Abstract
Objective
We sought to examine the relationship of seizure threshold (ST) to age and other demographic characteristics in a large sample where ST was determined by the dose titration (DT) method. We also compared the resulting stimulation levels to estimates predicted by an age-based formula, the half-age (HA) method.
Methods
In a multicenter prospective study patients received a standardized course of bilateral ECT for major depression using a brief pulse device. The ST was determined at the first treatment using a fixed algorithm of stimulations. Subsequent seizures were induced at a level 50% higher than the empirically determined ST. We only included data from subject receiving methohexital anesthesia. We correlated ST with demographic and clinical characteristics of the sample. The actual dosing levels at the second treatment were compared to estimates based on HA.
Results
Of the original 531 subjects, 402 met criteria for the current analysis. The ST was positively correlated with age. Male patients had slightly higher ST than female patients. Neither race, severity of illness, psychosis, nor use of psychotropic medications affected ST. Little variability in titrated ST was observed among our patients. An ST of 40 (“percent of charge”) or lower was found in 97.5% of patients, with either 20 or 40 in 80% of patients. Ninety-six percent of the patients were treated at the three levels of 15%, 30% or 60%. Estimated HA stimulus levels offered a wider range of choices compared to this particular algorithm used for ST determination at an average level of 18% above the determined ST.
Conclusions
ST correlates strongly with age, while there is weaker relation between ST and sex. There was little individual variation of ST determined by DT method among subjects, possibly because of the wide spacing between steps of this particular titration algorithm. HA estimates were 18% above the empirically determined ST. This suggests that the use of the HA estimates at the first treatment may result in fewer stimulations compared to the DT method.
Keywords: ECT, seizure threshold, dosing strategies
Introduction
Early electroconvulsive therapy (ECT) techniques aimed solely at the production of a generalized grand-mal seizure at the first stimulus regardless the amount of charge or type of electricity. Modern techniques seek to maximize efficacy and minimize side effects by using dosing charges at as low as possible levels, as some studies suggest that the level of the electrical charge above the seizure threshold is closely related to the cognitive adverse effects of the treatment. Much of the debate about modern ECT technique is centered on the amount of charge to be used at the first and subsequent treatments. With right unilateral (RUL) ECT, stimuli much higher than seizure threshold (ST) are necessary for effective treatment.1,2 In bilateral ECT though, they add little to efficacy while increasing the cognitive burden of the treatment.3 Therefore, methods to determine the adequate stimulus levels are directly or indirectly based on ST estimates.
ST varies among individuals and among clinical and treatment conditions in the same individual. This variation has been described to be as wide as 40-fold.2,4 Main factors identified as influencing ST include age and electrode placement while sex, concomitant medications, and electrical parameters such as pulse width, frequency and stimulus duration also seem to play an important role. 5-7
Two strategies for estimating charge levels are in common use in ECT: dose titration (DT) that requires direct determination of ST, and age-based formulas relying on the relationship between ST and patient's age.
The DT method was suggested as a way to offer an individualized approach.2 The estimation of the seizure threshold is based on stepwise applications of stimuli at starting levels that are anticipated to be sub-threshold and then at increasing levels until a seizure is elicited. Because the devices offer fixed increments, estimates are based on 25 mC or 50 mC increments, offering rough estimates of the ST. These estimates are used to guide dosing levels in subsequent sessions. In bilateral ECT, after determination of the ST at the first treatment, subsequent treatments are commonly administered at a level of 150% of the determined ST.8 The DT method has been criticized as being cumbersome, often prolonging the treatment time, as well as exposing patients to the risks of bradycardia caused by subthreshold stimuli.6
Alternative methods to select the stimulus charge are based on the relationship of ST to age, with very low levels in children and adolescents and higher levels in the elderly.6 An age related method9 and the half-age method6 have been described. The half-age (HA) method estimates the stimulating dose according to the patient's chronological age, using half this age in “percent of charge” for the Thymatron device or the equivalent in milicoulombs for the MECTA device as starting point at the first session. It has been criticized as a gross estimate unaccommodating to individual variations of ST and less sensitive than DT method.10
Most recommendations about dosing strategies are based on retrospective studies or prospective studies with small subject samples. We had the opportunity to study the characteristics of ST in a large cohort of patients who participated in a collaborative multicenter study funded by the National Institute of Mental Health (NIMH), conducted by the Consortium for Research in ECT (CORE).11 The study was conducted at five clinical sites (the New Jersey Medical School - University of Medicine and Dentistry of New Jersey in Newark, the Medical University of South Carolina in Charleston, The Zucker Hillside Hospital of the Northshore-Long Island Jewish Medical Center in New York, the University of Southwestern Texas Medical Center in Dallas, and the Mayo clinic in Rochester, Minnesota) over a 6 year period (1997 - 2003). The primary results of the study are reported,11 as are the effects of psychosis,12 age,13 melancholia,14 and the impact on suicide.15 In this report we aim to describe the ST characteristics of a large sample of patients treated with bilateral ECT. Furthermore, we assessed the clinical-demographic characteristics, and compared the calibrated ST dosing levels to HA method estimates.
Methods
The CORE study “continuation ECT vs. continuation pharmacotherapy” compared the efficacy of continuation ECT and continuation lithium plus nortriptyline in patients who responded to an acute course of ECT.11 In the acute phase, patients with unipolar major depression referred for ECT received a course of thrice-weekly bilateral (bifrontotemporal) ECT, delivered following a standardized protocol with a Thymatron DGx device (Somatics, Inc., Lake Bluff, Ill.). The patients who met remission criteria and remained remitted for 1 week were randomly assigned to receive either continuation ECT or continuation medication.
Seizure threshold was titrated at the first session using a fixed algorithm as described on table 1.5 Subsequent treatments were performed at a dose level 50% higher than the ST estimated at treatment 1.
Table 1.
Titration Schedule
| Patient Age |
Threshold at Treatment #1 (% of Charge)* |
Dose at Treatment #2 (% of Charge)* |
|---|---|---|
| Under 50 years | 5 | 10 |
| 10 | 15 | |
| 20 | 30 | |
| 40 | 60 | |
| 80 | 100 | |
| 50 years and older | ||
| 10 | 15 | |
| 20 | 30 | |
| 40 | 60 | |
| 80 | 100 | |
| 100 | 100 |
1 % of charge roughly corresponds to 5 mC of charge
Seizure threshold was defined as the lowest stimulation level required to elicit an adequate seizure, defined as at least 25 seconds of EEG duration and at least 20 seconds of motor duration. If an adequate seizure was not produced, stimulation at the next higher level was given after 30-40 seconds under the same anesthesia. Two different determination schedules were used to avoid excessive number of stimulations in older patients as several studies have shown that these patients have a higher ST.2,4,6
The medications used were glycopyrrolate 0.2 mg IV, methohexital 0.5-1.5 mg/kg IV, and succinylcholine 0.5-1.0 mg/kg IV.
Subjects
The original inclusion and exclusion criteria for the study are described elsewhere.11 Of note, patients with history of ECT within 6 months prior to enrollment, history of neurological illness (including head trauma) and recent substance use disorder were excluded. The primary sample for the current report comprises all patients who had valid ST determinations at their first ECT session and who received methohexital for anesthesia (402/531; 76%). Subjects were excluded for titration algorithm violations (n=12), use of anesthetic medications other than methohexital (n=83), diagnosis other than major depressive disorder (N=30), and other reasons (N=4). (For a period during the study, methohexital was unavailable and treatments were administered using other sedative agents.) There were no differences in basic demographic and baseline clinical characteristics between those included and those excluded from the sample (Table 2).
Table 2.
Patient Characteristics for 402 patients included in the study and 129 who were excluded.
| Characteristics | 402 Patients with ST treated with Methohexital | 129 Patients without ST or not treated with Methohexital | p-value |
|---|---|---|---|
| Frequency (%) or Mean ± sd | Frequency (%) or Mean ± sd | X2 or t-test | |
| Age | 55.2 ± 16.5 | 55.2 ± 17.2 | 0.972 |
| Sex | 0.231* | ||
| Female | 282 (70.1%) | 83 (64.3%) | |
| Ethnicity | 0.872 | ||
| Caucasian | 365 (90.8%) | 118 (91.5%) | |
| African-American | 24 (6.0%) | 8 (6.2%) | |
| Psychosis Status | |||
| Psychotic | 122 (30.4%) | 36 (27.9%) | 0.658 |
| HAM-D24 Total Score at Baseline | 35.2 ± 7.0 | 36.0 ± 7.4 | 0.230 |
| Seizure threshold | 25.2 ± 13.9 | - | - |
| Stimulus level at 2nd ECT (1.5 × ST) | 39.0 ± 21.7 | - | - |
| Half Age stimulus level (estimated) | 29.8 ± 8.4 | ||
| Number of stimuli to reach threshold | 2.5 ± 0.7 | - | - |
| Use of benzodiazepines** | 77 (19.2%) | 23 (17.8%) | 0.797 |
| Use of antidepressants** | 78 (19.4%) | 24 (18.6%) | 0.898 |
| Use of antipsychotics** | 44 (11.0%) | 21 (16.3%) | 0.123 |
| Use of anticonvulsants** | 29 (7.2%) | 13 (10.1%) | 0.348 |
Fisher's Exact Test
on day prior to first treatment.
A determined effort was made to discontinue psychotropic medications before initiating ECT. The protocol did not allow the use of psychotropic medications after the fifth day other than lorazepam (to 3mg/day) or diphenhydramine (50mg/day), as needed. Many patients however, had received various medications close to the day of the first ECT. We documented the use of any psychotropic medication and examined possible effects on ST.
For simplicity, and to facilitate comparisons between the dose levels estimated by DT and HA methods, we report dose levels in units of “percent of charge” as marked by the stimulating device. Roughly 1 unit of “percent of charge” corresponds to 5 mC of charge.
Statistical analyses
Demographic, baseline clinical, and treatment history variables were described using mean and standard deviations (mean ± sd) for continuous variables (age, baseline 24-item Hamilton depression scale (HAMD24), total number ECT in acute phase, ST, and number of stimuli to reach threshold) and percentages for categorical variables (sex, ethnicity, psychosis status, prior use of benzodiazepines, antidepressants, antipsychotics, and anticonvulsants). The demographic and baseline clinical and treatment history variables were compared across ST categories using analysis of variance for continuous variables and chi-square (or Fisher's Exact Test) for categorical variables.
ST-Age Relationship
The relationship between age and ST was evaluated using several approaches. For these analyses, age was considered both as a categorical variable and, in the modeling approach, as a continuous variable. For categorical analyses, we considered several age cutpoints to evaluate the sensitivity of results to choice of age categories, defined as 18-44, 45-64, and 65-85 years. Analysis of variance followed by Tukey's test for post hoc comparisons was used to compare logarithm of ST (log ST) across age categories. To evaluate the more complex multivariable relationship between ST, age, and sex, we used multivariable linear regression with log ST as the dependent variable, age as the primary independent variable (considered both as categorical and continuous), and sex and clinical center (site) as covariables. The consistency of the age-ST association across sex categories was evaluated via inclusion of a sex by age interaction term in the model. The interaction term was not significant in any of the models and was not included in the final models. As an additional modeling approach, we used a proportional odds model with ST treated as a categorical dependent variable (rather than a continuous variable) to evaluate sensitivity of conclusions to the analysis method. Results for the proportion odds model were similar to those for the linear regression approach and, based on ease of interpretation, we focus on results of the linear regression approach.
Additional predictors of ST
This modeling approach was also used to evaluate whether additional variables were predictive of ST. The additional variables were the presence of psychosis, disease severity (as measured by HAMD24 baseline), ethnicity (Caucasian/not Caucasian), and prior use (yes/no) of benzodiazepines, antidepressants, antipsychotics, and anticonvulsants.
Relationship between HA method and ST estimates
We estimated the stimulus dose for each patient if HA method had been used at the first and following ECT sessions. We compared these estimates to the actual stimulus doses used following DT method at treatment number 2. The degree to which the HA method agrees with that of the DT method was evaluated by determination of the Spearmen rank order correlation coefficient. The difference between the value of 150% ST and the estimated HA method was determined and the frequency distribution of the difference measures described.
Results
Patient Characteristics
The sample of 402 participants had valid ST determinations and received methohexital during the ECT procedure; 129 were excluded from these analyses (Table 2). The seizure threshold (5, 10, 20, 40 and 80% of charge) varied with average age (ANOVA, p<0.0001) and sex (χ2, p ≤ 0.05), with approximately 38% (46/120) of men having ST ≥ 40 compared to 27% (76/282) of women. The average ST level for men was 27.9% (±14.9) compared to 24.0% (±13.6) for women (tdf=400=2.57, p=0.01). There were no differences in ST levels with psychosis status, ethnicity, illness severity (baseline HAMD-24).
Using regression modeling with log of seizure threshold as the dependent variable we found no relationship between seizure threshold and use of benzodiazepines (n=77; p=0.49), anticonvulsants (n=29; p=0.95), antidepressants (n=78; p=0.17) or antipsychotics (n=44; p=0.32).
Distribution of Seizure Threshold Levels
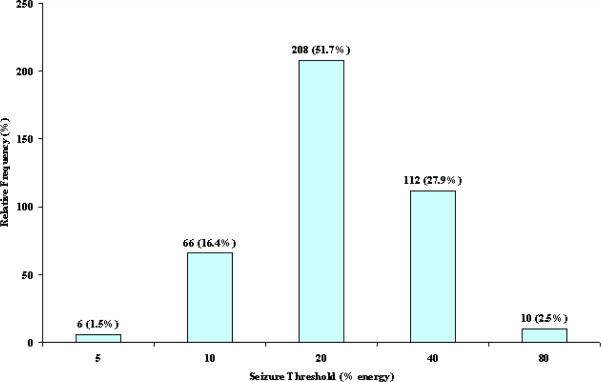
Approximately 80% (320/402) of patients had a ST of either 20 (51.7%) or 40 (27.9%). The percentage increased to 85.2% among 45-64 year olds (87.5% among 50-64 year olds) and 89.7% among those over 65 years of age (Figure 1, Table 3). In patients aged between 18 and 44, 90.6% (106/117) had a seizure threshold of 10 (35.9%) or 20 (54.7%). Further subdividing the youngest group, the proportion having an ST of 10 or 20 increased to 97.6% (40/41) for those between 18 and 34 years of age.
Figure 1.
Titrated Seizure Threshold Levels (N=402)
Table 3.
Patient Characteristics by Determined Seizure Threshold (N= 402)
| Characteristics | Frequency (%) or Mean ± sd | p-value | ||||
|---|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | 80 | X2 or ANOVA | |
| Age | 38.8 ± 7.6 | 42.3 ± 17.6 | 53.3 ± 14.9 | 66.3 ± 11.1 | 66.5 ± 8.4 | <0.0001 |
| Sex | 0.0151 | |||||
| % Female | 6 (100%) | 53 (80.3%) | 147 (70.7%) | 70 (62.5%) | 6 (60.0%) | |
| % Caucasian | 5 (83.3%) | 60 (93.8%) | 183 (90.1%) | 108 (96.4%) | 9 (100%) | |
| % Psychotic | 0 (0%) | 21 (31.8%) | 66 (31.7%) | 31 (27.7%) | 4 (40.0%) | |
| HAM-D24 Total Baseline Score | 35.3 ± 5.8 | 35.2 ± 7.9 | 35.2 ± 6.7 | 34.7 ± 7.3 | 37.4 ± 7.1 | 0.825 |
| Use of benzodiazepines2 | 0.7911 | |||||
| % Yes | 2 (33.3%) | 13 (19.7%) | 41 (19.7%) | 21 (18.8%) | 0 (0%) | |
| Use of antidepressants2 | 0.5731 | |||||
| % Yes | 2 (33.3%) | 12 (18.2%) | 44 (21.2%) | 19 (17.0%) | 1 (10.0%) | |
| Use of antipsychotics2 | 0.8971 | |||||
| % Yes | 1 (16.7%) | 8 (12.1%) | 22 (10.6%) | 13 (11.6%) | 0 (0%) | |
| Use of anticonvulsants2 | 0.1451 | |||||
| % Yes | 1 (16.7%) | 3 (4.5%) | 20 (9.6%) | 5 (4.5%) | 0 (0%) | |
Due to small expected frequencies, ST categories 5-10 and 40-80 were combined for chi-square test
Medication used on day prior to first treatment.
ST-Age Relationship
The mean ST increases with age [ANOVA, p ≤ 0.0001], with the mean of each age group differing significantly from the means of the other age groups (all means differed by Tukey's post hoc pairwise comparisons). Using regression modeling and treating age as a continuous variable, we found a positive linear relationship with ST (log) [beta coefficient (slope) =0.016, t=11.4, p ≤ 0.0001]. Variation in age, however, accounted for only 25% of the variation in ST levels [model r2=0.246]. Carrying out these analyses within age strata, the ST increased with age through the 18-44 and 45-64 years age groups, but did not increase with age beyond age 64 [18-44 years: beta coefficient for age=0.024, t=4.5, r2=0.15, p<0.0001; 45-64 years: beta coefficient for age=0.037, t=5.8, r2=0.19, p ≤ 0.0001; 65-85 years: beta coefficient for age=-0.007,
t=-1.0, r2=0.01, p≤ ns]. These relationships persisted after adjustment for sex, clinical center, psychosis status, baseline HAM-D, and prior use of benzodiazepines, anticonvulsants, antidepressants, or antipsychotics in the multivariable linear model and were also observed using ST as a categorical variable in a proportional odds regression model.
Sex and Age ST relationships
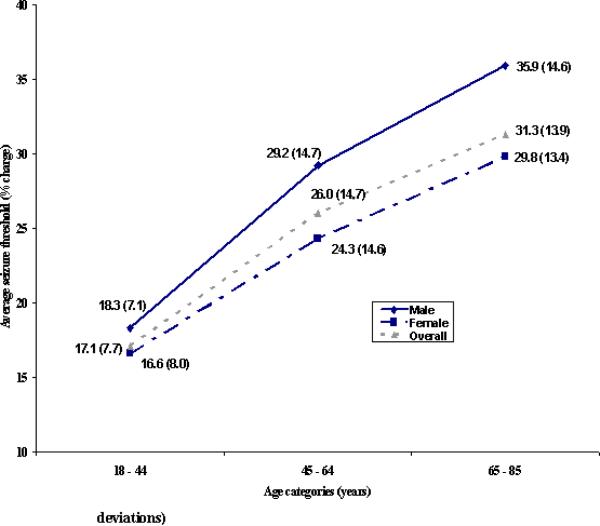
The relationship between mean ST and age for men and women is shown in Figure 2. Comparisons of mean ST across age categories reveals similar results for the two sex groups with both reflecting the same pattern of significance as described for the total sample. In regression modeling [with age (continuous), clinical center, sex, and sex by age interaction as independent variables and log ST as dependent variable], the sex by age interaction term was not significant which supported this observation of a similar age-ST relationship for men and women [age by sex interaction: beta coefficient =-0.005, t=-1.5, p=ns].
Figure 2.
Comparison of Average Titrated Seizure Threshold (in % charge) Across Age Categories and by Sex* (Values in parentheses are standard deviations)
*Females: p-value<0.0001, ANOVA for testing difference in average log seizure threshold across three age categories; 18-44 years group significantly different from 45-64, 65-85 years groups; 45-64 years group significantly different from 65-85 years group by Tukey's multiple comparison procedure
Males: p-value<0.0001, ANOVA for testing difference in average log seizure threshold across five age categories; 18-44 years group significantly different from 45-64, 65-85 years groups by Tukey's multiple comparison procedure
Relationship between the half-age method and titration methods
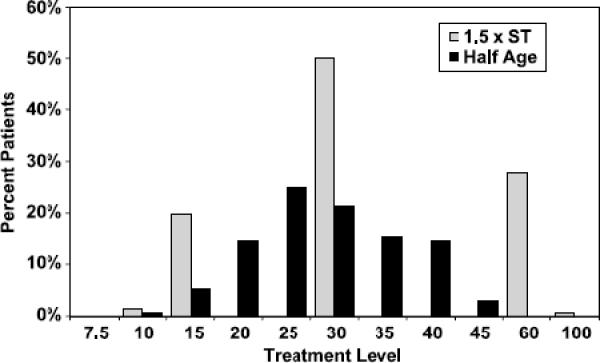
The correlation between the HA method and DT method estimates was 0.51 [Spearman's rank order correlation, p≤0.0001]. When considering the difference measure [1.5xST minus half-age], there was perfect agreement (difference=0) between the two methods for 14.7% of the measurements; the outcomes were within ±5 for 42.8% and within ±10 for 59.3% of cases. DT method resulted in treatment levels more than 10 units higher than half-age in 33% of patients and in treatment levels 30 or more units higher in approximately 11% of cases. Figure 3 depicts the actual treatment doses at treatment #2 and the distribution of stimulus doses suggested by the HA method.
Figure 3.
Distribution of 1.5 X Titrated Seizure Threshold and Estimated Half Age Dosing
Discussion
Our data confirm the strong positive relationship between age and ST as well as the weaker one of sex and ST. Other parameters such as diagnosis and severity of illness did not affect ST. We did not find a significant effect on ST by psychotropic medications used the day before ECT, but only a small number of our patients were on psychotropic medications at that time.
The purpose of determining ST prior to selecting a stimulus level is to obtain more accurate and individualized information in our efforts to maximize the efficacy and minimize the cognitive effects of bilateral ECT. The procedure to determine ST at the first session was generally well tolerated. The procedure to determine ST at the first session was generally well tolerated. These data, however, show that DT method based on this particular algorithm to determine ST does not offer an individualized approach in stimulus dose selection. Eighty percent of our patients had a ST estimated at 20 or 40 and 96% between 10 and 40. In contrast to earlier studies, the ST varied only by 4 times, from 10 to 40 “percent of charge” with very few outliers at 5 and 80. Following the DT method determination, only five choices of stimulus levels were possible for the second treatment: 10, 15, 30, 60 and 90. Ninety-six percent of our patients were treated at 15, 30 or 60 “percent of charge” levels, hardly indicative of an individualized approach. This is partially due to the nature of the incremental stepwise algorithm that lumps several patients who could possibly have ST between the 2 steps to the next higher value. For example, in our paradigm, all patients who could potentially seize with a stimulus from 21 to 40 were considered to have ST of 40 and were treated at 60 at the second treatment. An algorithm with smaller increments (such as 5-10-15-20-25-30) might have offered greater individualization of ST estimates. Its use, however, would be most likely limited by the increased number of stimulations that it would require.
By contrast to the empirically determined DT method levels, the HA method estimates might have offered a wider range of stimulation options in our sample (Figure 3). This suggests that HA method may be more individualized than previously thought. Assertions that HA method would result in unnecessarily high stimulations were not confirmed by our analysis. In fact, HA method would result in doses about 18% above the empirically determined ST. At the second treatment, treatment charges based on DT method were higher by about 31% above the HA method estimates, a reflection of the arbitrarily chosen 50% increment at the second treatment. Our data indicates that in most patients the HA method can be used as a starting point of treatment without concerns of over-stimulation. For the few patients who would not seize at their HA method level, treatment could be performed with restimulation at a higher point.
The strengths of this study, besides the large number of subjects, include the use of a well-characterized sample and the standardization of treatment procedures. Limitations include the use of various psychotropic medications, including benzodiazepines and anticonvulsants, by some patients the day before the first treatment. Furthermore, it is possible that the discontinuation of psychotropic medications and the presumed withdrawal just prior to first treatment may have influenced ST, either by decreasing (e.g benzodiazepines) or increasing it (e.g. antipsychotics). Unfortunately, these data were not available for analysis. Nevertheless, our sample is representative of “real world” conditions where most patients do not undergo a complete medication washout before the first ECT session. Still, our calculations showed no differences in ST between patients who received medications and those who did not. It is conceivable that the lack of medication effect on ST is due to the inability of the DT algorithm used to determine small variations in ST.
Another limitation is that our calculations and comparisons with the HA method are based on the assumption that all patients would have had an adequate seizure at the first ECT, if they were stimulated at the HA level. In clinical practice, however, if an adequate seizure is not elicited with the first stimulus, the use of the HA method offers the advantage of a higher starting point to determine ST, thus requiring fewer stimuli in one session.
The limitations and risks of the DT method, and the relative independence of treatment efficacy on the stimulus dose, do not encourage its use in bilateral ECT. It does not seem to offer clear advantage for effective dosing over the HA method. However, the DT method may be necessary for RUL ECT where the relationship between dosing charge and outcome is critical. Our data suggest that the HA method may offer a practical alternative to DT method and could be possibly used effectively as a starting point at the first ECT session. However, further prospective research directly comparing the two approaches for stimulus dosing is needed.
REFERENCES
- 1.McCall WV, Reboussin DM, Weiner RD, et al. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen. Psychiatry. 2000;57:438–444. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 2.Sackeim HA, Devanand DP, Prudic J. Stimulus intensity, seizure threshold, and seizure duration: impact on the efficacy and safety of electroconvulsive therapy. Psychiatr Clin. North Am. 1991;14:803–843. [PubMed] [Google Scholar]
- 3.Abrams R. Stimulus titration and ECT dosing. J. ECT. 2002;18:3–9. doi: 10.1097/00124509-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N. Engl. J. Med. 1993;328:839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 5.Beale MD, Kellner CH, Pritchett JT, et al. Stimulus dose-titration in ECT: a 2-year clinical experience. Convuls. Ther. 1994;10:171–176. [PubMed] [Google Scholar]
- 6.Petrides G, Fink M. The “half-age” stimulation strategy for ECT dosing. Convuls. Ther. 1996;12:138–146. [PubMed] [Google Scholar]
- 7.Sackeim HA, Decina P, Kanzler M, et al. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am. J. Psychiatry. 1987;144:1449–1455. doi: 10.1176/ajp.144.11.1449. [DOI] [PubMed] [Google Scholar]
- 8.Beale MD, Kellner CH. Proposed titration schedule. Convuls. Ther. 1997;13:44. [PubMed] [Google Scholar]
- 9.Abrams R. Electroconvulsive Therapy. Oxford University Press; New York: 2002. [Google Scholar]
- 10.Tiller JW, Ingram N. Seizure threshold determination for electroconvulsive therapy: stimulus dose titration versus age-based estimations. Aust. N. Z. J. Psychiatry. 2006;40:188–192. doi: 10.1080/j.1440-1614.2006.01773.x. [DOI] [PubMed] [Google Scholar]
- 11.Kellner CH, Knapp RG, Petrides G, et al. Continuation electroconvulsive therapy vs pharmacotherapy for relapse prevention in major depression: a multisite study from the Consortium for Research in Electroconvulsive Therapy (CORE) Arch. Gen. Psychiatry. 2006;63:1337–1344. doi: 10.1001/archpsyc.63.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrides G, Fink M, Husain MM, et al. ECT remission rates in psychotic versus nonpsychotic depressed patients: a report from CORE. J ECT. 2001;17:244–253. doi: 10.1097/00124509-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor MK, Knapp R, Husain M, et al. The influence of age on the response of major depression to electroconvulsive therapy: a C.O.R.E. Report. Am. J Geriatr. Psychiatry. 2001;9:382–390. [PubMed] [Google Scholar]
- 14.Fink M, Rush AJ, Knapp R, et al. DSM melancholic features are unreliable predictors of ECT response. J ECT. 2007 doi: 10.1097/yct.0b013e3180337344. In Press. [DOI] [PubMed] [Google Scholar]
- 15.Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am. J Psychiatry. 2005;162:977–982. doi: 10.1176/appi.ajp.162.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]