individuals using erectile dysfunction medication (data not included in the table).
Since testosterone levels decrease with age (Orwoll et al., 2006), and both CAG repeat length and age contribute to changes in muscle strength and function, we used a multivariate regression analysis to control for the other variables when assessing the relationship of repeat length, age and testosterone levels to strength and function. From the regression analysis, CAG repeat length, age at evaluation and total testosterone levels were each independently associated with strength and ADL in our SBMA cohort (Table 7). Repeat length and age correlated inversely and total testosterone correlated directly with strength and ADL. Total QMA, timed 2 min walk, and total ADL scores were significantly associated with CAG repeat length, age and total testosterone, when corrections for each of the other variables were made. The total IIEF score was also associated with CAG repeat length and age. Age was the only variable to significantly associate with the peroneal CMAP, average SNAP and MUNE. Total testosterone was the only variable with a significant association with quality of life, as measured by the SF-36v2 PCS. As with muscle strength and ADL, the correlation of total testosterone with quality of life was positive, i.e. higher total testosterone levels were associated better physical condition scores on the SF-36v2 questionnaire. Similar results were obtained when duration of weakness was substituted for age in the regression analysis (Supplementary Table 2), except that the associations of CAG repeat length corrected for age were not found with correction for disease duration. This is consistent with an effect of repeat length on the age of onset but not on the rate of disease progression. Disease duration, like age, was negatively associated with measures of muscle strength and function when corrected for repeat length and total testosterone. Total testosterone was positively associated with muscle strength and function when corrected for repeat length and disease duration, just as it was when corrected for repeat length and age.
Discussion
SBMA is a rare, debilitating, neurodegenerative disorder with no effective treatment. Although the cause of SBMA is known, there are unanswered questions about its natural history and the factors that determine its clinical course. Information regarding those prognostic factors and the clinical manifestations of SBMA may be essential for the design and conduct of future therapeutic trials.
In the development of a treatment for SBMA, early intervention may also be important to affect disease progression. The subjects in our study did not seek medical attention until an average of 2 years after symptom onset, and there was an additional 3 years from first medical evaluation until a clinical diagnosis of SBMA was made. Increased knowledge about SBMA and its clinical manifestations, together with increased availability of confirmatory genetic testing, should speed the diagnosis of SBMA.
The principal clinical features of SBMA in this study confirm the findings of other retrospective analyses (Kennedy et al., 1968; Lieberman et al., 2000). Muscle cramps were often reported as a first symptom, but leg weakness, tremor and bulbar involvement were also common. Sixteen of 50 subjects had no known family history, which is likely to be due to inheritance through non-manifesting female carriers.
The weakness affected upper and lower extremities and proximal and distal muscles similarly. A majority of the patients had some asymmetry in muscle strength, and interestingly 69% of these had greater weakness on the dominant side. Such dominant-side predominant weakness has been described in other neuromuscular disorders, particularly facioscapulohumeral dystrophy, and it may reflect asymmetric muscle use.
We found an inverse association between CAG repeat length and age at evaluation as reported previously (Atsuta et al., 2006). Androgen levels and strength both correlated inversely with the age of the subjects in this study. However, after adjusting for age (or disease duration) and repeat length, serum testosterone still correlated positively with muscle strength, as indicated by QMA and timed 2 min walk (Table 7).
In contrast to previous reports, our SBMA subjects had mean cholesterol and glucose levels similar to a national sample of men in the same age range, although 53% had elevated cholesterol and 12% increased fasting glucose levels relative to the laboratory reference standards (Table 3).
As shown in Tables 4–6, the SBMA population scored consistently lower in functional, physiological, and self-reported quality of life measures than age-matched healthy controls. SBMA scores resembled those reported in other impaired populations, e.g. the timed 2 min walk in 12 subjects (7 men and 5 women) with Parkinson's disease (Light et al., 1997), and the IIEF scores in patients with symptomatic erectile dysfuction (Dinsmore et al., 1999).
The decrease in physical function in SBMA is associated with lower scores in ADL and quality of life. The activity scores in the ADL questionnaire were decreased 5%-47% from maximal (normal). The mean ambulation velocities, both with and without gait aids, were less than what is normally required to cross a street in a pedestrian crosswalk (1.2 m/s) (http://mutcd.fhwa.dot.gov/pdfs/2003r1r2/mutcd2003r1r2complet.pdf). The divergence of the PCS and MCS scores on the SF-36v2 points to the physical burden of SBMA and indicates the patients' relatively normal psychological well being.
Reliable outcome measures will be needed to assess therapeutic efficacy in future clinical trials of SBMA. In this analysis, we examined several different measures used to quantify disease related deficits. Our cross-sectional analysis indicates that the QMA and timed 2 min walk appear promising as feasible, quantitative, and potentially meaningful measures of clinical status in SBMA. Each correlated with self-assessed quality of life as indicated by ADL, SF-36v2 and IIEF scores (Supplementary Table 2). The statistical MUNE may also be useful as a relatively objective, easily blinded, physiological biomarker of motor neuron degeneration. Additional research will be necessary to establish the reliability and sensitivity of these measures in SBMA, and to define the clinical course of the disease prospectively.
Trials of anti-androgen treatment in SBMA have been based on evidence of efficacy from animal models (Katsuno et al., 2002, 2003; Chevalier-Larsen et al., 2004; Banno et al., 2009). The animal studies indicate that the toxicity of the mutant androgen receptor protein in SBMA is ligand-dependent, and that reducing testosterone levels may be beneficial. However, our finding that higher, not lower, testosterone levels are associated with better muscle strength and function indicates that it may be necessary to balance the anabolic strengthening effects of androgens against any potentially deleterious effects of androgen-activated mutant androgen receptor toxicity, such as can be inferred from the animal studies. A limitation of this interpretation is that it is based on a cross-sectional analysis. The therapeutic implications are best addressed in prospective, randomized clinical trials.
Funding
Supported by intramural research funds from the National Institute of Neurological Disorders and Stroke and the National Institutes of Health Clinical Center. B.K.F. was a participant in the Clinical Research Training Program, a public–private partnership supported jointly by the National Institutes of Health and Pfizer Inc. (via a grant to the Foundation for the National Institutes of Health from Pfizer Inc).
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We would like to thank Dr Wilson Bryan for his help with the manuscript and the staff of the Epidemiology and Biostatistics Branch of the Rehabilitation Medicine Department and the Neurology Outpatient Unit of the National Institutes of Health Clinical Center for their involvement and support.
Glossary
Abbreviations
- ADL
activities of daily living
- CI
confidence interval
- CMAP
compound motor action potential
- IIEF
International Index of Erectile Function questionnaire
- MCS
mental component summary of the SF-36v2
- MUNE
motor unit nerve estimation
- NIH
the National Institutes of Health
- PCS
physical component summary of the SF-36v2
- QMA
quantitative muscle assessment
- SBMA
spinal and bulbar muscular atrophy
- SD
standard deviation
- SE
standard error
- SF-36v2
Medical Outcomes Study 36-item Short Form Version 2 questionnaire
- SNAP
sensory nerve action potential
References
- Andres PL, Skerry LM, Thornell B, Portney LG, Finison LJ, Munsat TL. A comparison of three measures of disease progression in ALS. J Neurol Sci. 1996;139 Suppl:64–70. doi: 10.1016/0022-510x(96)00108-6. [DOI] [PubMed] [Google Scholar]
- Antonini G, Gragnani F, Romaniello A, Pennisi EM, Morino S, Ceschin V, et al. Sensory involvement in spinal-bulbar muscular atrophy (Kennedy's disease) Muscle Nerve. 2000;23:252–8. doi: 10.1002/(sici)1097-4598(200002)23:2<252::aid-mus17>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Atsuta N, Watanabe H, Ito M, Banno H, Suzuki K, Katsuno M, et al. Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain. 2006;129:1446–55. doi: 10.1093/brain/awl096. [DOI] [PubMed] [Google Scholar]
- Banno H, Katsuno M, Suzuki K, Takeuchi Y, Kawashima M, Suga N, et al. Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann Neurol. 2009;65:140–50. doi: 10.1002/ana.21540. [DOI] [PubMed] [Google Scholar]
- Brooks D, Davis AM, Naglie G. The feasibility of six-minute and two-minute walk tests in in-patient geriatric rehabilitation. Can J Aging. 2007;26:159–62. doi: 10.3138/cja.26.2.009. [DOI] [PubMed] [Google Scholar]
- Chevalier-Larsen ES, O'Brien CJ, Wang H, Jenkins SC, Holder L, Lieberman AP, et al. Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci. 2004;24:4778–86. doi: 10.1523/JNEUROSCI.0808-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejager S, Bry-Gauillard H, Bruckert E, Eymard B, Salachas F, LeGuern E, et al. A comprehensive endocrine description of Kennedy's disease revealing androgen insensitivity linked to CAG repeat length. J Clin Endocrinol Metab. 2002;87:3893–901. doi: 10.1210/jcem.87.8.8780. [DOI] [PubMed] [Google Scholar]
- Dinsmore WW, Hodges M, Hargreaves C, Osterloh IH, Smith MD, Rosen RC. Sildenafil citrate (Viagra) in erectile dysfunction: near normalization in men with broad-spectrum erectile dysfunction compared with age-matched healthy control subjects. Urology. 1999;53:800–5. doi: 10.1016/s0090-4295(98)00586-x. [DOI] [PubMed] [Google Scholar]
- Ferrante MA, Wilbourn AJ. The characteristic electrodiagnostic features of Kennedy's disease. Muscle Nerve. 1997;20:323–9. doi: 10.1002/(SICI)1097-4598(199703)20:3<323::AID-MUS9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Finas D, Bals-Pratsch M, Sandmann J, Eichenauer R, Jocham D, Diedrich K, et al. Quality of life in elderly men with androgen deficiency. Andrologia. 2006;38:48–53. doi: 10.1111/j.1439-0272.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Doyu M, Minamiyama M, Sang C, Kobayashi Y, et al. Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med. 2003;9:768–73. doi: 10.1038/nm878. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Kume A, Li M, Nakagomi Y, Niwa H, et al. Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron. 2002;35:843–54. doi: 10.1016/s0896-6273(02)00834-6. [DOI] [PubMed] [Google Scholar]
- Katsuno M, Adachi H, Waza M, Banno H, Suzuki K, Tanaka F, et al. Pathogenesis, animal models and therapeutics in spinal and bulbar muscular atrophy (SBMA) Exp Neurol. 2006;200:8–18. doi: 10.1016/j.expneurol.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18:671–80. doi: 10.1212/wnl.18.7.671. [DOI] [PubMed] [Google Scholar]
- La Spada androgen receptor, Roling DB, Harding AE, Warner CL, Spiegel R, Hausmanowa-Petrusewicz I, et al. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992;2:301–4. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- La Spada androgen receptor, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352:77–9. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Lehky T, Chen C, Di Prospero N, Rhodes L, Fischbeck K, Floeter MK. Standard and modified statistical MUNE evaluations in spinal-bulbar muscular atrophy. Muscle Nerve. 2009 doi: 10.1002/mus.21399. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman AP, Fischbeck KH. Triplet repeat expansion in neuromuscular disease. Muscle Nerve. 2000;23:843–50. doi: 10.1002/(sici)1097-4598(200006)23:6<843::aid-mus2>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Light KE, Behrman AL, Thigpen M, Triggs WJ. The 2-minute walk test: A tool for evaluating walking endurance in clients with Parkinson's disease. Neurol Rep. 1997;21:136–9. [Google Scholar]
- Litman HJ, Bhasin S, O'Leary MP, Link CL, McKinlay JB. An investigation of the relationship between sex-steroid levels and urological symptoms: results from the Boston Area Community Health survey. BJU Int. 2007;100:321–6. doi: 10.1111/j.1464-410X.2007.06938.x. [DOI] [PubMed] [Google Scholar]
- Liveson JA, Ma DM. Laboratory reference for clinical neurophysiology. New York, NY: Oxford University Press; 1992. [Google Scholar]
- Lund A, Udd B, Juvonen V, Andersen PM, Cederquist K, Ronnevi LO, et al. Founder effect in spinal and bulbar muscular atrophy (SBMA) in Scandinavia. Eur J Hum Genet. 2000;8:631–6. doi: 10.1038/sj.ejhg.5200517. [DOI] [PubMed] [Google Scholar]
- Mathieu J, Boivin H, Richards CL. Quantitative motor assessment in myotonic dystrophy. Can J Neurol Sci. 2003;30:129–36. doi: 10.1017/s0317167100053397. [DOI] [PubMed] [Google Scholar]
- Miller P, Moreland J, Stevenson T. Measurement properties of a standardized version of the two-minute walk test for individuals with neurological dysfunction. Physiother Can. 2002;54:241–58. [Google Scholar]
- Orwoll E, Lambert LC, Marshall LM, Phipps K, Blank J, Barrett-Connor E, et al. Testosterone and estradiol among older men. J Clin Endocrinol Metab. 2006;91:1336–44. doi: 10.1210/jc.2005-1830. [DOI] [PubMed] [Google Scholar]
- Riazi A, Hobart JC, Lamping DL, Fitzpatrick R, Freeman JA, Jenkinson C, et al. Using the SF-36 measure to compare the health impact of multiple sclerosis and Parkinson's disease with normal population health profiles. J Neurol Neurosurg Psychiatry. 2003;74:710–4. doi: 10.1136/jnnp.74.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RC, Cappelleri JC, Gendrano N., III The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226–44. doi: 10.1038/sj.ijir.3900857. [DOI] [PubMed] [Google Scholar]
- Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13. doi: 10.1053/apmr.2001.9396. [DOI] [PubMed] [Google Scholar]
- Schmidt BJ, Greenberg CR, Allingham-Hawkins DJ, Spriggs EL. Expression of X-linked bulbospinal muscular atrophy (Kennedy disease) in two homozygous women. Neurology. 2002;59:770–2. doi: 10.1212/wnl.59.5.770. [DOI] [PubMed] [Google Scholar]
- Shefner JM, Cudkowicz ME, Zhang H, Schoenfeld D, Jillapalli D. The use of statistical MUNE in a multicenter clinical trial. Muscle Nerve. 2004;30:463–9. doi: 10.1002/mus.20120. [DOI] [PubMed] [Google Scholar]
- Sobue G, Doyu M, Kachi T, Yasuda T, Mukai E, Kumagai T, et al. Subclinical phenotypic expressions in heterozygous females of X-linked recessive bulbospinal neuronopathy. J Neurol Sci. 1993;117:74–8. doi: 10.1016/0022-510x(93)90157-t. [DOI] [PubMed] [Google Scholar]
- Subramony SH, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, et al. Measuring Friedreich ataxia: Interrater reliability of a neurologic rating scale. Neurology. 2005;64:1261–2. doi: 10.1212/01.WNL.0000156802.15466.79. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Katsuno M, Banno H, Takeuchi Y, Atsuta N, Ito M, et al. CAG repeat size correlates to electrophysiological motor and sensory phenotypes in SBMA. Brain. 2008;131:229–39. doi: 10.1093/brain/awm289. [DOI] [PubMed] [Google Scholar]
- Tanaka F, Doyu M, Ito Y, Matsumoto M, Mitsuma T, Abe K, et al. Founder effect in spinal and bulbar muscular atrophy (SBMA) Hum Molec Genet. 1996;5:1253–7. doi: 10.1093/hmg/5.9.1253. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H, Nakanishi T, Kondo K, Tsubaki T. Hereditary proximal neurogenic muscular atrophy in adult. Arch Neurol. 1965;12:597–603. doi: 10.1001/archneur.1965.00460300045005. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User's manual for the SF-36v2 Health Survey. Lincoln, RI: QualityMetric Incorporated; 2007. [Google Scholar]
- Wu AH, Whittemore AS, Kolonel LN, John EM, Gallagher RP, West DW, et al. Serum androgens and sex hormone-binding globulins in relation to lifestyle factors in older African-American, white, and Asian men in the United States and Canada. Cancer Epidemiol Biomarkers Prev. 1995;4:735–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.