Abstract
Cyclic nucleotide-gated channels localize exclusively to the plasma membrane of photosensitive outer segments of rod photoreceptors where they generate the electrical response to light. Here we found that targeting of cyclic nucleotide-gated channels to the rod outer segment required their interaction with ankyrin-G. Ankyrin-G localized exclusively to rod outer segments, coimmunoprecipitated with the cyclic nucleotide-gated channel, and bound to the C-terminal domain of the β1-subunit. Ankyrin-G depletion in neonatal mouse retinas markedly reduced cyclic nucleotide-gated channel expression. Transgenic expression of cyclic nucleotide-gated channel β-subunit mutants in Xenopus rods showed that ankyrin-G binding was necessary and sufficient for targeting of the β1-subunit to outer segments. Thus ankyrin-G is required for transport of cyclic nucleotide-gated channels to the plasma membrane of rod outer segments.
Cyclic nucleotide gated (CNG) channels initiate the electrical responses to light in photoreceptors and to chemical stimuli in olfactory neurons (1). CNG channels are segregated to sensory cilia, where visual and olfactory signal transduction takes place. This precise intracellular localization is dependent on the channel's β-subunit (CNG-β1) in both classes of neurons (2-4). However, the molecular mechanism(s) of CNG channel targeting to the plasma membrane of sensory cilia, where this channel normally functions, are unclear.
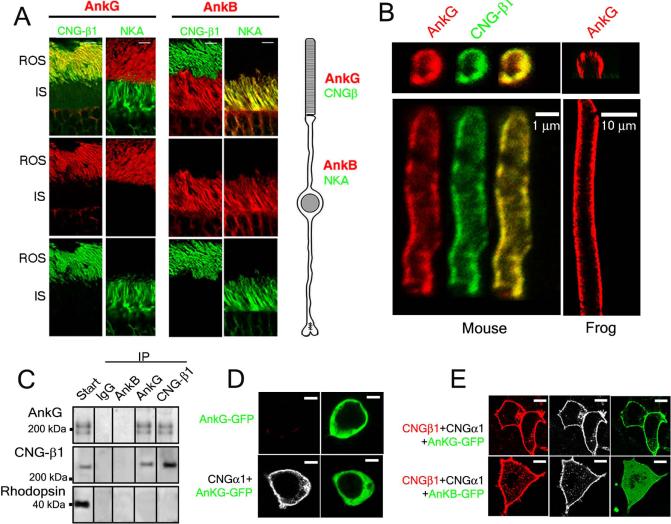
Ankyrin-G is a versatile membrane adaptor involved in the formation and maintenance of diverse specialized membrane domains (5-9). Ankyrin-G is localized exclusively to rod outer segments (ROS), where it was found along with CNG channels which have been localized to the ROS plasma membrane (10) (Fig. 1A, B). In contrast, the plasma membrane of the inner segment was lined with ankyrin-B, which is required for the coordinated expression of the Na/K ATPase, Na/Ca exchanger, and beta-2 spectrin ((11); Fig. 1A). Localization of ankyrin-G to the plasma membrane was evident in isolated mouse ROS, but was better demonstrated in frog ROS, which are 3-4-fold larger in diameter (Fig. 1B). Ankyrin-G also localized in the olfactory sensory cilia and the principal piece of sperm flagella, together with CNG-β1 (4, 12) (fig. S1). When we treated isolated bovine ROS with a cleavable cross-linker, solubilized them in 0.1% SDS, and immunoprecipitated using anti-ankyrin or anti-CNG-β1 antibodies, reciprocal CNG-β1/ankyrin-G co-immunoprecipitation was observed (Fig. 1C). The interaction with ankyrin-G was specific, because CNG-β1 was not precipitated by non-immune or anti-ankyrin-B antibodies, and the major ROS-specific protein, rhodopsin, was not precipitated in either case (Fig. 1C).
Fig 1. Ankyrin-G is restricted to photoreceptor outer segments and binds the rod CNG channel.
(A) Co-localization of ankyrin-G (AnkG, red, left 2 columns) with CNG channel (CNG-β1, green) in ROS and co-localization of ankyrin-B (AnkB, red, right 2 columns) with NKA (green) in inner segments (IS). A schematic of a rod cell is shown to the right. (B) Ankyrin-G (red) localizes to the plasma membrane of isolated mouse and frog ROS labeled with anti-CNG-β1 antibody (green). ROS tangential sections are shown in upper panels and longitudinal sections in lower panels. (C) Co-immunoprecipitation of ankyrin-G with CNG-β1 channels from bovine ROS extracts. Antibodies used for precipitations are indicated on the top, antibodies used for protein detection are indicated on the left. (D) CNG-α1 (white) alone does not recruit ankyrin-G-GFP (green) to the plasma membrane of HEK 293 cells. (E) Ankyrin-G-GFP (green) is recruited to the plasma membrane of HEK 293 cells co-expressing CNG-β1 (red) and CNG-α1 (white). Scale bars are: 5 μm in A, 10 μm in D and E.
The CNG channel binding to ankyrin-G was further evaluated using a HEK 293 cell-based assay for detecting ankyrin-membrane protein interactions (13). In this assay, over-expressed exogenous ankyrin-G fused to the C-terminus of green fluorescent protein (GFP; ankyrin-G-GFP) normally localized to the cytoplasm, is recruited to the plasma membrane upon co-expression of ankyrin binding partners such as neurofascin. CNG-α1 expressed in HEK 293 cells localized to the plasma membrane but did not recruit ankyrin-G-GFP (Fig. 1D), whereas CNG-β1 failed to localize to the plasma membrane of these cells when expressed by itself (fig. S2). Co-expression of CNG-α and CNG-β does yield functional heterotetrameric channels in the plasma membrane of HEK 293 cells (14). Such co-expression also resulted in efficient plasma membrane recruitment of ankyrin-G-GFP (Fig. 1E; complete recruitment was observed in 65% cells co-expressing the three proteins, n = 50). Ankyrin-B-GFP was not recruited to the plasma membrane under similar conditions (Fig. 1E), indicating that CNG-β1 interacts with ankyrin-G.
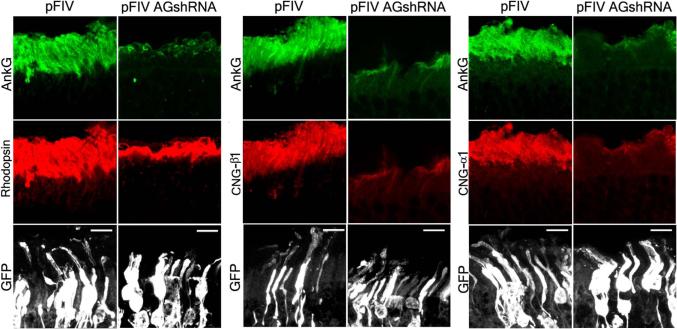
We next sought to evaluate whether ankyrin-G is required for localization of CNG channels to ROS in vivo, using shRNA to knockdown ankyrin-G expression in neonatal mouse retinas. We injected a mixture of a plasmid encoding shRNA targeting mouse ankyrin-G in 10-fold excess over a plasmid encoding GFP into the eyes of newborn pups followed by electroporation (15). Under these conditions, rods expressing GFP are typically co-transfected with the shRNA plasmid. Two weeks post-injection, photoreceptors transfected with control shRNA expressed GFP, displayed normal morphology and were robustly immunostained with ankyrin-G (Fig. 2). In contrast, photoreceptors transfected with ankyrin-G shRNA (GFP-positive) displayed a major reduction in the ankyrin G immunofluorescence in ROS (20-30% of control, based on immunofluorescence intensity of samples on the same slide, fig. S3) and their ROS were significantly shortened (average length of 4.7 μm vs. 15.5 μm in control rods; n=25; compare rhodopsin labeled sections). The immunofluorescence levels of both CNG-β1 and CNG-α1 were also markedly reduced, to a degree comparable with the ankyrin-G reduction (Fig. 2, fig. S3). In contrast, rhodopsin levels estimated by fluorescence intensities of samples on the same slide were similar between control and ankyrin-G shRNA expressing ROS (Fig. 2, fig. S3). The shortened ROS phenotype in ankyrin-G-depleted retina was more severe than reported for mice lacking the CNG-β1 subunit (2). Thus, ankyrin-G plays role(s) in either assembly and/or maintenance of ROS in addition to localization of the CNG channel. This result is similar to the requirement of ankyrin-G for biogenesis of the lateral membrane in cultured columnar epithelial cells (16).
Fig 2. Ankyrin-G is required for ROS morphogenesis.
Retinas of newborn mice were electroporated with either ankyrin-G shRNA or control pFIV (3μg/μl) plasmid, each mixed with pCAGGS-GFP (0.3μg/μl). Ankyrin-G (AnkG) staining is shown in green. The staining of rhodopsin (left), CNG-β1 (center) and CNG-α1 (right) is shown in red. GFP staining is shown in white. Scale bars are 10 μm.
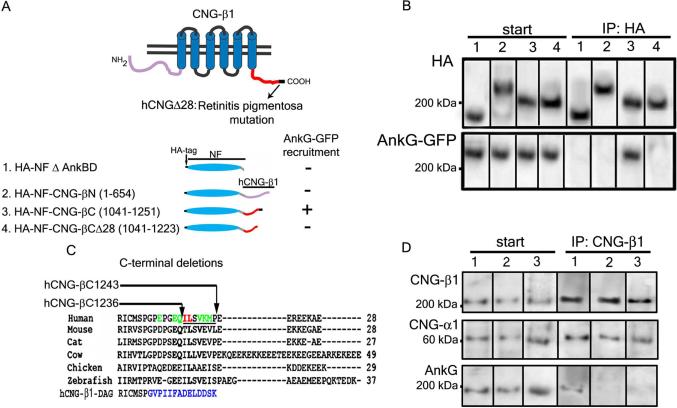
The reduction in CNG channel expression in ankyrin-G-depleted rods could be explained by a requirement for ankyrin-G in targeting the channel to ROS from the ER or Golgi located in the inner segment. Indeed, ankyrin-G is required for both post-Golgi transport and immobilization of its binding partner E-cadherin in epithelial cells (6). To test this hypothesis, we needed to identify CNG-β1 mutants lacking ankyrin-G-binding. We first determined if ankyrin-G bound to either the N- or C-terminal cytoplasmic domain of CNG-β1 (Fig. 3A). Ankyrin-G–GFP was co-expressed with protein constructs in which the ankyrin-binding domain of neurofascin was replaced with either the entire cytoplasmic N- or C-terminus of human CNG-β1 (NF-CNG-βN (amino acids 1-654) and NF-CNG-βC (amino acids 1041-1251), respectively; Fig. 3A). Ankyrin-G interacted only with the C-terminal domain of CNG-β1, both in the HEK 293-based plasma membrane recruitment assay (fig. S4) and co-immunoprecipitation experiments (Fig. 3B). Immunoprecipitation experiments in HEK cells were performed in the absence of a crosslinking reagent.
Fig 3. The ankyrin-G binding site resides in a C-terminal motif of CNG-β1.
(A) Schematic diagrams of rod CNG-β1 (top) and HA-tagged neurofascin (HA-NF) chimeras with CNG-β1 (bottom). Number within brackets indicate the amino acids stretches of the CNG-β1 polypeptide that were fused to neurofascin. The abilities of chimeras to recruit ankyrin-G-GFP to plasma membrane of HEK 293 cells is indicated by + or - (AnkBD, ankyrin-binding domain). (B) Ankyrin-G-GFP was co-expressed in HEK 293 cells with the chimeras shown in panel A, cells were lysed and proteins were immunoprecipitated by anti-HA antibodies. Immuno blots of samples from the starting material (left) and precipitated proteins (right) were probed with anti-HA or anti-GFP antibodies. Lane numbers correspond to the numbered chimeras in panel A. (C) Sequence of the 28 C-terminal amino acids of human CNG-β1 and homologous regions from other vertebrates. Arrows indicate sites of C-terminal deletions hCNG-βC1243 and hCNG-βC1236 used to identify residues critical for ankyrin-G binding. Colored residues were mutated to alanine with those in red being critical for ankyrin-G binding and those in green being neutral. The human CNG-β1/β-dystroglycan chimera (hCNGβ-DAG) is shown at the bottom with the dystroglycan sequences marked in blue. (D) CNG-β1 (lane 1) CNG-β28 (lane 2) and CNG-β1 IL1237AA (lane 3) were coexpressed with CNG-α1 in HEK 293 cells and immunoprecipitated using the anti-CNG-β1 antibodies. Each CNG-β1 mutant normally co-precipitated with CNG-α1, but failed to bind endogenous ankyrin-G. Starting material is shown on the left and immunoprecipitates on the right.
A truncation of the C-terminal 28 residues of CNG-β1 is associated with retinitis pigmentosa (RP; hCNG-βΔ28, Fig. 3A (17)). Indeed, neurofascin fused to CNG-β1 C-terminal domain bearing this deletion failed either to recruit or to co-immunoprecipitate ankyrin-G-GFP (fig. S2A, Fig. 3B). Additional deletion mutagenesis (hCNG-βC1243 and hCNG-βC1236, Fig. 3C) narrowed the interaction site to a 7-amino acid stretch in this region (underlined, Fig 3C, fig. S4B) and alanine-scanning mutagenesis revealed that the highly conserved residues I1237 and L1238 were essential for ankyrin-G binding (fig. S4B and Fig. 3C). Co-expressed CNG-α1 with mutant CNG-β1 (RP deletion or IL1237AA mutation) in HEK 293 cells abolished ankyrin-G binding without affecting the normal CNG-α1 and CNG-β1 association (Fig. 3D).
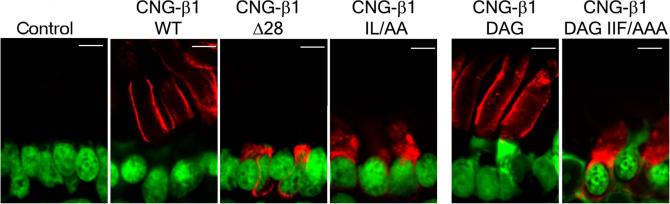
To test whether ankyrin-G binding was required for delivery of the channel to outer segments, human CNG-β1, wild type or mutants unable to interact with ankyrin-G, were expressed in the rods of transgenic Xenopus laevis (18). We used a specific antibody against human CNG-β1 (19) to distinguish it from the endogenous Xenopus CNG-β1 (Fig. 4). Wild type human CNG-β1 (WT) was found in ROS in a distinctive pattern consistent with its plasma membrane localization (Fig. 4). In marked contrast, both the RP (Δ28) and IL1237AA mutants were confined to perinuclear sites within rod cell bodies and were completely absent from ROS (Fig. 4).
Fig 4. Ankyrin-G binding is necessary and sufficient for CNG-β1 transport to ROS of transgenic Xenopus.
Retina sections in each panel are stained with anti-CNG-β1 antibody (red) and TOTO-3 to label the nuclei. Non-transgenic tadpole control on the left demonstrates that this antibody does not recognize the endogenous channel. Other panels depict the localization of wildtype (WT) human CNG-β1 or its mutants (indicated above each panel; see results for abbreviations). Scale bar 5 μm.
We next tested if an ankyrin-G-binding site from an unrelated protein was sufficient for targeting CNG-β1 to ROS. The native site in CNG-β1 required for interaction with ankyrin-G was substituted with 14-amino acids from beta-dystroglycan which binds ankyrin-G directly(5), and has little sequence similarity with the CNG-β1 motif (Fig. 3C). The CNG-β1/dystroglycan (CNG β-DAG) chimera associated with ankyrin-G when co-expressed with CNG α in HEK 293 cells (fig. S5). When expressed in transgenic Xenopus, this chimera was targeted to the ROS plasma membrane, however, the mutant CNG β-DAG IIF/AAA chimera lacking the ankyrin-binding site (fig. S5; (5)) was retained in the photoreceptor cell body (Fig. 4). Because there is no retrograde movement of membrane proteins from ROS back into the cell body (20), we conclude that ankyrin-G-binding is both necessary and sufficient for trafficking CNG-β1 to the outer segment. The ankyrin-G pathway could intersect with the microtubule motor Kif17/osm3, which is found in ROS (21) and is required for ciliary transport of olfactory CNG channels expressed in MDCK cells (3). Another question relates to the specific beta spectrin partner of ankyrin-G in ROS. Ankyrins partner with beta-spectrins in performing their scaffolding roles in the membrane cytoskeleton and in mediating post-Golgi transport through interactions with phospholipids and motor proteins (22-25). Beta-2 spectrin found in inner segments(26) is reduced in ankyrin-B (+/−) retina (11) and thus is a likely partner for ankyrin-B there. Beta-4 spectrin is associated with ankyrin-G in axon initial segments, and is present in rod inner and outer segments (fig. S4) making it a plausible ankyrin-G partner in ROS.
Ankyrin-G accomplishes two critical functions in photoreceptors: it is required for transport of CNG-beta1 from its site of synthesis and the assembly and/or maintenance of ROS. This resembles the role of ankyrin-G in axon initial segments where it binds to and coordinates the localization of three proteins required for the initiation and regulation of action potentials (Nav1.6, KCNQ2/3 channels, and 186 kDa neurofascin) (27, 28). Without ankyrin-G, axon initial segments lose these proteins and express dendritic markers (29). In epithelial cells ankyrin-G is required both for targeting E-cadherin to the plasma membrane and for biogenesis of the lateral membrane (6, 16). We hypothesize that, in addition to targeting the CNG channel, ankyrin-G can interact with other ROS membrane proteins as well as proteins required for their ROS trafficking and these interactions are essential for ROS morphogenesis. A conserved ankyrin-G-based mechanism may thus be shared by photoreceptors, neurons, and epithelial cells that accomplishes both the targeting of membrane-spanning proteins to specialized plasma membrane domains as well as assembly and/or maintenance of these domains.
Supplementary Material
SUPPORTING ONLINE MATERIAL
Materials and Methods
Animals and tissue collection: Animal care was in accordance with Duke University Medical Center institutional guidelines. Eyes were enucleated and quickly immersed in cold 2% paraformaldehyde (PFA) for 1 h. A slit was made in the enucleated eye by cutting around the ora serrata and fixation was continued in fresh 2% PFA at 4°C for another 1 h. The fixed eyecups were immersed in 30% sucrose (w/v) overnight at 4°C. The eyecups were embedded in OCT and cut on a cryostat to obtain 10 μm thick sections that were collected on slides coated with Vectabond (Vector labs). In in vivo knockdown experiments, eyecups from control and shRNA injected mice were embedded side by side in OCT, cut and collected on the same slide to minimize variations in quantification of immunofluorescence intensities. For observation of the olfactory epithelia, heads of neonatal mouse pups were first fixed in 2% PFA at 4°C for 3h, immersed in 30% sucrose, embedded in OCT and cut to obtain 15 μm thick coronal sections. Mouse sperm was isolated from the epididymis and vas deferens of adult males that had been housed separately from females. Transgenic Xenopus tadpoles at development stages 43-54 were fixed in 4% PFA, immersed in 30% sucrose, embedded in OCT and cut on a cryostat to obtain 12 μm thick sections for immunofluorescence analysis.
cDNA: cDNA encoding human CNG-β1(S1) was isolated by PCR from a retinal cDNA library (Clontech) and introduced into the XhoI and Not I sites of pEGFPN1 (Clontech). The sequence encoding the 28 C-terminal amino acids was deleted using PCR. Neurofascin fusion proteins were constructed by introducing PCR fragments coding for the cytoplasmic CNG-β1 N-terminus (amino acids 1-654), C-terminus (amino acid 1041-1251) or mutated C-terminus using ApaI and Not I sites in a HA-neurofascin plasmid described in an earlier study (S2). Deletion mutations were obtained by PCR. Alanine mutations were introduced using the Quickchange mutagenesis kit (Stratagene). PCR was used to introduce either the 14 amino acids encoding the ankyrin binding site of human β-dystroglycan (DAG) (see Fig 3 C)) or the DAG ankyrin binding site with the mutation IIF798AAA which disrupts binding to ankyrin-G (S3), were introduced downstream of P1217 of the CNG-β1 polypeptide. All mutations were confirmed by sequencing. For transgenic expression in Xenopus, the wild type and mutant CNG-β1 cDNA were subcloned behind the 5.5 kb opsin promoter in a vector containing a γ-crystallin-GFP cassette to facilitate identification of transgenic animals (S4). Mouse ankyrin-G shRNA pFIV plasmid and control pFIV plasmid have been described before (S3). A cDNA encoding bovine CNG-α1 in pcDNA3 was a gift of Dr. Robert Molday (University of British Columbia, Canada).
Retinal electroporation: Electroporation of newborn CD-1 mouse pups was performed as described (S5). Briefly, mice were anesthetized by chilling on ice and a small incision was made in the sclera near the lens using a 30-gauge needle. Plasmid either encoding ankyrin-G shRNA or control pFIV (3μg/μl; (S3)) was mixed with pCAGGS-GFP (0.3μg/μl; (S5)) in PBS containing 0.1% fast green as a tracer. Then, under a dissecting microscope, 0.5 μl of the DNA mixture was injected into the subretinal space using a 33-gauge blunt-ended needle fitted to a Nanofil syringe (WPI, Inc) through the previously made incision. After DNA injection, tweezer-type electrodes (BTX, model 520, 7 mm diameter) smeared with a thin coating of a conductive gel (Signa Gel, Parker Laboratories) was placed gently on either side of the heads of injected pups, and five 80V square pulses of 50 ms duration with 950 ms intervals were applied using a BTX ECM 830 pulse generator (Harvard Apparatus).
Antibodies: The rabbit polyclonal antibodies against Aqueoria Victoria green fluorescence protein (GFP), ankyrin-G, and ankyrin-B have been described before (S6-8). Monoclonal NKA pan alpha subunit antibody was from Affinity Bioreagents. Hybridoma supernatants containing monoclonal antibody against CNG-β1 (Garp 8G8; (S9)), CNG-α1 (Pmc1D1; (S10)) and rhodopsin (1D4; (S11)) were a generous gift from Robert Molday (University of British Columbia, Canada). Goat polyclonal CNG-α1 antibody (C-20) was from Santa Cruz Biotechnology, Inc. Rabbit polyclonal antibody against the HA epitope was from Covance.
Immunofluorescence: Immunofluorescence analysis of eye sections was performed as described before (S12). Briefly, mouse retina sections were permeabilized using 0.1% triton X-100, with 5% normal goat serum, and incubated with the appropriate primary and secondary antibodies. Immunofluorescence of HEK 293 cells was performed as described (S2). In recruitment assays, the cells were immunostained using the rabbit anti-GFP to detect ankyrin-G-GFP or ankyrin-B-GFP, the monoclonal Garp8G8 to detect CNG-β1 and a goat polyclonal antibody to detect CNG-α1. Images were collected using a 100 X objective (N.A. 1.45). To detect CNG-β1 constructs in Xenopus retina, tissue sections were permeabilized with 0.5% Triton X-100, blocked with 5% normal goat serum, incubated with a mouse monoclonal anti-CNG-β1 antibody (Garp 8G8), rinsed in PBST and incubated with anti-mouse IgG secondary antibodies conjugated to Alexa fluor 594 (Invitrogen) and the DNA dye TOTO-3 (Invitrogen). Images were collected using a 100 X objective (N.A. 1.45). Mouse sperm was immobilized on Cell-Tak (BD Biosciences) coated MatTek plates (MatTek Corp), stained with antibodies against ankyrin-G and acetylated tubulin, and images were collected using a 63X objective, N.A. 1.4.
Immunoprecipitation and immunoblotting: Bovine rod outer segments were isolated from fresh bovine eyes by sucrose gradient centrifugation as described (S13). Our preliminary studies showed that ankyrin-G is very poorly solubilized by 1% Triton X-100. For this reason we adopted a strategy where we solubilized ankyrin-G present in ROS with sodium dodecyl sulfate (SDS) after performing chemical crosslinking to preserve protein interactions. To perform crosslinking, the ROS pellet was resuspended in phosphate buffered saline (PBS) containing 20% sucrose w/v, 1 mM NaEDTA , 0.1% Triton X-100, 2 mM AEBSF, 10 μM bestatin, 10 μM E-64, 10 μM leupeptin, 10 μM pepstatin (all protease inhibitors from EMD Biosciences) and 10 mM DTSSP cleavable crosslinking reagent (Pierce). Crosslinking was carried out on ice for 30 min and the reaction was quenched by 150 mM Tris-Cl. SDS was then added to a final concentration of 0.1 % to completely solubilize ankyrin-G and other protein complexes present in the mixture. Triton X-100 was added to the SDS lysates to a final concentration of 1% and the samples were centrifuged at 60,000 × g. The supernatant was subjected to immunoprecipitation using 10 μg of ankyrin-G, ankyrin-B or non-specific rabbit immunoglobulin and immunoprecipitates were collected on protein-G Dynabeads (Dynal). CNG-β1 was immunoprecipitated with 20 μg of the monoclonal antibody Garp 8G8 coupled to sepharose beads.
For immunoprecipitation from cells, HEK 293 cells (seeded at 2×107 cells/10 cm plate) were co-transfected with 400 ng of ankyrin-G-GFP plasmid and 2.6 μg of plasmids encoding either HA-neurofascin or HA-neurofascin-CNG-β1 fusion proteins using 120 μg of Effectene (Qiagen). For co-expression of channel subunits, cells were transfected with 3 μg of CNG-α1 and 1 μg of CNG-β1. Cells were lysed 48 h later using 1% Triton X-100 in PBS containing 20% sucrose w/v, 1 mM EDTA, 2 mM AEBSF, 10 μM bestatin, 10 μM E-64, 10 μM leupeptin, and 10 μM pepstatin (EMD Biosciences). The cell lysate was cleared of insoluble material by centrifugation at 100,000 × g. HA-neurofascin and HA-neurofascin-CNG-β1 fusion proteins were immunoprecipitated using a polyclonal anti-HA antibody. CNG-β1 was immunoprecipitated as described above. The supernatants and pellets from each immunoprecipitation of ROS lysates were resolved by SDS-PAGE and then immunoblotted with antibodies against ankyrin-G, CNG-β1 (Garp 8G8) and rhodopsin (1D4). Neurofascin fusion proteins and ankyrin-G-GFP from transfected HEK 293 cells were detected in immunoblots using anti-HA and anti-GFP antibodies, respectively. CNG-α1 and CNG-β1 were immunoblotted with the corresponding Pmc1D1 and Garp 8G8 antibodies. The antibodies were detected using 125I-labeled protein A/G (Pierce).
HEK 293 based membrane recruitment assay: The assay was performed as described in a previous study (S2). Briefly, 20 ng of plasmid encoding ankyrin-G-GFP and 180 ng of HA-neurofascin, HA-neurofascin CNG fusion protein or CNG-α1 were co-transfected using Effectene (5 μg, Qiagen) into HEK 293 cells grown on Matek Plates (3×104 cell/1.4 mm2). For probing CNG-β1 interaction with ankyrin-G, a mixture of plasmids encoding CNG-β1 (50 ng), CNG-α1 (130 ng) and ankyrin-GFP (20 ng) was transfected into HEK 293 cells. Immunofluorescence was performed as described above.
Generation of Transgenic Tadpoles
Transgenic Xenopus laevis tadpoles were produced using the restriction enzyme-mediated integration method described before (S14, S15) with modifications described in (S16, S17). Transgenic tadpoles, identified by expression of the GFP reporter in the lens, were collected at development stages 43-54 and immunodetection of proteins expressed in their rods was performed as described above. A minimum of four individual transgenic animals were evaluated for every DNA construct.
SUPPLEMENTARY FIGURE LEGENDS
Fig S1. Ankyrin-G localizes to the principle piece of the sperm flagellum and to olfactory cilia. (A) Ankyrin-G (AnkG, red) localizes to the proximal part of the principle piece (PP) of sperm flagellum, the same region where CNG-β1 has previously been shown to localize (S18). The sperm flagellum is marked by acetylated tubulin (Ac-tub, green). The DNA in the head of sperm is labeled in blue. Middle piece is abbreviated as MP. Upper panels show multiple sperm and the lower panel is an enlargement of the single sperm indicated in the box in the upper panel. Scale bars are 20 μm in the upper panels and 10 μm in the lower panels. (B) Ankyrin-G localizes to the olfactory cilia where CNG-β1 has previously been shown to localize (S19), but not to the respiratory cilia. Confocal section of mouse olfactory epithelium (upper panels) and respiratory epithelium (lower panels) in the nasal septum of a three day old mouse was immunostained with antibodies against acetylated tubulin (Ac-tub, green) to label cilia and against ankyrin-G (AnkG, red). Merged panels are shown to the right. Ankyrin-G co-stains the cilia of the olfactory epithelium but not that of the respiratory epithelium. Scale bars are 5 μm.
Fig S2. Expression of the beta subunit of CNG channel at the plasma membrane requires the co-expression of the CNG alpha subunit. The upper panels show the intracellular localization of CNG-β1 (red) subunit when expressed alone in HEK 293 cells. Lower panels show the plasma membrane localization of CNG-β1 (red) when coexpressed with CNG-α1 in HEK 293 cells (white). Scale bars are 10 μm.
Fig S3. Effect of ankyrin-G depletion in rods on the levels of ROS resident proteins as measured by fluorescence intensity. Fluorescence intensity of ankyrin-G (AnkG), CNG-β1, CNG-α1 and rhodopsin (Rho) in either pFIV control transfected (black bars) rod photoreceptor outer segments or pFIV ankyrin-G shRNA transfected (grey bars) rod photoreceptor outer segments. Transfected cells were identified by expression of GFP from a pCAAGS-GFP plasmid mixed in with the control or shRNA plasmids at a 10-fold lower concentration. Data are mean +/- SD (n= 50 GFP positive ROS). An important caveat regarding interpretation of fluorescence intensity of rhodopsin is as follows. Rhodopsin in the outer segment represents a very specific case in which the fluorescence intensity may not accurately reflect its concentration. This is because rhodopsin concentration in the outer segment is ~3 mM which corresponds to ~100,000 molecules in each of 800 discs. For this reason its immunostaining signal can be saturated and therefore changes in rhodopsin density may not be accurately reflected by immunostaining intensity.
Fig S4. Mapping of the ankyrin-G binding site within the 28 C-terminal amino acids ofCNG-β1. The data in all panels represent results from the HEK 293 plasma membrane recruitment assays using AnkG-GFP and HA-neurofascin/CNG-β1 fusion proteins. cDNAs transfected into HEK 293 cells in addition to AnkG-GFP are listed on the left of each panel. The HA-neurofascin/CNG-β1 fusion proteins were detected using the anti-HA antibody and ankyrin-G-GFP was detected using the antibody against GFP. Scale bars are 10 μm. (A) The ankyrin-G binding site maps to the 28 C-terminal amino acids of CNG-β1. The results are summarized in Fig. 3A with the numbers on the right side of each panel corresponding to the schematic diagrams in Fig. 3A. (B) Identification of the IL1237 residues of CNG-β1 as the amino acids critical for ankyrin-G binding. The two upper panels illustrate the hCNG-β1243 and hCNG-β1236 deletion mutations shown in Fig. 3C. Other panels illustrate the results of the alanine-scanning mutagenesis of individual amino acid residues within the CNG-β1 C-terminus. The ankyrin-G binding residues IL1237 are shown in red.
Fig S5. CNG-β1/dystroglycan chimera co-immunoprecipitates CNG-α1 subunit and endogenous ankyrin-G in HEK 293 cells. cDNA encoding one of three CNG-β1 constructs was co-transfected into HEK 293 cells along with the cDNA encoding the CNG-α1 subunit. The first construct was human CNG-β1, used as a control. The second encoded a CNG-β1/dystroglycan (DAG) chimera in which the ankyrin-G binding site of CNG-β1 was substituted with 14-amino acids containing the ankyrin-G binding site of beta-dystroglycan. The third construct encoded a CNGβ1/DAG chimera bearing the IIF/AAA mutation in the ankyrin binding site. Immunoprecipitation was performed using an N-terminal specific CNG-β1 antibody. Starting material is shown on the left and the immunoprecipitates on the right.
Fig S6. Localization of β-4-spectrin in rod photoreceptors. Beta-4-spectrin (red) was labeled using a rabbit antibody (gift of Michele Solimena, Medical Faculty Carl Gustav Carus of the University of Technology, Dresden, Germany (S20)) and is expressed in both inner segments (IS) and ROS of mouse rod photoreceptors. ROS were labeled using an antibody against CNG β1 (green). The scale bar is 5 μm.
References
1. C. A. Colville, R. S. Molday, J Biol Chem 271, 32968 (1996).
2. X. Zhang, J. Q. Davis, S. Carpenter, V. Bennett, J Biol Chem 273, 30785 (1998).
3. G. Ayalon, J. Q. Davis, P. B. Scotland, V. Bennett, Cell 135, 1189 (2008).
4. S. A. Baker et al., J Cell Biol 183, 485 (2008).
5. T. Matsuda, C. L. Cepko, Proc Natl Acad Sci U S A 101, 16 (2004).
6. R. J. Hu, S. Moorthy, V. Bennett, J Cell Biol 128, 1069 (1995).
7. K. Kizhatil, V. Bennett, J Biol Chem 279, 16706 (2004).
8. P. J. Mohler, A. O. Gramolini, V. Bennett, J Biol Chem 277, 10599 (2002).
9. A. Poetsch, L. L. Molday, R. S. Molday, J Biol Chem 276, 48009 (2001).
10. N. J. Cook, L. L. Molday, D. Reid, U. B. Kaupp, R. S. Molday, J Biol Chem 264, 6996 (1989).
11. R. S. Molday, D. MacKenzie, Biochemistry 22, 653 (1983).
12. K. Kizhatil, N. K. Sandhu, N. S. Peachey, V. Bennett, Exp Eye Res 88, 57 (2009).
13. M. A. Livrea, C. Nicotra, A. Bongiorno, G. Ciaramitaro, M. Romano, Experientia 36, 894 (1980).
14. E. Amaya, K. L. Kroll, Methods Mol Biol 97, 393 (1999).
15. K. L. Kroll, E. Amaya, Development 122, 3173 (1996).
16. S. Batni, S. S. Mani, C. Schlueter, M. Ji, B. E. Knox, Methods Enzymol 316, 50 (2000).
17. S. L. Whitaker, B. E. Knox, J Biol Chem 279, 49010 (2004).
18. B. Wiesner et al., J Cell Biol 142, 473 (1998).
19. W. Bonigk et al., J Neurosci 19, 5332 (1999).
20. S. Lacas-Gervais et al., J Cell Biol 166, 983 (2004).
References and notes
- 1.Matulef K, Zagotta WN. Annu Rev Cell Dev Biol. 2003;19:23. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- 2.Huttl S, et al. J Neurosci. 2005;25:130. doi: 10.1523/JNEUROSCI.3764-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jenkins PM, et al. Curr Biol. 2006;16:1211. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Michalakis S, et al. J Biol Chem. 2006;281:35156. doi: 10.1074/jbc.M606409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayalon G, Davis JQ, Scotland PB, Bennett V. Cell. 2008;135:1189. doi: 10.1016/j.cell.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 6.Kizhatil K, et al. J Biol Chem. 2007;282:26552. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 7.Lowe JS, et al. J Cell Biol. 2008;180:173. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohler PJ, et al. Proc Natl Acad Sci U S A. 2004;101:17533. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou D, et al. J Cell Biol. 1998;143:1295. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wohlfart P, Haase W, Molday RS, Cook NJ. J Biol Chem. 1992;267:644. [PubMed] [Google Scholar]
- 11.Kizhatil K, Sandhu NK, Peachey NS, Bennett V. Exp Eye Res. 2009;88:57. doi: 10.1016/j.exer.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner B, et al. J Cell Biol. 1998;142:473. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Davis JQ, Carpenter S, Bennett V. J Biol Chem. 1998;273:30785. doi: 10.1074/jbc.273.46.30785. [DOI] [PubMed] [Google Scholar]
- 14.Chen TY, et al. Nature. 362:764. 993. [Google Scholar]
- 15.Matsuda T, Cepko CL. Proc Natl Acad Sci U S A. 2004;101:16. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kizhatil K, Bennett V. J Biol Chem. 2004;279:16706. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- 17.Kondo H, et al. Invest Ophthalmol Vis Sci. 2004;45:4433. doi: 10.1167/iovs.04-0544. [DOI] [PubMed] [Google Scholar]
- 18.Baker SA, et al. J Cell Biol. 2008;183:485. doi: 10.1083/jcb.200806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poetsch A, Molday LL, Molday RS. J Biol Chem. 2001;276:48009. doi: 10.1074/jbc.M108941200. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen-Legros J, Hicks D. Int Rev Cytol. 2000;196:245. doi: 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- 21.Insinna C, Pathak N, Perkins B, Drummond I, Besharse JC. Dev Biol. 2008;316:160. doi: 10.1016/j.ydbio.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett V, Healy J. Trends Mol Med. 2008;14:28. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Kizhatil K, et al. J Biol Chem. 2007;282:2029. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 24.Mohler PJ, Yoon W, Bennett V. J Biol Chem. 2004;279:40185. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 25.Muresan V, et al. Mol Cell. 2001;7:173. doi: 10.1016/s1097-2765(01)00165-4. [DOI] [PubMed] [Google Scholar]
- 26.Madreperla SA, Edidin M, Adler R. J Cell Biol. 1989;109:1483. doi: 10.1083/jcb.109.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins SM, Bennett V. J Cell Biol. 2001;155:739. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan Z, et al. J Neurosci. 2006;26:2599. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hedstrom KL, Ogawa Y, Rasband MN. J Cell Biol. 2008;183:635. doi: 10.1083/jcb.200806112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.We thank J. Hoffman for constructing the plasmids used in the study. V.Y.A was funded by NIH grant EY12859. V.B is an investigator of Howard Hughes Medical Institute (HHMI) and was funded by HHMI.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING ONLINE MATERIAL
Materials and Methods
Animals and tissue collection: Animal care was in accordance with Duke University Medical Center institutional guidelines. Eyes were enucleated and quickly immersed in cold 2% paraformaldehyde (PFA) for 1 h. A slit was made in the enucleated eye by cutting around the ora serrata and fixation was continued in fresh 2% PFA at 4°C for another 1 h. The fixed eyecups were immersed in 30% sucrose (w/v) overnight at 4°C. The eyecups were embedded in OCT and cut on a cryostat to obtain 10 μm thick sections that were collected on slides coated with Vectabond (Vector labs). In in vivo knockdown experiments, eyecups from control and shRNA injected mice were embedded side by side in OCT, cut and collected on the same slide to minimize variations in quantification of immunofluorescence intensities. For observation of the olfactory epithelia, heads of neonatal mouse pups were first fixed in 2% PFA at 4°C for 3h, immersed in 30% sucrose, embedded in OCT and cut to obtain 15 μm thick coronal sections. Mouse sperm was isolated from the epididymis and vas deferens of adult males that had been housed separately from females. Transgenic Xenopus tadpoles at development stages 43-54 were fixed in 4% PFA, immersed in 30% sucrose, embedded in OCT and cut on a cryostat to obtain 12 μm thick sections for immunofluorescence analysis.
cDNA: cDNA encoding human CNG-β1(S1) was isolated by PCR from a retinal cDNA library (Clontech) and introduced into the XhoI and Not I sites of pEGFPN1 (Clontech). The sequence encoding the 28 C-terminal amino acids was deleted using PCR. Neurofascin fusion proteins were constructed by introducing PCR fragments coding for the cytoplasmic CNG-β1 N-terminus (amino acids 1-654), C-terminus (amino acid 1041-1251) or mutated C-terminus using ApaI and Not I sites in a HA-neurofascin plasmid described in an earlier study (S2). Deletion mutations were obtained by PCR. Alanine mutations were introduced using the Quickchange mutagenesis kit (Stratagene). PCR was used to introduce either the 14 amino acids encoding the ankyrin binding site of human β-dystroglycan (DAG) (see Fig 3 C)) or the DAG ankyrin binding site with the mutation IIF798AAA which disrupts binding to ankyrin-G (S3), were introduced downstream of P1217 of the CNG-β1 polypeptide. All mutations were confirmed by sequencing. For transgenic expression in Xenopus, the wild type and mutant CNG-β1 cDNA were subcloned behind the 5.5 kb opsin promoter in a vector containing a γ-crystallin-GFP cassette to facilitate identification of transgenic animals (S4). Mouse ankyrin-G shRNA pFIV plasmid and control pFIV plasmid have been described before (S3). A cDNA encoding bovine CNG-α1 in pcDNA3 was a gift of Dr. Robert Molday (University of British Columbia, Canada).
Retinal electroporation: Electroporation of newborn CD-1 mouse pups was performed as described (S5). Briefly, mice were anesthetized by chilling on ice and a small incision was made in the sclera near the lens using a 30-gauge needle. Plasmid either encoding ankyrin-G shRNA or control pFIV (3μg/μl; (S3)) was mixed with pCAGGS-GFP (0.3μg/μl; (S5)) in PBS containing 0.1% fast green as a tracer. Then, under a dissecting microscope, 0.5 μl of the DNA mixture was injected into the subretinal space using a 33-gauge blunt-ended needle fitted to a Nanofil syringe (WPI, Inc) through the previously made incision. After DNA injection, tweezer-type electrodes (BTX, model 520, 7 mm diameter) smeared with a thin coating of a conductive gel (Signa Gel, Parker Laboratories) was placed gently on either side of the heads of injected pups, and five 80V square pulses of 50 ms duration with 950 ms intervals were applied using a BTX ECM 830 pulse generator (Harvard Apparatus).
Antibodies: The rabbit polyclonal antibodies against Aqueoria Victoria green fluorescence protein (GFP), ankyrin-G, and ankyrin-B have been described before (S6-8). Monoclonal NKA pan alpha subunit antibody was from Affinity Bioreagents. Hybridoma supernatants containing monoclonal antibody against CNG-β1 (Garp 8G8; (S9)), CNG-α1 (Pmc1D1; (S10)) and rhodopsin (1D4; (S11)) were a generous gift from Robert Molday (University of British Columbia, Canada). Goat polyclonal CNG-α1 antibody (C-20) was from Santa Cruz Biotechnology, Inc. Rabbit polyclonal antibody against the HA epitope was from Covance.
Immunofluorescence: Immunofluorescence analysis of eye sections was performed as described before (S12). Briefly, mouse retina sections were permeabilized using 0.1% triton X-100, with 5% normal goat serum, and incubated with the appropriate primary and secondary antibodies. Immunofluorescence of HEK 293 cells was performed as described (S2). In recruitment assays, the cells were immunostained using the rabbit anti-GFP to detect ankyrin-G-GFP or ankyrin-B-GFP, the monoclonal Garp8G8 to detect CNG-β1 and a goat polyclonal antibody to detect CNG-α1. Images were collected using a 100 X objective (N.A. 1.45). To detect CNG-β1 constructs in Xenopus retina, tissue sections were permeabilized with 0.5% Triton X-100, blocked with 5% normal goat serum, incubated with a mouse monoclonal anti-CNG-β1 antibody (Garp 8G8), rinsed in PBST and incubated with anti-mouse IgG secondary antibodies conjugated to Alexa fluor 594 (Invitrogen) and the DNA dye TOTO-3 (Invitrogen). Images were collected using a 100 X objective (N.A. 1.45). Mouse sperm was immobilized on Cell-Tak (BD Biosciences) coated MatTek plates (MatTek Corp), stained with antibodies against ankyrin-G and acetylated tubulin, and images were collected using a 63X objective, N.A. 1.4.
Immunoprecipitation and immunoblotting: Bovine rod outer segments were isolated from fresh bovine eyes by sucrose gradient centrifugation as described (S13). Our preliminary studies showed that ankyrin-G is very poorly solubilized by 1% Triton X-100. For this reason we adopted a strategy where we solubilized ankyrin-G present in ROS with sodium dodecyl sulfate (SDS) after performing chemical crosslinking to preserve protein interactions. To perform crosslinking, the ROS pellet was resuspended in phosphate buffered saline (PBS) containing 20% sucrose w/v, 1 mM NaEDTA , 0.1% Triton X-100, 2 mM AEBSF, 10 μM bestatin, 10 μM E-64, 10 μM leupeptin, 10 μM pepstatin (all protease inhibitors from EMD Biosciences) and 10 mM DTSSP cleavable crosslinking reagent (Pierce). Crosslinking was carried out on ice for 30 min and the reaction was quenched by 150 mM Tris-Cl. SDS was then added to a final concentration of 0.1 % to completely solubilize ankyrin-G and other protein complexes present in the mixture. Triton X-100 was added to the SDS lysates to a final concentration of 1% and the samples were centrifuged at 60,000 × g. The supernatant was subjected to immunoprecipitation using 10 μg of ankyrin-G, ankyrin-B or non-specific rabbit immunoglobulin and immunoprecipitates were collected on protein-G Dynabeads (Dynal). CNG-β1 was immunoprecipitated with 20 μg of the monoclonal antibody Garp 8G8 coupled to sepharose beads.
For immunoprecipitation from cells, HEK 293 cells (seeded at 2×107 cells/10 cm plate) were co-transfected with 400 ng of ankyrin-G-GFP plasmid and 2.6 μg of plasmids encoding either HA-neurofascin or HA-neurofascin-CNG-β1 fusion proteins using 120 μg of Effectene (Qiagen). For co-expression of channel subunits, cells were transfected with 3 μg of CNG-α1 and 1 μg of CNG-β1. Cells were lysed 48 h later using 1% Triton X-100 in PBS containing 20% sucrose w/v, 1 mM EDTA, 2 mM AEBSF, 10 μM bestatin, 10 μM E-64, 10 μM leupeptin, and 10 μM pepstatin (EMD Biosciences). The cell lysate was cleared of insoluble material by centrifugation at 100,000 × g. HA-neurofascin and HA-neurofascin-CNG-β1 fusion proteins were immunoprecipitated using a polyclonal anti-HA antibody. CNG-β1 was immunoprecipitated as described above. The supernatants and pellets from each immunoprecipitation of ROS lysates were resolved by SDS-PAGE and then immunoblotted with antibodies against ankyrin-G, CNG-β1 (Garp 8G8) and rhodopsin (1D4). Neurofascin fusion proteins and ankyrin-G-GFP from transfected HEK 293 cells were detected in immunoblots using anti-HA and anti-GFP antibodies, respectively. CNG-α1 and CNG-β1 were immunoblotted with the corresponding Pmc1D1 and Garp 8G8 antibodies. The antibodies were detected using 125I-labeled protein A/G (Pierce).
HEK 293 based membrane recruitment assay: The assay was performed as described in a previous study (S2). Briefly, 20 ng of plasmid encoding ankyrin-G-GFP and 180 ng of HA-neurofascin, HA-neurofascin CNG fusion protein or CNG-α1 were co-transfected using Effectene (5 μg, Qiagen) into HEK 293 cells grown on Matek Plates (3×104 cell/1.4 mm2). For probing CNG-β1 interaction with ankyrin-G, a mixture of plasmids encoding CNG-β1 (50 ng), CNG-α1 (130 ng) and ankyrin-GFP (20 ng) was transfected into HEK 293 cells. Immunofluorescence was performed as described above.
Generation of Transgenic Tadpoles
Transgenic Xenopus laevis tadpoles were produced using the restriction enzyme-mediated integration method described before (S14, S15) with modifications described in (S16, S17). Transgenic tadpoles, identified by expression of the GFP reporter in the lens, were collected at development stages 43-54 and immunodetection of proteins expressed in their rods was performed as described above. A minimum of four individual transgenic animals were evaluated for every DNA construct.
SUPPLEMENTARY FIGURE LEGENDS
Fig S1. Ankyrin-G localizes to the principle piece of the sperm flagellum and to olfactory cilia. (A) Ankyrin-G (AnkG, red) localizes to the proximal part of the principle piece (PP) of sperm flagellum, the same region where CNG-β1 has previously been shown to localize (S18). The sperm flagellum is marked by acetylated tubulin (Ac-tub, green). The DNA in the head of sperm is labeled in blue. Middle piece is abbreviated as MP. Upper panels show multiple sperm and the lower panel is an enlargement of the single sperm indicated in the box in the upper panel. Scale bars are 20 μm in the upper panels and 10 μm in the lower panels. (B) Ankyrin-G localizes to the olfactory cilia where CNG-β1 has previously been shown to localize (S19), but not to the respiratory cilia. Confocal section of mouse olfactory epithelium (upper panels) and respiratory epithelium (lower panels) in the nasal septum of a three day old mouse was immunostained with antibodies against acetylated tubulin (Ac-tub, green) to label cilia and against ankyrin-G (AnkG, red). Merged panels are shown to the right. Ankyrin-G co-stains the cilia of the olfactory epithelium but not that of the respiratory epithelium. Scale bars are 5 μm.
Fig S2. Expression of the beta subunit of CNG channel at the plasma membrane requires the co-expression of the CNG alpha subunit. The upper panels show the intracellular localization of CNG-β1 (red) subunit when expressed alone in HEK 293 cells. Lower panels show the plasma membrane localization of CNG-β1 (red) when coexpressed with CNG-α1 in HEK 293 cells (white). Scale bars are 10 μm.
Fig S3. Effect of ankyrin-G depletion in rods on the levels of ROS resident proteins as measured by fluorescence intensity. Fluorescence intensity of ankyrin-G (AnkG), CNG-β1, CNG-α1 and rhodopsin (Rho) in either pFIV control transfected (black bars) rod photoreceptor outer segments or pFIV ankyrin-G shRNA transfected (grey bars) rod photoreceptor outer segments. Transfected cells were identified by expression of GFP from a pCAAGS-GFP plasmid mixed in with the control or shRNA plasmids at a 10-fold lower concentration. Data are mean +/- SD (n= 50 GFP positive ROS). An important caveat regarding interpretation of fluorescence intensity of rhodopsin is as follows. Rhodopsin in the outer segment represents a very specific case in which the fluorescence intensity may not accurately reflect its concentration. This is because rhodopsin concentration in the outer segment is ~3 mM which corresponds to ~100,000 molecules in each of 800 discs. For this reason its immunostaining signal can be saturated and therefore changes in rhodopsin density may not be accurately reflected by immunostaining intensity.
Fig S4. Mapping of the ankyrin-G binding site within the 28 C-terminal amino acids ofCNG-β1. The data in all panels represent results from the HEK 293 plasma membrane recruitment assays using AnkG-GFP and HA-neurofascin/CNG-β1 fusion proteins. cDNAs transfected into HEK 293 cells in addition to AnkG-GFP are listed on the left of each panel. The HA-neurofascin/CNG-β1 fusion proteins were detected using the anti-HA antibody and ankyrin-G-GFP was detected using the antibody against GFP. Scale bars are 10 μm. (A) The ankyrin-G binding site maps to the 28 C-terminal amino acids of CNG-β1. The results are summarized in Fig. 3A with the numbers on the right side of each panel corresponding to the schematic diagrams in Fig. 3A. (B) Identification of the IL1237 residues of CNG-β1 as the amino acids critical for ankyrin-G binding. The two upper panels illustrate the hCNG-β1243 and hCNG-β1236 deletion mutations shown in Fig. 3C. Other panels illustrate the results of the alanine-scanning mutagenesis of individual amino acid residues within the CNG-β1 C-terminus. The ankyrin-G binding residues IL1237 are shown in red.
Fig S5. CNG-β1/dystroglycan chimera co-immunoprecipitates CNG-α1 subunit and endogenous ankyrin-G in HEK 293 cells. cDNA encoding one of three CNG-β1 constructs was co-transfected into HEK 293 cells along with the cDNA encoding the CNG-α1 subunit. The first construct was human CNG-β1, used as a control. The second encoded a CNG-β1/dystroglycan (DAG) chimera in which the ankyrin-G binding site of CNG-β1 was substituted with 14-amino acids containing the ankyrin-G binding site of beta-dystroglycan. The third construct encoded a CNGβ1/DAG chimera bearing the IIF/AAA mutation in the ankyrin binding site. Immunoprecipitation was performed using an N-terminal specific CNG-β1 antibody. Starting material is shown on the left and the immunoprecipitates on the right.
Fig S6. Localization of β-4-spectrin in rod photoreceptors. Beta-4-spectrin (red) was labeled using a rabbit antibody (gift of Michele Solimena, Medical Faculty Carl Gustav Carus of the University of Technology, Dresden, Germany (S20)) and is expressed in both inner segments (IS) and ROS of mouse rod photoreceptors. ROS were labeled using an antibody against CNG β1 (green). The scale bar is 5 μm.
References
1. C. A. Colville, R. S. Molday, J Biol Chem 271, 32968 (1996).
2. X. Zhang, J. Q. Davis, S. Carpenter, V. Bennett, J Biol Chem 273, 30785 (1998).
3. G. Ayalon, J. Q. Davis, P. B. Scotland, V. Bennett, Cell 135, 1189 (2008).
4. S. A. Baker et al., J Cell Biol 183, 485 (2008).
5. T. Matsuda, C. L. Cepko, Proc Natl Acad Sci U S A 101, 16 (2004).
6. R. J. Hu, S. Moorthy, V. Bennett, J Cell Biol 128, 1069 (1995).
7. K. Kizhatil, V. Bennett, J Biol Chem 279, 16706 (2004).
8. P. J. Mohler, A. O. Gramolini, V. Bennett, J Biol Chem 277, 10599 (2002).
9. A. Poetsch, L. L. Molday, R. S. Molday, J Biol Chem 276, 48009 (2001).
10. N. J. Cook, L. L. Molday, D. Reid, U. B. Kaupp, R. S. Molday, J Biol Chem 264, 6996 (1989).
11. R. S. Molday, D. MacKenzie, Biochemistry 22, 653 (1983).
12. K. Kizhatil, N. K. Sandhu, N. S. Peachey, V. Bennett, Exp Eye Res 88, 57 (2009).
13. M. A. Livrea, C. Nicotra, A. Bongiorno, G. Ciaramitaro, M. Romano, Experientia 36, 894 (1980).
14. E. Amaya, K. L. Kroll, Methods Mol Biol 97, 393 (1999).
15. K. L. Kroll, E. Amaya, Development 122, 3173 (1996).
16. S. Batni, S. S. Mani, C. Schlueter, M. Ji, B. E. Knox, Methods Enzymol 316, 50 (2000).
17. S. L. Whitaker, B. E. Knox, J Biol Chem 279, 49010 (2004).
18. B. Wiesner et al., J Cell Biol 142, 473 (1998).
19. W. Bonigk et al., J Neurosci 19, 5332 (1999).
20. S. Lacas-Gervais et al., J Cell Biol 166, 983 (2004).