Abstract
Four N-(benzenesulfonyl)-L-glutamic acid bis(p-substituted phenylhydrazides) were synthesized and evaluated for anticancer activity in vitro in DU-145 and PC-3 prostate cancer and in COLO-205 colon cancer cell lines by MTT assay. The analog with the nitro group substitution exhibited potent activity (% Inhibition 84.7 and 72.0 in DU-145 and PC-3 respectively at 80 μg/ml concentration). Another series of substituted 1-(benzenesulfonyl)-5-oxopyrrolidine 2-carboxamides (11a-f) were synthesized and evaluated for anticancer activity in vitro in colon (COLO-205), breast (Zr-75-1) and prostate (PC-3) cancer cell lines by MTT assay using adriamycin as standard. Test compounds 11a-c showed potent activity (% Inhibition 61.2 to 79.2 at 20 μg/ml and 67.2 to 87.2 at 40 μg/ml) in PC-3 cell line which is superior to the activity of Adriamycin. In comparison compounds 11d-f were less potent. In Zr-75-1 cell line 11a-e showed % inhibition ranging from 32.4 to 54.9 at 10 μg/ml concentration while in COLO-205 cell line 11a-f showed poor activity.
Keywords: L-glutamic amides, L-glutamic acid hydrazides, anticancer activity
L-Glutamic acid plays an important role in the biosynthesis of purine and pyrimidine bases of DNA and RNA1. It is metabolized to L-glutamine by L-glutamine synthetase and this metabolic process is essential for normal maintenance of cells. The synthesis of L-glutamine is hindered in neoplastic cells due to lower reactivity of L-glutamine synthetase. Thus antagonists of this enzyme can interfere with the metabolic role of L-glutamine and act as anti-cancer agents2. Azaserine and 6-diaza-5-oxo-L-norleucine antagonized the metabolic process involving L-glutamine and exhibited antitumor activity in animal models3. L-glutamic acid γ-(4-hydroxyanilide) a growth regulatory substance isolated from mushroom Agaricus bisporouswas found to inhibit B16 mouse melanoma cells in culture4.
Similarly an arylamide derivative of L-threo-γ-hydroxy glutamic acid isolated from Justica ghiesbreghtiana was found to be active against various tumors5. The synthetic amides of L-glutamic acid also exhibited activity against Ehrlich ascites carcinoma6. In the present work it is proposed to synthesize novel N-(benzenesulfonyl)-L-glutamic acid bis(p-substituted phenylhydrazides) and substituted 1-(benzenesulfonyl)-5-oxopyrrolidine-2-carboxamides and evaluate them in cancer cell lines to ascertain their anticancer activity.
All melting points were recorded on Thermonik melting point apparatus and are uncorrected; Fourier-Transform Infrared (FT-IR) spectra (KBr discs) were recorded in Jasco FT-IR 5300 instrument. 1H-NMR spectra were recorded in Varian spectrophotometer at 60 MHz for first set of compounds and at 300 MHz for second set of compounds. The chemical shift values are reported in δ units (ppm) relative to internal standard tetramethylsilane (TMS). Elemental analysis values for final compounds were within ±0.4 % of theoretical value.
A group of four N-(benzenesulfonyl)-L-glutamic acid bis(p-substituted phenylhydrazides) were synthesized by condensing L-glutamic acid with benzenesulfonyl chloride in presence of aqueous NaOH at 70ºfollowed by acidification with aqueous HCl and extraction in dry ethyl acetate. The sulfonamide formed was converted to acid chloride using thionyl chloride and then condensed with excess of p-substituted phenylhydrazines to get bisamides (1-4). The physical constant and spectral characteristics are given in Table 1.
TABLE 1.
PHYSICAL AND SPECTRAL DATA OF N-(BENZENESULFONYL)-L-GLUTAMIC ACID BIS (P-SUBSTITUTED PHENYLHYDRAZIDES) (1-4)
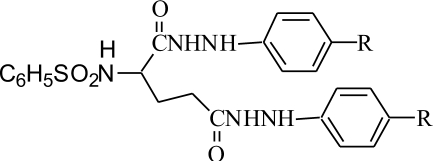
| Compound | R | M.P.º | Yield % | IR (KBr cm-1) | 1H-NMR (DMSO-D6) δ ppm |
|---|---|---|---|---|---|
| 1 | H | 190 | 65 | 3341, 3221 (N-H stretch), 1684 (C=O stretch), 1358, 1168 (S=O stretch). | 1.7-2.7 (5H, m, -CH CH2 CH2), 4.3 (2H, s, 2 Ar-NH-), 6.9-8.0 (15 H, m, Ar-H), 9.3 (1H, s, -SO2NH, 10.1 (2H,s, 2 HN-CO) |
| 2 | NO2 | 202 | 67 | 3200 (N-H stretch), 1672 (C=O stretch), 1342, 1172 (S=O stretch). | 1.8-3.5 (5H, m, -CH CH2 CH2), 4.4 (2H,s, 2 Ar-NH-), 6.92-8.30 (13 H, m, Ar-H), 9.5 (1H, -SO2NH, 10.1 (2H, s, 2 HN-CO) |
| 3 | Cl | 113-114 | 41 | 3312 (N-H stretch), 1703 (C=O stretch), 1356, 1170 (S=O stretch). | 2.07-3.5 (5H, m, -CHCH2CH2), 4.2 (2H, s, 2 Ar-NH-), 6.6-7.93 (13 H, m, Ar-H), 8.9 (1H, s, -SO2NH, 10.2 (2H, s, 2 HN-CO) |
| 4 | CH3 | 212-214 | 57 | 3225, 3209 (N-H stretch), 1684 (C=O stretch), 1361, 1168 (S=O stretch). | 2.35 (6H, s, 2CH3), 2.50-3.56 (5H, -CHCH2CH2), 4.0 (2H, s, 2 Ar-NH-), 6.54-7.93 (13H, m, Ar-H), 9.5 (1H, -SO2NH, 10.0 (2H, s, 2 HN-CO) |
General procedure used for the synthesis of N-substituted-5-oxopyrrolidine-2-carboxylic acid is as follows, in a 250 ml round bottom flask fitted with a CaCl2 guard tube, 20 g (0.066 mol) of N-p-toluenesulfonyl-L-glutamic acid (7) was taken and 25 ml (0.264 mol) of freshly distilled POCl3was added to it gradually with cooling of the flask in ice bath. Then 2-3 drops of DMF was added and the reaction mixture stirred for 3-4 h at room temperature. The reaction mixture was poured with stirring into crushed ice and the precipitate formed was extracted in chloroform. Chloroform extract dried over anhydrous MgSO4. Removal of solvent gave 8 in pure form. Compound 8 (R=CH3), Yield: 95 %; Mp 178-180°. IR (KBr, cm-1): 3414 (O-H stretch), 2932 (C-H stretch), 1736 (C=O stretch, lactam), 1707 (C=O stretch, acid), 1356 (S=O stretch). 1H NMR (CDCl3) δ ppm: 1.9 (2H, m, -CH2), 2.1 (3H, s, -CH3), 2.2 (2H, t, -CH2 -C=O), 4.9 (1H, t, NC-H), 7.3 (2H, d, Ar-H), 8.0 (2H, Ar-H), 9.0 (1H, s, -OH).
The above formed acid was converted to acid chloride by heating for an hour with 15 ml of SOCl2and with addition of 2 drops of DMF and removing the excess of SOCl2by vacuum distillation. Compound 9 (R=CH3), Yield: 95 %; Mp 71-72°. Synthesis of substituted 1-(benzenesulfonyl)-5-oxopyrrolidine 2-carboxamides (11a-f) was achieved by adding gradually to a solution of 3.9 g (0.012 mol) of 9 (R=CH3) in 5 ml of chloroform 1.46 g (0.020 mol) t-butylamine (10b) dissolved in 5 ml of chloroform with temperature maintained at 0°. The temperature was then raised to 50° and the mixture heated for 5-6 h. The chloroform solution was washed several times with water to remove unreacted amine, dried and then concentrated in vacuum. The amide obtained was purified by recrystallization with aqueous methanol or with ethyl acetate-hexane mixture (4:1). Compound 11b: Yield: 78 %; Mp 202-203°. IR (KBr, cm-1): 3290 (N-H stretch), 2966 (C-H stretch); 1751 (C=O stretch, lactam), 1651 (C=O amide). 1H NMR (CDCl3) δ ppm: 1.0 (3H, t, -CH3), 1.2 (2H, m, -CH2), 1.4 (2H, q, -CH2), 1.8 (2H, m, -CH2), 2.2 (2H, t, H2 C-CO), 2.4 (2H, t, N-CH2), 2.5 (3H, s, Ar-CH3), 5.3 (1H, m, -N-C-H), 7.3 (2H, d, Ar-H), 8.0 (2H, dd, Ar-H).
Test compounds 1-4 were evaluated for cytotoxicity in DU-145, PC-3 (prostate) and colo-205 (colon) cancer cell lines, while test compounds 11a-f were tested in PC-3, colo-205 and Zr-75-1 (breast) human cancer cell lines. All compounds were evaluated in vitro at 10, 20, 40 and 80 mg/ml concentrations using Adriamycin as standard and using MTT assay7.
Cell suspension 90 μl /well (5×103cells) was added to 96 wells and incubated at 37° for 24 h in 5% CO2incubator. Ten microlitres of either solutions of test compounds or solution of Adriamycin were added to the wells and the plates were incubated at 37° for 48 h in 5 % CO2 incubator. MTT reagent 10 μl (0.5%) was then added to the wells and further incubated for 6 h. The supernatant was then carefully removed and the formazan crystals formed were dissolved in 100 μl of acidified isopropanol (0.04 N HCl in isopropanol) and the absorbance of the solution was then read on spectrophotometer at a wavelength of 540 nm. The absorbance values were used to calculate % inhibition at various concentrations. The results are average values of three experiments.
A series of substituted 1-(benzenesulfonyl)-5-oxopyrrolidine-2-carboxamides (11a-f) were synthesized by cyclizing compound 7 using PCl5 and reacting with amines (10a-f). This method gave colored impurities which were difficult to remove. The above reaction when carried out with excess POCl3, instead of PCl5, generated less colored impurities but complete removal of excess POCl 3 at the end of the reaction was difficult. Hence in the reaction with POCl3after cyclisation of 7, the reaction mixture was poured into crushed ice to destroy excess POCl3and the product isolated was the free acid (8). The free acid was then converted to the acid chloride using SOCl2 (Scheme 1). The structure & physical constants & yields of the final steps are given in Table 2.
Scheme 1.
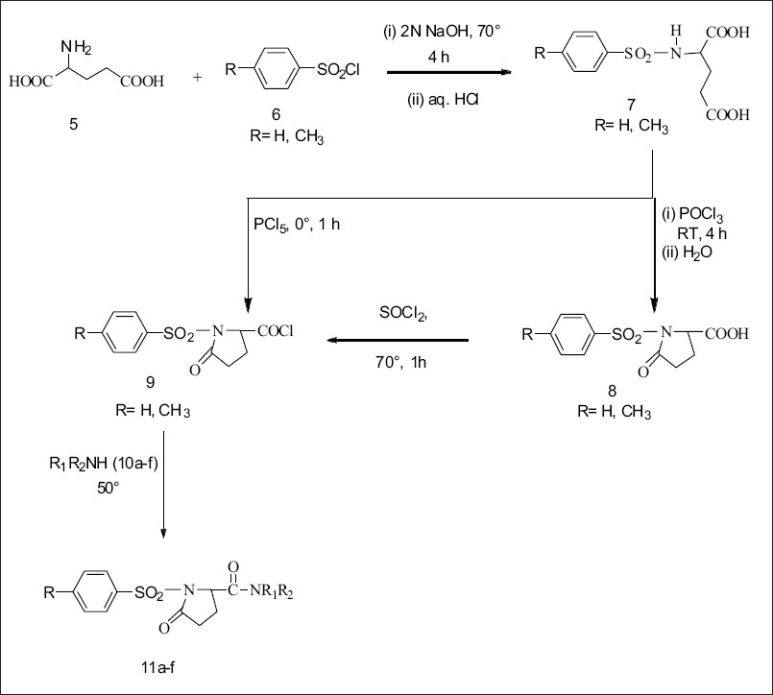
Synthesis of substituted 1-(benzylsulfonyl)-5-oxopyrrolidine-2-carboxamides.
11a: R -CH3, R1 -H, R2 n-C3H7; 11b: R -CH3, R1 -H, R2 n-C4H9; 11c: R -CH3, R1 -H, R2 -NH2; 11d: R -CH3, R1 -C2H5, R2 -C2H5; 11e: R -H, R1 -H, R2 n-C3H7; 11f: R -H, R1 -H, R2 n-C4H9.
TABLE 2.
PHYSICAL AND SPECTRAL DATA OF 1-(BENZENESULFONYL)-5-OXOPYRROLIDINE-2-CARBOXAMIDES (11a-f)
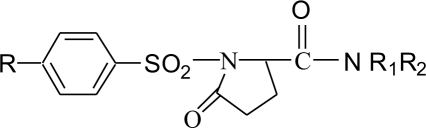
| Compound | R | R1 | R2 | Yield % | M.P.º | IR (KBr cm-1) |
|---|---|---|---|---|---|---|
| 11a | CH3 | H | n-C3H7 | 76 | 194-195 | 3300 (N-H stretch), 1751 (C=O stretch, lactam), 1653(C=O stretch), 1359(S=O stretch). |
| 11b | CH3 | H | n-C4H9 | 78 | 202-203 | 3290 (N-H stretch), 1751 (C=O stretch, lactam), 1651 (C=O stretch), 1356 (S=O stretch). |
| 11c | CH3 | H | -NH2 | 91 | 178-179 | 3321(N-H stretch), 1732 (C=O stretch, lactam), 1648 (C=O stretch), 1350 (S=O stretch). |
| 11d | CH3 | C2H5 | C2H5 | 89 | 143-145 | 3375(N-H stretch), 1738 (C=O stretch, lactam), 1660 (C=O stretch), 1358 (S=O stretch) |
| 11e | H | H | n-C3H7 | 82 | 205-208 | 3362(N-H stretch), 1732 (C=O stretch, lactam), 1664 (C=O stretch), 1348 (S=O stretch). |
| 11f | H | H | n-C4H9 | 79 | 214-215 | 3369(N-H stretch), 1718 (C=O, stretch lactam), 1687 (C=O stretch), 1356 (S=O stretch). |
Test compound 2 was found to be potent with % inhibition of 84.7, 86.6 and 42.2 in DU-145, PC-3 colo-205 cancer cell lines respectively at 80 μg/ml concentration, but was less potent at lower concentrations. Compounds 1, 3 and 4 exhibited poor activity at all doses studied (Table 3).
TABLE 3.
IN VITRO ANTICANCER ACTIVITY OF TEST COMPOUNDS (1-4) ON DU-145, PC-3 AND COLO-205 CELL LINES
| Compound | % Inhibition at 80 μg/ml in | ||
|---|---|---|---|
| DU-145 | PC-3 | COLO-205 | |
| 1 | 1.3 | 0.0 | 7.9 |
| 2 | 84.7 | 72.0 | 42.2 |
| 3 | 0.5 | 0.0 | 10.7 |
| 4 | 0.0 | 0.0 | 28.1 |
| Adriamycin | 86.6 | 98.3 | 97.8 |
In vitro anticancer activity of test compounds (1-4) using MTT colourimetric assay in DU-145, PC-3 (prostate) and COLO-205 (colon) human cancer cell lines
Test compounds 11a-f exhibited significant inhibitory activity in PC-3 cell line and 11a-c were more active than Adriamycin at 10, 20 and 40 μg/ml concentrations. In Zr-75-1 cell line the % inhibition ranged from 31.5 to 54.9 at 10 μg/ml concentrations but activity dropped with increasing concentrations. In colo-205 cell line, test compounds 11a-f as well as Adriamycin showed reduced activity at all concentrations. The percentage inhibition determined is reported in Table 4. The present work led to synthesis of two series of L-glutamic acid amides and among these, tests compounds 2 and 11a-c exhibited potent anticancer activity in some of the cell lines tested.
TABLE 4.
IN VITRO ANTICANCER ACTIVITY OF TEST COMPOUNDS (11a-f) IN PC-3, COLO-205 AND ZR-75-1 CANCER CELL LINES
| Compound | % Inhibition at various concentrations in μg/ml in | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC-3 | Zr-75-1 | COLO-205 | ||||||||||
| 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | 10 | 20 | 40 | 80 | |
| 11a | 52.1 | 70.7 | 81.1 | 45.5 | 32.4 | 0.0 | 0.5 | 0.0 | 1.3 | 2.0 | 4.9 | 1.5 |
| 11b | 63.6 | 79.2 | 67.2 | 55.9 | 31.5 | 11.8 | 5.6 | 5.9 | 2.1 | 3.2 | 4.6 | 2.3 |
| 11c | 59.1 | 61.2 | 82.9 | 45.9 | 53.8 | 5.8 | 2.3 | 3.4 | 3.9 | 3.8 | 3.0 | 1.2 |
| 11d | 27.9 | 40.9 | 45.6 | 23.3 | 51.6 | 10.0 | 2.7 | 6.1 | 2.2 | 1.6 | 0.8 | 0.9 |
| 11e | 31.9 | 65.2 | 19.3 | 05.3 | 54.9 | 12.9 | 6.4 | 5.3 | 0.4 | 1.1 | 0.6 | 0.7 |
| 11f | 19.2 | 0.73 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.9 | 11.4 | 3.7 | 5.2 |
| Adriamycin | 56.0 | 59.2 | 61.2 | 97.2 | 60.9 | 78.1 | 83.8 | 87.8 | 50.7 | 52.6 | 54.9 | 57.6 |
In vitro anticancer activity of test compounds (11a-f) using MTT colourimetric assay in PC-3 (prostate), COLO-205 (colon) and ZR-75-1 (breast) human cancer cell lines
Acknowledgments
Authors are thankful to Amrut Mody Research Foundation (AMRF), Mumbai for financial support for this project.
REFERENCES
- 1.Rodwell VW. Metabolism of purine and pyrimidine nucleotides. In: Murray KR, Granner DK, Mayes PA, Rodwell VW, editors. Harper's biochemistry. 25th ed. Stamford, Connecticut: Appleton and Lange; 2000. pp. 386–401. [Google Scholar]
- 2.Livingston RB, Venditti JM, Cooney DA, Carter SK. Glutamine antagonists in chemotherapy. In: Garattini S, Goldin A, Hawking F, Kopin IJ, editors. Advances in pharmacology and chemotherapy. Vol. 8. New York: Academic Press; 1970. pp. 57–120. [DOI] [PubMed] [Google Scholar]
- 3.Dewald HA, Moore AM. 6-Diazo-5-oxo-L-norleucine, a New Tumor-inhibitory Substance1a Preparation of L-, D- and DL-Forms1b. J Am Chem Soc. 1958;80:3941–5. [Google Scholar]
- 4.Rosowsky A, Wick MM, Kim SH. Structural analogs of L-glutamic acid gamma-(4-hydroxyanilide) and gamma-(3,4-dihydroxyanilide) as potential agents against melanoma. J Med Chem. 1979;22:1034–7. doi: 10.1021/jm00195a004. [DOI] [PubMed] [Google Scholar]
- 5.Luzzio FA, Thomas EM, Figg WD. Thalidomide metabolites, Part 1: Derivatives of (+)-2-(N-phthalimido)-γ-hydroxyglutamic acid. Tetrahedron Lett. 2000;41:2275–8. [Google Scholar]
- 6.Srikanth K, Kumar CA, Ghosh B, Jha T. Synthesis, screening and quantitative structure-activity relationship (QSAR) studies of some glutamine analogues for possible anticancer activity. Bioorg Med Chem. 2002;10:2119–31. doi: 10.1016/s0968-0896(02)00079-2. [DOI] [PubMed] [Google Scholar]
- 7.Twentyman PR, Fox NE, Rees JK. Chemosensitivity testing of fresh leukemia cells using the MTT colourimetric assay. Br J Haematol. 1989;71:19–24. doi: 10.1111/j.1365-2141.1989.tb06268.x. [DOI] [PubMed] [Google Scholar]


