Abstract
Background
Mammary ductoscopy allows direct visualization of ductal epithelium using a fibreoptic microendoscope. As the first centre in Canada to apply ductoscopy to surgical practice, we report our experience with this technology.
Methods
Between 2004 and 2008, 65 women with pathologic nipple discharge underwent ductoscopy before surgical duct excision under general anesthetic. Prospective data collection included cannulation and complication rates, procedure length and lesion visualization rate compared with preoperative ductography, if performed. In addition, we classified the endoscopic appearance according to Makita and colleagues and correlated it with surgical pathology.
Results
It took longer than 6 months to overcome technical problems before the routine use of ductoscopy in the operating room. The ductoscope was easy to use: we achieved cannulation in 63 of 66 breast ducts (95%) and we visualized a lesion in 52 of 63 breast ducts (83%). The mean procedure length was 5.1 minutes, with no complications. Lesions seen on ductography were seen endoscopically 30 of 33 (91%) times. All 3 malignancies were seen: invasive carcinoma in 1 of 62 (1.6%) and in situ disease in 2 of 62 (3.2%) patients. Surgeons found ductoscopy helpful in defining the extent of duct excision. Except for the “polypoid solitary” class, which accurately predicted a papilloma (23/23), we found poor correlation between Makita and colleague’s endoscopic classification and final pathology.
Conclusion
Ductoscopy is feasible, safe and practical. Our surgeons routinely use it to identify the location and extent of duct excision without ordering preoperative ductography. Identifying pathology based on the endoscopic appearance is unreliable unless the lesion is solitary and polypoid.
Abstract
Contexte
La ductoscopie mammaire permet une visualisation directe de l’épithélium des canaux à l’aide d’un microendoscope à fibres optiques. En tant que premier centre au Canada à appliquer la ductoscopie à la pratique chirurgicale, nous faisons état de l’expérience que nous avons acquise avec cette technologie.
Méthodes
Entre 2004 et 2008, 65 femmes présentant un écoulement pathologique au niveau du mamelon ont subi une ductoscopie avant une excision canalaire chirurgicale sous anesthésie générale. La collecte prospective des données a inclus les taux de canulation et de complications, la durée de l’intervention et le taux de visualisation des lésions, comparativement à la ductographie préopératoire, le cas échéant. De plus, nous avons classifié l’aspect à l’endoscopie selon les critères de Makita et coll. et nous avons établi sa corrélation avec la pathologie chirurgicale.
Résultats
Il a fallu plus de six mois pour surmonter certains problèmes techniques avant de pouvoir utiliser de routine la ductoscopie au bloc opératoire. Facile d’emploi, le ductoscope nous a permis d’effectuer avec succès la canulation de 63 canaux mammaires sur 66 (95 %) et de visualiser une lésion dans 52 canaux mammaires sur 63 (83 %). La durée moyenne de l’intervention a été de 5,1 minutes, sans complications. Les lésions perceptibles à la ductographie s’observaient à l’endoscopie 30 fois sur 33 (91 %). Les 3 néoplasies ont été aperçues : un carcinome envahissant dans 1 cas sur 62 (1,6 %) et une maladie in situ dans 2 cas sur 62 (3,2 %). Les chirurgiens ont trouvé la ductoscopie utile pour déterminer l’étendue de l’excision canalaire. À l’exception de la classe « solitaire et polypoïde » qui permettait de prévoir avec précision un papillome (23/23), nous avons observé une faible corrélation entre la classification endoscopique de Makita et coll. et la pathologie finale.
Conclusion
La ductoscopie est une intervention faisable, sécuritaire et pratique. Nos chirurgiens l’utilisent de routine pour localiser l’excision canalaire et en déterminer l’ampleur, sans demander de ductographie préopératoire. La reconnaissance de la pathologie à partir de l’aspect endoscopique est peu fiable, à moins que la lésion ne soit solitaire et polypoïde.
Pathologic nipple discharge is unilateral, persistent and spontaneous, and arises from a single duct.1 Discharge of this type is responsible for up to 5% of consultations at breast clinics.2 The etiology is usually benign: a papilloma is the most common cause (40%–70%), followed by adenomatous or papillary epithelial proliferations (14%). However, 1%–23% of women with pathologic nipple discharge are diagnosed with breast cancer or ductal carcinoma in situ;3 therefore, further investigations including a surgical duct excision are generally recommended.
Mammary ductoscopy has been described since 1989 to evaluate and diagnose nipple discharge in women.4,5 The development of a submillimetre fibreoptic microendoscope that directly inserts into the nipple orifice allows not only direct visualization of the mammary ductal epithelium,6 but also biopsy and cytological analysis of intraductal lesions.7 Despite this, ductoscopy has yet to be incorporated into routine surgical practice. As the first centre to introduce ductoscopy to Canada, our group at Princess Margaret Hospital prospectively collected data to report on our initial experience of bringing this new technology into surgical practice.
One of the suggested benefits of ductoscopy is the potential of avoiding a surgical duct excision, because most of these lesions are benign. If benign and malignant lesions could be differentiated based on their endoscopic appearance, as has been suggested by researchers in Japan,8,9 and because ductoscopy has been reported to be performed under local anesthetic,7 perhaps in the future most benign lesions could be excised through ductoscopy in the clinic. In 2001, the Japanese Association of Mammary Ductoscopy8 proposed a classification system based on the objective endoscopic appearance of intraductal lesions. There were 4 categories: polypoid, solitary; polypoid, multiple; superficial; and combined. Makita and colleagues9 showed that the polypoid solitary appearance was owing to benign papillary lesions, whereas combined and superficial lesions were more likely to be associated with breast cancer. The polypoid multiple types of lesions were found to be benign in some patients (35%) and malignant in others (65%). We therefore prospectively attempted to classify the lesions we saw based on their endoscopic appearance.
Methods
We obtained ethics approval from the Institutional Review Board of the University Health Network. From July 2004 to September 2008, women with pathologic nipple discharge in our 5-surgeon clinical practice were offered entry into this prospective study. Exclusion criteria included inability to provide informed consent or poor general health such that an additional 15–30 minutes of general anesthesia was not recommended. We obtained approval for the use of “medical devices for special access” for each patient from Health Canada; although approved in the United States, the ductoscope is not approved in Canada as a medical device.
Equipment included a ductoscope (MS-611, model #100–0201, 0.7 mm; FiberTech Co., Ltd.) attached to a fibre imaging system (FT-201, FiberTech Co., Ltd.) that projected an image onto a computer screen. Patients underwent ductoscopy after induction of general anesthesia. After identification of discharge from the duct, the surgeon gently dilated the duct orifice. For dilation we initially used a set of Bowman lacrimal probes (Karl Storz GmbH & Co., size 00-4); later, we worked with hospital engineers to design a cannula that gently increased in diameter over its length. The surgeon inserted and advanced the ductoscope under direct visualization, and manually injected normal saline through the biopsy channel to dilate the duct as the scope advanced. The scope was directed by moving the breast. When a lesion was identified, the surgeon marked the skin over the area. On removal of the ductoscope, the surgeon proceeded with standard duct excision, making sure to remove the marked area. The surgeon completed a data sheet that included an endoscopic description of any visualized lesion. We retrospectively compared the endoscopic appearance with the findings on ductography. We do not routinely order pre-operative ductography at our institution; it is usually performed at the discretion of the radiologist.
Results
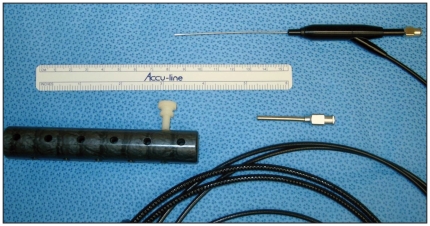
In all, 65 women with pathologic nipple discharge (patient age 25–76 yr) underwent ductoscopy before standard surgical duct excision from July 2004 to September 2008. Two surgeons performed the first 2 procedures to learn the procedure, with the assistance of a technical team from FiberTech Co., Ltd., Japan. The endoscope was easy to use, the ducts well visualized and lesions easily seen. However, it took longer than 6 months to overcome technical problems, which led to the early loss of at least 4 ductoscopes with a delivery time from Japan of 2 months. In Japan, each endoscope can be used at least 30–40 times before it needs to be replaced; at this point, the picture quality deteriorates as fibreoptic fibres get damaged. Our first endoscopes broke where the metal scope meets the plastic handle (Fig. 1) after only 1 or 2 uses during the sterilization process. After extensive consultation with our hospital engineers, the scopes are now fitted with a specially designed perforated silicon sleeve to protect this junction and to allow sterilization. Some surgeons also use a small metal cannula during the procedure to protect this junction. The light source needed to be refitted to Canadian electrical standards. Computer failure prevented the use of the equipment from September 2006 to January 2007.
Fig. 1.
Our first 4 FiberTech (0.7-mm external diameter) ductoscopes broke during the sterilization process at the junction of the metal scope and the plastic handle. Hospital engineers designed and built the perforated silicone sleeve (lower left), which fits over the scope when not in use to protect the junction and to allow for instrument sterilization. The metal cannula protects the junction when the surgeon uses the scope.
For 65 women, we performed ductoscopy on 66 breast ducts as 1 woman had discharge from 2 separate breast ducts. This particular patient had 2 ductoscopies, but both ducts were excised in the same specimen, with 1 pathology report. The cannulation rate, defined as the ability to enter the duct and visualize the epithelium, was successful in 63 of 66 (95%) breast ducts. Figure 2 and Figure 3 show select images obtained on ductoscopy. Reasons for failure were obstruction of the duct lumen at the nipple (1 patient), the ductoscope entered a seroma from previous surgery (1 patient) and video failure (1 patient). The surgeon visualized a lesion in 52 of 63 (83%) ducts. It took an average of 12.4 minutes per patient (recorded for 53 patients) to set up and conclude the procedure, with a mean endoscopy time of 5.1 minutes (recorded for 18 patients). There were no complications caused by the ductoscopy. After duct excision, 4 of 65 (6%) women experienced a seroma leak in the postoperative period, which resolved.
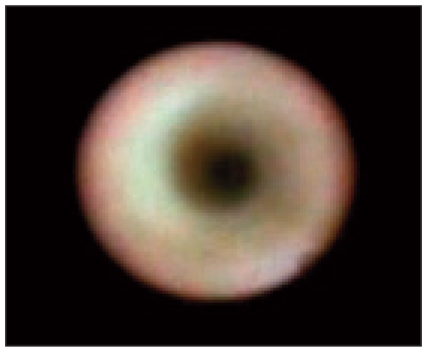
Fig. 2.
Ductoscopy image of a normal breast duct.
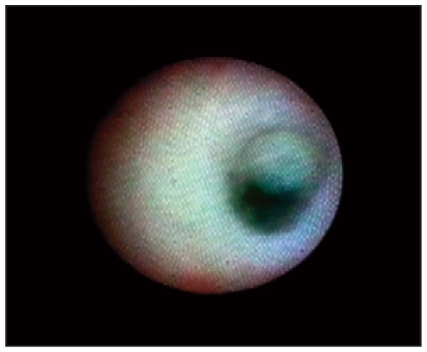
Fig. 3.
Ductoscopy image of a benign solitary papilloma.
We retrospectively compared preoperative ductograms with the surgeon’s ability to identify a lesion on ductoscopy (Table 1). Ductography was ordered for 47 patients, and a lesion defined as either a radiographic filling defect or a suspected mass. Ductography was technically not successful in 7 patients, contraindicated in 2 patients owing to radiographic contrast allergy, and did not show a lesion in 5 patients. Of the 33 lesions seen on preoperative ductograms, 30 were seen on ductoscopy (91%); the 3 not seen on ductoscopy were all benign papillomas. One lesion was seen on ductoscopy that was not seen on ductography (described as a polypoid multiple and pathologically a benign papilloma); in the other 4 cases in which no lesion was seen on ductography, no lesion was seen on ductoscopy either.
Table 1.
Comparison of the surgeon’s ability to identify lesions via ductoscopy versus preoperative radiographic ductography (n = 63)*
| Radiographic ductography | Mammary ductoscopy | |
|---|---|---|
| Lesion seen | Lesion not seen | |
| Lesion seen | 30 | 3 |
| Lesion not seen | 1 | 4 |
| Unsuccessful attempt at ductography or ductography contraindicated | 9 | — |
| Not ordered | 12 | 4 |
n = 63 because 1 woman had discharge from 2 separate breast ducts.
Table 2 describes the pathologic findings and compares them to the endoscopic appearance using the Japanese Association of Mammary Ductoscopy classification. No lesions were seen for 11 of 62 patients (18%); for 7 of 11 patients (64%), papillomas were seen on pathology. All 3 malignancies were seen; invasive carcinoma in 1 of 62 (2%) and in situ disease in 2 of 62 (3%). Of the 52 visualized lesions, 5 (10%) filled the duct entirely and could not be classified. Except for the “polypoid solitary” class, which accurately predicted a papilloma (23/23), we found a poor correlation between Makita’s endoscopic classification and final pathology.
Table 2.
Comparison of the endoscopic appearance to the final pathology (n = 62)
| Histologic diagnosis | Japanese Association of Mammary Ductoscopy classification8 | No lesion seen | Difficult to classify | |||
|---|---|---|---|---|---|---|
| Polypoid solitary | Polypoid multiple | Combined | Superficial spreading | |||
| Benign papilloma | 17 | 8 | 1 | 3 | 7 | 3 |
| No papilloma, no atypia | 2 | 4 | ||||
| Papilloma and atypical hyperplasia | 6 | 2 | 1 | 2 | 2 | |
| Hyperplasia with atypia, no papilloma | 1 | |||||
| Ductal carcinoma in situ | 1 | 1 | ||||
| Invasive ductal cell carcinoma | 1 | |||||
Discussion
We experienced considerable delays in adapting the Japanese technology to our Canadian operating room, primarily owing to differences in sterilization and electrical standards. Once these were overcome, however, we found the mammary ductoscopy easy to use, safe and a practical addition to the surgical duct excision. Surgeons found the ductoscopy made duct excision easier. Many papillomas are found within the first few centimetres of the nipple; visualization of the lesion allows the surgeon to limit the resection in these cases with confidence, without taking the entire duct. Dietz and colleagues10 noted that deep ductal lesions are less likely to be missed at surgical resection if resection is guided by the ductoscope as opposed to “blind” surgical resection. Furthermore, Moncrief and colleagues11 noted a nonsignificant trend in finding intraductal neoplasia among women who underwent ductoscopy-guided excision compared with women who underwent conventional retroareolar surgical terminal duct excision. Other advantages included conservation of breast tissue during resection and the ability to potentially preserve breast function, benefiting women who wish to breastfeed.11
Based on our experience, our surgeons now routinely use ductoscopy before surgical duct excision without ordering preoperative ductography. This practice is supported by Dietz and colleagues10 who, after reporting their experience with 70 patients, no longer order preoperative ductography because mammary ductoscopy has a higher lesion localization rate and allows for direct visualization.
We found it difficult to classify many of the lesions we saw into the discrete categories that had been proposed by Makita and colleagues.9 We confirm their finding that if a lesion is single and polypoid, it can be confidently declared a benign papilloma. The associated hyperplasia identified with 13 of the papillomas was likely an incidental finding. Any other appearance did not correlate with pathology. Other classification systems have been proposed. Al Sarakbi and colleagues12 graded lesions between 0 and 5 (D0-D5) based on the degree of suspicion of malignancy of the lesion. However, the classification system by Al Sarakbi and colleagues depends on surgeon experience rather than an objective description. We agree with the authors that verifying the endoscopic appearance of a lesion with histologic diagnosis continues to be a challenge unless the lesion is solitary and polypoid. We continue to offer surgical excision to all patients regardless of ductoscopic findings, but observe with interest reports such as that by Matsunaga and colleagues,13 who successfully therapeutically removed papillomas by intraductal biopsy 38 of 46 (82.6%) times. We have not begun ductoscopy under local anesthesia in a clinical office setting, but we recognize the potential advantages of affording women a rapid diagnosis.7
Mammary ductoscopy is safe, easy and practical, and is routinely used in the management of nipple discharge at our institution. Direct visualization of a lesion makes surgical excision easier and more reliable, decreasing the need for preoperative ductography. Despite the routine use of ductoscopy, we continue to offer surgical excision to all patients and await future studies to help define the role of mammary ductoscopy as a diagnostic and therapeutic tool for investigating pathologic nipple discharge. Until such gaps are filled, it is likely that mammary ductoscopy will remain experimental in Canada.
Acknowledgement
Funding for the ductoscope was provided by the Women’s Committee and the Amelia Plastina Breast Cancer Fund, Princess Margaret Hospital Foundation.
Footnotes
Presented in part at the Canadian Society of Surgical Oncology Meeting, Toronto, Ont., May 2, 2008.
Competing interests: None declared.
Contributors: Drs. Connolly, Leong and Easson designed the study. All authors acquired the data, which Drs. Simpson, Leong, Escallon, McCready, Reedijk and Easson analyzed. Drs. Simpson, Connolly, Leong, McCready, Reedijk and Easson wrote the article, which all authors reviewed and approved for publication.
References
- 1.Lang JE, Kuerer HM. Breast ductal secretions: clinical features, potential uses, and possible applications. Cancer Control. 2007;14:350–9. doi: 10.1177/107327480701400405. [DOI] [PubMed] [Google Scholar]
- 2.Okazaki A, Hirata K, Okazaki M, et al. Nipple discharge disorders: current diagnostic management and the role of fiber-ductoscopy. Eur Radiol. 1999;9:583–90. doi: 10.1007/s003300050715. [DOI] [PubMed] [Google Scholar]
- 3.Escobar PF, Crowe JP, Matsunaga T, et al. The clinical applications of mammary ductoscopy. Am J Surg. 2006;191:211–5. doi: 10.1016/j.amjsurg.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 4.Makita M, Sakamoto G, Akiyama F, et al. Duct endoscopy and endoscopic biopsy in the evaluation of nipple discharge. Breast Cancer Res Treat. 1991;18:179–88. doi: 10.1007/BF01990034. [DOI] [PubMed] [Google Scholar]
- 5.Pereira B, Mokbel K. Mammary ductoscopy: past, present, and future. Int J Clin Oncol. 2005;10:112–6. doi: 10.1007/s10147-004-0403-7. [DOI] [PubMed] [Google Scholar]
- 6.Berna JD, Garcia-Medina V, Kuni CC. Ductoscopy: a new technique for ductal exploration. Eur J Radiol. 1991;12:127–9. doi: 10.1016/0720-048x(91)90112-9. [DOI] [PubMed] [Google Scholar]
- 7.Dooley WC, Francescatti D, Clark L, et al. Office-based breast ductoscopy for diagnosis. Am J Surg. 2004;188:415–8. doi: 10.1016/j.amjsurg.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Association of Mammary Ductoscopy. Endoscopic classification of intraductal lesions. Proceedings of 6th annual meeting of Japanese Association of Mammary Ductoscopy; 2001; Tokyo. [Google Scholar]
- 9.Makita M, Akiyama F, Gomi N, et al. Endoscopic classification of intra-ductal lesions and histological diagnosis. Breast Cancer. 2002;9:220–5. doi: 10.1007/BF02967593. [DOI] [PubMed] [Google Scholar]
- 10.Dietz JR, Crowe JP, Grundfest S, et al. Directed duct excision by using mammary ductoscopy in patients with pathologic nipple discharge. Surgery. 2002;132:582–8. doi: 10.1067/msy.2002.127672. [DOI] [PubMed] [Google Scholar]
- 11.Moncrief RM, Nayar R, Diaz LK, et al. A comparison of ductoscopyguided and conventional surgical excision in women with spontaneous nipple discharge. Ann Surg. 2005;241:575–81. doi: 10.1097/01.sla.0000157371.10776.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Sarakbi W, Salhab M, Mokbel K. Does mammary ductoscopy have a role in clinical practice? Int Semin Surg Oncol. 2006;3:16. doi: 10.1186/1477-7800-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsunaga T, Ohta D, Misaka T, et al. Mammary ductoscopy for the diagnosis and treatment of intraductal lesions of the breast. Breast Cancer. 2001;8:213–21. doi: 10.1007/BF02967511. [DOI] [PubMed] [Google Scholar]