As long as we keratinocytes can remember, melanocytes have lived inconspicuously among us at the basal membrane and have been primarily known as meek and obedient pigment producers in the epidermis. However, recent investigations are suggesting that these fellows have several identities, work undercover in many other places in the human body and have functions we can only speculate about.
The purpose of this essay is to consider the various aspects of the well-known and the more obscure abilities of melanocytes.
First of all, how does one recognize a melanocyte if it is encountered somewhere outside the epidermis? They are usually identified by their expression of melanocyte-specific proteins e.g. tyrosinase (TYR), TYRP1, DCT, Pmel17/gp100, MART-1 and/or MITF. However, melanocyte precursors (known as melanoblasts) are more difficult to identify since they don’t produce melanin and therefore don’t usually express those markers, although occasionally DCT and/or KIT are detectable as specific markers.
The favorite habitat of melanocytes is the epidermis, but large numbers of them can be found in hair follicles and in the eyes where they manufacture melanin for hair and eye pigmentation, respectively, and in other, less well-known locations as discussed below. The fact that we keratinocytes control melanocytes in the skin via an armamentarium of growth factors [1] has led to the impression that keratinocytes and melanocytes live in a sort of master-slave relationship. However, the dependency is not unilateral at all; melanocytes transport melanin in membrane-bound organelles (termed melanosomes) via their elongated dendrites and then transfer them to us [2] whereupon we arrange them to form a critical protective barrier (known as supranuclear ‘caps’) to shield our DNA from UV radiation [3].
Melanocytes (and melanin) also function early during human development; they play critical roles during embryonic development as can be seen in individuals with oculocutaneous albinism type 1 (OCA1). OCA1 results from the dysfunction of TYR which leads to impaired pigmentation of skin, hair and eyes [4] but also to misrouting of the optic nerves at the chiasm [5]. Melanocytes express the melanocortin 1 receptor (MC1R) that regulates the quality and quantity of their melanin production. MC1R is controlled by the agonists melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH) [6], which stimulate the melanogenic cascade and thus the synthesis of eumelanin, as well as by the antagonist agouti signaling protein (ASP) [7]. It is known that ASP elicits the production of pheomelanin, but it was shown only recently that ASP also modulates the expression of genes involved in morphogenesis (especially in nervous system development) [8].
Besides their existence in the skin, melanin and melanocytes have been reported to appear in the stria vascularis of the cochlea [9], in the leptomeninges [10], substantia nigra and locus coerulus [11] of the brain, in the heart [12,13] and there is evidence that they operate even in such inhospitable territories as adipose tissue [14].
Note in passing that there are two distinct types of melanocytes: differentiated melanocytes that originate from the neural crest and can be found at various locations in the body, and a second type, the retinal pigment epithelium (RPE), specifically present only as a single layer of cells lying behind the retina that develop in situ from the optic cup of the brain [15]. The RPE plays a critical role in the active phagocytosis and turnover of the rod outer segments of the retina as well as in the uptake, processing and transport of retinoids and consequently has an important function in vision [16].
Melanocytes are also present as intermediate cells in the stria vascularis of the cochlea. Strial intermediate cells are required for the generation of endolymph-mediated action potentials that are necessary for normal hearing [9]. Hearing impairment can be associated with inherited pigmentary disorders, e.g. Waardenburg syndrome [17], and studies have shown that the extent of induced temporary hearing loss is inversely related to skin pigment type [18]. Melanin granules produced by melanocytes in the inner ear even play important roles in balance [19].
Extracutaneous melanocytes located in the brain may have several neuroendocrine functions. Human melanocytes express lipocalin-type prostaglandin D synthase (L-PGDS) which generates prostaglandin D2 (PGD2) [20]. Besides, in melanocytes, β-endorphin, an endogenous opioid, is generated from proopiomelanocortin (POMC) together with MSH and ACTH. Does this suggest that melanocytes regulate sleep? PGD2 is a potent sleep-inducing substance [21] and opioid receptors are located in the nuclei that are active in sleep regulation [19]. Also, there are indications that a certain melanocyte-derived factor might be involved in controlling the central chemosensor that generates the respiratory rhythm [19]. Pigment in the brain, termed neuromelanin, consists of a large, complex, eumelanin-covered pheomelanin core which may also contain aliphatics and peptides [22]. Neuromelanin is primarily localized in dopaminergic neurons of the substantia nigra and in the locus coerulus, and accumulates in the human substantia nigra with age [11]. A selective loss of dopaminergic neurons containing neuromelanin is associated with Parkinson’s disease [11]. Various studies support the concept that neuromelanins have a protective function by binding/removal of ROS and metals that would otherwise be highly toxic to neurons [11,23,24]. A recent study showed that virtually all brain tissues contain significant amounts of neuromelanin, which are thought to play important roles in reducing toxicity in those tissues [25].
It has also been brought to our attention that melanocytes are located in the valves and septa of the heart [12,13]. Mice presenting with hyper (hypo-) pigmented skin show increased (or decreased) heart pigmentation [13]. Cardiac melanocytes may originate from the same precursor population as skin melanocytes as they depend on the same the signaling molecules known to be required for proper skin melanocyte development [12], but their function in this location so far is obscure. It may well turn out that the production of melanin is not always of benefit, either in the heart or in other tissues, such as the lungs, where in a rare disease known as LAM [26], muscle cells revert towards their developmental origins and express some melanocyte markers, such as tyrosinase, Pmel17, etc. The resulting production and accumulation of melanin in lung tissues is eventually lethal.
Recently, we have learned that melanin biosynthesis also takes place in the visceral adipose tissue of morbidly obese humans [14]. Hypothetically, the ectopic synthesis of melanin in the cytosol of obese adipocytes may serve as a compensatory mechanism to act as an anti-inflammatory factor and to reduce oxidative damage. During increases of cellular fat deposition, adipocytes become more exposed to endogenous apoptotic signals, especially ROS, which could be counteracted by ectopically produced melanin. In addition, adipocytic melanin may also suppress the secretion of proinflammatory molecules [14].
In conclusion, we think it unfair that melanocytes reap all the glory for their role in pigmenting the skin and providing it critical protection against UV damage, when in fact it is us as keratinocytes that form the bulk population of that tissue and deserve all the credit. It adds insult to injury that melanocytes are now beginning to take more and more glory for their roles in other tissues of the body. Where will it all end?
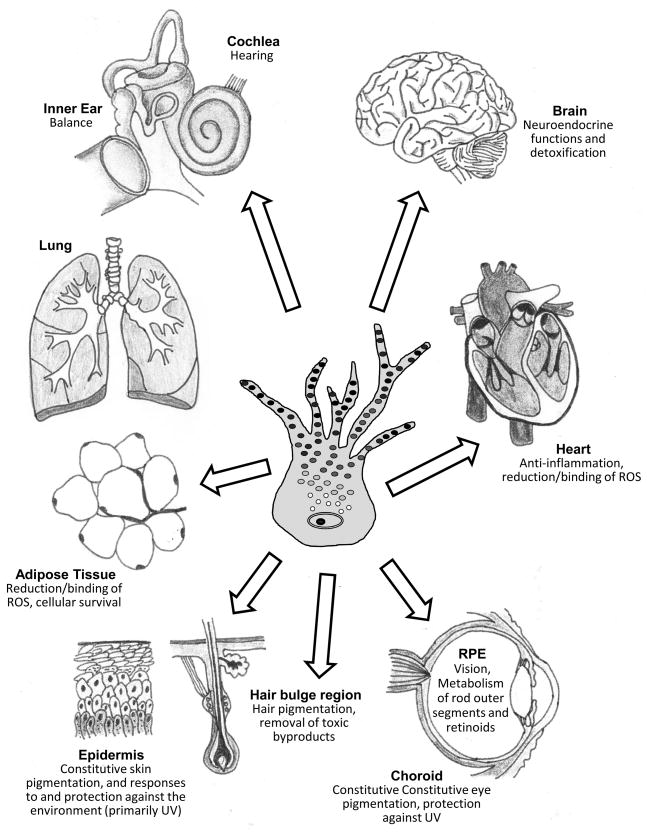
Figure.
Schematic showing the known distribution of melanocytes in various human tissues, including the skin (epidermis and hair bulbs, bottom left), adipose tissue (lower left), lung (upper left), ear (inner ear and cochlea, top left), brain (top right), heart (right) and eye (retinal pigment epithelium and choroid, bottom right).
Table 1.
Locations and Functions of Melanocytes (and Melanocyte Imitators)
| Location | Function(s) | Ref |
|---|---|---|
| Skin - epidermis | Constitutive skin pigmentation, and responses to and protection against the environment (primarily UV) | [1 – 3] |
| Skin – hair follicles | Hair pigmentation, removal of toxic byproducts | [2] |
| Skin – hair bulge region | Melanocyte stem cell reservoir for skin | |
| Eye – choroid | Constitutive eye pigmentation, protection against UV | [4, 5] |
| Eye – RPE | Vision, Metabolism of rod outer segments and retinoids | [15,16] |
| Ear – cochlea | Hearing | [9,17,18] |
| Inner ear – | Balance | [19] |
| Brain – all tissues | Neuroendocrine and detoxification | [10,11,20–25] |
| Heart - | Anti-inflammation, reduction/binding of ROS | [12,13] |
| Adipose Tissue - | Reduction/binding of ROS, cellular survival | [14] |
| Lung - | Unwanted? No known function; lethal consequences | [26] |
References
- 1.Yamaguchi Y, Brenner M, Hearing VJ. The regulation of skin pigmentation. J Biol Chem. 2007;282:27557–27561. doi: 10.1074/jbc.R700026200. [DOI] [PubMed] [Google Scholar]
- 2.Tolleson WH. Human melanocyte biology, toxicology, and pathology. J Environ Sci and Health. 2005;23:105–161. doi: 10.1080/10590500500234970. [DOI] [PubMed] [Google Scholar]
- 3.Montagna W, Carlisle K. The architecture of black and white facial skin. J Am Acad Dermatol. 1991;24:929–937. doi: 10.1016/0190-9622(91)70148-u. [DOI] [PubMed] [Google Scholar]
- 4.Spritz RA. Molecular genetics of oculocutaneous albinism. Hum Mol Genet. 1994;3:1469–1475. doi: 10.1093/hmg/3.suppl_1.1469. [DOI] [PubMed] [Google Scholar]
- 5.King RA, Hearing VJ, Creel DJ, Oetting WS. Albinism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 5587–5627. [Google Scholar]
- 6.Millington GW. Proopiomelanocortin (POMC): The cutaneous roles of its melanocortin products and receptors. Clin Exp Dermatol. 2006;31:407–412. doi: 10.1111/j.1365-2230.2006.02128.x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki IA, Tada A, Ollmann M, Barsh GS, Im S, Lamoreux ML, Hearing VJ, Nordlund JJ, Abdel-Malek ZA. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to α-melanotropin. J Invest Dermatol. 1997;108:838–842. doi: 10.1111/1523-1747.ep12292572. [DOI] [PubMed] [Google Scholar]
- 8.Le Pape E, Passeron T, Giubellino A, Valencia JC, Wolber R, Hearing VJ. Microarray analysis reveals the complex effects of MC1R signaling in melanocytes. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0806753106. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana M. Sound needs sound melanocytes to be heard. Pigment Cell Res. 1999;12:344–354. doi: 10.1111/j.1600-0749.1999.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldgeier MH, Klein LE, Klein-Angerer S, Moellmann G, Nordlund JJ. The distribution of melanocytes in the leptomeninges of the human brain. J Invest Dermatol. 1984;82:235–238. doi: 10.1111/1523-1747.ep12260111. [DOI] [PubMed] [Google Scholar]
- 11.Zecca L, Tampellini D, Gatti A, Crippa R, Eisner M, Sulzer D, Ito S, Fariello R, Gallorini M. The neuromelanin of human substantia nigra and its interaction with metals. J Neural Transm. 2002;109:663–672. doi: 10.1007/s007020200055. [DOI] [PubMed] [Google Scholar]
- 12.Brito FC, Kos L. Timeline and distribution of melanocyte precursors in the mouse heart. Pigment Cell Melanoma Res. 2008;21:464–470. doi: 10.1111/j.1755-148X.2008.00459.x. [DOI] [PubMed] [Google Scholar]
- 13.Yajima I, Larue L. The location of heart melanocytes is specified and the level of pigmentation in the heart may correlate with coat color. Pigment Cell Melanoma Res. 2008;21:471–476. doi: 10.1111/j.1755-148X.2008.00483.x. [DOI] [PubMed] [Google Scholar]
- 14.Randhawa M, Huff T, Valencia JC, Younossi Z, Chandhoke V, Hearing VJ, Baranova A. Evidence for the ectopic synthesis of melanin in human adipose tissue. FASEB J. 2008 doi: 10.1096/fj.08-116327. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bharti K, Nguyen MT, Skuntz S, Bertuzzi S, Arnheiter H. The other pigment cell: Specification and development of the pigmented epithelium of the vertebrate eye. Pigment Cell Res. 2006;19:380–394. doi: 10.1111/j.1600-0749.2006.00318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bok D. The retinal pigment epithelium: A versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 17.Tassabehji M, Newton VE, Read AP. Waardenburg syndrome type 2 caused by mutations in the human microphthalmia (MITF) gene. Nat Genet. 1994;8:251–255. doi: 10.1038/ng1194-251. [DOI] [PubMed] [Google Scholar]
- 18.Barrenas ML, Lindgren F. The influence of inner ear melanin on susceptibility to TTS in humans. Scand Audiol. 1990;19:97–102. doi: 10.3109/01050399009070759. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Takahashi NH, Shibahara S. Neuroendocrine functions of melanocytes: Beyond the skin-deep melanin maker. Tohoku J Exp Med. 2007;211:201–221. doi: 10.1620/tjem.211.201. [DOI] [PubMed] [Google Scholar]
- 20.Takeda K, Yokoyama S, Aburatani H, Masuda T, Han F, Yoshizawa M, Yamaki N, Yamamoto H, Eguchi N, Urade Y, Shibahara S. Lipocalin-type prostaglandin D synthase as a melanocyte marker regulated by MITF. Biochem Biophys Res Commun. 2006;339:1098–1106. doi: 10.1016/j.bbrc.2005.11.125. [DOI] [PubMed] [Google Scholar]
- 21.Urade Y, Hayaishi O. Biochemical, structural, genetic, physiological, and pathophysiological features of lipocalin-type prostaglandin D synthase. Biochim Biophys Acta. 2000;1482:259–271. doi: 10.1016/s0167-4838(00)00161-8. [DOI] [PubMed] [Google Scholar]
- 22.Bush WD, Garguilo J, Zucca FA, Albertini A, Zecca L, Edwards GS, Nemanich RJ, Simon JD. The surface oxidation potential of human neuromelanin reveals a spherical architecture with a pheomelanin core and a eumelanin surface. Proc Natl Acad Sci U S A. 2006;103:14785–14789. doi: 10.1073/pnas.0604010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulzer D, Bogulavsky J, Larsen KE, Behr G, Karatekin E, Kleinman MH, Turro N, Krantz D, Edwards RH, Greene LA, Zecca L. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc Natl Acad Sci U S A. 2000;97:11869–11874. doi: 10.1073/pnas.97.22.11869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zucca FA, Giaveri G, Gallorini M, Albertini A, Toscani M, Pezzoli G, Lucius R, Wilms H, Sulzer D, Ito S, Wakamatsu K, Zecca L. The neuromelanin of human substantia nigra: Physiological and pathogenic aspects. Pigment Cell Res. 2004;17:610–617. doi: 10.1111/j.1600-0749.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 25.Zecca L, Bellei C, Costi P, Albertini A, Monzani E, Casella L, Gallorini M, Bergamaschi L, Moscatelli A, Turro NJ, Eisner M, Crippa PR, Ito S, Wakamatsu K, Bush WD, Ward WC, Simon JD, Zucca FA. New melanic pigments in the human brain that accumulate in aging and block environmental toxic metals. Proc Natl Acad Sci U S A. 2008;105:17567–17572. doi: 10.1073/pnas.0808768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, Riemenschn W, Matsui K, Tavis WD, Moss J. Lymphangioleiomyomatosis (LAM). a review of clinical and morphological features. J Nippon Med Sch. 2000;67:311–329. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]