Abstract
Introduction:
The nuclear matrix protein 22 (NMP22) assay has been shown to have greater sensitivity for the diagnosis and detection of recurrent urothelial carcinoma of the bladder (UCB) over that of traditional urine cytology. We assessed the use of NMP22 to predict which high-risk superficial UCB patients will have recurrence, progression or disease-related death; we compared these results to standard urine cytology.
Methods:
One hundred consecutive patients with high-risk superficial UCB were enrolled. During surveillance, urine was collected for cytology and NMP22 testing. Patients were followed for at least 6 months. Retrospective chart review was undertaken to collect data on previous tumour history, tumour characteristics, disease recurrences, progression and death. Kaplan-Meier analyses were performed to determine the significance between NMP22-positive and -negative patients in terms of recurrence-free, progression-free and overall survival. Similar analyses were performed for urine cytology.
Results:
From 94 eligible patients, 15 and 79 were NMP22 positive and negative, respectively. The baseline characteristics between the 2 groups were not significantly different in terms of patient characteristics, prior tumour history or intravesical therapies received. Mean recurrence-free survival time was significantly lower in the NMP22 positive group (p = 0.038); however, mean progression-free and overall survival were not significantly different between the 2 groups (p = 0.297 and 0.519, respectively). Urine cytology demonstrated no significant predictive power for disease recurrence, progression or survival.
Conclusion:
The nuclear matrix protein 22 assay appears to have predictive value for future tumour recurrences, but not progression or overall survival in patients with high-risk superficial UCB.
Résumé
Introduction :
Il a été montré que le test de dépistage de la protéine 22 de la matrice nucléaire (NMP22) présentait une sensibilité supérieure pour le diagnostic et le dépistage du carcinome urothélial récurrent de la vessie, en comparaison avec la cytologie urinaire classique. Nous avons évalué l’emploi de la NMP22 pour prédire la récurrence, la progression de la maladie et le décès relié à la maladie chez des patients atteints de carcinome urothélial de la vessie (CUV) superficiel et présentant un risque élevé. Les résultats ont été comparés à ceux obtenus avec une épreuve de cytologie urinaire standard.
Méthodologie :
Cent patients consécutifs présentant un CUV superficiel à risque élevé ont été inscrits à l’étude. Pendant la période de surveillance, un échantillon d’urine a été recueilli en vue de l’épreuve de cytologie et du test de dépistage de la NMP22. Le suivi a duré au moins 6 mois. Un examen rétrospectif des dossiers a fourni des données concernant les antécédents tumoraux, les caractéristiques de la tumeur, les récurrences, la progression et les décès. Des analyses de Kaplan-Meier ont été effectuées afin de déterminer le niveau de signification entre les patients NMP22-positifs et négatifs en matière de délai sans récurrence, de délai sans progression et de survie globale. Des analyses similaires ont été réalisées pour les données obtenues par cytologie urinaire.
Résultats :
Sur les 94 patients admissibles, 15 patients étaient NMP22-positifs et 79, NMP22-négatifs. Les caractéristiques au départ entre les deux groupes n’étaient pas significativement différentes en ce qui concerne les caractéristiques des patients, les antécédents tumoraux et les antécédents de traitements intravésicaux. Le délai moyen de survie sans récurrence était significativement moins long dans le groupe de patients NMP22-positifs (p = 0,038); cela dit, le délai moyen sans progression et la survie globale moyenne n’étaient pas significativement différents entre les deux groupes (p = 0,297 et 0,519, respectivement). L’épreuve de cytologie urinaire n’a montré aucune puissance de prédiction significative concernant la récurrence de la maladie, la progression ou la survie.
Conclusion :
Le test de dépistage de la protéine 22 de la matrice nucléaire semble avoir une certaine valeur prédictive concernant les récurrences tumorales, mais non la progression ou la survie globale chez les patients atteints de CUV superficiel présentant un risque élevé de récurrence.
Introduction
In Canada, bladder cancer is the fourth most common cancer in men and eleventh most common cancer in women. It is predicted that 5100 men and 1700 women will be diagnosed with bladder cancer in 2008 and 1250 men and 530 women will succumb to this disease.1 Ninety percent of bladder cancer cases are classified as urothelial carcinoma of the bladder (UCB). Approximately 70% of newly diagnosed patients present with superficial disease that has not invaded into the muscularis propia.2 Superficial disease can be further divided into low-risk and high-risk groups. Patients having tumours that are high grade, T1, multiple, greater than 3 cm in size, quickly recurring at the 3-month surveillance cystoscopy or carcinoma in situ have high-risk superficial disease more prone to recur and progress.3 Standard treatments employ transurethral resection of bladder tumour (TURBT) and intravesical therapy. Despite following patients closely with routine surveillance cystoscopy, urine cytologies and radiologic examination, many of these patients recur and progress to muscle invasive or metastatic disease. This disease progression leads to reduced survival and has prompted treatments that may have a significant adverse impact on one’s quality of life. Thus, there is a need for alternative, noninvasive biologic tests that can identify recurrent or early progressive disease in patients with high-risk superficial UCB.
Recently, measurement of the nuclear matrix protein 22 (NMP22) in urine samples has come to the forefront as a cost-efficient point-of-care proteomic assay that could be used to detect UCB. The set of nuclear matrix proteins is involved in RNA splicing, transcription, DNA replication and regulation of gene expression.4 Importantly, changes in the composition of nuclear matrix proteins have been associated with the malignant transformation of cells.5 Furthermore, certain nuclear matrix proteins show cell specificity6 and become elevated in concordance with structural and morphological changes in cancer cell nuclei.7 Specifically, the concentration of NMP22 in the urine of a healthy patient is low, but becomes elevated in some patients with UCB.8 In fact, the NMP22 assay has been shown to have greater sensitivity for the diagnosis and detection of recurrent bladder tumours over that of traditional urine cytology.7,9 Additionally, measurements of NMP22 show increased predictive values if used in conjunction with behavioural and symptomatic data.10 These studies support the use of NMP22 as a detection tool for diagnosis and monitoring of UCB; however, to our knowledge, no investigations have been done assessing the use of NMP22 as a marker to predict which patients with high-risk superficial UCB will have recurrence, progression or disease-related death. We examine this question while also comparing results to standard urine cytology.
Methods
A total of 100 consecutive patients that presented for the first time with high-risk UCB (without any inflammatory condition of the urinary tract) from May 2006 to April 2008 were included in the study. The study protocol was approved by the institutional review board and informed consent was obtained from each participant in the study. Each patient enrolled provided a urine sample prior to their first cystoscopy following the pathologic diagnosis of a high-risk superficial UCB from their last TURBT. The urine sample was split for urine cytology and detection of NMP22 protein levels via the NMP22 point-of-care device (NMP22 BladderChek Test, Matritech Inc, Newton, Mass) as previously described.7
Study participants did not know the results of the NMP22 assay until after all 100 consecutive patients had been enrolled. After complete enrolment, retrospective chart reviews were undertaken documenting events from the time of diagnosis of the patient’s highest grade/stage tumour to the most recent follow-up. All patients had been followed for at least 6 months. Data were collected on presenting symptoms, bladder cancer history, tumour recurrence, progression and disease-related death. Patients who had a positive cystoscopy or malignant tumour on pathological examination after the enrolment routine surveillance were said to have recurred. Patients who demonstrated higher-stage and/or grade disease than was previously documented were identified to have disease progression. Follow-up was documented as the time from the previous highest stage/grade tumour to recurrence, progression, death or most recent follow-up.
Kaplan-Meier curves were constructed to compare tumour recurrence, progression and disease-related death rates between NMP22-positive and -negative patients. The log-rank test was used to determine if there was a significant difference between the 3 curves. A p-value less than 0.05 was defined as significant. Statistical analyses were performed using SPSS, version 16.0 (SPSS Inc, Chicago, Illinois).
Results
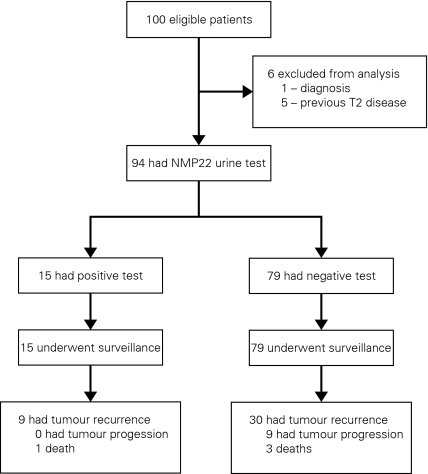
One participant originally thought to have UCB was excluded from the study after pathological examination revealed that the tumour was of lymphoma origin. Also, 5 patients who were subsequently discovered to have had previous T2 disease were also excluded (Fig. 1). In total, there were 94 patients in this study; 15 were in the NMP22-positive group and 79 were in the NMP22-negative group. There were no notable differences in baseline characteristics between NMP22-positive and NMP22-negative groups (Table 1).
Fig. 1.
Flow diagram of study documenting exclusions and number of recurrences, progressions and deaths in NMP22 positive and negative groups.
Table 1.
Baseline patient characteristics
| Variable | NMP22 positive (n = 15) | NMP22 negative (n = 79) | Overall (n = 94) |
|---|---|---|---|
| Age, yr. | |||
| Mean | 74.6 | 72.1 | 72.4 |
| Range | 47–87 | 44–94 | 44–94 |
| Sex | |||
| Male, no. (%) | 11 (73) | 60 (76) | 71 (76) |
| Female, no. (%) | 4 (27) | 19 (24) | 23 (24) |
| Presenting symptoms | |||
| Hematuria, no. (%) | 10 (67) | 48 (61) | 58 (62) |
| Voiding and irritative, no. (%) | 1 (6.5) | 12 (15) | 13 (14) |
| Asymptomatic, no. (%) | 3 (20) | 14 (18) | 17 (18) |
| Multiple symptoms, no. (%) | 0 (0) | 3 (4) | 3 (3) |
| Other symptoms, no. (%) | 1 (6.5) | 2 (2) | 3 (3) |
| Stage of initial tumour | |||
| Ta, no. (%) | 7 (47) | 35 (44) | 42 (45) |
| T1, no. (%) | 6 (40) | 36 (46) | 42 (45) |
| Tis, no. (%) | 2 (13) | 8 (10) | 10 (10) |
| Tumour with associated CIS | |||
| Yes, no. (%) | 2 (13) | 6 (8) | 8 (9) |
| No, no. (%) | 13 (87) | 73 (92) | 86 (91) |
| Multiple tumours | |||
| Yes, no. (%) | 9 (60) | 44 (54) | 53 (56) |
| No, no. (%) | 6 (40) | 35 (44) | 41 (44) |
| Intravesical BCG | |||
| Yes, no. (%) | 12 (80) | 61 (77) | 73 (78) |
| No, no. (%) | 3 (20) | 16 (20) | 21 (22) |
| Postoperative MMC | |||
| Yes, no. (%) | 8 (53) | 38 (48) | 46 (49) |
| No, no. (%) | 7 (47) | 41 (52) | 48 (51) |
Tis = tumour in situ; CIS = carcinoma in situ; BCG = bacillus Calmette–Guérin; MMC = mitomycin C.
Seven, 6 and 2 patients from the NMP22-positive group had pathological Ta, T1 and CIS, respectively. Conversely, 35, 36 and 8 patients from the NMP22-negative group had pathological Ta, T1 and CIS, respectively. Only 2 patients in the NMP22-positive group had associated CIS with a Ta or T1 tumour, while 9 patients in the same group possessed multiple tumours. Six patients from the NMP22-negative group had associated CIS, while 44 patients in the group had multiple tumours. Differences in baseline characteristics between cytology-positive and -negative groups were also insignificant. Eighty percent versus 77% of patients received intravesical bacilli Calmette-Guérin (BCG) and 53% versus 48% of patients in the NMP22-positive and -negative group, respectively, received intravesical mitomycin C chemotherapy immediately after TURBT. There were 26 patients (33%) in the NMP22-negative group that had prior lower-risk superficial tumours, while 6 patients (40%) in the NMP22-positive group had prior lower-risk superficial tumours.
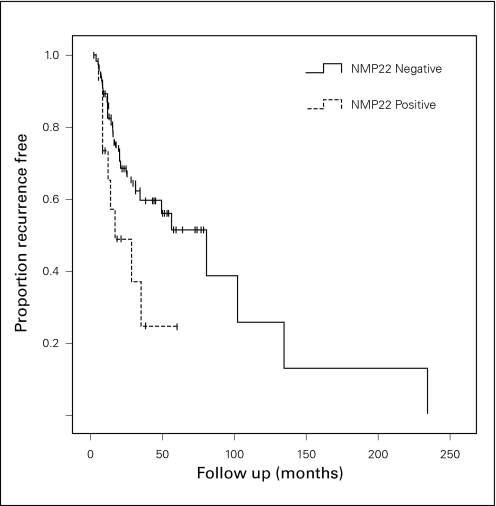
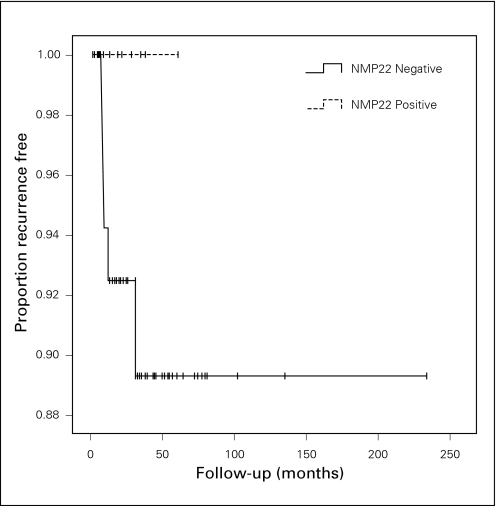
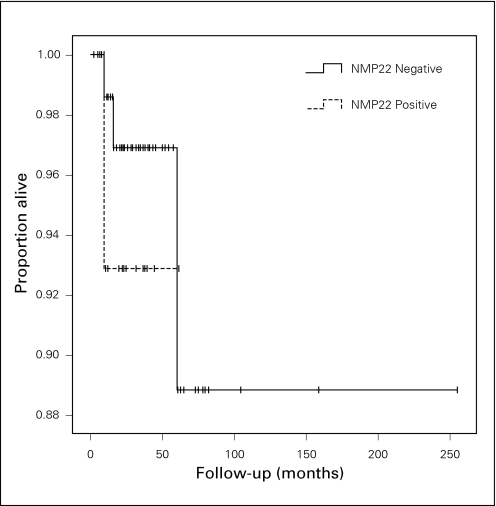
Kaplan-Meier analysis demonstrated a significant difference between NMP22-positive and NMP22-negative groups in terms of recurrence-free survival, p = 0.038 (Fig. 2). Sixty percent of NMP22-positive patients recurred (9/15) and 37.9% of NMP22-negative patients recurred (30/79). The mean recurrence-free survival times for NMP22-positive and -negative patients were 26.6 months (14.9–38.2) and 79.5 months (45.4–113.6), respectively. In contrast, progression-free and overall survival were not significantly different between NMP22-positive and -negative groups, p = 0.297 and p = 0.519, respectively (Fig. 3, Fig. 4). The median follow-up for the NMP22-negative and -positive groups was 26.5 and 21.0 months, respectively. The median follow-up for the cytology-negative and -positive groups was 25.6 and 26.3 months, respectively. Also, total follow-up time between the NMP22-positive and NMP22-negative groups was insignificant (p = 0.174). In this study, only 6 patients had progression from their highest-stage/grade tumour and all 6 were in the NMP22-negative group. Only 4 patients died and 2 of these deaths were disease related. Of these 2 disease-related deaths, one patient was in the NMP22-positive group while the other was not. Similar analysis of cytology positive and cytology negative groups showed no significant difference in terms of recurrence-free survival, progression-free survival and disease-related death (data not shown).
Fig. 2.
Kaplan-Meier curves showing recurrence-free survival in NMP22-positive and -negative groups, p = 0.038.
Fig. 3.
Kaplan-Meier curves showing progression-free survival in NMP22-positive and -negative groups, p = 0.297.
Fig. 4.
Kaplan-Meier curves showing overall survival in NMP22-positive and -negative groups, p = 0.519.
Discussion
With recurrence rates around 50% for noninvasive bladder cancers11 and progression rates varying from 10% to 50%,12 predicting these occurrences as early as possible is important to maintaining optimal outcomes. In this study, we demonstrate that high-risk patients with negative cystoscopy on surveillance, but positive urine NMP22, have a higher rate of recurrence and reduced recurrence-free survival time in comparison to patients who have a negative NMP22 test. In contrast, there was no evidence to suggest that urine cytology has the same predictive capability. Although controversial, many will follow a protocol whereby the follow-up of patients with a recent resection of bladder tumour(s) includes cystoscopy and urine cytology every 3 months for the first 2 years, then every 6 months for the next 2 years and then annually thereafter. If tumour recurrence is detected during follow-up, the protocol is reset and patients will have to undergo surveillance every 3 months.13 Although NMP22 has been approved for use in the detection of initial and recurrent bladder tumours, it has not been widely adopted. However, our data suggest that patients with high-risk superficial UCB who are NMP22 positive with negative cystoscopies are even more likely to recur than patients who are NMP22 negative. Thus, this may identify a select group of patients that should undergo intravesical therapy with maintenance. For those patients who cannot tolerate maintenance therapies of standard dosages of intravesical BCG, strategies such as reduced dosing, symptomatic therapy and using adjunctive immunotherapies (e.g., interferon), should be employed, knowing this group of high-risk patients will likely have tumour recurrences. Also, routine cytologies and cystoscopies should be employed, rather than relaxing the frequency of surveillance studies for this select group of high-risk patients that is at even higher risk of tumour recurrences. This select group of patients may also be a population to target for photodynamic therapy studies to identify occult tumour recurrences or occult tumour persistence.
In our data, we also observed that NMP22-positive patients did not progress more often than NMP22-negative patients. Interestingly, all 6 of the progressions seen in our patient population tested negative for NMP22; however, statistical analysis showed that no significant difference in progression-free survival time existed between the two groups. These results suggest that NMP22 as a marker may not be predictive of disease progression for stage or grade.
Finally, NMP22-positive patients show no difference in overall survival rates or survival time than NMP22-negative patients. There was a similar result when urine cytology underwent the same analysis. Certainly, the lack of an association between NMP22 and disease progression supports the notion that NMP22 may not be able to predict overall survival or alter the natural history of the disease. Kaplan-Meier analysis performed on disease-specific survival was not possible due to having only 1 event per group; however, the log-rank test suggested an insignificant result (p = 0.195). The relatively short follow-up may be a factor with regard to the lack of association between NMP22 detection and survival outcomes.
Limitations of the study involve the retrospective collection of clinical data. In addition, it has been noted that tumour recurrences are usually smaller than initial bladder tumours.14 With routine follow-up, presumably occult tumours have growth rates that reflect the eventual size of the lesion. As a mitotic apparatus protein facilitating proper segregation of chromosomes during cell division,15 NMP22 is elevated in tumours having more active cell division,16 and growth rates suggesting larger recurrences are more detectable by NMP22 in the preclinical stage. Similarly, the number of lesions is important, as more cells may be available to release NMP22. Therefore, small size and low number of tumours could potentially account for the high false-negative rate (38%) seen in this study when accessing NMP22’s ability to detect early carcinogenesis or subclinical recurrences.
Another concern is the high false-positive rate in terms of tumour recurrence seen in urine NMP22 testing. Our study showed that 40% of NMP22 positive patients were entirely recurrence free, as of their most recent follow-up. Although this is a significantly high false-positive rate, it is based on one urine test. Studies have shown that serial measurements of NMP22 can increase the sensitivity of detecting subclinical tumour recurrences before the next scheduled follow-up.17 Similarly, consistently negative results of NMP22 increases the negative predictive value of continued tumour-free survival; this observation addresses the notable false negative rate seen in our study. These data require validation using other data sets and, optimally, in a multicentre cohort.
Conclusion
The mean time to tumour recurrence is lower and the rate of tumour recurrence is higher for patients with high-risk superficial UCB who test positive for NMP22. In contrast, NMP22 was not predictive of progression-free or overall survival in this patient population. NMP22 may further stratify patients with high-risk superficial UCB requiring more frequent surveillance and continued maintenance intravesical therapies to reduce the risk of recurrence.
Acknowledgments
Matritech Inc donated the NMP22 BladderChek test kits for use in this study.
Footnotes
Competing interests: None declared.
This paper has been peer-reviewed.
References
- 1.Canadian Cancer Society. National Cancer Institute of Canada. Statistics Canada . Canadian cancer statistics; Toronto: 2008. [Google Scholar]
- 2.Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: Epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6suppl1):4–34. doi: 10.1016/j.urology.2005.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Pow-Sang JM, Seigne JD. Contemporary management of superficial bladder cancer. Cancer Control. 2000;7(4):335–9. doi: 10.1177/107327480000700402. [DOI] [PubMed] [Google Scholar]
- 4.Nickerson J. Experimental observations of a nuclear matrix. J Cell Sci. 2001;114:463–74. doi: 10.1242/jcs.114.3.463. [DOI] [PubMed] [Google Scholar]
- 5.Zink D, Fischer AH, Nickerson JA. Nuclear structure in cancer cells. Nat Rev Cancer. 2004;4:677–87. doi: 10.1038/nrc1430. [DOI] [PubMed] [Google Scholar]
- 6.Fey EG, Penman S. Nuclear matrix proteins reflect cell type of origin in cultured human cells. Proc Natl Acad Sci. 1988;85:11–5. doi: 10.1073/pnas.85.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005;293:810–6. doi: 10.1001/jama.293.7.810. [DOI] [PubMed] [Google Scholar]
- 8.Soloway MS, Briggman JV, Carpinito GA, et al. Use of a New Tumor Marker, Urinary NMP22, in the Detection of Occult or Rapidly Recurring Transitional Cell Carcinoma of the Urinary Tract Following Surgical Treatment. J Urol. 1998;156:363–7. doi: 10.1097/00005392-199608000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Grossman HB, Soloway M, Messing E, et al. Surveillance for recurrent bladder cancer using a point-of-care proteomic assay. JAMA. 2006;295:299–305. doi: 10.1001/jama.295.3.299. [DOI] [PubMed] [Google Scholar]
- 10.Lotan Y, Shariat SF. Impact of risk factors on the performance of the nuclear matrix protein 22 point-of-care test for bladder cancer detection. BJU Int. 2008;101:1362–7. doi: 10.1111/j.1464-410X.2008.07473.x. [DOI] [PubMed] [Google Scholar]
- 11.Holmang S, Hedelin H, Anderstron C, et al. The relationship among multiple recurrences, progression and prognosis of patients with Ta and T1 transitional cell cancer of the bladder followed for at least 20 years. J Urol. 1995;153:1823–6. [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network Available at: http://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf Accessed November 5, 2009.
- 13.Budman LI, Kassouf W, Steinberg JR. Biomarkers for detection and surveillance of bladder cancer. Can Urol Assoc J. 2008;2:212–221. doi: 10.5489/cuaj.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen CT, Jones JS. Defining the role of NMP22 in bladder cancer surveillance. World J Urol. 2008;26:51–8. doi: 10.1007/s00345-007-0226-z. [DOI] [PubMed] [Google Scholar]
- 15.Compton DA, Cleveland DW. NuMA is required for the proper completion of mitosis. J Cell Biol. 1993;120:947–57. doi: 10.1083/jcb.120.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taimen P, Viljamaa M, Kallajoki M. Preferential expression of NuMA in the nuclei of proliferating cells. Exp Cell Res. 2000;256:140–9. doi: 10.1006/excr.2000.4799. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Carbayo M, Urrutia M, Gonzalez de Buitrag JM, et al. Utility of serial urinary tumor markers to individualize intervals between cystoscopies in the monitoring of patients with bladder carcinoma. Cancer. 2001;92:2820–8. doi: 10.1002/1097-0142(20011201)92:11<2820::aid-cncr10092>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]