Abstract
The lateral line is a mechanoreceptive sensory system that allows fish to sense objects and motion in their local environment. Variation in lateral line morphology may allow fish in different habitats to differentially sense and respond to salient cues. Threespine sticklebacks (Gasterosteus aculeatus) occupy a diverse range of aquatic habitats; we therefore hypothesized that populations within the G. aculeatus species complex might show variation in the morphology of the lateral line sensory system. We sampled 16 threespine stickleback populations from marine, stream and lake (including benthic and limnetic) habitats and examined the distribution, type and number of neuromasts on different regions of the body. We found that the threespine stickleback has a reduced lateral line canal system, completely lacking canal neuromasts. Although the arrangement of lines of superficial neuromasts on the body was largely the same in all populations, the number of neuromasts within these lines varied across individuals, populations and habitats. In pairwise comparisons between threespine sticklebacks adapted to divergent habitats, we found significant differences in neuromast number. Stream residents had more neuromasts than marine sticklebacks living downstream in the same watershed. In two independent lakes, benthic sticklebacks had more trunk neuromasts than sympatric limnetic sticklebacks, providing evidence for parallel evolution of the lateral line system. Our data provide the first demonstration that the lateral line sensory system can vary significantly between individuals and among populations within a single species, and suggest that this sensory system may experience different selection regimes in alternative habitats.
Keywords: lateral line, stickleback, evolution, sensory system, diversity, adaptation
INTRODUCTION
Animals rely on their sensory systems to perceive biologically relevant stimuli in their environment. Natural selection favors sensory systems that are adapted to stimuli used for survival and reproduction (Endler, 1992; Dangles et al., 2009). Niche-dependent specialization of sensory systems can be seen throughout the animal kingdom and is likely to be important for many behaviors, including predator evasion, prey detection and mate choice. However, we know very little about the extent of sensory system diversity within species or how existing sensory systems evolve to match specific stimuli in the environment.
The mechanoreceptive lateral line is a sensory system that is unique to aquatic vertebrates and is used to detect water motion in aquatic environments (Dijkgraaf, 1963; Bleckmann, 1993). The lateral line system conveys information about local water displacement over the body surface via clusters of hair cells called neuromasts, which are organized in many anatomically distinct lines. All of the hair cells in a neuromast extend their cilia into a gelatinous cupula that projects into the surrounding water and is mechanically coupled to local water motion. There are two general types of neuromasts with different receptive capabilities. Superficial neuromasts sit on the surface of the skin and are in direct contact with external hydrodynamic flow fields near the body of the animal, allowing them to detect water velocity. By contrast, canal neuromasts occur under the skin within fluid-filled canals that contact the surrounding water via small pores. Hydrodynamic pressure differences at the canal pores induce the motion of fluid within the canals, enabling canal neuromasts to detect the acceleration of external water flow near the body (Münz, 1989; Bleckmann, 1993; Coombs and Montgomery, 1999).
In fishes, the lateral line system is important for a number of behaviors, including the ability to detect and localize prey (Montgomery and Macdonald, 1987; Bleckmann and Bullock, 1989; Montgomery, 1989; Janssen et al., 1999), navigate around stationary objects (Hassan, 1989), orient to currents (Montgomery et al., 1997; Baker and Montgomery, 1999), detect moving objects (Dijkgraaf, 1963; Blaxter and Fuiman, 1989), court and communicate with conspecifics (Satou et al., 1991; Bleckmann, 1993; Satou et al., 1994; Plath et al., 2006) and school with other fish (Pitcher et al., 1976; Partridge and Pitcher, 1980). Fishes living in diverse environments and displaying different behaviors have variation in the morphology of the lateral line system (Coombs et al., 1988; Webb, 1989b), including differences in the type (Coombs and Montgomery, 1994; Carton and Montgomery, 2004), arrangement (Dijkgraaf, 1963; Webb, 1989b), number (Vischer, 1990) and size (Teyke, 1990; Vischer, 1990; Coombs and Montgomery, 1994; Wada et al., 2008) of neuromasts. The diversity of the lateral line across species with unique habitats, behaviors and life histories suggests that divergence in this sensory system plays a role in adaptation to different environments (Braun and Grande, 2008). However, the existence of lateral line variation within and between populations of a single species has not been investigated.
The threespine stickleback (Gasterosteus aculeatus) provides a unique opportunity to study variability in the lateral line system within one species that utilizes different marine and freshwater habitats. This small teleost fish is found throughout the Northern hemisphere in thousands of diverse and isolated aquatic environments (ocean, estuaries, lakes and streams) that differ in hydrodynamic activity, habitat complexity, food sources and other ecological characteristics. The colonization of freshwater habitats by marine sticklebacks has occurred within the past 15,000 years since the end of the last ice age (Bell and Foster, 1994). These recently diverged populations can be hybridized in the laboratory, and genetic tools can be used to investigate the molecular basis of phenotypic variation that has arisen during adaptation to different environments (Peichel et al., 2001; Kingsley et al., 2004; Kingsley and Peichel, 2007). Furthermore, the well-described ecology and evolutionary history of sticklebacks, combined with its experimental tractability, make it one of the few systems in which it has been possible to identify the putative selective forces contributing to the evolution of diverse traits (Barrett et al., 2008; Kitano et al., 2008).
In the current study we compare the lateral line system across 16 threespine stickleback populations living in marine, lake and stream environments in the Pacific Northwest and in Japan to test the hypothesis that populations of G. aculeatus show differences in lateral line morphology related to their habitat and ecology. Using fluorescent vital dyes, we examine the peripheral morphology of the lateral line system and ask the following questions: what is the general pattern of the threespine stickleback lateral line? How diverse is lateral line morphology across habitats, populations and individuals? Is there evidence that natural selection has played a role in the evolution of the lateral line system in the threespine stickleback?
MATERIALS AND METHODS
Stickleback collection and care
Threespine sticklebacks (Gasterosteus aculeatus L.) were collected between May and August 2007 from a variety of locations around the Pacific Northwest and Japan. We used the following populations (number of specimens are indicated in parentheses): Washington state: Allen Creek-AC (6), Manchester Clam Bay-MC (10), Willapa Bay-WB (10); British Columbia: Beaver Lake-BL (6), Hotel Lake-HL (9), Little Campbell Marine-LM (10), Little Campbell Stream-LS (10), Misty Inlet-MI (10), Misty Lake-ML (10), Misty Outlet-MO (11), North Lake-NL (9), Paxton Benthic-PB (10), Paxton Limnetic-PL (6), Priest Benthic-RB (10), Priest Limnetic-RL (5); Japan: Japanese Pacific-JP (10). These populations were chosen to represent three broad categories of threespine stickleback habitat traditionally used by stickleback researchers: marine (MC, WB, LM, JP), stream (AC, LS, MI, MO) and lake (BL, HL, ML, NL, PB, PL, RB, RL). Marine sticklebacks are usually anadromous, spending most of the year in marine waters but breeding in estuaries and the lower portions of streams. Little is known about the habitats of marine sticklebacks outside of the breeding season. Stream sticklebacks are found year-round in slow-flowing regions of freshwater streams. The stream sticklebacks in the current study were collected from shallow, highly vegetated habitats. Lake sticklebacks are found in a variety of lake habitats that are hydrodynamically similar although they range in size, depth, bottom substrate, water chemistry and clarity, prey availability and predators. The lake sticklebacks in the current study represent two sub-groups: solitary lake populations where a single stickleback population occupies a lake (BL, HL, ML, NL) and sympatric-pair populations where two stickleback populations occupy alternative niches in a lake (PB, PL, RB, RL). The solitary lakes in the current study are all located at sea level (under 100 m elevation); they range in size from Misty Lake, which is the shallowest and widest, with a maximum depth of 6.1 m and surface area of 35.6 hectares (1 hectare=10,000 m2) (Moore and Hendry, 2005) to North Lake, which is the deepest and narrowest, with a maximum depth of 16.4 m and surface area of 12.8 hectares (http://www.fishwizard.com/).
In addition to representing these three broad habitat categories, the populations chosen for the current study also include four sets of well-studied populations that occupy alternative habitats within a common watershed: the Misty lake–stream system, the Little Campbell River marine–stream pair, and the Paxton Lake and Priest Lake benthic–limnetic pairs. For each system, differences in abiotic factors (salinity, water flow, water clarity), as well as biotic factors (vegetation, food source, predator abundance) between the alternative habitats have been described; they are briefly summarized here. The Misty Lake lake–stream system (ML, MI and MO) on northern Vancouver Island is situated in cedar forests and is consequently tannin-stained. The lake has a surface area of 35.6 hectares, a maximum depth of 6.1 m, and a mean depth of 1.7 m (Moore and Hendry, 2005). The stickleback population in the lake (ML) is reproductively isolated from the populations living in the inlet (MI) and outlet (MO) streams (Hendry et al., 2002). The stream habitats are similar to one another; both are shallower and narrower than the lake itself and are highly vegetated. The Little Campbell marine–stream pair is found in the Little Campbell River, which runs for 28 km in southern British Columbia and connects with the Pacific Ocean near the USA–Canadian border. Little Campbell stream sticklebacks (LS) live in the upper portion of the river in a shallow, tannin-stained and heavily vegetated habitat. They are reproductively isolated from the Little Campbell marine sticklebacks (LM), which migrate from the ocean into the lower tidal portion of the river during the breeding season (Hagen, 1967). Finally, Paxton and Priest Lakes, located on Texada Island in the Strait of Georgia, each contain a benthic and a limnetic stickleback population. The limnetic populations (PL and RL) occupy the pelagic, open-water zones of each lake where they feed on zooplankton. They are reproductively isolated from the benthic populations (PB and RB), which occupy the more heavily vegetated littoral (benthic) zones and feed on macroinvertebrates (McPhail, 1994).
The primary focus of the current study was the threespine stickleback but we also examined two other stickleback species to ensure that we could identify canal pores and distinguish canal and superficial neuromasts using our methods. Fourspine stickleback (Apeltes quadracus Mitchell) are exclusively anadromous (Wootton, 1976) and were collected during the breeding season from a small tidal stream that also contains breeding marine threespine sticklebacks in Demarest Lloyd State Park in Dartmouth, MA, USA, in May 2007. Brook stickleback (Culaea inconstans Kirtland) live exclusively in freshwater (Wootton, 1976) and were collected from a large sinkhole lake, Pine Lake in Wood Buffalo National Park, Alberta, Canada in June 2007. Pine Lake has a surface area of 335 hectares with a maximum depth of 24 m and a mean depth of 5.3 m (Nelson, 1971).
All fish were caught in unbaited minnow traps, with the exception of North Lake and Willapa Bay, which were collected by hand netting. Following transport to the lab, all animals were housed in standard 29 gallon (110 l) aquarium tanks under summer lighting conditions (16 h:8 h light:dark) at approximately 15.5°C. Sticklebacks were kept in groups of up to 20 and were fed live brine shrimp nauplii twice daily. Water in each tank was continuously oxygenated with an air stone and circulated through an external charcoal filter (AquaClear 20 Power Filter; Hagen, Montreal, Canada), creating a slow, continuous current around the tank. All fish were kept in 0.35% saltwater (3.5 g l–1 Instant Ocean salt, Aquarium Systems, Mentor, OH, USA, 0.4 ml l–1 NaHCO3), with the exception of Willapa Bay and Manchester marine sticklebacks, which were kept at a 3× higher salt concentration. With the exception of the adjustments to salinity, all fish were kept in identical laboratory conditions that have been optimized for the health of diverse stickleback populations. Therefore, these laboratory conditions do not recapitulate the diverse natural habitats of the individual populations we studied. All fish used in this study were wild-caught adults, except the Willapa Bay population, which was collected in the juvenile stage and reared to adulthood in the lab, and the Japanese Pacific Ocean population, which consisted of laboratory-reared animals derived from an in vitro cross between wild-caught adults from the Bekanbeushi River in Akkeshi on Hokkaido Island, Japan (Kitano et al., 2007).
To ensure that the groove morphology found in the Japanese Pacific population was not an artifact of being reared in the laboratory, we also examined 10 wild-caught Japanese Pacific sticklebacks from the Bekanbeushi River that had been fixed in 10% buffered formalin since 2005. For comparison, we also examined wild-caught, formalin-fixed Paxton Benthic sticklebacks. Fixed specimens were examined on a Nikon SMZ 1500 stereomicroscope (Nikon Inc., Melville, NY, USA).
Animals were collected with permission from the Washington State Department of Fish and Wildlife (07-047), Commonwealth of Massachusetts-Division of Marine Fisheries (152769), Wood Buffalo National Park (WB-2007-1007) and the British Columbia Ministry of Environment (NA/SU07-31839 and NA07-31713). All animal procedures were approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee (#1575).
Neuromast staining
To visualize neuromasts, we stained live fish with the fluorescent vital dye 2-[4-(dimethylamino)styrl]-N-ethylpyridinium iodide (DASPEI; Invitrogen/Molecular Probes, Carlsbad, CA, USA) using a protocol adapted from Harris et al. (Harris et al., 2003). The dye was suspended in dH2O to a concentration of 0.038%. Immediately before use, this solution was diluted to a working concentration of 0.025% with fresh fish-tank water. Fish were allowed to swim freely in the staining solution for 15 min. Following two brief rinses in fresh fish-tank water, fish were anesthetized in 0.016% MS-222 (tricaine methylsulfonate; Fisher Scientific, Pittsburgh, PA, USA) for approximately two minutes or until the fish were motionless and breathing shallowly. For visual analysis and photography, fish were immersed in a Petri dish containing 0.005% MS-222. Neuromasts were counted on the left side of each fish under a Leica fluorescence dissecting scope with a FITC filter set (Leica Microsystems Inc., Bannockburn, IL, USA). Images were captured using a SPOT CCD camera (Diagnostic Instruments, Sterling Heights, MI, USA) with Metamorph software (Molecular Devices, Sunnyvale, CA, USA). The contrast of all images was adjusted uniformly using the automated ‘Levels’ function in Adobe Photoshop (San Jose, CA, USA). Each fish was stained and photographed only once for this study.
Scanning election microscopy (SEM)
A portion of the operculum and cheek was removed from four Little Campbell marine threespine sticklebacks and fixed in half-strength Karnovsky's fixative (2.5% glutaraldehyde, 2% paraformaldehyde in cacodylate buffer) at 4°C for 4 days. Samples were washed in cacodylate buffer and then immersed in increasingly concentrated ethanol baths (50%, 70%, 95%, 100%, 100%) for 30 min each. Tissue was then infiltrated with hexamethyldisilazone (HMDS; Ted Pella, Redding, CA, USA), dried in a fume hood, mounted on a stub and sputter coated with gold palladium. SEM images were captured with a JEOL 5800 electron microscope (JEOL, Tokyo, Japan).
Statistical analysis
All statistical analyses were conducted using SPSS 13.0 software (SPSS, Chicago, IL, USA). Multivariate analysis of variance (MANOVA) was used to compare neuromast numbers in each anatomical line as a function of population. For MANOVA, overall differences among groups were tested with the Wilks' lambda multivariate test. For both MANOVA and ANOVA, Tukey's post-hoc tests were used to determine pairwise differences among groups. To reduce multivariate data into fewer dimensions two methods were used: Principal Component Analysis (PCA) and Discriminant Function Analysis (DA). PCA regression scores were compared between habitat groups by analysis of variance (ANOVA). DA was applied to the Paxton–Priest data set in order to ask whether lateral line phenotypes were better differentiated by habitat or by lake. Discriminant functions were computed using simultaneous independent data entry (as opposed to sequential) and prior probabilities were adjusted to account for unequal group sizes. Functions were tested for significance using Wilks' lambda test statistic.
In some individuals, DASPEI staining was unclear in certain body regions due to high background, epidermal deformity or parasitism. For these individuals, neuromast counts for obscured lines were not included in the data set. In order to perform statistical tests, the missing data points were replaced with population means. This ‘supplemented’ data accounted for less than 7% of the data set.
RESULTS
The threespine stickleback has a reduced lateral line with no canal neuromasts
To characterize the threespine stickleback lateral line system, we collected samples from 16 populations from the Pacific Northwest and Japan. We chose four marine, four stream and eight lake sites for our collections (Fig. 1). Using the fluorescent dye DASPEI (Fig. 2A) we examined individuals from each population for neuromast type (canal vs superficial), neuromast arrangement, and neuromast number.
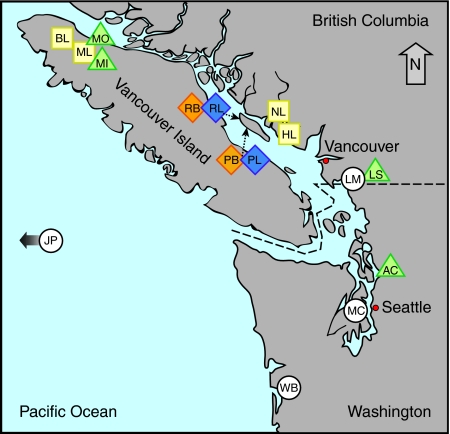
Fig. 1.
Map of threespine stickleback populations examined in the present study. Symbols represent the type of habitat sampled: white circle=marine, green triangle=stream, yellow square=lake, interlocked orange and blue diamonds=benthic–limnetic pair lakes. Population abbreviations are as follows: Allen Creek-AC, Beaver Lake-BL, Hotel Lake-HL, Japanese Pacific-JP, Little Campbell Marine-LM, Little Campbell Stream-LS, Manchester Clam Bay-MC, Misty Inlet-MI, Misty Lake-ML, Misty Outlet-MO, North Lake-NL, Paxton Benthic-PB, Paxton Limnetic-PL, Priest Benthic-RB, Priest Limnetic-RL, Willapa Bay-WB.
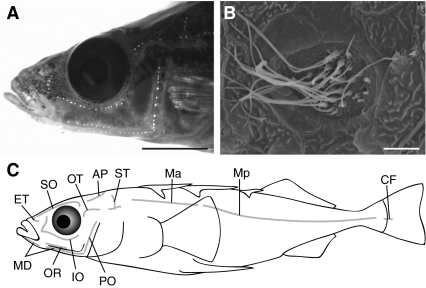
Fig. 2.
Arrangement of superficial neuromasts on the threespine stickleback. (A) View of a representative threespine stickleback head stained with DASPEI to highlight superficial neuromasts. Scale bar=5 mm. (B) Scanning electron micrograph of a preopercular superficial neuromast on a Little Campbell marine threespine stickleback. Scale bar=5 μm. (C) Schematic representation of neuromast arrangement. Abbreviations for line names: infraorbital (IO), oral (OR), mandibular (MD), preopercular (PO), otic (OT), supratemporal (ST), main trunk line anterior (Ma), main trunk line posterior (Mp), caudal fin (CF), ethmoid (ET), supraorbital (SO) and anterior pit (AP).
In all populations examined in this study, threespine sticklebacks had a reduced lateral line canal system, lacking canals and thus canal neuromasts (Fig. 2). Although threespine sticklebacks have been reported to lack canal neuromasts (Honkanen, 1993), potential population differences had never been examined, and one other stickleback species (Pungitius pungitius) had been reported to have canal neuromasts (Honkanen, 1993). We examined DASPEI-stained samples of two additional stickleback species: the fourspine stickleback (A. quadracus) and the brook stickleback (C. inconstans). In both species, canal pores were visible, and canal and superficial neuromasts were easily distinguished (see Fig. S1 in supplementary material). Using both DASPEI and SEM, we did not observe canal pores or canal neuromasts in any of the specimens from the Little Campbell marine threespine stickleback population; instead, we only observed superficial neuromasts even in regions where A. quadracus and C. inconstans had canal neuromasts, such as the preopercular (PO) line (Fig. 2). Thus, our evidence suggests that threespine sticklebacks have exclusively superficial neuromasts.
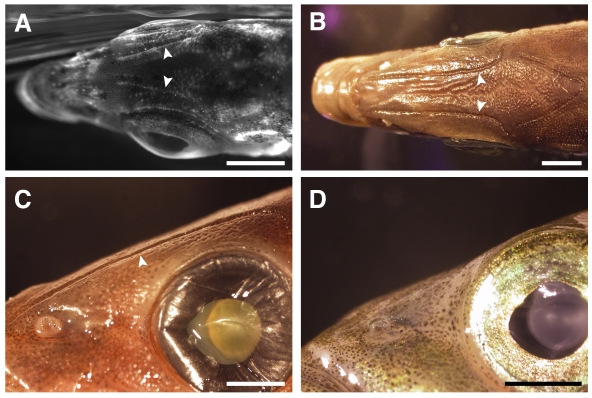
The Japanese Pacific lateral line had an anatomical feature that was unique among the populations examined (Fig. 3). In Japanese Pacific sticklebacks, neuromasts on the head were situated within long, linear depressions running along the rostro-caudal axis. This feature was particularly noticeable on the dorsal surface in the supraorbital (SO) lines (Fig. 3) and on the ventral surface in the mandibular (MD) lines (data not shown). In these regions, bony depressions ran for the entire length of the neuromast line, situating SO and MD neuromasts below the level of the skin surface. We call these neuromasts ‘groove’ neuromasts (Coombs et al., 1988) to distinguish them from canal or superficial neuromasts. We also examined 10 wild-caught, formalin-fixed Japanese Pacific specimens. Grooves were present in all 10 wild-caught Japanese Pacific samples we examined (Fig. 3B,C); however, they were not present in wild-caught, formalin-fixed Paxton Benthic specimens (Fig. 3D). In addition, we have not observed groove neuromasts in any other lab-reared threespine stickleback populations.
Fig. 3.
Superficial neuromasts on the heads of Japanese Pacific marine sticklebacks lie in bony grooves. (A) Dorsal view of a DASPEI-stained, lab-reared Japanese Pacific marine stickleback head showing faintly stained superficial neuromasts of the supraorbital (SO) line lying in grooves (arrowheads). Scale bar=2.5 mm. (B) Dorsal view of a wild-caught Japanese Pacific marine stickleback head showing SO grooves (arrowheads). Scale bar=2.5 mm. (C) Lateral view of groove (arrowhead) morphology on a wild-caught Japanese Pacific marine stickleback. Scale bar=2 mm. (D) Lateral view of a wild-caught Paxton Benthic stickleback showing an absence of grooves. Scale bar=2 mm.
Arrangement of neuromast lines does not vary among threespine sticklebacks
We defined 12 lines of neuromasts on threespine sticklebacks based on body position (Fig. 2C). Our nomenclature follows Northcutt (Northcutt, 1989) and Webb (Webb, 1989b); however, the nomenclature is not necessarily intended to reflect homology with other fishes. We identified the following neuromast lines (Fig. 2C): infraorbital (IO), oral (OR), mandibular (MD), preopercular (PO), otic (OT), supratemporal (ST), main trunk line anterior (Ma), main trunk line posterior (Mp), caudal fin (CF), ethmoid (ET), supraorbital (SO) and anterior pit (AP). The arrangement of lines was constant across threespine stickleback populations, with the exception of North Lake, which had no neuromasts in the CF line (Table 1).
Table 1.
Number of neuromasts in all 12 lines for 16 stickleback populations
| IO | OR | MD | PO | OT | ST | Ma | Mp | CF | ET | SO | AP | Total | CV | |
| JPM | 22.1 (3.5) | 4.9 (2.2) | 25.7 (3.9) | 10.5 (2.6) | 4.7 (1.9) | 7.6 (2.8) | 11.8 (1.9) | 29.0 (3.1)* | 1.0 (0.8) | 5.6 (1.2) | 20.2 (3.2) | 6.3 (2.1) | 149.3 (19.0) | 12.7% |
| LMM | 22.0 (5.7) | 5.7 (1.3) | 26.7 (4.8) | 14.4 (2.9) | 6.7 (2.1) | 9.8 (4.1) | 17.9 (3.8) | 29.6 (6.5)* | 1.2 (1.2) | 6.1 (1.7) | 22.4 (1.8) | 6.8 (2.0) | 169.3 (19.5) | 11.5% |
| MCM | 30.6 (5.6) | 7.0 (1.6) | 30.4 (5.5) | 17.5 (2.3) | 9.2 (1.5) | 14.7 (3.0) | 24.1 (5.2) | 34.8 (8.2)* | 2.9 (1.3) | 5.8 (1.7) | 25.7 (3.6) | 10.0 (2.3) | 212.7 (27.5) | 12.9% |
| WBM | 23.2 (5.1) | 5.7 (2.3) | 18.5 (7.8) | 12.2 (4.1) | 6.0 (2.6) | 7.2 (5.4) | 20.2 (8.7) | 31.5 (7.1)* | 1.7 (1.6) | 5.3 (2.2) | 20.0 (2.3) | 5.9 (1.9) | 157.3 (35.0) | 22.2% |
| ACS | 24.7 (4.6) | 7.7 (1.0) | 21.7 (3.9) | 15.7 (2.9) | 8.7 (1.4) | 11.8 (5.0) | 23.8 (9.8) | 37.5 (10.3) | 1.5 (2.1) | 5.8 (1.0) | 21.7 (2.7) | 6.7 (2.7) | 187.2 (38.0) | 20.3% |
| LSS | 27.9 (3.0) | 9.2 (1.4) | 30.5 (2.0) | 16.8 (3.7) | 9.3 (1.6) | 14.4 (2.3) | 29.3 (5.0) | 58.4 (12.9) | 4.2 (2.0) | 6.6 (1.3) | 23.1 (2.9) | 8.4 (1.2) | 238.2 (19.2) | 8.1% |
| MIS | 26.4 (4.9) | 6.0 (1.3) | 27.0 (4.2) | 15.9 (2.6) | 7.6 (1.3) | 12.3 (2.7) | 24.9 (7.1) | 26.9 (5.2) | 2.9 (1.8) | 5.8 (0.6) | 21.9 (3.6) | 6.3 (1.7) | 183.9 (27.7) | 15.1% |
| MOS | 30.5 (2.8) | 9.1 (3.5) | 29.6 (3.3) | 16.5 (3.1) | 7.9 (1.0) | 14.5 (2.2) | 25.4 (5.5) | 36.6 (9.4) | 6.1 (1.9) | 6.7 (1.7) | 25.0 (3.1) | 8.1 (1.5) | 215.9 (22.1) | 10.2% |
| BLL | 26.0 (3.7) | 7.2 (2.5) | 24.8 (6.2) | 15.3 (2.0) | 8.3 (1.8) | 11.7 (3.8) | 21.2 (5.6) | 41.7 (8.4) | 3.7 (1.3) | 5.8 (1.6) | 23.4 (2.7) | 8.4 (1.9) | 197.5 (28.2) | 14.3% |
| HLL | 22.7 (3.5) | 6.6 (2.3) | 23.6 (3.6) | 11.6 (4.4) | 6.9 (1.9) | 11.8 (2.5) | 17.6 (3.8) | 17.5 (7.0) | 1.2 (0.8) | 5.0 (1.7) | 16.5 (5.9) | 4.8 (2.0) | 145.7 (20.1) | 13.8% |
| MLL | 28.8 (3.4) | 6.8 (3.1) | 26.5 (6.6) | 17.7 (4.0) | 7.8 (2.3) | 12.7 (4.0) | 21.0 (6.3) | 37.0 (14.7) | 4.1 (2.3) | 5.8 (1.4) | 23.8 (2.0) | 7.2 (1.8) | 199.2 (33.1) | 16.6% |
| NLL | 28.4 (4.6) | 5.9 (1.3) | 28.6 (5.2) | 14.9 (3.0) | 6.4 (2.4) | 11.5 (2.2) | 22.1 (4.6) | 28.1 (3.3)* | 0.0 (0.0) | 6.4 (1.6) | 20.0 (4.7) | 6.5 (2.3) | 178.9 (15.9) | 8.9% |
| PBBL | 31.8 (4.5) | 11.2 (3.4) | 38.8 (6.4) | 19.9 (4.0) | 11.8 (2.2) | 18.0 (4.7) | 34.2 (10.7) | 84.3 (14.0) | 2.8 (1.7) | 8.5 (2.9) | 31.5 (3.0) | 12.7 (2.2) | 305.5 (34.4) | 11.2% |
| RBBL | 32.6 (6.1) | 9.8 (2.8) | 32.6 (7.1) | 18.5 (3.5) | 12.8 (4.4) | 16.2 (6.0) | 35.9 (13.0) | 74.6 (11.1) | 3.6 (1.6) | 10.2 (3.2) | 30.8 (7.4) | 11.3 (3.3) | 288.8 (36.4) | 12.6% |
| PLLL | 28.3 (5.5) | 8.5 (2.1) | 27.4 (6.6) | 16.3 (2.9) | 9.0 (2.4) | 14.0 (3.6) | 16.5 (4.0) | 49.3 (8.5) | 2.0 (1.7) | 6.7 (2.8) | 26.3 (2.9) | 6.5 (1.5) | 210.8 (29.9) | 14.2% |
| RLLL | 40.8 (4.2) | 9.4 (3.8) | 40.2 (7.2) | 21.2 (8.4) | 12.0 (1.9) | 19.6 (6.7) | 35.2 (8.7) | 53.4 (14.7) | 4.3 (2.7) | 8.6 (2.5) | 35.2 (5.3) | 10.0 (3.5) | 289.8 (48.9) | 16.9% |
| Overall | 27.7 (6.1) | 7.5 (2.9) | 28.2 (7.3) | 15.8 (4.4) | 8.3 (3.0) | 12.8 (4.9) | 23.8 (9.4) | 41.6 (20.4) | 2.7 (2.2) | 6.5 (2.3) | 24.0 (5.8) | 7.9 (3.0) | 206.8 (55.5) | 26.8% |
Mean (standard deviation) number of neuromasts is shown for each neuromast line (top row) and population (center column). Population abbreviations are given in the text. Superscript notations following each population name indicate habitat groups (M=marine, S=stream, L=lake, BL=benthic lake, LL=limnetic lake). Asterisks in Mp column mark fully plated populations. Individual variation in total neuromast number for each population is shown using the coefficient of variation (CV) in the far right column.
Although threespine sticklebacks have one main trunk line, we have defined an anterior (Ma) and a posterior (Mp) subregion to accommodate the unique arrangement of bony lateral plates along the flank. Most marine and freshwater stickleback populations differ in lateral plate morphology (Bell and Foster, 1994). Marine fish generally have a ‘complete’ set of plates, with one plate on each body segment and a bony keel along the caudal peduncle. By contrast, most lake and stream fish, including those used in the current study (with the exception of the North Lake population), are ‘low’ plate morphs, meaning that their plates extend caudally only as far as the second dorsal spine. In the absence of lateral plates, neuromasts exist in a single row along the flank. However, in the presence of a plate, we observe a pair of neuromasts sitting on top of every plate, with one neuromast situated on the dorsal portion of the plate and one situated on the ventral portion (see Fig. S2 in supplementary material). Due to these differences in lateral line morphology between the plated and unplated regions of the anterior and posterior segments of the trunk line, we designate the main trunk line anterior to the second dorsal spine as the Ma line and the main trunk line posterior to the second dorsal spine as the Mp line.
Neuromast number varies among threespine sticklebacks
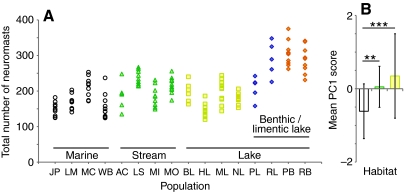
We observed substantial variation in neuromast number across individuals, populations and habitats (Fig. 4A). Relative degrees of individual variation in total neuromast number were compared using the coefficient of variation (standard deviation reflected as a percentage of the mean for each population), which ranged from ±8.1% in Little Campbell stream to ±22.2% in Willapa Bay (Table 1). Population also has a significant effect on neuromast number, as revealed by MANOVA (F180,1109=4.203; P<0.0001). Post-hoc tests found 335 pairwise differences among all of the 12 lines of the 16 populations we compared. To reduce the complexity of this data set, we performed a PCA. All of the 12 lines loaded positively onto a single principal component (PC1), explaining 56.15% of total variance in the data set (Fig. 4B). To test whether the number of neuromasts differed as a function of marine, stream or lake habitat, we performed an ANOVA on the PC1 regression scores. Habitat has a significant effect on neuromast number (F2,141=13.581, P<0.0001): marine sticklebacks have fewer neuromasts than stream (P<0.005) or lake (P<0.0001) sticklebacks but stream sticklebacks and lake sticklebacks do not differ (P=0.267).
Fig. 4.
Neuromast number differs between individuals, populations and habitats. (A) Individual fish are represented by single points and are organized by population on the x-axis (see Fig. 1 for population abbreviations). Habitat is indicated by symbol: white circle=marine, green triangle=stream, yellow square=solitary lake, blue diamonds=limnetic lake, orange diamonds=benthic lake. (B) Mean Principal Component scores differ between marine (white), stream (green) and lake (yellow) habitats. The lake category includes solitary, benthic and limnetic lake populations. Error bars indicate ± s.d. Asterisks indicate significant differences (***P<0.001, **P<0.01).
Comparisons between ecologically divergent threespine stickleback populations
Because there is some heterogeneity in the populations chosen to represent each habitat, population-level variation might have a greater effect on neuromast number than marine, stream or lake habitat. To better understand the relationship between habitat and lateral line phenotype, we compared ecologically divergent but closely related populations found within the same watershed. Our data set contained four such population comparisons that have been well characterized: a lake–stream system, a stream–marine pair and two sympatric benthic–limnetic lake pairs.
No difference in neuromast number between lake and stream sticklebacks
For our lake–stream comparison, we focused on the Misty Lake system on northern Vancouver Island, British Columbia. Misty Lake is a shallow lake with a mean depth of 1.7 m. The Misty Inlet and Misty Outlet streams are slow-flowing (mean flow rate of 8 cm s–1 in the Outlet and 3 cm s–1 in the Inlet) and are highly vegetated (Moore and Hendry, 2005). Both streams contain stickleback populations that are morphologically and genetically distinct from the lake population despite the lack of physical barriers separating the three groups from one another (Hendry et al., 2002). Although the lake and stream habitats differ, there were no differences in neuromast number in any line between Misty Inlet, Outlet and Lake (F24,34=1.530, P=0.125).
Stream-resident sticklebacks have more neuromasts than marine sticklebacks
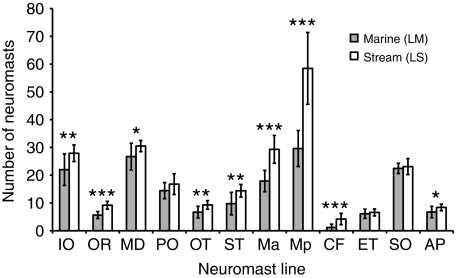
Next we compared a stream-resident population and a marine population from the Little Campbell River, British Columbia. The marine population is anadromous and lives in the marine environment for the majority of the year, only moving into the lower tidal portion of the stream to breed. The stream resident population lives in the slow-moving (approximately 3 cm s–1), densely vegetated upper regions of the stream all year round, upstream from the marine breeding zone (Hagen, 1967). We compared the number of neuromasts in each line between the stream and marine populations using MANOVA (F12,7=10.878, P<0.002) (Fig. 5). Nine out of the 12 lines differed between the two groups [OR, Ma, Mp, CF (P<0.001); IO, OT, ST (P<0.01); MD, AP (P<0.05)], while three lines did not differ [PO, ET and SO (P>0.05)]. In all of the nine lines with significant differences, the stream resident population had more neuromasts than the marine population.
Fig. 5.
Stream and marine sticklebacks differ in lateral line morphology. Differences in neuromast number between stream resident (LS) and marine (LM) sticklebacks from the Little Campbell River. Shaded bars represent the marine population; open bars represent the stream population. The total number of neuromasts is plotted for each line. Error bars indicate ± s.d. Asterisks indicate lines that differ between populations (***P<0.001, **P<0.01, *P<0.05).
Benthic–limnetic pairs exhibit parallel differences in neuromast number
Finally, we compared neuromast number between sympatric stickleback populations from two lakes. Paxton and Priest lakes are located on Texada Island in the Strait of Georgia, British Columbia (Fig. 1). Both lakes contain two populations of threespine sticklebacks: a limnetic form living in the open water and a benthic form living near the substrate. Limnetic sticklebacks have an elongated, streamlined body shape and feed on zooplankton in the open-water column. Benthic sticklebacks are deep-bodied and feed on macroinvertebrates in the littoral zone among lake vegetation (McPhail, 1994).
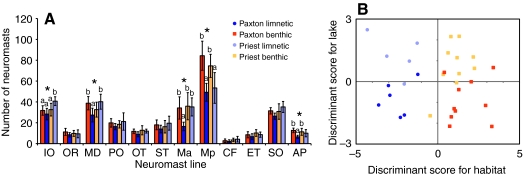
We compared the number of neuromasts in each line for Priest and Paxton benthic and limnetic fish by MANOVA (Fig. 6A). Neuromast number differed among these four populations (F36,48=2.609, P<0.001), and significant pairwise differences occurred in five lines: IO, MD, Ma, Mp and AP (P<0.01). In Paxton Lake, benthic fish had more neuromasts than limnetic fish in the MD, Ma and AP lines. In Priest Lake, limnetic fish had more neuromasts than benthic fish in the IO line. In both Paxton and Priest Lakes, benthic fish had more neuromasts than limnetic fish in the Mp line.
Fig. 6.
Parallel differences in lateral line phenotypes between benthic–limnetic pairs. In both panels, the Paxton populations are represented in saturated colors, with benthic sticklebacks shown as orange squares and limnetic sticklebacks as blue circles; the Priest populations are represented with pastel colors, with benthic sticklebacks shown as yellow squares and limnetic sticklebacks as purple circles. (A) Differences in neuromast number between sympatric benthic and limnetic populations in Paxton and Priest Lakes. The total number of neuromasts is plotted for each line. Error bars indicate ± s.d. Asterisks indicate lines that differ between populations labeled as ‘a’ and ‘b’ (P<0.05). (B) Discriminant Function Analysis of benthic and limnetic lateral line phenotypes in Paxton and Priest Lakes. Discriminant scores for habitat are plotted on the x-axis and discriminant scores for lake are plotted on the y-axis. Each symbol represents an individual stickleback.
Because the differences in neuromast number between benthic and limnetic sticklebacks were not always the same in the two lakes, we used DA to clarify the effect of habitat and lake on neuromast number. DA generated two functions, one that classified the data set by lake and one by habitat (Fig. 6B). The habitat function successfully discriminates benthic and limnetic sticklebacks based on neuromast number (Wilks' lambda=0.213, P<0.0001) with the Ma, Mp and AP lines contributing significantly to this function. The lake function cannot successfully discriminate Paxton and Priest sticklebacks using the same data set (Wilks' lambda=0.530, P=0.265). Therefore, we conclude that habitat, rather than lake origin, better distinguishes the lateral line phenotypes between these four populations.
DISCUSSION
We have demonstrated that G. aculeatus has a reduced lateral line canal system and completely lacks canal neuromasts. In all of the 16 populations examined, superficial neuromasts are arranged in the same 12 lines on the head and body. The arrangement of neuromasts in the trunk line is noteworthy for two reasons. First, many teleosts have several trunk lines of neuromasts, including lines that are dorsal and/or ventral to the main line. Threespine sticklebacks have only a single main trunk line, lacking additional dorsal and ventral trunk lines. Second, threespine sticklebacks are scaleless and they lack the proliferated ‘stitches’ of superficial neuromasts that are frequently observed on the lateral scales of the trunk in some other fish. Instead, they have paired neuromasts on each bony lateral plate and a single line of neuromasts in unplated regions (see Fig. S2 in supplementary material).
The threespine stickleback lateral line system is also unusual in its absence of canal neuromasts. Previously, Honkanen (Honkanen, 1993) demonstrated the absence of canals in a single European population of threespine sticklebacks but suggested that populations differing in plate morphology might show variability in the presence of canals. However, we find that all of the 16 populations examined in the present study have exclusively superficial neuromasts regardless of habitat or plate morphology. The developmental and evolutionary history of these neuromasts is unknown; therefore, we cannot currently distinguish whether they are ‘accessory,’ ‘replacement’ or any other class of free neuromast (Coombs et al., 1988; Webb, 1989b).
The lack of canal neuromasts has predictable effects on mechanoreception based on the different response properties of superficial and canal neuromasts (Coombs and Montgomery, 1994). Differences in the filtering properties (Münz, 1989; Vischer, 1990; Montgomery et al., 1994), physical fragility or sensitivity (Dijkgraaf, 1963) of canal and superficial neuromasts might be adaptations to different levels of hydrodynamic noise. For example, having more superficial neuromasts makes a fish more sensitive to water disturbances, particularly in slow flow environments, whereas having more canal neuromasts attenuates low-frequency noise (including background water flow) and improves detection of high-frequency stimuli such as prey (Engelmann et al., 2000). Dijkgraaf (Dijkgraaf, 1963) predicted that actively swimming fish living in high flow environments will have reduced superficial neuromasts and narrower, more highly branched canals whereas fish that are less active swimmers or that live in slower moving water will have proliferated superficial neuromasts and reduced canals or may lack canals entirely (Dijkgraaf, 1963; Coombs et al., 1988; Northcutt, 1989). Consistent with this hypothesis, threespine sticklebacks are a fairly slow swimming fish that live in characteristically low-flow habitats. However, other stickleback species live in very similar habitats to threespine sticklebacks and have canal neuromasts (Honkanen, 1993) (see Fig. S1 in supplementary material), suggesting hydrodynamic regime in the environment is not the only factor affecting neuromast evolution in sticklebacks.
Behavioral adaptations may also play an important role in shaping the lateral line. In contrast to Dijkgraaf's prediction (Dijkgraaf, 1963) that the proliferation of superficial neuromasts and loss of canals will be found in low flow environments, Carton and Montgomery (Carton and Montgomery, 2004) found that torrent fish occupying fast-flow streams exhibit the opposite morphology, with many superficial neuromasts and a reduced canal system; Carton and Montgomery suggest that this is related to their ability to feed nocturnally. The absence of head canals in many surface-feeding fish is thought to be an adaptation to detection of surface waves (Coombs et al., 1988), while the loss of a trunk canal is common in benthic, planktivorous or schooling fishes (Webb, 1989b). Similar behavioral specializations may be playing a role the evolution of the threespine stickleback lateral line system, as populations of G. aculeatus can be benthic or planktivorous (McPhail, 1994), and some threespine stickleback populations also school (A.R.W., unpublished).
Alternatively, loss of canal neuromasts may not always result directly from selection on the lateral line. For example, Antarctic notothenioid fishes may have lost canals as a result of paedomorphosis, the retention of juvenile characteristics, which has allowed these fish to retain a pelagic, rather than a benthic, lifestyle as adults (Northcutt, 1989; Webb, 1989a; Coombs and Montgomery, 1994). Thus, in threespine sticklebacks, hydrodynamic activity, behavioral specializations, feeding habits and/or developmental constraints might have all played a role in the loss of canal neuromasts.
Groove neuromasts
In Japanese Pacific marine threespine sticklebacks, we observed bony grooves running the length of the SO and MD lines on the dorsal and ventral aspects of the head, respectively. This morphology could have a significant effect on the perception of hydrodynamic stimuli. Because these neuromasts lie within grooves that run along the long axis of the head, they may be protected from stimulation by water moving obliquely to the groove. Therefore, they may be differentially sensitive to water motion in the axis of the groove. They may also respond to different types of stimuli than superficial neuromasts, as has been shown for canal neuromasts (Coombs and van Netten, 2006). Thus, the presence of grooves might affect lateral line-associated behaviors such as rheotaxis, schooling or prey detection. Developmentally, grooves may be intermediate stages in canal formation (Webb et al., 2008); canals are present in the SO and MD lines of the related sticklebacks C. inconstans and A. quadracus (see Fig. S1 in supplementary material). Because the Japanese Pacific population was the only population in our study that was reared in the lab, we verified that grooves are also present in wild-caught Japanese Pacific sticklebacks (Fig. 3B,C), indicating that grooves are not an artifact of laboratory rearing. It remains to be determined why the Japanese Pacific marine threespine stickleback has this particular morphology.
Divergence in the threespine stickleback lateral line
We also examined the extent of diversity in neuromast number among threespine stickleback populations. Our results show that neuromast number is highly variable, both within and between populations. Previous studies of the lateral line have typically failed to note or failed to find any variability in neuromast number within species (Coombs and Montgomery, 1994; Carton and Montgomery, 2004). One exception is a recent study by Schmitz et al. who found 9% variability in total number of superficial neuromasts in aquarium-stocked goldfish (Schmitz et al., 2008). We find that intra-population variation in total neuromast number ranges from 8.1% to 22.2% (Table 1). Because most of the fish we studied were wild-caught, we do not know whether this variation is due to environmental or genetic effects. However, the extent of within-species variability in sensory receptor number is significant for two reasons. First, if this variation is genetically controlled, it provides a substrate upon which natural selection can act. Second, because changes in the sensory periphery are capable of changing perception of external stimuli (van Staaden and Romer, 1998; Jacobs et al., 2007), the amount of variability we observe has the potential to shape individual perception and consequent behavior to a greater extent than previously thought. Our future studies will take advantage of the genetic tools available for sticklebacks to identify the genetic contributions to lateral line variation at the individual and population levels.
We hypothesized that broadly defined habitat differences might be associated with differences in neuromast number in the lateral line as a result of natural selection. We compared fish from habitats that differ in hydrodynamic activity: marine sticklebacks inhabit both tidal zones and open-water environments, stream sticklebacks inhabit shallow, narrow waterways with slow currents, and lake sticklebacks live in a range of habitats where water currents are minimal. Our analysis showed that there is a correlation between habitat and neuromast number but that there is also substantial variation between populations. The use of simplistic habitat categories (lake, stream, marine) may have masked relevant ecological differences among our populations from the perspective of lateral line diversity. For example, the lakes we have chosen differ in size, depth, vegetation, bottom substrate, water clarity and food sources. Any of these factors could be playing a role in lateral line diversity and evolution. Thus, the results of this broad-scale habitat comparison are difficult to interpret. Consequently, we performed a series of targeted comparisons between well-studied ecological ‘pairs’ of populations that have diverged from one another in habitat-use, behavior and other characteristics.
Although we found no difference in neuromast number between the lake and two stream populations in the Misty watershed, we did find differences between marine and stream resident sticklebacks from the Little Campbell watershed and between benthic and limnetic sticklebacks from both Paxton and Priest Lakes. The stream population had more neuromasts than the marine population in 9 out of the 12 lines across the body. In Paxton and Priest Lakes, neuromast number differed on the jaw line, face, dorsal aspect of the head and the trunk. Paxton limnetic sticklebacks generally had fewer neuromasts than the other three groups, and both limnetic populations had fewer neuromasts than the benthic populations in the Mp line. Because these lakes were independently colonized by marine ancestors (McPhail, 1994; Taylor and McPhail, 2000), the existence of similar phenotypes suggests they were shaped by similar selective forces, a phenomenon called parallel evolution. Thus, we used this unique natural experiment to ask whether the lateral line has diverged in parallel between the benthic and limnetic populations. DA suggested that limnetic and benthic sticklebacks in these two lakes have experienced common selective pressures, resulting in divergent lateral line phenotypes based on habitat. Habitat-based selection may thus be an important force shaping lateral line evolution in these stickleback populations.
Lake and stream sticklebacks are derived from ancestral marine sticklebacks, with benthic and stream forms showing the most divergence from the marine form in many structural and behavioral traits (McPhail, 1994). Our finding that both benthic and stream-dwelling sticklebacks have more neuromasts than limnetic or marine relatives suggests that this may be adaptive in these habitats. Previous work indicates that having a greater number of neuromasts may increase sensitivity and aid in detecting stimuli, and that having more neuromasts in a given body area (a higher density of neuromasts) may increase resolution and aid in deciphering stimuli (Coombs and Montgomery, 1999). Stream-resident and benthic sticklebacks share several ecological features that may make improved mechanoreceptive sensitivity or resolution advantageous. Both populations feed on benthic prey and live in highly vegetated, complex habitats where visual cues are somewhat scarce (Hagen, 1967; McPhail, 1994). The lateral line system is known to play roles in prey detection (Montgomery and Macdonald, 1987; Montgomery, 1989; Janssen et al., 1999) and spatial navigation (Hassan, 1989), particularly for fish that are benthic (Coombs and Janssen, 1989; Montgomery, 1989) or that do not rely on visual cues (Saunders and Montgomery, 1985; Plath et al., 2006; Bassett et al., 2007).
From these studies, we conclude that the lateral line sensory system of threespine sticklebacks living in diverse habitats is being shaped by natural selection. In the future, the experimental tractability of sticklebacks will make it possible to clarify what selective pressures (social, predatory, prey- or navigation-related) are relevant for the evolution of this sensory system. In the meantime, this work provides a first glimpse into lateral line diversity and functional evolution within a single species and sheds light on the process of sensory system evolution on a fine scale.
Supplementary Material
Acknowledgments
We are grateful to Matt Arnegard, Susan Foster, Andrew Hendry, Jun Kitano, Jean-Sebastien Moore, Dolph Schluter and Mike Shapiro for help collecting sticklebacks; Cecilia Moens and Greg Walsh for use of their microscope; Anne Knecht for DASPEI-staining advice; Tiffany Malek, Bobbie Schneider and Franque Remington for help with electron microscopy; and especially Matt Arnegard, Anna Greenwood, Joe Sisneros, James Urton and Barry Wark for helpful suggestions on the manuscript.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/213/1/108/DC1
This work was supported by a Burroughs Wellcome Fund Career Award in the Biomedical Sciences (C.L.P.) and National Institutes of Health grant P50 HG02568 (C.L.P.). Deposited in PMC for release after 12 months.
- AC
- Allen Creek
- AP
- anterior pit line
- BL
- Beaver Lake
- CF
- caudal fin line
- DA
- Discriminant Function Analysis
- DASPEI
- 2-[4-(dimethylamino)styrl]-N-ethylpyridinium iodide
- ET
- ethmoid line
- HL
- Hotel Lake
- IO
- infraorbital line
- JP
- Japanese Pacific
- LM
- Little Campbell Marine
- LS
- Little Campbell Stream
- M
- main trunk line
- Ma
- main trunk line anterior
- MC
- Manchester Clam Bay
- MD
- mandibular line
- MI
- Misty Inlet
- ML
- Misty Lake
- MO
- Misty Outlet
- Mp
- main trunk line posterior
- NL
- North Lake
- OR
- oral line
- OT
- otic line
- PB
- Paxton Benthic
- PCA
- Principal Component Analysis
- PL
- Paxton Limnetic
- PO
- preopercular line
- RB
- Priest Benthic
- RL
- Priest Limnetic
- SO
- supraorbital line
- ST
- supratemporal line
- WB
- Willapa Bay
REFERENCES
- Baker C. F., Montgomery J. C. (1999). The sensory basis of rheotaxis in the blind Mexican cave fish, Astyanax fasciatus. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 184, 519-527 [Google Scholar]
- Barrett R. D. H., Rogers S. M., Schluter D. (2008). Natural selection on a major armor gene in threespine stickleback. Science 322, 255-257 [DOI] [PubMed] [Google Scholar]
- Bassett D. K., Carton A. G., Montgomery J. C. (2007). Saltatory search in a lateral line predator. J. Fish Biol. 70, 1148-1160 [Google Scholar]
- Bell M. A., Foster S. A. (1994). The Evolutionary Biology of the Threespine Stickleback. Oxford: Oxford University Press; [Google Scholar]
- Blaxter J. H. S., Fuiman L. A. (1989). Function of the free neuromasts of marine teleost larvae. InThe Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 481-499 New York: Springer-Verlag; [Google Scholar]
- Bleckmann H. (1993). Role of the lateral line in fish behaviour. In Behaviour of Teleost Fishes (ed. Pitcher T. J.), pp. 201-246 London: Chapman & Hall; [Google Scholar]
- Bleckmann H., Bullock T. H. (1989). Central nervous physiology of the lateral line, with special reference to cartilaginous fishes. In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 387-408 New York: Springer-Verlag; [Google Scholar]
- Braun, C. B. and Grande, T. (2008). Evolution of peripheral mechanics for the enhancement of sound reception. In Fish Bioacoustics (ed. Webb J. F., Fay R. R. and Popper A. N.), pp. 99-144 New York: Springer; [Google Scholar]
- Carton A. G., Montgomery J. C. (2004). A comparison of lateral line morphology of blue cod and torrentfish: two sandperches of the family Pinguipedidae. Environ. Biol. Fishes 70, 123-131 [Google Scholar]
- Coombs S., Janssen J. (1989). Peripheral processing by the lateral line system of the mottled sculpin (Cottus bairdi). In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 299-319 New York: Springer-Verlag; [Google Scholar]
- Coombs S., Montgomery J. (1994). Function and evolution of superficial neuromasts in an Antarctic notothenioid fish. Brain Behav. Evol. 44, 287-298 [DOI] [PubMed] [Google Scholar]
- Coombs S., Montgomery J. C. (1999). The enigmatic lateral line system. In Comparative Hearing in Fish and Amphibians (ed. Fay R. R. and Popper A. N.), pp. 319-362 New York: Springer-Verlag; [Google Scholar]
- Coombs S., van Netten S. (2006). The hydrodynamics and structural mechanics of the lateral line system. In Fish Biomechanics (ed. Shadwick R. E. and Lauder G. V.), pp. 103-139 San Diego: Elsevier; [Google Scholar]
- Coombs S., Janssen J., Webb J. F. (1988). Diversity of lateral line systems: evolutionary and functional considerations. In Sensory Biology of Aquatic Animals (ed. Atema J., Fay R. R., Popper A. N. and Tavolga W. N.), pp. 553-593 New York: Springer-Verlag; [Google Scholar]
- Dangles O., Irschick D., Chittka L., Casas J. (2009). Variability in sensory ecology: expanding the bridge between physiology and evolutionary biology. Q. Rev. Biol. 84, 51-74 [DOI] [PubMed] [Google Scholar]
- Dijkgraaf S. (1963). The functioning and significance of the lateral-line organs. Biol. Rev. Camb. Philos. Soc. 38, 51-105 [DOI] [PubMed] [Google Scholar]
- Endler J. A. (1992). Signals, signal conditions, and the direction of evolution. Am. Nat. 139, S125-S153 [Google Scholar]
- Engelmann J., Hanke W., Mogdans J., Bleckmann H. (2000). Hydrodynamic stimuli and the fish lateral line. Nature 408, 51-52 [DOI] [PubMed] [Google Scholar]
- Hagen D. W. (1967). Isolating mechanisms in threespine sticklebacks (Gasterosteus). J. Fish. Res. Bd. Canada 24, 1637-1692 [Google Scholar]
- Harris J. A., Cheng A. G., Cunningham L. L., MacDonald G., Raible D. W., Rubel E. W. (2003). Neomycin-induced hair cell death and rapid regeneration in the lateral line of zebrafish (Danio rerio). J. Assoc. Res. Otolaryngol. 4, 219-234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan E. S. (1989). Hydrodynamic imaging of the surroundings by the lateral line of the blind cave fish Anoptichthys jordani. In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 217-227 New York: Springer-Verlag; [Google Scholar]
- Hendry A. P., Taylor E. B., McPhail J. D. (2002). Adaptive divergence and the balance between selection and gene flow: lake and stream stickleback in the Misty system. Evolution 56, 1199-1216 [DOI] [PubMed] [Google Scholar]
- Honkanen T. (1993). Comparative study of the lateral-line system of the three-spined stickleback (Gasterosteus aculeatus) and the nine-spined stickleback (Pungitius pungitius). Acta Zool. 74, 331-336 [Google Scholar]
- Jacobs G. H., Williams G. A., Cahill H., Nathans J. (2007). Emergence of novel color vision in mice engineered to express a human cone photopigment. Science 315, 1723-1725 [DOI] [PubMed] [Google Scholar]
- Janssen J., Sideleva V., Biga H. (1999). Use of the lateral line for feeding in two Lake Baikal sculpins. J. Fish Biol. 54, 404-416 [Google Scholar]
- Kingsley D. M., Peichel C. L. (2007). The molecular genetics of evolutionary change in sticklebacks. In Biology of the Three-Spined Stickleback (ed. Östlund-Nilsson S., Mayer I. and Huntingford F.), pp. 41-81 Boca Raton: CRC Press; [Google Scholar]
- Kingsley D. M., Zhu B., Osoegawa K., de Jong P. J., Schein J., Marra M., Peichel C., Amemiya C., Schluter D., Balabhadra S., et al. (2004). New genomic tools for molecular studies of evolutionary change in threespine sticklebacks. Behaviour 141, 1331-1344 [Google Scholar]
- Kitano J., Mori S., Peichel C. L. (2007). Phenotypic divergence and reproductive isolation between sympatric forms of Japanese threespine sticklebacks. Biol. J. Linn. Soc. 91, 671-685 [Google Scholar]
- Kitano J., Bolnick D. I., Beauchamp D. A., Mazur M. M., Mori S., Nakano T., Peichel C. L. (2008). Reverse evolution of armor plates in the threespine stickleback. Curr. Biol. 18, 769-774 [DOI] [PubMed] [Google Scholar]
- McPhail J. D. (1994). Speciation and the evolution of reproductive isolation in the sticklebacks (Gasterosteus) of south-western British Columbia. In The Evolutionary Biology of the Threespine Stickleback (ed. Bell M. A. and Foster S. A.), pp. 399-437 Oxford: Oxford University Press; [Google Scholar]
- Montgomery J. C. (1989). Lateral line detection of planktonic prey. In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 561-574 New York: Springer-Verlag; [Google Scholar]
- Montgomery J. C., Macdonald J. A. (1987). Sensory tuning of lateral line receptors in Antarctic fish to the movements of planktonic prey. Science 235, 195-196 [DOI] [PubMed] [Google Scholar]
- Montgomery J., Coombs S., Janssen J. (1994). Form and function relationships in lateral line systems: comparative data from six species of Antarctic notothenioid fish. Brain Behav. Evol. 44, 299-306 [DOI] [PubMed] [Google Scholar]
- Montgomery J. C., Baker C. F., Carton A. G. (1997). The lateral line can mediate rheotaxis in fish. Nature 389, 960-963 [Google Scholar]
- Moore J., Hendry A. P. (2005). Both selection and gene flow are necessary to explain adaptive divergence: evidence from clinal variation in stream stickleback. Evol. Ecol. Res. 7, 871-886 [Google Scholar]
- Münz H. (1989). Functional organization of the lateral line periphery. In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 285-297 New York: Springer-Verlag; [Google Scholar]
- Nelson J. S. (1971). Absence of the pelvic complex in ninespine sticklebacks, Pungitius pungitius, collected in Ireland and Wood Buffalo National Park, Canada, with notes on meristic variation. Copeia 4, 707-717 [Google Scholar]
- Northcutt R. G. (1989). The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 17-78 New York: Springer-Verlag; [Google Scholar]
- Partridge B. L., Pitcher T. J. (1980). The sensory basis of fish schools: relative roles of lateral line and vision. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 135, 315-325 [Google Scholar]
- Peichel C. L., Nereng K. S., Ohgi K. A., Cole B. L. E., Colosimo P. F., Buerkle C. A., Schluter D., Kingsley D. M. (2001). The genetic architecture of divergence between threespine stickleback species. Nature 414, 901-905 [DOI] [PubMed] [Google Scholar]
- Pitcher T. J., Partridge B. L., Wardle C. S. (1976). A blind fish can school. Science 194, 963-965 [DOI] [PubMed] [Google Scholar]
- Plath M., Seggel U., Burmeister H., Heubel K. U., Schlupp I. (2006). Choosy males from the underground: male mating preferences in surface- and cave-dwelling Atlantic mollies (Poecilia mexicana). Naturwissenschaften 93, 103-109 [DOI] [PubMed] [Google Scholar]
- Satou M., Shiraishi A., Matsushima T., Okumoto N. (1991). Vibrational communication during spawning behavior in the hime salmon (landlocked red salmon, Oncorhynchus nerka). J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 168, 417-428 [Google Scholar]
- Satou M., Takeuchi H. A., Nishii J., Tanabe M., Kitamura S., Okumoto N., Iwata M. (1994). Behavioral and electrophysiological evidences that the lateral line is involved in the inter-sexual vibrational communication of the hime salmon (landlocked red salmon, Oncorhynchus nerka). J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 174, 539-549 [Google Scholar]
- Saunders A. J., Montgomery J. C. (1985). Field and laboratory studies of the feeding behaviour of the piper Hyporhamphus ihi with reference to the role of the lateral line in feeding. Proc. R. Soc. Lond., B, Biol. Sci. 224, 209-221 [DOI] [PubMed] [Google Scholar]
- Schmitz A., Bleckmann H., Mogdans J. (2008). Organization of the superficial neuromast system in goldfish, Carassius auratus. J. Morphol. 269, 751-761 [DOI] [PubMed] [Google Scholar]
- Taylor E. B., McPhail J. D. (2000). Historical contingency and ecological determinism interact to prime speciation in sticklebacks, Gasterosteus. Proc. Biol. Sci. 267, 2375-2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyke T. (1990). Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain Behav. Evol. 35, 23-30 [DOI] [PubMed] [Google Scholar]
- van Staaden M. J., Romer H. (1998). Evolutionary transition from stretch to hearing organs in ancient grasshoppers. Nature 394, 773-775 [Google Scholar]
- Vischer H. A. (1990). The morphology of the lateral line system in 3 species of Pacific cottoid fishes occupying disparate habitats. Cell. Mol. Life Sci. 46, 244-250 [Google Scholar]
- Wada H., Hamaguchi S., Sakaizumi M. (2008). Development of diverse lateral line patterns on the teleost caudal fin. Dev. Dyn. 237, 2889-2902 [DOI] [PubMed] [Google Scholar]
- Webb J. F. (1989a). Developmental constraints and evolution of the lateral line system in teleost fishes. In The Mechanosensory Lateral Line (ed. Coombs S., Görner P. and Münz H.), pp. 79-97 New York: Springer-Verlag; [Google Scholar]
- Webb J. F. (1989b). Gross morphology and evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav. Evol. 33, 34-53 [DOI] [PubMed] [Google Scholar]
- Webb J. F., Montgomery J. C., Mogdans J. (2008). Bioacoustics and the lateral line system of fishes. In Fish Bioacoustics (ed. Webb J. F., Fay R. R. and Popper A. N.), pp. 145-182 New York: Springer; [Google Scholar]
- Wootton R. J. (1976). The Biology of the Sticklebacks. London: Academic Press; [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.