Abstract
Focal brain lesions are assumed to produce language deficits by two basic mechanisms: local cortical dysfunction at the lesion site, and remote cortical dysfunction due to disruption of the transfer and integration of information between connected brain regions. However, functional imaging studies investigating language outcome after aphasic stroke have tended to focus only on the role of local cortical function. In this positron emission tomography functional imaging study, we explored relationships between language comprehension performance after aphasic stroke and the functional connectivity of a key speech-processing region in left anterolateral superior temporal cortex. We compared the organization of left anterolateral superior temporal cortex functional connections during narrative speech comprehension in normal subjects with left anterolateral superior temporal cortex connectivity in a group of chronic aphasic stroke patients. We then evaluated the language deficits associated with altered left anterolateral superior temporal cortex connectivity in aphasic stroke. During normal narrative speech comprehension, left anterolateral superior temporal cortex displayed positive functional connections with left anterior basal temporal cortex, left inferior frontal gyrus and homotopic cortex in right anterolateral superior temporal cortex. As a group, aphasic patients demonstrated a selective disruption of the normal functional connection between left and right anterolateral superior temporal cortices. We observed that deficits in auditory single word and sentence comprehension correlated both with the degree of disruption of left-right anterolateral superior temporal cortical connectivity and with local activation in the anterolateral superior temporal cortex. Subgroup analysis revealed that aphasic patients with preserved positive intertemporal connectivity displayed better receptive language function; these patients also showed greater than normal left inferior frontal gyrus activity, suggesting a possible ‘top-down’ compensatory mechanism. These results demonstrate that functional connectivity between anterolateral superior temporal cortex and right anterior superior temporal cortex is a marker of receptive language outcome after aphasic stroke, and illustrate that language system organization after focal brain lesions may be marked by complex signatures of altered local and pathway-level function.
Keywords: aphasia, post-stroke recovery, functional neuroimaging, neural networks, anterior temporal lobe
Introduction
Stroke-related aphasia is a significant clinical problem, persisting in around one in eight long-term stroke survivors (Wade et al., 1986). Despite the availability of functional neuroimaging techniques enabling direct investigation of language architecture, the cortical processes underlying natural restoration of language function after aphasic stroke remain poorly understood. From the earliest days of nineteenth-century aphasiology, language deficits due to focal brain lesions have been attributed to two basic mechanisms: local cortical dysfunction at the lesion site, and remote cortical dysfunction due to disruption of information transfer along pathways between connected brain regions (Wernicke, 1874; Geschwind, 1965a, b). These two mechanisms are given equal prominence in modern conceptual frameworks of focal lesion effects (Catani and Ffytche, 2005). Nevertheless, the functional imaging literature on aphasic stroke is dominated by studies investigating relationships between local cortical function and language performance (see Heiss et al., 2003; Price and Crinion, 2005 for reviews). Studies taking this approach have established several local cortical mechanisms that contribute to post-stroke language outcome, including the recruitment of areas within, or adjacent to, left perisylvian language cortex (e.g. Cao et al., 1999; Cardebat et al., 2003; Saur et al., 2006), and the re-lateralization of language processing to intact homotopic right-hemisphere regions (e.g. Cardebat et al., 2003; Sharp et al., 2004; Saur et al., 2006). In contrast, the relationship between functional integrity of cortico-cortical pathways and behavioural outcome after aphasic stroke has not been directly investigated.
The concept of functionally connected cortical regions is integral to contemporary models of the normal language system as a distributed network of regions organized into parallel, functionally specialized and interdependent processing pathways (Mesulam, 1998; Vigneau et al., 2006; Hickok and Poeppel, 2007; Saur et al., 2008). Functional connections between large-scale neuronal assemblies (cortical regions) can be investigated by connectivity analysis of functional neuroimaging data. Functional connectivity is defined as temporal correlation (covariation) between neurophysiological (e.g. haemodynamic) responses in anatomically distinct brain regions (Friston, 1994). The presence of a functional connection between two regions indicates that responses in these regions show a consistent relationship over time. Correlated activity can result from direct integration of information between two regions, or may be the consequence of modulatory input from a third region (Friston, 1994). Distinguishing between these two interpretations must rely on converging evidence from other sources. Functional connectivity analysis has previously been used to study the organization of inter-regional functional connections in the normal brain during written language processing (Horwitz et al., 1998; Just et al., 2004a), speech production (Paus et al., 1996; Horwitz and Braun, 2004; Schulz et al., 2005), speech perception (Husain et al., 2006; Obleser et al., 2007), verbal imagery (Just et al., 2004b), voice recognition (von Kriegstein and Giraud, 2004), and verbal working memory (Buchsbaum et al., 2005). Altered patterns of functional connectivity during language processing have been successfully demonstrated in developmental dyslexia (Horwitz et al., 1998), but functional connectivity methods have not been applied to the study of aphasic stroke.
Classical models of speech comprehension focus on the role of Wernicke's area in posterolateral temporal cortex. Although this region would seem an obvious starting point for investigation of pathway-level function in aphasic stroke, in practice technical factors hamper connectivity analysis of Wernicke's area in receptive aphasia. Haemodynamic responses, the crucial variable for analyses of functional connectivity, are abolished in lesioned cortex: posterolateral temporal cortex is involved in the majority of stroke lesions associated with speech-specific comprehension deficits (Kreisler et al., 2000). However, cumulative evidence from functional imaging studies in normal subjects (see Scott and Johnsrude, 2003) indicates that speech comprehension engages anterolateral as well as posterolateral superior temporal cortex (STC). Left anterolateral STC, in particular the anterior superior temporal sulcus, responds preferentially to intelligible speech in comparison to unintelligible speech-like stimuli (Scott et al., 2000; Crinion et al., 2003; Spitsyna et al., 2006). Although the precise role of this region in speech comprehension is debated, in broad terms left anterolateral STC appears to act as an interface between speech-sound representations and word meaning (Scott et al., 2000). Responses in both left anterolateral STC and its right hemisphere homologue have previously been shown to correlate with spoken sentence comprehension performance in aphasic stroke patients (Crinion and Price, 2005; Crinion et al., 2006). Left anterolateral STC is rarely involved in stroke lesions associated with speech comprehension deficits (Kreisler et al., 2000) and most middle cerebral artery territory infarcts spare this region (Caviness et al., 2002; Hosoda et al., 2007), so that investigation of left anterolateral STC functional connectivity is technically feasible in receptive aphasia. In the normal brain, left anterolateral STC is postulated to interact with other speech-responsive regions, including left inferior frontal gyrus (IFG) and anterior basal temporal cortex (BTC), during the course of speech comprehension (Spitsyna et al., 2006; Vigneau et al., 2006). However, the normal functional connectivity of left anterolateral STC has not been formally investigated.
Investigation of left anterolateral STC and its connectivity is also prompted by findings from semantic dementia and repetitive transcranial magnetic stimulation in normal participants. The selective comprehension deficit observed in semantic dementia (the temporal lobe variant of frontotemporal dementia: Hodges et al., 1992) is associated with circumscribed bilateral temporal lobe atrophy that is most severe in anterior and ventral temporal cortex (Patterson et al., 2007; Lambon Ralph and Patterson, 2008; Rohrer et al., 2009; Seeley et al., 2009). The potential importance of left and right anterior temporal lobe regions and their interconnectivity in normal comprehension has been underlined by recent repetitive transcranial magnetic stimulation studies: stimulation of either left or right anterior temporal regions produces a selective semantic effect in line with the semantic dementia data (Pobric et al., 2007, Lambon Ralph et al., 2009).
The aims of the present study were to establish the normal organization of left anterolateral STC functional connections during speech comprehension, to compare this with left anterolateral STC connectivity in a group of aphasic stroke patients with impaired speech comprehension, and to examine links between integrity of left anterolateral STC connectivity and language performance in the aphasic group. We investigated two hypotheses: (i) that left anterolateral STC connectivity would be abnormal in the aphasic subjects; and (ii) that alterations in left anterolateral STC connectivity in the aphasic patients would correlate with speech comprehension deficits.
Materials and methods
Subjects
Raw PET and neuropsychological data were drawn from a previously reported functional imaging study of narrative comprehension that investigated 24 aphasic stroke patients and 11 normal control subjects (Crinion et al., 2006). Lesion extent was evaluated in these 24 aphasic patients from volumetric T1-weighted MRI brain scans by a neurologist (J.E.W.) blinded to patients' PET and behavioural data. Patients were included in the aphasic group of the present study only if their stroke lesions did not involve the left anterolateral STC, defined as lateral superior temporal gyrus and sulcus anterior to the rostral border of Heschl's gyrus. Sixteen patients [11 males; mean age ± standard error (SEM) = 65.8 ± 2.0 years] fulfilled this criterion. All were right-handed native English speakers with acquired speech comprehension deficits resulting from a single left hemisphere ischaemic stroke (mean time since stroke onset ± SEM = 28.8 ± 9.2 months). Lesion site varied between patients; lesion overlap was maximal in posterior STC (Fig. 1C). The 11 subjects in the normal control group were right-handed native English speakers with no history of neurological illness (9 males; mean age ± SEM = 54.6 ± 4.3 years). Mean age of the normal control group was significantly lower than that of the aphasics group (independent-samples t-test; P = 0.03). Data collection was preceded by local ethics committee approval and provision of informed consent from all subjects according to the Declaration of Helsinki; radioisotope administration was approved by the UK Department of Health.
Figure 1.
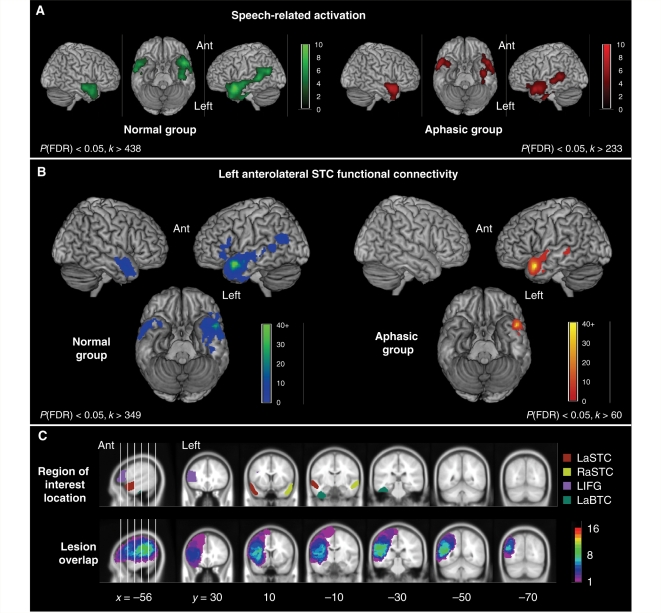
Functional imaging data in the normal and aphasic groups. (A) Activation related to speech comprehension in the normal (green) and aphasic (red) groups, determined by the contrast of narrative and control conditions. Statistical parametric maps are displayed using a voxel-level statistical threshold of P < 0.05, corrected for false discovery rate (FDR), with a cluster extent threshold equivalent to 5% of the total number of suprathreshold voxels (see Experimental Methods). SPMs have been rendered onto a template brain in standard MNI stereotactic space, with intensity scales representing T values. Ant = anterior. (B) Left anterolateral superior temporal functional connectivity in the normal (green-blue) and aphasic (red-yellow) groups. Statistical thresholding and display procedures are the same as in panel A. (C) Location of anatomical regions used in the region of interest analyses (upper row), displayed for comparison with the distribution and overlap of stroke lesions in the aphasic group (lower row). To assess lesion overlap, hand-drawn images of stroke lesions were normalized into standard MNI stereotactic space; regions of interest and lesion images are displayed on coronal slices of a canonical averaged T1-weighted MNI-space brain image available in SPM99. The intensity scale refers to the number of patients with lesions at a particular voxel.
Behavioural assessment of language
All aphasic subjects underwent comprehensive neuropsychological testing around the time of PET scanning. Auditory, written single word and sentence comprehension were evaluated using subtests from the Comprehensive Aphasia Test battery (Swinburn et al., 2004), and auditory syntactic comprehension was evaluated using the Test of Reception of Grammar (Bishop, 1989). At the time of PET scanning, all but one of the patients showed evidence of a persistent auditory comprehension deficit on these assessments.
PET data acquisition
Experimental stimuli and PET data acquisition methods have been described previously (Crinion et al., 2006; see Supplementary Methods for details). In all subjects, PET scanning involved two experimental conditions (see Supplementary Methods for details). In the spoken narrative (Sp) condition, subjects listened to narratives consisting of high-frequency vocabulary in simple sentence structures, designed to maximize comprehensibility in the aphasic patients. A naturalistic implicit comprehension paradigm was used to avoid task difficulty confounds associated with performance of explicit verbal tasks by aphasic subjects (Price et al., 2006). In the control condition, subjects listened to temporally reversed versions of the narrative stimuli (RevSp condition). Time-reversed speech is unintelligible and disrupts normal prosodic patterns, but preserves the vocal quality and the overall acoustic complexity of normal speech (Crinion et al., 2003). Subjects were informed prior to scanning that the control stimuli would be unintelligible.
During PET data acquisition, 15O-labelled water (H215O), administered intravenously, was used to demonstrate changes in regional cerebral blood flow, equivalent to changes in tissue concentration of H215O. Normal subjects underwent eight scans for each condition. Aphasic subjects underwent either six, seven or eight scans for each condition, depending on radiation dosage limits and individual tolerance of scanning. Stimuli were presented binaurally via headphones, with sound volume individually adjusted to optimize comfort and perceptual clarity. Presentation order of the two experimental conditions was randomized within and between subjects. During each scan, stimulus delivery began approximately 10 s prior to the phase of rapid uptake of tracer into cerebral tissue and continued throughout the period of data acquisition. After measured attenuation correction, images were reconstructed by filtered back projection.
Data analysis
We conducted preprocessing and analysis of PET data using the Statistical Parametric Mapping-99 (SPM99) statistical software package (Wellcome Department of Imaging Neuroscience, UCL, London, UK; http://www.fil.ion.ucl.ac.uk/spm). Image preprocessing involved realignment to correct for head motion between scans, normalization into standard Montreal Neurological Institute (MNI) stereotactic brain space, and smoothing with an isotropic 10 mm full-width-at-half-maximum Gaussian kernel. For the aphasic subjects, the cost function masking method of normalization was employed (Brett et al., 2001), in which a hand-drawn stroke mask, derived from the T1-weighted volumetric MRI scan, prevents the normalization algorithm from interpreting the infarct's edge as part of the brain surface.
T1-weighted volumetric MRI images from each aphasic subject were also normalized into MNI stereotactic space using the cost function masking method. Normalization parameters were then applied to the hand-drawn stroke mask of each subject to create normalized images of lesion extent. The MarsBaR software toolbox within SPM99 (Brett et al., 2002) was used to assess overlap between these normalized lesion images and to obtain measures of normalized lesion volume (in mm3) for each subject.
Significance thresholds for all whole-brain analyses were set at P < 0.05, corrected for multiple comparisons using the false discovery rate (Genovese et al., 2002). Application of this correction limits false positive activations to a maximum of 5% of the total number of suprathreshold voxels; to ensure that no false positive clusters were reported, customized cluster extent thresholds corresponding to 5% of the total number of suprathreshold voxels were applied to each contrast of interest.
Whole-brain analyses of speech-related activation
Regions engaged in speech comprehension in the normal group were identified with a whole-brain fixed-effects subtractive analysis that included scan order as a nuisance variable and blocked ANCOVA with global counts as confound. The contrast of interest [Sp–RevSp] was used to demonstrate regions responding preferentially to intelligible speech. The same method was used to identify speech-responsive regions in the aphasic group.
Whole-brain analyses of left anterolateral STC functional connectivity
The results of the initial within-group analyses of speech-related activation were used as the basis for whole-brain analyses of left anterolateral STC functional connectivity. In the normal group, the most significant left anterolateral STC activation peak for the contrast of interest [Sp–RevSp] was identified from the group-specific subtractive analysis. The first eigenvariate (adjusted for effects of interest) was extracted from a 5 mm radius spherical source region centred on this peak, as a representative measure of regional cerebral blood flow responses within that region across all scans [Sp and RevSp]. The eigenvariate data were used as the covariate of interest (predictor variable) in a first-level whole-brain linear regression analysis. Individual-subject contrast images drawn from this analysis were entered into a second-level (random effects) analysis to identify voxels demonstrating a positive linear relationship with regional cerebral blood flow responses in the left anterolateral STC source region. The whole-brain connectivity analysis in the aphasic subjects employed the same method, using a group-specific source region centred on the most significant left anterolateral STC activation peak from the aphasic group subtractive analysis. This approach enabled an anatomically unconstrained search within each group for voxels where regional cerebral blood flow responses across all scans co-varied positively with responses in a speech-responsive left anterolateral STC source region. Using scans from both conditions effectively investigated covariance with left anterolateral STC responses during processing of complex speech-like sounds, regardless of intelligibility. Although between-condition differences will influence covariance, as voxels demonstrating significantly greater activation for the Sp condition will tend to co-vary with left anterolateral STC, this method will also detect regions that co-vary with left anterolateral STC but do not show significant differences in activation between conditions. Moreover, regions showing significantly greater mean activation for the Sp condition will not necessarily co-vary significantly with left anterolateral STC activity on a scan-by-scan basis. Differences in the magnitude of left anterolateral STC functional connectivity between the normal and aphasic groups were investigated in a second-level analysis with an independent-samples t-test model, using an F-contrast to probe two-tailed differences.
Region of interest analyses
Left anterolateral STC connectivity was explored further using region of interest analyses. A left anterior superior temporal cortex (LalSTC) region of interest, defined anatomically as cortex in left superior temporal sulcus and lateral left superior temporal gyrus, was created manually from SPM99’s canonical averaged T1-weighted magnetic resonance image using ANALYZE software (Biomedical Imaging Resource, Mayo Clinic). The posterior border of the left anterolateral STC region of interest was the anterior border of Heschl's gyrus, the anterior border was the anterior termination of the superior temporal sulcus, the superior border was the junction between lateral surface of the superior temporal gyrus and the supratemporal plane, and the inferior border was the junction between the inferior bank of the superior temporal sulcus and the lateral surface of the middle temporal gyrus. The selection of additional regions of interest was based on the results of the whole-brain analysis of left anterolateral STC connectivity in the normal group: anatomically defined regions of interest were created for each cortical region demonstrating a significant functional connection with left anterolateral STC in the normal group. Construction of all regions of interest was based on anatomical criteria rather than peak activation coordinates in order to avoid possible sampling bias associated with between-group differences in peak location. Individual-subject data for region of interest analyses were obtained using the MarsBaR software toolbox within SPM99 (Brett et al., 2002), and statistical tests outside SPM99 were conducted using the Statistical Package for the Social Sciences-14 software package (SPSS Inc., Chicago).
Region of interest-based analyses of functional connectivity used correlation coefficients as measures of inter-regional connectivity (Horwitz et al., 1998). The first eigenvariate (adjusted for effects of interest) was extracted from each region of interest, in each subject, as a representative measure of regional cerebral blood flow responses in each region. Because we wished to explore meaningful relationships between connectivity and behavioural performance in the aphasic group, eigenvariate data from the baseline (RevSp) scans were discarded in all subjects. Only data from the intelligible speech (Sp) condition were used to determine connection strength, resulting in between six and eight regional cerebral blood flow measurements per subject for each region of interest. In each subject, regional cerebral blood flow responses to speech in the LalSTC region of interest were correlated separately with responses in regions of interest from each connected region, generating Pearson correlation coefficients as individual-subject measures of connectivity strength for each LalSTC connection. After application of Fisher's r-to-Z transform to generate normalized correlation coefficients (r′′), independent-samples t-tests were used to identify differences in connectivity strength between the normal and aphasic groups for each left anterolateral STC connection.
In addition, measures of mean regional cerebral blood flow responses (mean effect size) for the contrast [Sp–RevSp] were obtained from each region of interest, for each subject. Independent-samples t-tests were used to identify differences in the magnitude of mean speech-related activation between the normal and aphasic groups in each region of interest.
In the aphasic group, relationships between functional connectivity and behavioural performance were investigated for each LalSTC connection by correlating language test scores with connectivity measures (r′). Links between local cortical function and behavioural performance were investigated for each region of interest by correlating language test scores with speech-related responses (mean effect size). The behavioural measures of primary interest were the auditory single word, sentence and syntactic comprehension scores. When significant correlations between physiological responses and auditory comprehension measures were identified, correlations with written single word and sentence comprehension scores were also investigated in order to determine modality specificity.
Results
Left anterolateral STC functional connectivity
The normal and aphasic groups both demonstrated significant activation for Sp comprehension in similar bilateral temporal regions that included left anterolateral STC (Fig. 1A; Supplementary Table 1). Source regions for whole-brain analyses of left anterolateral STC connectivity were centred on the most significant left anterolateral STC speech-related responses in each group. These responses were located at similar coordinates within the superior temporal sulcus; [−52, 10, −18] for the normal group and [−50, 14, −22] for the aphasic group. In the normal group, regions demonstrating significant positive functional connectivity with left anterolateral STC were: anterior left superior temporal gyrus and sulcus, and left temporal pole adjacent to the source region; mid and posterior left superior temporal sulcus; left anterior BTC, centred on the fusiform gyrus; anterior left IFG, predominantly within pars triangularis; and homotopic right anterolateral STC (Fig. 1B; Supplementary Table 2). In the aphasic group, voxels demonstrating significant functional connectivity with left anterolateral STC were limited to left anterior superior temporal gyrus and superior temporal sulcus adjacent to the source region, and a small area in left mid superior temporal sulcus (Fig. 1B; Supplementary Table 2). Unlike the normal group, no significant functional connection was seen with voxels in left anterior BTC, left IFG or right anterolateral STC in the aphasic group. However, these differences were not statistically significant when connection strength was compared directly between the two groups in a whole-brain analysis.
Region of interest analyses
We created anatomically defined regions of interest for left anterolateral STC (LalSTC region of interest; see Materials and methods section for details) and for cortical areas demonstrating a significant functional connection with this region in the normal group's whole-brain connectivity analysis. These additional regions of interest were: LIFG (left IFG pars triangularis), LaBTC (left anterior fusiform gyrus in basal temporal cortex), and RalSTC (right anterior superior temporal gyrus and sulcus) (Fig. 1C; see Supplementary Methods for details of region of interest construction). LalSTC, left anterior fusiform gyrus and right anterior superior temporal gyrus and sulcus regions of interest were intact in all aphasic subjects (Fig. 1C). Four aphasic patients had lesions extending into the LIFG region of interest; analyses involving this region of interest excluded these subjects. Left mid and posterior STC were not included in the region of interest analysis, despite showing significant connectivity with left anterolateral STC in the normal group, as lesions extended into these regions in the majority of aphasic subjects.
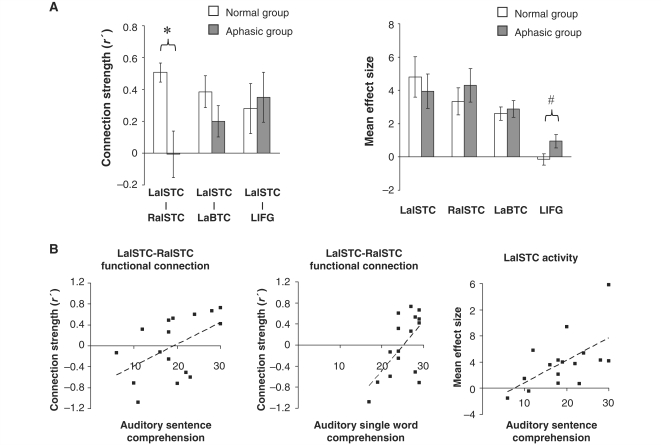
Investigating between-group differences in region of interest-based connection strength measures (normalized correlation coefficients), we found that connectivity between the LalSTC and right anterior superior temporal gyrus and superior temporal sulcus regions of interest was significantly lower in the aphasic than the normal group (P = 0.004) (Fig. 2A). Within the aphasic group, there was a significant positive correlation between measures of LalSTC–RalSTC functional connection strength and behavioural measures of auditory single word (r = 0.61, P = 0.01) and sentence comprehension (r = 0.51, P = 0.04) (Fig. 2B), but not syntactic comprehension (r = 0.28, P = 0.32). The association between inter-temporal connectivity and language comprehension was specific to the auditory modality; connection strength did not correlate with comprehension scores for written single words (r = −0.14, P = 0.61) or sentences (r = −0.02, P = 0.94). LalSTC–LaBTC and LalSTC–LIFG connectivity did not differ significantly between groups, and did not correlate significantly with language performance in the aphasic group.
Figure 2.
Region of interest analyses in the normal and aphasic groups. (A) Mean connection strength (normalized correlation coefficient, r′) (left) and mean speech-related activity (effect size) (right) in the normal and aphasic groups. Units of measurement for effect size are arbitrary. Error bars represent standard error of the mean. In the aphasic group, data relating to the LIFG region of interest are based on the 12 subjects with lesions sparing this region; data relating to all other regions are based on the entire group of 16 subjects. Significant between-group differences and non-significant trends are indicated: *P < 0.005; #P < 0.1 (see text for exact P-values). (B) Statistically significant correlations between physiological markers and behavioural measures of speech comprehension in the aphasic group. Dashed lines represent lines of best fit.
When investigating group differences in region of interest-based measures of speech-related activation (mean effect sizes), we found that LalSTC region of interest activation did not differ significantly between the normal and aphasic groups (Fig. 2A). However, there was a significant positive association between the magnitude of speech-related activation in the LalSTC region of interest and auditory sentence comprehension scores in the aphasic group (r = 0.59, P = 0.02) (Fig. 2B). This behavioural association was modality-specific, with no correlation between LalSTC activity and written sentence comprehension scores (r = 0, P = 1.0). There were strong but non-significant trends towards positive correlations between LalSTC activation and auditory single word (r = 0.45, P = 0.08) and auditory syntactic comprehension scores (r = 0.45, P = 0.09). In addition, there was a borderline-significant increase in speech-related LIFG region of interest activity in the aphasic group compared with the normal group (P = 0.053) (Fig. 2A). A positive relationship between LIFG activation and auditory single word comprehension scores approached statistical significance (r = 0.57, P = 0.056). Right anterior superior temporal gyrus and sulcus and LaBTC region of interest activation measures did not differ significantly between groups, nor did they correlate significantly with language performance.
In the aphasic group, inter-temporal connectivity was not significantly related to the magnitude of speech-related activation in either the LalSTC region of interest (Pearson r = 0.38, P = 0.15) or the RalSTC region of interest (Pearson r = 0.36, P = 0.17); therefore stronger connectivity was not simply a product of greater local activation, nor was weaker connectivity the result of weaker local activation. There was no significant relationship between either LalSTC–RalSTC connectivity or LalSTC activation and patient age (r = −0.06, P = 0.81; r = −0.39, P = 0.14, respectively), time post stroke onset (r = 0.25, P = 0.35; r = −0.11, P = 0.69, respectively), or lesion size (r = −0.14, P = 0.61; r = 0.22, P = 0.42, respectively). In the normal group, there was a borderline-significant positive relationship between patient age and LalSTC–RalSTC connectivity (r = 0.60, P = 0.052).
To investigate the possibility that post-stroke reorganization had resulted in functional alterations in homotopic right hemisphere activity (for example, Leff et al., 2002) or connections, we repeated between-group comparisons and behavioural correlations using measures of right anterior BTC and right IFG speech-related activation, and measures of RalSTC–RaBTC and RalSTC–RIFG connectivity obtained from right hemisphere regions of interest that mirrored those in left anterior BTC and left IFG. The normal and aphasic groups did not differ significantly with respect to right anterior BTC activation, right IFG activation, RalSTC–RaBTC connectivity or RalSTC–RIFG connectivity, nor did any of these measures correlate significantly with speech comprehension performance in the aphasic group.
Subgroup analysis of aphasic patients
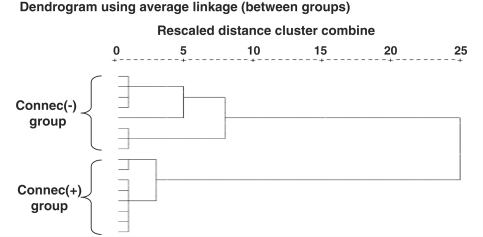
Region of interest analysis identified a selective abnormality of inter-temporal functional connectivity in the aphasic group. However, the strength of the LalSTC–RalSTC functional connection varied across the aphasic group from strongly positive to strongly negative. To investigate the hypothesis that the aphasic group contained subsets of patients with normal and abnormal inter-temporal connectivity, we performed a hierarchical cluster analysis using an agglomerative algorithm with LalSTC–RalSTC connectivity measures as the sole defining variable. Separation between clusters was determined by squared Euclidean distance, with the optimal cluster number solution determined by inspection of distance measures at each stage of the analysis. Hierarchical cluster analysis is an exploratory data analysis technique that seeks to organize a set of observations into groups such that differences between same-group observations are minimized while between-group differences are maximized (Everitt et al., 2001). Thus cluster analysis provided a data-driven criterion for dividing aphasic patients into subgroups with similar inter-temporal connectivity.
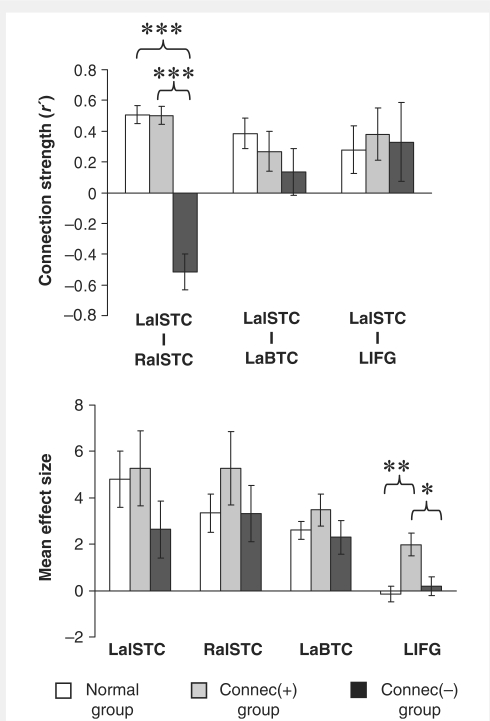
Cluster analysis indicated a clear division of the aphasic group into two subgroups, each containing eight patients (Fig. 3). One group, designated the Connec(+) patient group, demonstrated positive mean LalSTC–RalSTC connectivity, while the other group, designated the Connec(−) patient group, demonstrated negative mean inter-temporal connectivity (Table 1; Fig. 4). One-way ANOVA involving the two patient groups and the normal group showed a significant effect of group on LalSTC–RalSTC connectivity measures [F(2,24) = 51.7, P < 0.0005]. Inter-temporal connectivity in the normal and Connec(+) groups did not differ (P = 0.96), but each was significantly greater than the Connec(−) group (P < 0.0005 in both cases) (Fig. 4). ANOVA also demonstrated a significant between-group difference in mean LIFG speech-related activation [F(2,20) = 6.5, P = 0.007]. The Connec(+) patients with intact LIFG (n = 5) showed greater mean LIFG activation than either the normal group or the LIFG-intact Connec(−) patients (n = 7) (P = 0.007 and P = 0.022, respectively); LIFG activation in the latter two groups did not differ significantly (P = 0.53) (Fig. 4). ANOVAs performed on LalSTC–LIFG and LalSTC–LaBTC connectivity measures, and on LalSTC, RalSTC and LaBTC speech-related responses showed no significant between-group differences. Additional direct comparison of LalSTC activation in the Connec(+) and Connec(−) patients did not show a significant difference between LalSTC speech-related responses in the two subgroups (independent-samples t-test, P = 0.21; see Fig. 4). Finally, independent-samples t-tests demonstrated that the Connec(+) group possessed better language function than the Connec(−) group, with significantly higher auditory single word comprehension scores (P = 0.027), and a strong trend towards higher auditory sentence comprehension scores (P = 0.057).
Figure 3.
Cluster analysis in the aphasic group. The cluster dendrogram demonstrates separation of the aphasic group into two subgroups on the basis of LalSTC–RalSTC functional connection strength.
Table 1.
Comparison of characteristics of the Connec(+) and Connec(−) patient subgroups
| Connec(+) group | Connec(−) group | |
|---|---|---|
| Number of patients | 8 | 8 |
| LalSTC–RalSTC connection strength (r')** | 0.50 (0.06) | −0.52 (0.12) |
| LIFG speech-related activity* | 1.98 (0.49) | 0.20 (0.41) |
| Age (years) | 65.6 (2.9) | 65.9 (3.1) |
| Time post stroke onset (months) | 36.1 (16.0) | 21.5 (9.5) |
| Lesion size (mm3) | 93 341 (24 017) | 96 090 (27 629) |
| Auditory comprehension | ||
| Single word* | 27.1 (0.7) | 23.1 (1.4) |
| Sentence† | 22.4 (2.3) | 15.8 (2.2) |
Data are given as mean values (±SEM). Significant differences between subgroups are indicated in bold, with non-significant trends indicated in italics. Data relating to the LIFG region of interest are based on the five Connec(+) subjects and seven Connec(−) subjects with lesions sparing this region.
**P < 0.0005; *P < 0.05; †P < 0.1 (see text for exact P values).
Figure 4.
Region of interest-based functional connectivity and speech-related activity in the aphasic subgroups. Mean connection strength (normalized correlation coefficient, r′) (top) and mean speech-related activity (effect size) (bottom) in the Connec(+), Connec(−) and normal groups. In the aphasic subgroups, data relating to the LIFG regions of interest are based on the five Connec(+) subjects and seven Connec(−) subjects with lesions sparing this region; data relating to all other regions are based on the full number of eight subjects in each subgroup. Normal data are identical to that in Fig. 2A. Units of measurement for effect size are arbitrary. Error bars represent standard error of the mean. Significant between-group differences are indicated: ***P < 0.0005; **P < 0.01, *P < 0.05 (see text for exact P-values).
The two aphasic subgroups did not differ significantly in terms of patient age (P = 0.95), time post stroke onset (P = 0.45) or lesion size (P = 0.94) (see Table 1). In addition, there was no evidence of between-group differences in the degree of reorganization in the contralateral hemisphere: measures of connectivity and speech-related activation relating to homotopic RaBTC and RIFG regions did not differ significantly between patient groups.
In summary, speech comprehension performance in the aphasic patient group was linked to both the strength of intertemporal functional connectivity and the magnitude of left anterolateral lSTC speech-related activation. These two physiological markers were not themselves correlated; this is not statistically inconsistent, since a parameter may demonstrate separate significant correlations with two parameters that are not themselves significantly correlated. Examination of R2 values indicated that left anterolateral STC activity and intertemporal connection strength accounted for different proportions of the variance in auditory sentence comprehension scores, with left anterolateral STC activity accounting for slightly more of the variance than inter-temporal connectivity (R2 values of 0.34 and 0.26, respectively). Subgroup analysis indicated that when aphasic patients were divided on the basis of inter-temporal connection strength, the resulting subgroups did not differ in terms of the magnitude of left anterolateral STC activation. Overall, these results suggest that left anterolateral STC activity and inter-temporal functional connectivity make distinct contributions to speech comprehension performance; the relative importance of each of these factors cannot be determined from the current data.
Relationship between inter-temporal connectivity and lesion location
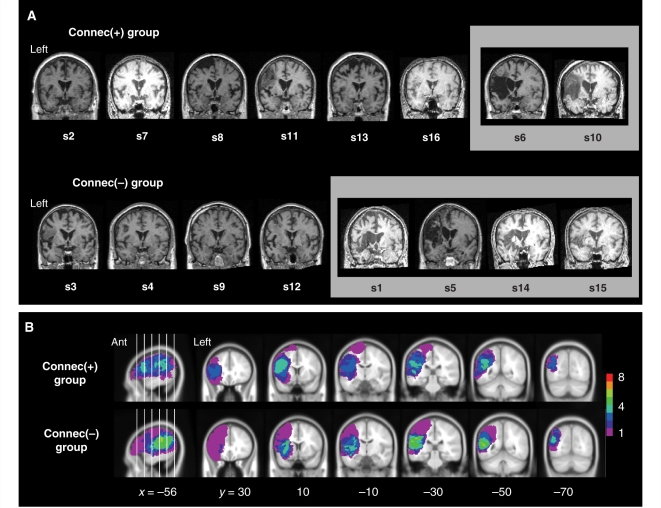
Human and non-human primate neuroanatomical studies indicate that left and right anterior temporal lobes, including anterior STC, are directly connected via the white matter tract of the anterior commissure (Demeter et al., 1990; Catani et al., 2002; Schmahmann and Pandya, 2006). In order to test the hypothesis that the strength of LalSTC–RalSTC connectivity was influenced by the presence or absence of a lesion involving white matter along the known trajectory of the anterior commissural pathway leading from anterior STC. (Catani et al., 2002; Peuskens et al., 2004), we inspected normalized T1-weighted MRI images from each aphasic patient. Anterior commissural pathway involvement was determined from normalized images in order to minimize interindividual differences in temporal lobe orientation and because in MNI stereotactic space, the anterior commissure is an easily identifiable anatomical landmark coincident with the image origin. Visual inspection of individual lesion extent demonstrated no consistent pattern of anterior commissural pathway involvement in stroke lesions: lesions involving the temporal stem at the level of the anterior commissure where seen in four out of eight Connec(−) patients, but also in two out of eight Connec(+) patients (Fig. 5A).
Figure 5.
Lesion distribution in the aphasic subgroups. (A) T1-weighted structural images from aphasic patients in the Connec(+) and Connec(−) groups are displayed in the coronal plane at the level of the anterior commissure in order to demonstrate the structural integrity of interhemispheric white matter projections from anterolateral STC via the anterior commissure. Structural images have been normalized into MNI stereotactic brain space to facilitate identification of the anterior commissure, which is located at the image origin in MNI space. Images outlined in grey show lesion involvement of the temporal stem at the level of the anterior commissure, and therefore probable disruption of white matter tracts projecting from anterolateral STC to right hemispheric homotopic cortex. (B) Lesion overlap in the Connec(+) and Connec(−) patient subgroups, displayed as normalized lesion images in the same manner as in Fig. 1C. Ant = anterior.
Visual inspection of lesion overlap in the two patient subgroups suggested more consistent involvement of posterior superior and middle temporal cortex in the Connec(−) group (Fig. 5B). In order to test the hypothesis that inter-temporal connection strength was influenced by lesion location, we used an adaptation of voxel-based lesion-symptom mapping methods. Voxel-based lesion-symptom mapping compares neuropsychological scores of patients with and without lesions at a particular voxel in order to identify voxels where lesion status has a significant influence on behaviour (Bates et al., 2003; Rorden et al., 2007). We used LalSTC–RalSTC connectivity measures (normalized correlation coefficients) as the predictor (‘symptom’) variable in a non-parametric voxel-based lesion-symptom mapping analysis (see Supplementary Methods for details). This analysis demonstrated no brain regions where the presence of a lesion significantly influenced the strength of LalSTC–RalSTC connectivity (5% false discovery rate significance threshold; see Supplementary Methods). In particular, there were no voxels demonstrating a significant relationship between lesion status and inter-temporal connectivity in posterior superior and middle temporal cortex, or in white matter along the trajectory of the anterior commissural pathways. A further analysis using left anterolateral STC activation measures (mean effect sizes) as the predictor variable failed to demonstrate any brain regions where the presence of a lesion significantly influenced the strength of left anterolateral STC activation during speech comprehension. Finally, to investigate the possibility that auditory comprehension performance was influenced by lesion location, we conducted additional voxel-based lesion-symptom mapping analyses using auditory single word and sentence comprehension measures as predictor variables. There were no brain regions where the presence of a lesion significantly influenced auditory comprehension performance.
Discussion
For the first time, this study demonstrates a link between functional integrity of cortico-cortical language pathways and behavioural outcome after aphasic stroke. During normal narrative speech comprehension, dominant anterolateral STC demonstrated strong positive functional connections with other speech-responsive temporal cortical regions, including ipsilateral basal temporal cortex, homotopic non-dominant anterior temporal cortex; and ipsilateral inferior frontal cortex. As a group, aphasic stroke patients with receptive language impairment showed a selective loss of positive functional connectivity between left and right anterior STCs during narrative speech comprehension. Other left anterolateral STC connections were unaffected, meaning that this alteration in inter-temporal connectivity is unlikely to represent an epiphenomenal consequence of ‘noisier’ neural signals or decoupled vascular reactivity throughout a lesioned left hemisphere. Although the aphasic patients were significantly older than the normal subjects, this age difference cannot explain the reduction in LalSTC-RalSTC connectivity in the aphasic group, since inter-temporal connectivity tended to increase in strength with increasing age in the normal subjects.
Our data demonstrate the novel finding of a strong functional connection between left and right anterolateral STCs during normal narrative speech comprehension. Functional connectivity between two brain regions may reflect direct neural communication between two regions, or may be the consequence of a common ‘driving’ input (Friston, 1994). These two interpretations are not mutually exclusive; both mechanisms may contribute to functional connectivity. Because functional connectivity data alone cannot determine which explanation is most likely, interpretation of the left-right anterolateral STC functional connection must rely on convergent evidence from other sources. Current evidence provides little support for the idea that inter-temporal functional connectivity during normal speech comprehension is driven largely by common perceptual input from an external stimulus. Auditory information is relayed to anterolateral STC along a hierarchical processing pathway originating in more posterior early auditory association cortex (Rauschecker and Tian, 2000; Scott and Johnsrude, 2003), but these early auditory association regions demonstrate different response profiles to complex sounds in the left and right hemispheres (Scott et al., 2000; Scott and Johnsrude, 2003). Parallel ‘bottom-up’ relaying of auditory information along left and right hemisphere auditory processing pathways would therefore be unlikely to result in correlated responses in left and right anterolateral STC. Nor does existing evidence support the contention that inter-temporal functional connectivity during normal speech comprehension is driven principally by common neural input from a region elsewhere in left hemisphere language cortex. Comparative anatomical data from non-human primates suggest that the majority of contralateral afferent connections with human anterolateral STC arise from homotopic cortex, with only limited interhemispheric afferent input from non-homotopic regions (Cipolloni and Pandya, 1989; Gloor, 1997). Input from a left hemisphere region outside anterolateral STC is therefore unlikely to have an overriding influence on right anterolateral STC activation. Moreover, voxel-based lesion-symptom mapping analysis in our patient group failed to demonstrate a region outside anterolateral STC where lesion involvement was consistently associated with disrupted inter-temporal connectivity. Overall, converging evidence is most consistent with the idea that functional connectivity between left and right anterolateral STCs during normal narrative speech comprehension is mediated predominantly by direct neural communication via substantial interhemispheric anatomical connections linking homotopic cortical regions.
Several mechanisms could account for disruption of left-right anterolateral STC connectivity after focal brain injury. The direct interhemsipheric anatomical connection between left and right anterolateral STCs, which appears likely to provide the greatest contribution to normal inter-temporal functional connectivity, is located in the anterior commissure (Demeter et al., 1990; Catani et al., 2002; Schmahmann and Pandya, 2006). A white matter lesion involving anterior commissure tracts could disrupt interhemispheric transfer of neural information, leading to reduced functional connectivity between left and right anterolateral STCs. In the present study, half of the patients with abnormal inter-temporal connectivity demonstrated lesion involvement of white matter in the anterior portion of the temporal stem, where fibre tracts from anterior STC exit towards the anterior commissure. Alternatively, de-correlation of responses in left and right anterolateral STCs could occur as a remote consequence of a lesion elsewhere in left hemisphere language cortex, in the absence of structural disruption of anterior commissure tracts. If critical input into left anterolateral STC is lost as result of a focal lesion, either the quantity or quality of left anterolateral STC activity may be impaired due to changes in the size or the selectivity of the activated neuronal subpopulation, respectively. Effectively, abnormal input may result in ‘noisy’ left anterolateral STC activity that disrupts functional connectivity with right anterolateral STC. We found no evidence that changes in the quantity of left anterolateral STC activity after focal left hemisphere injury could account for disruption of inter-temporal connectivity: left anterolateral STC activation did not differ in the Connec(+) and Connec(−) subgroups, and inter-temporal connection strength in the patient group was unrelated to the magnitude of left anterolateral STC activity. Therefore, we hypothesize that remote lesion effects on the quality, rather than quantity, of left anterolateral STC activity may have contributed to loss of inter-temporal connectivity. Voxel-based lesion-symptom mapping analysis failed to demonstrate that altered left-right anterolateral STC connectivity was associated with a consistent lesion site elsewhere in left hemisphere language cortex, which suggests that lesions at multiple sites in the left hemisphere language network have the potential to disrupt functional connectivity between left and right anterolateral STC by this mechanism.
Across our aphasic group, inter-temporal connection strength was correlated with spoken single word and sentence comprehension performance. Subgroup analysis demonstrated that patients with significantly altered connectivity also demonstrated poorer receptive language function. Voxel-based lesion-symptom mapping analysis did not demonstrate any evidence that altered connectivity and impaired language comprehension were by-products of a consistent lesion elsewhere in left hemisphere language cortex. These data alone do not prove a causal relationship between altered inter-temporal connectivity and impaired speech comprehension. This is, however, the most plausible interpretation, which is strengthened by the abundance of published converging evidence indicating that function of the left anterolateral STC, and co-operation between left and right anterolateral STCs, are major contributors to normal language comprehension. In the normal brain, left anterolateral STC responds preferentially to the presence of intelligible verbal information (Scott et al., 2000; Belin et al., 2002; Davis and Johnsrude, 2003). Left anterolateral STC is activated by single-word stimuli (e.g. Price et al., 1996; Wise et al., 2001), but appears to play a greater role in the processing of connected language (Mazoyer et al., 1993; Stowe et al., 1998; Vandenberghe et al., 2002; Xu et al., 2005). In general, connected language is richer in both semantic and syntactic content than single-word stimuli. Left anterolateral STC activity is influenced by semantic processes operating over supralexical time-scales, such as the degree of semantic coherence within (Vandenberghe et al., 2002; Ferstl et al., 2008) and between sentences (Fletcher et al., 1995; Maguire et al., 1999), and the integration of lexical-semantic information into local context (Marinkovic et al., 2003). Data on the role of left anterolateral STC in syntactic processing are less straightforward. Some of the studies supporting a specific role in syntactic processing for the left anterior temporal lobe implicate the supratemporal plane (planum polare) rather than anterolateral STC (Meyer et al., 2000; Friederici, 2002; Friederici et al., 2003). Left anterolateral STC activation is modulated by the presence of syntactic structure (Humphries et al., 2006; Stowe et al., 1998), but not by syntactic complexity (Stowe et al., 1998). In aphasic stroke patients, lesion-symptom mapping data suggest that left anterolateral STC is involved in ‘very basic morphosyntactic aspects of sentence comprehension’ (Dronkers et al., 2004). However, in patients with semantic dementia, a disease marked by significant atrophy of superior as well as inferior left anterior temporal cortical structures (Chan et al., 2001; Rohrer et al., 2009; Seeley et al., 2009), syntactic comprehension is intact despite striking impairments in lexical-semantic processing (Schwartz et al., 1979; Hodges et al., 1992; Gorno-Tempini et al., 2004).
Taken together, these findings suggest that left anterolateral STC processes word-level information relevant to sentence context (Stowe et al., 1998), binding meaningful lexical information together over time into a coherent message (Vandenberghe et al., 2002). The results of the present study support this view. Aphasic patients showed a significant correlation between left anterolateral STC activity during comprehension of sentence-based stimuli and performance on auditory sentence comprehension testing outside the scanner. Associations between left anterolateral STC activation and auditory single word and syntactic comprehension were weaker, implying that neither single word nor syntactic processing alone accounts for the contribution of left anterolateral STC to sentence-level processing. Rather, left anterolateral STC activation during sentence comprehension appears to reflect the computational demands of processing and integration of meaningful information from multiple words across a sentence.
As discussed above, the positive functional connection between normal left and right anterolateral STCs demonstrated in the present study appears most likely to reflect direct neural communication between these two regions. Although functional connectivity analysis alone cannot determine the direction of information flow along a pathway, most cortico-cortical connections support bidirectional transmission of neural information. Existing evidence supports more than one possible account of mutual information exchange between the anterior STCs. One body of functional imaging data suggests that these two anterior temporal regions are engaged in distinct aspects of speech processing, with left anterolateral STC supporting the integrative processing of meaningful verbal information (as discussed above), and right anterolateral STC processing non-verbal voice-related information such as intonation and speaker identity (Scott et al., 2000; Belin et al., 2002; von Kriegstein et al., 2003). Positive functional connectivity between left and right anterolateral STCs during normal narrative speech comprehension may represent the integration of verbal and non-verbal information, a process considered essential for speech comprehension (Friederici and Alter, 2004). An alternative model of anterior temporal function, based on lesion and repetitive transcranial magnetic stimulation data, proposes that semantic processing is bilaterally distributed across both anterior temporal cortices rather than left-lateralized. In patients with semantic dementia, significant deterioration in semantic processing is linked to the progression from left-lateralized to bilateral anterior temporal atrophy (Lambon Ralph et al., 2001; Patterson et al., 2007; Lambon Ralph and Patterson, 2008). Patients with early-onset chronic temporal lobe epilepsy demonstrate impairments in semantic knowledge regardless of whether the seizure focus is left- or right-lateralized (Bell et al., 2001). In normal subjects, semantic decision times are selectively slowed after application of repetitive transcranial magnetic stimulation to either left or right anterolateral STC (Pobric et al., 2007; Lambon Ralph et al., 2009). These findings suggest that semantic processing is supported by interaction between left and right anterior temporal regions. Integration of semantic information between left and right anterior temporal cortices may account more plausibly for the role of the inter-temporal functional connection in single-word comprehension.
Within our aphasic cohort, we identified two patient subgroups with distinct physiological and behavioural profiles. In the Connec(+) group, better receptive language function was associated with maintenance of normal positive inter-temporal connectivity, coupled with increased activation in anterior left IFG. In the absence of longitudinal data, it is difficult to ascertain whether preservation of inter-temporal connectivity in this subgroup of aphasic patients represented sparing at the time of lesion onset, or post-lesional reorganization and recovery. However, we speculate that the association of ‘good’ left-right anterolateral STC connectivity with increased left IFG activation may be more consistent with a recovery process. Anterior IFG possesses direct white matter connections with anterior STC (Anwander et al., 2006) and has a widely-acknowledged role in ‘top-down’ modulation of language comprehension processes (Thompson-Schill et al., 1997; Wagner et al., 2001), including the facilitation of context-dependent selection and integration of word meaning (Swaab et al., 1998; Bedny et al., 2007). In the present study, we demonstrated a significant functional connection between left anterior IFG and anterolateral STC during normal narrative speech comprehension. Although the data need to be interpreted with caution, increased left IFG top-down input into the left anterior temporal lobe after aphasic stroke may serve as a compensatory mechanism to stabilize or ‘de-noise’ anterolateral STC signals during the processing of speech for meaning, restoring normal functional connectivity between left and right anterolateral STCs. In the Connec(−) group, poorer receptive language function was associated with negative inter-temporal connectivity and a failure to increase left IFG activation. On the basis of functional imaging evidence, inhibition of the lesioned left hemisphere by the intact right hemisphere has been proposed as a maladaptive reorganization process after aphasic stroke (Rosen et al., 2000; Naeser et al., 2004; Saur et al., 2006). A similar mechanism may account for the negative inter-temporal connectivity observed in the subgroup with poorer language outcome.
Our claim that inter-temporal connectivity is critical to speech comprehension might appear to be challenged by evidence that removal of the left anterior temporal lobe for surgical treatment of intractable temporal lobe epilepsy is unlikely to result in significant speech comprehension deficits (Davies et al., 1995, 1998). However, left anterior temporal lobectomy rarely involves the removal of normally functioning left anterior temporal cortex from a normally organized language system. Temporal lobe epilepsy is associated with significant interictal hypofunction of anterior temporal cortex ipsilateral to the seizure focus (Ryvlin et al., 1991; Semah et al., 1995). Functional imaging and cortical stimulation studies provide evidence of both intra- and inter-hemispheric reorganization of language cortex in left temporal lobe epilepsy patients (Billingsley et al., 2001; Adcock et al., 2003; Thivard et al., 2005; Köylü et al., 2006; Hamberger et al., 2007). Atypical language lateralization is significantly more common in left temporal lobe epilepsy patients than healthy controls (Springer et al., 1999), and is associated with more frequent interictal epileptiform activity (Janszky et al., 2003, 2006). In most published series investigating language function after ATL, the majority of patients underwent surgery in adulthood after onset of seizures years previously in childhood or adolescence (e.g. Hermann et al., 1991; Davies et al., 1995, 1998). Thus the chronic intermittent electrical insult inflicted by abnormal ictal and interictal activity in left temporal lobe epilepsy contributes to reduced left anterolateral STC function and significant reorganization of the neural systems supporting language. Such reorganization cannot be excluded even in adult patients with relatively recent onset of seizures prior to ATL, or in those with rapidly progressive structural lesions: patterns of functional reorganization differ fundamentally between acute-onset lesions (minutes to hours) and those that evolve over more extended time scales (Desmurget et al., 2007). The absence of significant speech comprehension deficits after left anterior temporal lobectomy occurs in the context of probable preoperative redistribution of cortical function away from left anterolateral STC, and reorganization of functional pathways linked to this region. Our findings demonstrate that when an acute focal lesion in a previously healthy brain leaves left anterolateral STC intact, functional connectivity between left and right anterolateral STC is a marker of speech comprehension performance, and by extension, that functional connectivity LalSTC plays a key role in normal speech comprehension.
In conclusion, this study demonstrates that speech comprehension performance after aphasic stroke is linked to both left anterolateral STC activity and left-right anterolateral STC connection strength, while increased ‘top-down’ input from LIFG may act as a compensatory mechanism to maintain inter-temporal connectivity. These findings confirm the dual contribution of local cortical and pathway-level function to focal lesion effects on language processing, and illustrate that language system organization after focal brain lesions may be marked by complex signatures of altered intra- and inter-regional function. By establishing functional connectivity between left and right anterolateral STC as a novel marker of receptive language function after aphasic stroke, this study paves the way for future work investigating the role of inter-temporal functional connectivity in the recovery and treatment of a variety of aphasic disorders.
Funding
This work was funded by grants from Action Medical Research; the Barnwood House Trust; the Wellcome Trust, UK; Medical Research Council, UK.
Supplementary Material
Acknowledgements
The authors thank Dr Elizabeth Warburton for assistance with patient recruitment and PET scanning.
Glossary
Abbreviations
- BTC
basal temporal cortex
- IFG
inferior frontal gyrus
- LaBTC
left anterior fusiform gyrus region of interest
- LalSTC
left anterolateral STC region of interest
- LIFG
left IFG pars triangularis region of interest
- MNI
Montreal Neurological Institute
- RalSTC
right anterior superior temporal gyrus and suslcus region of interest
- SPM
statistical parametric mapping
- STC
superior temporal cortex
Supplementary material
Supplementary material is available at Brain online.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. NeuroImage. 2003;18:423–38. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knösche TR. Connectivity-based parcellation of Broca's area. Cereb Cortex. 2006;17:816–25. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6:448–50. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Bedny M, Hulbert JC, Thompson-Schill SL. Understanding words in context: the role of Broca's area in word comprehension. Brain Res. 2007;1146:101–14. doi: 10.1016/j.brainres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Ahad P. Human temporal-lobe response to vocal sounds. Cogn Brain Res. 2002;13:17–26. doi: 10.1016/s0926-6410(01)00084-2. [DOI] [PubMed] [Google Scholar]
- Bell BD, Hermann BP, Woodard AR, Jones JE, Rutecki PA, Sheth R, et al. Object naming and semantic knowledge in temporal lobe epilepsy. Neuropsychology. 2001;15:434–43. doi: 10.1037//0894-4105.15.4.434. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, McAndrews MP, Crawley AP, Mikulis DJ. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–27. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- Bishop DVM. Test for the Reception of Grammar. Manchester, England: University of Manchester Age and Cognitive Performance Research Centre; 1989. [Google Scholar]
- Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. NeuroImage. 2001;14:486–500. doi: 10.1006/nimg.2001.0845. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. [Abstract]. Presented at the 8th International Conference on Functional Mapping of the Human Brain, June 2–6, 2002, Sendai, Japan. NeuroImage. 2002;16:497. [Google Scholar]
- Buchsbaum BR, Olsen RK, Koch P, Berman KF. Human dorsal and ventral auditory streams subserve rehearsal-based and echoic processes during verbal working memory. Neuron. 2005;48:687–97. doi: 10.1016/j.neuron.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Cao Y, Vikingstad EM, George KP, Johnson AF, Welch KMA. Cortical language activation in stroke patients recovering from aphasia with functional MRI. Stroke. 1999;30:2331–40. doi: 10.1161/01.str.30.11.2331. [DOI] [PubMed] [Google Scholar]
- Cardebat D, Démonet JF, de Boissezon X, Marie N, Marié RM, Lambert J, et al. Behavioral and neurofunctional changes over time in healthy and aphasic subjects: a PET language activation study. Stroke. 2003;34:2900–6. doi: 10.1161/01.STR.0000099965.99393.83. [DOI] [PubMed] [Google Scholar]
- Catani M, ffytche DH. The rises and falls of disconnection syndromes. [Review] Brain. 2005;128:2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Makris N, Montinaro E, Sahin BA, Bates JF, Schwamm L, et al. Anatomy of stroke, part I: an MRI-based topographic and volumetric system of analysis. Stroke. 2002;33:2549–56. doi: 10.1161/01.str.0000036083.90045.08. [DOI] [PubMed] [Google Scholar]
- Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer's disease. Ann Neurol. 2001;49:433–42. [PubMed] [Google Scholar]
- Cipolloni PB, Pandya DN. Connectional analysis of the ipsilateral and contralateral afferent neurons of the superior temporal region in the rhesus monkey. J Comp Neurol. 1989;281:567–85. doi: 10.1002/cne.902810407. [DOI] [PubMed] [Google Scholar]
- Crinion J, Price CJ. Right anterior superior temporal activation predicts auditory sentence comprehension following aphasic stroke. Brain. 2005;128:2858–71. doi: 10.1093/brain/awh659. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Lambon-Ralph MA, Warburton EA, Howard D, Wise RJS. Temporal lobe regions engaged in normal speech comprehension. Brain. 2003;126:1193–1201. doi: 10.1093/brain/awg104. [DOI] [PubMed] [Google Scholar]
- Crinion JT, Warburton EA, Lambon-Ralph MA, Howard D, Wise RJS. Listening to narrative speech after aphasic stroke: the role of the left anterior temporal lobe. Cereb Cortex. 2006;16:1116–25. doi: 10.1093/cercor/bhj053. [DOI] [PubMed] [Google Scholar]
- Davies KG, Maxwell RE, Beniak TE, Destafney E, Fiol ME. Language function after temporal lobectomy without stimulation mapping of cortical function. Epilepsia. 1995;36:130–6. doi: 10.1111/j.1528-1157.1995.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Davies KG, Bell BD, Bush AJ, Hermann BP, Dohan FC, Jaap AS. Naming decline after left anterior temporal lobectomy correlates with pathological status of resected hippocampus. Epilepsia. 1998;39:407–19. doi: 10.1111/j.1528-1157.1998.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Davis MH, Johnsrude IS. Hierarchical processing in spoken language comprehension. J Neurosci. 2003;23:3423–31. doi: 10.1523/JNEUROSCI.23-08-03423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter S, Rosene DL, Van Hoesen GW. Fields of origin and pathways of the interhemispheric commissures in the temporal lobe of macaques. J Comp Neurol. 1990;302:29–53. doi: 10.1002/cne.903020104. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. [Review] Brain. 2007;130:898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–77. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Everitt BS, Landau S, Leese M. Cluster Analysis. 4th. London: Hodder Arnold; 2001. [Google Scholar]
- Ferstl EC, Neumann J, Bogler C, von Cramon DY. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Hum Brain Mapp. 2008;29:581–93. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, et al. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: A dynamic dual pathway model. Brain Lang. 2004;89:267–76. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Rüschmeyer SA, Hahne A, Fiebach CJ. The role of left inferior frontal and superior temporal cortex in sentence comprehension: localizing syntactic and semantic processes. Cereb Cortex. 2003;13:170–77. doi: 10.1093/cercor/13.2.170. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–78. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965a;88:237–94. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965b;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- Gloor P. The temporal lobe and limbic system. New York: Oxford University Press; 1997. [Google Scholar]
- Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, Wiliiams A, Perrine K, Devinsky O, et al. Evidence for cortical reorganization of language in patients with hippocampal sclerosis. Brain. 2007;130:2942–50. doi: 10.1093/brain/awm187. [DOI] [PubMed] [Google Scholar]
- Heiss WD, Thiel A, Kessler J, Herholz K. Disturbance and recovery of language function: correlates in PET activation studies. Neuroimage. 2003;20:S42–49. doi: 10.1016/j.neuroimage.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Somes G. Language function following anterior temporal lobectomy. J Neurosurg. 1991;74:560–6. doi: 10.3171/jns.1991.74.4.0560. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. [Review] Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Braun AR. Brain network interactions in auditory, visual and linguistic processing. Brain Lang. 2004;89:377–84. doi: 10.1016/S0093-934X(03)00349-3. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci USA. 1998;95:8939–44. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda K, Ishii K, Minoshima S, Kohmura E. Probabilistic cortical surface map of the middle cerebral artery territory for single-photon emission computed tomography studies. J Neurosurg. 2007;106:119–27. doi: 10.3171/jns.2007.106.1.119. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006;18:665–79. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain FT, McKinney CM, Horwitz B. Frontal cortex functional connectivity changes during sound categorization. NeuroReport. 2006;17:617–21. doi: 10.1097/00001756-200604240-00012. [DOI] [PubMed] [Google Scholar]
- Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–51. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided interictal epileptic activity indices shift of language lateralization in temporal lobe epilepsy: an fMRI study. Epilepsia. 2006;47:921–7. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004a;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Just MA, Newman SD, Keller TA, McEleney A, Carpenter PA. Imagery in sentence comprehension: an fMRI study. NeuroImage. 2004b;21:112–24. doi: 10.1016/j.neuroimage.2003.08.042. [DOI] [PubMed] [Google Scholar]
- Köylü B, Trinka E, Ischebeck A, Visani P, Trieb T, Kremser C, et al. Neural correlates of verbal semantic memory in patients with temporal lobe epilepsy. Epilepsy Res. 2006;72:178–91. doi: 10.1016/j.eplepsyres.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Kreisler A, Godefroy O, Delmaire C, Debachy B, Leclercq M, Pruvo JP, et al. The anatomy of aphasia revisited. Neurology. 2000;54:1117–23. doi: 10.1212/wnl.54.5.1117. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K. Generalisation and differentiation in semantic memory: Insights from semantic dementia. Ann NY Acad Sci. 2008;1124:61–76. doi: 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci. 2001;13:341–56. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Ralph MA, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cer Cor. 2009;19:832–8. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Leff A, Crinion J, Scott S, Turkheimer F, Howard D, Wise R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Ann Neurol. 2002;51:553–8. doi: 10.1002/ana.10181. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Morris RGM. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122:1839–50. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38:487–97. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazoyer BM, Tzourio N, Frak V, Syrota A, Murayama N, Levrier O, et al. The cortical representation of speech. J Cogn Neurosci. 1993;5:467–79. doi: 10.1162/jocn.1993.5.4.467. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. From sensation to cognition. [Review] Brain. 1998;121:1013–52. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Meyer M, Friederici AD, von Cramon DY. Neurocognition of auditory sentence comprehension: event related fMRI reveals sensitivity to syntactic violations and task demands. Cogn Brain Res. 2000;9:19–33. doi: 10.1016/s0926-6410(99)00039-7. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. NeuroImage. 2004;22:29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Obleser J, Wise RJS, Dresner MA, Scott SK. Functional integration across brain regions improves speech perception under adverse listening conditions. J Neurosci. 2007;27:2283–9. doi: 10.1523/JNEUROSCI.4663-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. [Review] Nat Rev Neurosci. 2007;8:976–87. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Paus T, Perry DW, Zatorre RJ, Worsley KJ, Evans AC. Modulation of cerebral blood flow in the human auditory cortex during speech: role of motor-to-sensory discharges. Eur J Neurosci. 1996;8:2236–46. doi: 10.1111/j.1460-9568.1996.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Peuskens D, van Loon J, Van Calenbergh F, van den Bergh R, Goffin J, Plets C. Anatomy of the anterior temporal lobe and the frontotemporal region demonstrated by fiber dissection. Neurosurgery. 2004;55:1174–84. doi: 10.1227/01.neu.0000140843.62311.24. [DOI] [PubMed] [Google Scholar]
- Pobric G, Jefferies E, Lambon Ralph MA. Anterior temporal lobes mediate semantic representation: mimicking semantic dementia by using rTMS in normal participants. Proc Natl Acad Sci USA. 2007;104:20137–41. doi: 10.1073/pnas.0707383104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Crinion J. The latest on functional imaging studies of aphasic stroke. [Review] Curr Opin Neurol. 2005;18:429–34. doi: 10.1097/01.wco.0000168081.76859.c1. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ. Hearing and saying. The functional neuro-anatomy of auditory word processing. Brain. 1996;119:919–31. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Price CJ, Crinion J, Friston KJ. Design and analysis of fMRI studies with neurologically impaired patients. [Review] J Magn Reson Imaging. 2006;23:816–26. doi: 10.1002/jmri.20580. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Tian B. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA. 2000;97:11800–6. doi: 10.1073/pnas.97.22.11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, et al. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72:1562–9. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19:1081–8. doi: 10.1162/jocn.2007.19.7.1081. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Petersen SE, Linenweber MR, Snyder AZ, White DA, Chapman L, et al. Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology. 2000;55:1883–94. doi: 10.1212/wnl.55.12.1883. [DOI] [PubMed] [Google Scholar]
- Ryvlin P, Cinooti L, Froment JC, Le Bars D, Landais P, Chaze M, et al. Metabolic patterns associated with non-specific magnetic resonance imaging abnormalities in temporal lobe epilepsy. Brain. 1991;114:2363–83. doi: 10.1093/brain/114.6.2363. [DOI] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, et al. Dynamics of language reorganization after stroke. Brain. 2006;129:1371–84. doi: 10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Saur D, Kreher BW, Schnell S, Kümmerer D, Kellmeyer P, Vry MS, et al. Ventral and dorsal pathways for language. Proc Natl Acad Sci USA. 2008;105:18035–40. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford University Press; 2006. [Google Scholar]
- Schulz GM, Varga M, Jeffires K, Ludlow CL, Braun AR. Functional neuroanatomy of human vocalization: an H2150 PET study. Cereb Cortex. 2005;15:1835–47. doi: 10.1093/cercor/bhi061. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Marin OS, Saffran EM. Dissociations of language function in dementia: a case study. Brain Lang. 1979;7:277–306. doi: 10.1016/0093-934x(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Scott SK, Johnsrude IS. The neuroanatomical and functional organization of speech perception. [Review] Trends Neurosci. 2003;26:100–7. doi: 10.1016/S0166-2236(02)00037-1. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank SC, Rosen S, Wise RJS. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123:2400–6. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Crawford RK, Zhou J, Miller BL, Grecius MD. Neurodegenerative diseases target large-scale human brain networks. Neuron. 2009;62:42–52. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semah F, Baulac M, Hasboun D, Frouin V, Mangin J-F, Papageorgiou S, et al. Is interictal temporal hypometabolism related to mesial temporal sclerosis? A positron emission tomography/magnetic resonance imaging confrontation. Epilepsia. 1995;36:447–56. doi: 10.1111/j.1528-1157.1995.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Wise RJS. Retrieving meaning after temporal lobe infarction: the role of the basal language area. Ann Neurol. 2004;56:836–46. doi: 10.1002/ana.20294. [DOI] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–36. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PSF, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–45. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Stowe LA, Broere CAJ, Paans AMJ, Wijers AA, Mulder G, Vaalburg W, et al. Localizing components of a complex task: sentence processing and working memory. NeuroReport. 1998;9:2995–9. doi: 10.1097/00001756-199809140-00014. [DOI] [PubMed] [Google Scholar]
- Swaab TY, Brown C, Hagoort P. Understanding ambiguous words in sentence contexts: electrophysiological evidence for delayed contextual selection in Broca's aphasia. Neuropsychologia. 1998;36:737–61. doi: 10.1016/s0028-3932(97)00174-7. [DOI] [PubMed] [Google Scholar]
- Swinburn K, Porter G, Howard D. The comprehensive aphasia test. Hove, UK: Psychology Press; 2004. [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, Samson S, et al. Productive and perceptive language reorganization in temporal lobe epilepsy. NeuroImage. 2005;24:841–51. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, D’Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieval of semantic knowledge: A reevaluation. Proc Natl Acad Sci USA. 1997;94:14792–7. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. J Cogn Neurosci. 2002;14:550–60. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Vigneau M, Beaucousin V, Hervé PY, Duffau H, Crivello F, Houdé O, et al. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30:1414–32. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Giraud AL. Distinct functional substrates along the right superior temporal sulcus for the processing of voices. NeuroImage. 2004;22:948–55. doi: 10.1016/j.neuroimage.2004.02.020. [DOI] [PubMed] [Google Scholar]
- von Kriegstein K, Eger E, Kleinschmidt A, Giraud AL. Modulation of neural responses to speech by directing attention to voices or verbal content. Cogn Brain Res. 2003;17:48–55. doi: 10.1016/s0926-6410(03)00079-x. [DOI] [PubMed] [Google Scholar]
- Wade DT, Hewer RL, David RM, Enderby PM. Aphasia after stroke: natural history and associated deficits. J Neurol Neurosurg Psychiatry. 1986;49:11–6. doi: 10.1136/jnnp.49.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Paré-Blagoev EJ, Clark J, Poldrack RA. Recovering meaning: left prefrontal cortex guides controlled semantic retrieval. Neuron. 2001;31:329–38. doi: 10.1016/s0896-6273(01)00359-2. [DOI] [PubMed] [Google Scholar]
- Wernicke K. Breslau: Cohn & Weigert; 1874. The aphasia symptom complex: a psychological study of an anatomic basis. Translated in: Eggert GH. Wernicke's Works on Aphasia: A Sourcebook and Review. The Hague: Mouton Publishers, 1977. [Google Scholar]
- Wise RJ, Scott SK, Blank SC, Mummery CJ, Murphy K, Warburton EA. Separate neural subsystems within 'Wernicke's area'. Brain. 2001;124:83–95. doi: 10.1093/brain/124.1.83. [DOI] [PubMed] [Google Scholar]
- Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. NeuroImage. 2005;25:1002–15. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.