Abstract
Objective
To quantify the frequency and type of new safety information arising from studies performed under the auspices of the Pediatric Exclusivity Program, to describe the dissemination of these findings in the peer-reviewed literature and compare this with the FDA review, and to describe their effect on pediatric labeling.
Design
Cohort study of the 365 trials performed for 153 drugs.
Setting
The Pediatric Exclusivity incentive from December 1997 through September 2007.
Participants
Food and Drug Administration publicly available records and peer-reviewed literature retrievable by Medline search.
Main Exposures
New safety findings obtained from the trials completed for exclusivity.
Main Outcome Measures
Concordance of the information highlighted in the peer-reviewed article abstracts with the information in the FDA labeling and drug reviews.
Results
There were 137 labeling changes; we evaluated 129 of these (the 8 selective serotonin reuptake inhibitors were excluded from review). Thirty-three products (26%) had pediatric safety information added to the labeling. Of these, 12 products had neuropsychiatric safety findings, and 21 had other important safety findings. Only 16/33 (48%) of these trials were reported in the peer-reviewed literature; however, 7/16 of these publications focused on findings substantively different from those highlighted in the FDA reviews and labeling changes.
Conclusions
Medication adverse events in children often differ from those in adults, particularly those that are neuropsychiatric in nature. Labeling changes for pediatric use demonstrate that pediatric drug studies provide valuable and unique safety data that can guide the use of these drugs in children. Unfortunately, most these articles are not published, and almost half of the published articles focus their attention away from the crucial safety data.
The majority of prescription drugs on the market do not contain adequate information in their labeling regarding their pediatric use. The longstanding and widespread nature of this problem was demonstrated by 2 surveys of drug monographs in the Physicians’ Desk Reference, in which 78% of products in 1973 and 81% in 1991 lacked sufficient pediatric use information or labeling.1,2 Without the provision of appropriate information concerning pediatric dosing safety or efficacy, physicians who treat children must decide between withholding treatment proven effective in older patients or participating in the practice of off-label use by prescribing to children products not studied in pediatrics. Off-label use—with dosing being based on untested hypotheses—puts children at an increased risk of adverse events in exchange for often unproven potential therapeutic benefit.
In 1994, in an effort to improve pediatric use information in product labeling, the US Food and Drug Administration (FDA) defined additional approaches that could establish pediatric indications. Although controlled pediatric efficacy studies were encouraged, they had not been not required by law.3 Unfortunately, this voluntary program did not result in an increase in the number of pediatric studies. Of the 430 drugs for which supplements were submitted, only 15% supplied sufficient pediatric information for labeling, and most of these submissions targeted narrow age ranges (e.g., studies limited to adolescents).4
In 1997, the President signed the US Food and Drug Administration Modernization Act into law.5 This act included a Pediatric Exclusivity Provision, under which a sponsor could be granted an additional 6 months of marketing exclusivity for conducting pediatric studies specified in a FDA written request. These incentives were maintained under the Best Pharmaceuticals for Children Act (BPCA) of 2002.6 This program and the Food and Drug Administration Amendments Act (FDAAA) of 2007 have significantly increased pediatric drug research. As of September 2007, 153 drugs have received a pediatric exclusivity determination, and 137 drugs have received pediatric labeling changes.7 However, the extent and quality of new pediatric safety information established through pediatric exclusivity have not previously been described in detail. Of note, most of the safety data have not been disseminated in the peer-reviewed literature.8
The Pediatric Exclusivity Program was reauthorized as part of the Food and Drug Administration Amendments Act of 2007; however, several policy questions remain unanswered. This program is a substantial investment,9 children cannot give consent, the trials are technically challenging, and thus how to best transform these data into public health policy, beyond modification of the existent labeling, is vital to public health. We address these questions by assessing the availability of safety information resulting from pediatric trials conducted in response to the legislative efforts.
We examined the clinical trials analyzed in the FDA reviews and subsequent labeling changes that incorporated new pediatric safety information resulting from the exclusivity incentive between December 1997 and September 2007. We then compared the review conducted by the FDA with the data available in the corresponding peer-reviewed publications. In contrast to the medical reviewers at the FDA, journal editors, referees, and readers rarely have access to the individual patient data obtained in the clinical trial. We sought not only to report the safety results derived from these studies (many of which are unpublished), but also to examine the nuances of what has been put forth in the peer-reviewed literature and to comment on the implications of these findings for current and future public policy.
METHODS
Clinical Trials Overview
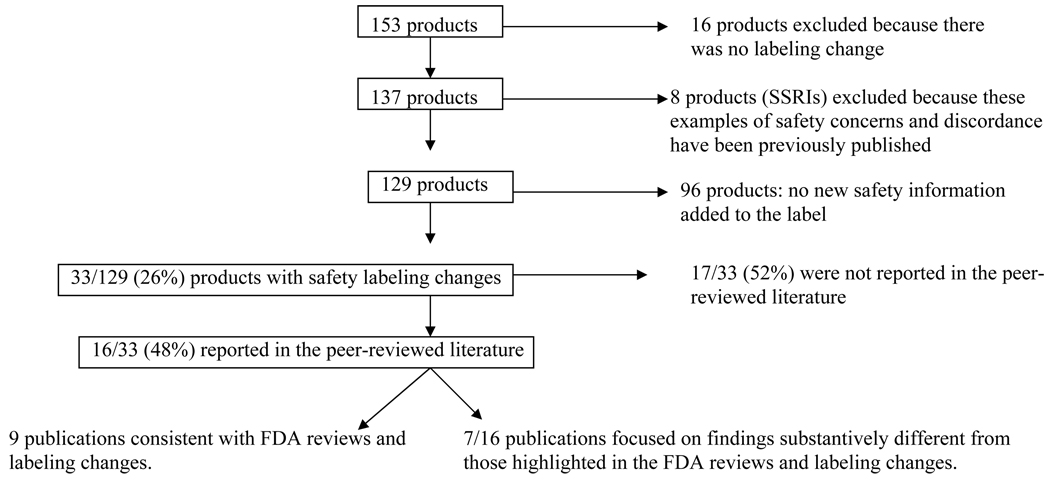
We identified all drugs that received pediatric labeling changes as a result of the Pediatric Exclusivity Program through September 2007 (Figure 1). Studies for exclusivity were solicited by the FDA in the form of written requests issued to pharmaceutical companies. The written requests specified required elements of the studies that were performed, including age ranges, sample sizes, study design, and trial end points. Companies were required to submit to the FDA all of the data from these trials.
Figure 1.
Products studied for Pediatric Exclusivity and subsequent disposition. FDA = Food and Drug Administration; SSRI = selective serotonin reuptake inhibitor.
The Safety Cohort
Of the products submitted for pediatric exclusivity (n=153), new pediatric information was included in the labeling for 137 (89.5%, Figure 1). We excluded selective serotonin reuptake inhibitors (n=8; Figure 1); these products had a black box warning added to their product labeling detailing an increased risk of suicidality in children and adolescents. This warning was the result of trials conducted in children for exclusivity. Ultimately, additional antidepressant drug trials contributed to the final analysis, and these data have been previously analyzed and disseminated.10 We analyzed the FDA reviews and labeling of the drugs for which new pediatric safety data were elucidated (n=33; Figure 1); these included those with neuropsychiatric findings (n=12) that were unexpected or greater in frequency than anticipated from studies completed in adults, and products that had other safety findings (n=21).
Data Transparency and Peer Review
We have previously reported on the fraction of studies published in the peer-reviewed literature across the Exclusivity Program.8 In the present study, we used similar search strategies to obtain publication status, including: 1) searching Medline with the product generic name, “all child (0–18 years),” “1998–2008,” and “English language”; 2) generic name, “1998–2008,” “English language,” and ages of trial participants; 3) use of key words from the study design provided by the written request and the generic name (allowing capture of manuscripts prior to 1998). We then compared the text of the FDA labeling and medical review to the abstract and text of the peer-reviewed article. When the FDA review and article abstract differed markedly, we copied the reports of both the FDA and the article for side-by-side comparison.
We received a waiver of review from the Duke University Medical Center Institutional Review Board and a letter of exempt status from the FDA’s Research Involving Human Subjects Committee because there were no associated patient identifiers in any of the patient-level data that we analyzed.
RESULTS
The Pediatric Exclusivity Program
In the first 10 years of the Pediatric Exclusivity Program, a pediatric exclusivity determination was made for 153 products. Over 95,000 children were in enrolled in 365 trials, from which there were 137 pediatric labeling changes. Of the 365 trials, 67 had 1 or more safety events as their primary end points, 197 trials were well-powered efficacy trials, and 109 trials focused on the pharmacokinetic end points.
Unexpected Safety Findings and the Central Nervous System
Twelve products with unexpected and important neuropsychiatric safety findings have been elucidated (TABLE 1). FDA medical reviewers found an increase in suicidal ideation as compared with adults in a trial of ribavirin and interferon alpha. Agitation was observed in young children exposed to famotidine; this resolved upon discontinuation of the product. Aggressive and hyperactive behavior was more frequently observed in children exposed to tolterodine compared with placebo, and post-marketing experience with sumatriptan demonstrated serious adverse events rarely reported in adults, including stroke, vision loss, and death.
Table 1.
Key Central Nervous System Safety Findings, 1997–2007
| Drug name | Indication studied | Key central nervous system safety findings per FDA medical reviewer |
|---|---|---|
| Brimonidine | Prevention of post-operative IOP elevations | Increased incidence of somnolence in patients 2–6 years of age (50–83%) vs. patients >7 years of age (25%). |
| Famotidine | Gastroesophageal reflux | Agitation was observed in 5/35 (14%) patients and resolved upon discontinuation of the drug. |
| Gabapentin | Adjunctive therapy for partial seizures | Neuropsychiatric adverse events identified in 3–12-year-olds included emotional lability, hostility/aggression, thought disorder, hyperkinesia. |
| Levetiracetam | Adjunctive therapy for partial seizures | 37.6% of pediatric patients reported behavioral symptoms compared with 13.3% in adults. Somnolence occurred in 22.8% of pediatric patients compared with 14.8% of adults. |
| Oxcarbazepine | Adjunctive therapy for children with epilepsy | Approximately 11% of pediatric patients < 4 years of age discontinued treatment because of adverse events including convulsions, status epilepticus, and ataxia. |
| Ribavirin/Intron A | Chronic hepatitis C | An increased incidence of suicidal ideation or attempts was observed among pediatric patients compared with adults. Also noted were decreases in the rate of linear growth and weight gain. |
| Sibutramine* | Obesity | Of 368 obese adolescents treated with sibutramine and 130 patients with placebo, 1 patient in each group attempted suicide, and 2 sibutramine-treated patients reported suicidal ideation. It is unknown if sibutramine increases the risk of suicidal behavior or thinking in pediatric patients. The data are inadequate to recommend the use of sibutramine for the treatment of obesity in pediatric patients. |
| Tolterodine* | Urinary frequency, urge incontinence | Aggressive, abnormal, and hyperactive behavior and attention disorders occurred in 2.9% of children treated with tolterodine vs. 0.9% treated with placebo. Increased incidence of urinary tract infections also occurred compared with placebo. In addition to safety concerns, efficacy was not established. |
| Sevoflurane | Induction/ maintenance of general anesthesia | Rare cases of seizures have been reported in pediatric patients in association with sevoflurane use. The majority of cases were in children and young adults, most of whom had no medical history of seizures. |
| Sumatriptan* | Acute migraine | Post-marketing experience documented serious adverse events rarely reported in adults, including stroke, visual loss, and death, in children after using subcutaneous, oral, and/ or nasal sumatriptan. Efficacy was not established. |
| Zolpidem | Insomnia associated with ADHD | In an 8-week controlled study in 201 patients ages 6–17 years, > 5% of treatment-emergent adverse events were of neuropsychiatric origin, including dizziness (23.5%), headache (12.5%), and hallucinations (7.4% vs. 0% in placebo group). |
| Modafinil* | Narcolepsy | Treatment emergent adverse events included Tourette’s syndrome, insomnia, hostility, increased cataplexy, increased hypnagogic hallucinations, and suicidal ideation. Serious rash, including Stevens-Johnson Syndrome, requiring hospitalization and discontinuation of treatment has been reported in adults and children in association with the use of modafinil. Modafinil is not approved for use in pediatric patients for any indication. |
Product did not demonstrate efficacy for the indication studied in addition to the safety concerns listed.
ADHD = attention deficit hyperactivity disorder, BMD = bone mineral density, FDA = US Food and Drug Administration, IOP = intraocular pressure.
Other Key Safety Findings
In addition to the 12 products with notable neuropsychiatric adverse events, 21 products had substantive safety concerns when tested in children. Use of several of these products (TABLE 2) resulted in adverse events related to growth, including suppression of the hypothalamic–pituitary–adrenal axis (betamethasone, mometasone) and musculoskeletal events (ciprofloxacin and levofloxacin). Two anti-infectives (ertapenem and linezolid) failed to achieve reliable concentrations in the cerebrospinal fluid, and 2 products that were used for anesthesia (desflurane and propofol) were found to result in severe laryngeal spasm and increased mortality, respectively. Also noteworthy was the early discontinuation of a trial evaluating the use of irinotecan for the treatment of refractory tumors and untreated rhabdomyosarcoma due to progressive disease and early deaths.
Table 2.
Other Key Safety Findings, 1997–2007
| Product | Indication studied | Finding per FDA medical reviewer |
|---|---|---|
| Betamethasone and Betamethasone/ Clotrimazole | Atopic dermatitis | Diprolene AF cream: 32% of children <13 years of age treated for atopic dermatitis had HPA axis suppression. Diprosone: HPA axis suppression with each formulation: cream—23% (2yr–12yr); ointment—28% (6mo–12yr); and lotion—73% (6yr–12yr) Lotrisone: 40% of 12–16-year-olds treated for tinea pedis, and 47% of 12–16-year-olds treated for tinea cruris demonstrated adrenal suppression by cosyntropin testing. |
| Budesonide | Asthma | A dose-dependent effect on growth was observed. Pneumonia was observed more frequently (3 vs. 0) in patients treated with Pulmicort. |
| Calcitriol | Hypocalcemia management in patients on hemodialysis | Transient hypercalcemia was seen in 1 of 16 calcitriol-treated patients; 6 of 16 (38%) calcitriol-treated patients and 2 of 19 (11%) placebo-treated patients had Ca × P >75. |
| Celecoxib | JRA | Celecoxib should be used only with caution in patients with systemic onset JRA due to the risk for serious adverse reactions, including the risk of disseminated intravascular coagulation. |
| Ciprofloxacin | Complicated UTI, acute pyelonephritis | Not drug of first choice due to increased adverse events compared with controls, including events related to joints and/or surrounding tissues. |
| Desflurane | Anesthesia | Higher rates of coughing, laryngospasm, and secretions: respiratory AE in 39%, and 5% of children exposed to desflurane experienced severe laryngospasm. |
| Ertapenem | Anti-infective | Not recommended in the treatment of meningitis in the pediatric population due to lack of sufficient CSF penetration. |
| Fentanyl | Management of chronic pain | Duragesic should be administered to children only if they are opioid-tolerant and aged 2 years or older. |
| Fluticasone | Corticosteroid-responsive dermatoses | Cutivate: In a study of 35 pediatric patients treated for atopic dermatitis, subnormal adrenal function was observed with cosyntropin stimulation testing. |
| Irinotecan | Refractory tumors | Accrual for phase 2 study with 21 children with previously untreated rhabdomyosarcoma halted due to high rate (23.6%) of progressive disease and early deaths (14%). |
| Isotretinoin | Severe recalcitrant nodular acne | An increased incidence of back pain, arthralgia, and myalgia observed in pediatric patients. In a study of pediatric patients given a single course of therapy, 7.9% had decreases in lumbar spine BMD >4%, 10.6% had decreases in total hip BMD >5% (both adjusted for body mass index). |
| Lamotrigine | Adjunctive therapy for partial seizures | Approximately 11.5% of the 1081 pediatric patients who received the drug as adjunctive therapy in clinical trials discontinued treatment because of an AE. |
| Leflunomide | JRA | 14/74 patients experienced ALT and/or AST elevations. |
| Levofloxacin | Anti-infective | In a prospective, long-term, surveillance study, levofloxacin-treated children had a significantly higher incidence of musculoskeletal disorders (arthralgia, arthritis, tendinopathy, and gait abnormality) compared with non–fluoroquinolone-treated children. |
| Linezolid | Anti-infective | Use of linezolid for the empiric treatment of pediatric patients with central nervous system infections is not recommended: therapeutic concentrations were not consistently achieved or maintained in the CSF. |
| Midazolam | Sedation/anxiolysis/ amnesia | Identified a subpopulation (children with congenital heart disease and pulmonary hypertension) at higher risk for AEs and the need to start therapy at the lower end of the dosing range. |
| Mometasone | Corticosteroid responsive dermatoses/ allergic rhinitis | Elocon cream and ointment: evidence of HPA axis suppression in pediatric patients 6–23 months of age. Elocon lotion: should not be used for the treatment of diaper dermatitis. |
| Pimecrolimus | Atopic dermatitis | Not recommended for use in children <2 years of age. Infants on Elidel cream had an increased incidence of infections compared with vehicle. |
| Propofol | Anesthetic | Propofol is not indicated for pediatric ICU sedation as safety has not been established; in a multicenter trial, the incidence of mortality (causality not established) was 9% in the propofol arm versus 4% in the standard sedative agents arm. |
| Sirolimus | Prevention of rejection after renal transplantation | The use of sirolimus in combination with calcineurin inhibitors and corticosteroids was associated with an increased risk of deterioration of renal function, lipid abnormalities, and urinary tract infections. |
| Sotalol | Arrhythmias | Smaller children (BSA < 0.33 m2) showed tendency for larger change in QTc and increased frequency of prolongation of the QTc interval, as well as greater beta-blocking effects. |
AE =adverse event, BSA = body surface area, CSF = cerebrospinal fluid, HPA = hypothalamic–pituitary–adrenal, ICU = intensive care unit, JRA = juvenile rheumatoid arthritis, UTI = urinary tract infection.
Trial Publications
Information on only 16/33 (48%; Figure 1) of the products with neuropsychiatric and other safety concerns has been published in the peer-reviewed literature retrievable by Medline search. Nine articles had abstracts and article text that accurately reflected the FDA clinical review and labeling change. Seven articles substantially differed in their presentation and interpretation of the data submitted to the FDA.11–17 In TABLE 3, the text from the labeling change (available at http://www.fda.gov/cder/pediatric/labelchange.htm) is shown alongside the abstract conclusions and relevant quotes from the article text.
Table 3.
Discordant Results
| Drug | FDA labeling change with respect to safety and efficacy | Article abstract conclusions (and text, where noted) |
|---|---|---|
| Budesonide | A dose-dependent effect on growth was observed in the 12-week trial which supports the finding that the use of Pulmicort Respules in infants 6 to 12 months of age may result in systemic effects and is consistent with the findings of growth suppression in other studies with inhaled corticosteroids. Pneumonia was observed more frequently in patients treated with Pulmicort Respules than in patients treated with placebo. | Article: "no suppression of adrenal function with once-daily treatment; " mentions the abnormal responses in the body of text and briefly mentions reduced growth velocity, but does not report pneumonias separately from “respiratory infections.” |
| Glimepiride | Data are insufficient to recommend pediatric use of glimepiride. Trial suggested differences favoring metformin. AE profile in the pediatric population was similar to that for adults. | Glimepiride reduced A1C similarly to metformin with greater weight gain, and there was comparable safety over 24 weeks in the treatment of pediatric subjects with type 2 diabetes. |
| Levetiracetam | Safety and effectiveness have not been established in patients less than 4 years of age. 37.6% of pediatric patients reported behavioral symptoms compared with 13.3% in adults. Somnolence occurred in 22.8% in pediatric patients compared with 14.8 in adults. | Levetiracetam adjunctive therapy administered at 60mg/kg/day is efficacious and well-tolerated in children with treatment-resistant partial seizures. The article states that "the incidence of many of the common adverse events including infection, fever, abdominal pain, nausea, diarrhea, increased cough, rhinitis, and otitis media that were seen in both the levetiracetam and placebo groups is consistent with the expected incidence for school-age children." |
| Oxcarbazepine | Extended adjunctive therapy age range from 4 years down to 2 years. No evidence that drug was effective as adjunctive therapy in patients < 2 years. Approximately 11% of pediatric patients < 4 years discontinued treatment because of adverse events including convulsions, status epilepticus, and ataxia. | Article: Did not present subgroup analysis, and described most frequent adverse events as somnolence and pyrexia, with AEs also including ataxia and vomiting, similar to database findings by FDA. |
| Ribavirin/Intron A | Increased incidence of suicidal ideation or attempts (2.4% versus 1%) among pediatric patients compared with adult patients. Decrease in rate of linear growth (mean percentile assignment decrease of 9%) and in rate of weight gain (mean percentile assignment decrease of 13%) during 48 weeks of treatment; a general reversal was noted during the 24-week post-treatment period. Patients with viral genotype 1 had a lower response rate to combination therapy compared with patients with genotype non-1, 36% versus 81%. | "Interferon alfa-2b in combination with ribavirin is effective and safe in children with chronic hepatitis C virus.” Article text mentions all suicidal ideation and attempts, and offers this explanation: "The presence of a chronic illness and a history of depression or behavior disorder are also associated with an increased risk of suicide. It is therefore possible that study medications were not directly responsible for suicidal ideation, but rather uncovered underlying psychological problems in predisposed individuals. Nevertheless, this highlights the importance of carefully monitoring children and adolescents given interferon and ribavirin for the development of depressive symptoms, particularly in those with ‘at-risk’ comorbid conditions." |
| Sibutramine | The data are inadequate to recommend the use of sibutramine for the treatment of obesity in pediatric patients. Efficacy in obese adolescents has not been adequately studied. Sibutramine's mechanism of action inhibiting the reuptake of serotonin and norepinephrine is similar to that of some antidepressants. It is unknown if sibutramine increases the risk of suicidal behavior or thinking in pediatric patients. In a study of adolescents with obesity in which 368 patients were treated with sibutramine and 130 patients with placebo, 1 patient in each group attempted suicide. Suicidal ideation was reported by 2 sibutramine-treated patients and none of the placebo patients | "Sibutramine added to a behavior therapy program reduced BMI and body weight more than placebo and improved the profile of several metabolic risk factors in obese adolescents." The article text mentions blood pressure and tachycardia as statistically significant events, and mentions 2 suicide attempts (1 in each group). The article does not mention suicidal ideation, depressed state, or accidental injuries. States that rates of growth and maturation did not differ between groups. |
| Tolterodine | Efficacy in pediatric population has not been demonstrated. 710 pediatric patients ages 5–10 years with urinary frequency and urge incontinence were studied in 2 randomized placebo controlled trials. Urinary tract infections were higher in patients treated with Detrol LA (6.6%) compared with placebo (4.5%). Aggressive, abnormal, and hyperactive behavior and attention disorders occurred in 2.9% of children treated with Detrol LA compared with 0.9% treated with placebo. | Data presented in 2 articles: "Analysis of the primary efficacy outcome did not reveal a statistically significant effect of treatment. However, secondary analyses demonstrated that tolterodine was well tolerated among 5–10-year-old children with diurnal incontinence." The article also mentions, "Differences in the number of incontinence episodes per week, voids per 24 hours, and volume of urine per void between tolterodine and placebo did not reach statistical significance. This finding may be explained by a high placebo response and under-dosage of tolterodine among children with greater body weight. Tolterodine was well tolerated." Mentions increased incidence of UTI: "Although a larger percentage of tolterodine vs. placebo recipients experienced urinary tract infection, there were no reports of urinary retention." No mention of behavioral or attention adverse events. |
AE =adverse event, FDA = US Food and Drug Administration, UTI = urinary tract infection.
COMMENT
Exclusivity and Pediatric Drug Development Today
In 2007, Congress renewed the Pediatric Exclusivity Program for an additional 5 years. With this extension, there are 3 major mechanisms of pediatric drug development in the United States: the Pediatric Research Equity Act (PREA), the Best Pharmaceuticals for Children Act Pediatric Exclusivity incentive, and the off-patent process.
The Pediatric Research Equity Act, originally signed into law in 2003 and reauthorized by the FDAAA of 2007, requires the study of certain drugs and biologicals in children. This mechanism has some limitations: 1) the requirements apply only to an indication that exists in both adults and children, and 2) many of the studies completed for this mechanism are small in size and scope (e.g., bioequivalence, single-dose pharmacokinetics, and small safety trials).18
The Best Pharmaceuticals for Children Act off-patent process, originally outlined in 2002, allows the National Institutes of Health to sponsor studies for pediatric labeling for products that no longer have marketing exclusivity protection. This mechanism has never been appropriately funded, and, though a number of studies are ongoing, as of the time of this report, no studies have been submitted that have resulted in pediatric labeling.
Pediatric exclusivity has been extremely successful in ensuring the completion of many pediatric trials and subsequent labeling concerning pediatric use. The paucity of pediatric trials makes dissemination of the outcomes and data from all trials in this program important because of the frequent off-label use of these products. A detailed description and analysis of each trial—including outcomes of the trials, case report forms, and tabulations—and any supplemental information are compiled into a final study report, which must be submitted to the FDA in a manner that is appropriate to support new pediatric labeling. The FDA then reviews all of the available data and negotiates any new labeling modifications with the company. Historically, the FDA did not put information about failed studies in labeling. As of 2007, the FDA requires information to be added to product labeling on studies done in response to either BPCA or PREA, including information concerning negative studies.
Exclusivity and its Effects on Public Policy
Pediatric drug trials are often conducted after a product has been developed for adults, and information developed from previous adult trials is often used to design pediatric trials. In addition, because of the small number of pediatric patients with a given disease and the ethical mandate that children should not be exposed to additional risks without potential benefit, pediatric studies tend to be smaller in size. However, well-powered safety and efficacy trials for therapeutics are a critical component of pediatric health.
We have highlighted 12 products with neuropsychiatric safety findings and 21 other products with crucial safety concerns such as laryngospasm, increased rate of progression of cancer, and increased risk of death. From these studies, it is noted that adverse events and serious adverse events (SAEs) in the Pediatric Exclusivity Program were commonly localized to the central nervous system. This finding is of special public health concern given the gravity of findings (e.g., suicidality, death) and the potential impact of these products on the developing brains of children. The high frequency of neuropsychiatric adverse event findings in these trials demonstrates the public health need for the continued conduct of well-powered safety trials in children.
These findings are especially remarkable given the relative size of the trials. If the expected SAE rate in the placebo group is 10%, in order to detect an absolute increase of 10% in incidence of SAE (20% incidence in the product group and thus a number needed to harm=10), then a trial of 400 children provides 80% power. In order to detect an absolute increase of 5% in SAE, a trial of 1370 provides 80% power: a sample size that is larger than all but 2 of the trials conducted for exclusivity. In order to detect an increase of 2%, a trial of 7682 provides 80% power. Of the studies completed for exclusivity, 25% enrolled ≤30 children (mostly pharmacokinetic studies); the median sample size was 103, 25% of trials enrolled ≥214, and 2 studies enrolled >1000 children.
Potential Improvements to Pediatric Exclusivity and Future Steps
The Pediatric Exclusivity Program has greater transparency of data than that available for adult patient populations. This increase in transparency mandates the public dissemination of the results of clinical and pharmacologic trials submitted to the FDA in response to a written request; FDAAA requires that written requests, as well as medical reviews, clinical pharmacology reviews, and statistical reviews, be made available on the FDA Web site for applications in response to BPCA and PREA. Transparency in the Exclusivity Program can be further improved by increasing the fraction of studies published in the peer-reviewed literature and providing greater access to study data.
Greater access to study data is essential for both public health and the integrity of the program. Because there is no incentive for additional studies to answer questions that arise from the limited pediatric therapeutic studies conducted, many questions concerning why trials failed or why products have higher adverse event rates in children remain unaddressed. Furthermore, the studies with the greatest potential for public health impact are, on average, the studies least likely to be published. Specifically, trials that uncover new safety findings are less likely to be published than other types of trials, and trials that uncover results unfavorable to a company (or its product) are less likely to be published than those with favorable results.8
These data advance previous findings by showing that the few studies that are published often emphasize results that are discordant from the findings viewed as important to public health by the FDA reviewer. The FDA reviewers, as part of their employment, are vetted and cleared of conflicts of interest. The decision to grant exclusivity is a team effort in which multiple staff members of the agency participate. These members have expertise in clinical pediatric medicine, ethics, epidemiology, clinical trials, pharmacology, toxicology, and statistics.
Some of the discordant results are among the most notable findings of the program. Differences between peer-reviewed published articles and FDA reviews may reflect incomplete access to data by journal editors and referees. Other reasons include the lack of consistent numeric or clinical threshold or criteria for inclusion of adverse events in drug labeling. This can lead to different interpretations of the same set of data by FDA reviewers and other researchers.
The Pediatric Exclusivity Program grants marketing protection, which, in turn, leads to higher prices for drugs that are bought (at least for the elderly) by Medicare dollars. We, and others, have shown a low incidence of publication, and we have previously provided evidence that if data are not published within 3 years of being submitted to the FDA, they are unlikely to ever be published.8,19–21 It might be proposed that all data collected during these trials and submitted to the FDA also be submitted in a public manner similar to the approach provided for the “off-patent” studies that are conducted under the second mechanism discussed above. More importantly, greater access to data will result in greater dissemination of findings and thus improve children’s health.
ACKNOWLEDGMENTS
Drs. Benjamin, Smith, and Li received support from NICHD 1U10-HD45962-04 and 1UL 1RR024128-02; Dr. Califf receives support from 1UL 1RR024128-02. The views expressed are those of the authors. No official endorsement by the US Food and Drug Administration is provided or should be inferred. Dr. Benjamin receives research support from Pfizer, Astellas, and Biosynexus.
Footnotes
Please see http://archpedi.ama-assn.org/ for the published manuscript.
REFERENCES
- 1.Gilman JT, Gal P. Pharmacokinetic and pharmacodynamic data collection in children and neonates. A quiet frontier. Clin Pharmacokinet. 1992;23:1–9. doi: 10.2165/00003088-199223010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104(3 Pt 2):585–590. [PubMed] [Google Scholar]
- 3.Specific requirements on content and format of labeling for human prescription drugs: revision of “pediatric use” subsection in the labeling. Federal Regist. 1994;59:64240. 21 CFR Part 201. [Google Scholar]
- 4.Regulations requiring manufacturers to assess the safety and effectiveness of new drugs and biological products in pediatric patients. Federal Regist. 1998;63:66632–66672. 21 CFR Parts 201, 312, 314, and 601. [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Modernization Act of 1997. 1997. Pub L No. 105–115, 111 Stat 2296. [Google Scholar]
- 6.Best Pharmaceuticals for Children Act of 2002. 2002. Pub L No. 107–109. [Google Scholar]
- 7. [Accessed March 9, 2009];BPCA labeling changes. Available at: http://www.fda.gov/cder/pediatric/labelchange.htm.
- 8.Benjamin DK, Jr, Smith PB, Murphy MD, et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296:1266–1273. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JS, Eisenstein EL, Grabowski HG, et al. Economic return of clinical trials performed for pediatric exclusivity. JAMA. 2007;297:480–488. doi: 10.1001/jama.297.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressants. Arch Gen Psychiatry. 2006;63:332–339. doi: 10.1001/archpsyc.63.3.332. [DOI] [PubMed] [Google Scholar]
- 11.Gottschalk M, Danne T, Aleksandra V, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes. Diabetes Care. 2007;30:790–794. doi: 10.2337/dc06-1554. [DOI] [PubMed] [Google Scholar]
- 12.Glauser TA, Ayala R, Elterman RD, et al. Double-blind placebo-controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology. 2006;66:1654–1660. doi: 10.1212/01.wnl.0000217916.00225.3a. [DOI] [PubMed] [Google Scholar]
- 13.Pina-Garza JE, Espinoza R, Nordli D, et al. Oxcarbazepine adjunctive therapy in infants and young children with partial seizures. Neurology. 2005;65:1370–1375. doi: 10.1212/01.wnl.0000186800.18456.72. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Peralta RP, Kelly DA, Haber B, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: efficacy, safety, and pharmacokinetics. Hepatology. 2005;42:1010–1018. doi: 10.1002/hep.20884. [DOI] [PubMed] [Google Scholar]
- 15.Berkowitz RI, Fujioka K, Daniels SR, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145:81–90. doi: 10.7326/0003-4819-145-2-200607180-00005. [DOI] [PubMed] [Google Scholar]
- 16.Nijman RJM, Borgstein NG, Ellsworth P, Djurhuus JC. Tolerodine treatment for children with symptoms of urinary urge incontinence suggestive of detrusor overactivity: results from 2 randomized, placebo controlled trials. J Urology. 2005;173:1334–1339. doi: 10.1097/01.ju.0000152322.17542.63. [DOI] [PubMed] [Google Scholar]
- 17.Berger WE, Qaqundah PY, Blake KB, et al. Safety of budesonide suspension in infants six to twelve months with mild to moderate persistent asthma or recurrent wheeze. J Pediatr. 2005;146:91–95. doi: 10.1016/j.jpeds.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 18. [Accessed March 9, 2009];PREA labeling changes. Available at http://www.fda.gov/cder/pediatric/Prea_label_post-mar_2_mtg.htm.
- 19.Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170:477–480. [PMC free article] [PubMed] [Google Scholar]
- 20.Iodannes JPA. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279:281–289. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 21.Uppal NK, Dupuis LL, Parshuram CS. Documentation of pediatric drug safety in manufacturers' product monographs: a cross-sectional evaluation of the Canadian compendium of pharmaceuticals and specialities. Paediatr Drugs. 2008;10:193–197. doi: 10.2165/00148581-200810030-00007. [DOI] [PubMed] [Google Scholar]