Abstract
In insects, a family of peptides with sequence homology to the vertebrate calcitonins has been implicated in the control of diuresis, a process that includes mixing of the hemolymph. Here, we show that a member of the insect calcitonin-like diuretic hormone (CLDH) family is present in the American lobster, Homarus americanus, serving, at least in part, as a powerful modulator of cardiac output. Specifically, during an ongoing EST project, a transcript encoding a putative H. americanus CLDH precursor was identified; a full-length cDNA was subsequently cloned. In silico analyses of the deduced prepro-hormone predicted the mature structure of the encoded CLDH to be GLDLGLGRGFSGSQAAKHLMGLAAANFAGGPamide (Homam-CLDH), which is identical to a known Tribolium castaneum peptide. RT-PCR tissue profiling suggests that Homam-CLDH is broadly distributed within the lobster nervous system, including the cardiac ganglion (CG), which controls the movement of the neurogenic heart. RT-PCR analysis conducted on pacemaker neuron- and motor neuron-specific cDNAs suggests that the motor neurons are the source of the CLDH message in the CG. Perfusion of Homam-CLDH through the isolated lobster heart produced dose-dependent increases in both contraction frequency and amplitude and a dose-dependent decrease in contraction duration, with threshold concentrations for all parameters in the range 10–11 to 10–10 mol l–1 or less, among the lowest for any peptide on this system. This report is the first documentation of a decapod CLDH, the first demonstration of CLDH bioactivity outside the Insecta, and the first detection of an intrinsic neuropeptide transcript in the crustacean CG.
Keywords: calcitonin-like diuretic hormone (CLDH), cardiac ganglion (CG), cardioactive peptide, diuretic hormone 31 (DH31), expressed sequence tag (EST), heart, neurohormone, neuromodulator, neuropeptide, reverse transcriptase polymerase chain reaction (RT-PCR), transcriptomics, Homarus americanus
INTRODUCTION
In hematophagous insects, and likely sap feeders as well, a rapid, post-feeding diuresis is needed to concentrate and process an ingested blood meal, the vast majority of which is water (Te Brugge et al., 2008; Coast, 2009). This diuretic event involves the coordination of a number of physiological processes that are under hormonal control, including transport of water and ions across the epithelium of the gut and Malpighian tubules, mixing of the blood meal within the digestive tract, mixing of the hemolymph, and, ultimately, expulsion of the waste derived from the fully digested food bolus (Coast, 2009). At present, a number of hormones have been implicated in the control of diuresis in insects, including the biogenic amine serotonin and several peptides (Coast, 2009), one of which shows structural similarity to members of the vertebrate calcitonin family (Furuya et al., 2000) and another to the corticotropin-releasing factor family of peptides (Kataoka et al., 1989).
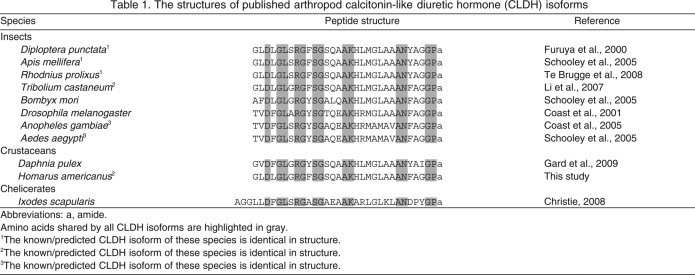
Originally isolated from neural tissues of the cockroach Diploptera punctata (Furuya et al., 2000), isoforms of calcitonin-like diuretic hormone (CLDH) have subsequently been identified in a number of insect species (Coast et al., 2001; Coast et al., 2005; Schooley et al., 2005; Li et al., 2007; Te Brugge et al., 2008). These peptides are all 31 amino acids in overall length in the Insecta, hence their alternative name, diuretic hormone 31 (DH31); they display a high degree of amino acid identity, and are amidated at their carboxyl (C)-termini (Table 1). In at least some insects, these CLDHs have been shown to increase the rate of fluid secretion by the Malpighian tubules (Furuya et al., 2000), to possess potent natriuretic potential (Coast et al., 2005), to be cardioactive (Te Brugge et al., 2008), and/or to modulate hindgut contractility (Te Brugge et al., 2008), all of which are important components of post-feeding diuresis.
Table 1.
The structures of published arthropod calcitonin-like diuretic hormone (CLDH) isoforms
Recently, a putative member of the CLDH family was identified via transcriptome analysis from the cladoceran crustacean Daphnia pulex (Gard et al., 2009). Like its insect counterparts, this Dappu-CLDH peptide is 31 amino acids in length, amidated at its C-terminus, and possesses extensive sequence similarity to the other known CLDHs. For example, Dappu-CLDH differs from the CLDH of D. punctata at only five residues (Table 1). In addition, an amino (N)-terminally extended isoform of CLDH (34 amino acids in length) was recently predicted from the deer tick Ixodes scapularis, a member of the arthopod subphylum Chelicerata (Christie, 2008a). These data suggest that members of the CLDH family are not restricted to insects, but may well be broadly conserved within the Arthropoda. However, it is unknown what function(s) these peptides play in non-insects.
Here, using transcriptomics and molecular cloning, we have identified the first CLDH from a member of the crustacean order Decapoda. Specifically, a 31 amino acid, C-terminally amidated peptide, identical in structure to that of red flour beetle Tribolium castaneum DH31 (Li et al., 2007), was predicted from the American lobster Homarus americanus (Table 1). Reverse transcriptase polymerase chain reaction (RT-PCR) tissue profiling suggests that Homam-CLDH is broadly distributed within the lobster nervous system, including the cardiac ganglion (CG), which controls the movement of the neurogenic heart (reviewed in Cooke, 2002). RT-PCR analysis conducted on the two classes of neurons present in the CG, the pacemaker neurons and the motor neurons (Cooke, 2002), suggests that it is the latter population of somata that express the CLDH transcript. Perfusion of the lobster cardiac neuromuscular system with Homam-CLDH elicited dose-dependent increases in both contraction amplitude and frequency and a dose-dependent decrease in burst duration, with threshold concentrations of less than 10–10 mol l–1 for each parameter, among the lowest seen for any peptide modulator on the heart. Collectively, our results show that an isoform of the CLDH family is present in decapod crustaceans, that this peptide is identical in structure to an insect CLDH, and that the peptide can function, at least in part, as a potent modulator of cardiac output. These data provide the first demonstration of bioactivity for a member of the CLDH family outside the Insecta. Moreover, our identification of Homam-CLDH transcript within the lobster CG, specifically in the motor neurons of the ganglion, is the first detection of an intrinsic peptide paracrine/hormone in this portion of the nervous system in any crustacean.
MATERIALS AND METHODS
Animals and tissue collection
American lobsters H. americanus Milne-Edwards were purchased from local suppliers in the Stonington and Brunswick areas of Maine (USA) and were maintained for up to 2 weeks in flow-through or recirculating natural seawater aquaria at 10–12°C. For tissue collection, animals were anesthetized by being packed in ice for 30–60 min, after which tissues [eyestalk ganglia, supraesophageal ganglion (brain), commissural ganglion, CG, heart muscle, midgut, and posterior midgut caecum] were collected via manual micro-dissection in chilled physiological saline [composition in mmol l–1: NaCl 479.12, KCl 12.74, CaCl2 13.67, MgSO4 20.00, Na2SO4 3.91, Trizma base 11.45 and maleic acid 4.82 (pH 7.45)].
Molecular cloning
cDNA library construction and EST submission
The construction and normalization of the H. americanus cDNA library used here has been described in detail in a previous report (Towle and Smith, 2006). In brief, multiple tissues (brain, gill, epipodite, branchiostegite, heart, ovary, testis, antennal gland, skeletal muscle and hepatopancreas) from four individuals (two males and two females) were collected; total RNA samples were prepared individually from each tissue, checked for quality, then pooled for construction and normalization of a cDNA library in the pCMV.SPORT6.1 vector by Invitrogen Corporation (Carlsbad, CA, USA). Plasmids were isolated and inserts single-pass sequenced from their 5′ end using SP6 primer (5′-ATTTAGGTGACACTATAG-3′) at the Marine DNA Sequencing and Analysis Facility at Mount Desert Island Biological Laboratory (Salisbury Cove, ME, USA). Sequence traces were processed for submission to dbEST [National Center for Biotechnology Information (NCBI); Bethesda, MD, USA] using the trace2dbest component of PartiGene software (University of Edinburgh, Edinburgh, UK). Prior to submission, all ESTs were subjected to blastx analysis, i.e. translated nucleotide sequence versus protein sequence (Altschul et al., 1997), and annotated accordingly.
cDNA sequence analysis
To further characterize the clone putatively identified by blastx analysis as encoding CLDH (i.e. clone HA_MX1_53h01), a sample of the bacteria (Escherichia coli) possessing the insert-containing vector was cultured overnight in LB-medium at 37°C. Plasmid containing the cDNA was subsequently isolated using a Wizard Plus Miniprep DNA Purification System (Promega, Madison, WI, USA; catalog no. A7500). The vector insert was then sequenced on an ABI 3100 16-capillary sequencer (Applied Biosystems Incorporated, Foster City, CA, USA) using both vector- and insert-specific forward and reverse sequencing primers (Integrated DNA Technologies, Inc., Coralville, IA, USA; Table 2). The sequence trace files resulting from each round of sequencing were analyzed using 4Peaks software (mekentosj.com), and the high quality nucleotide sequences were aligned using Lasergene software (DNASTAR Inc., Madison, WI, USA).
Table 2.
Vector- and insert-specific primers used for sequencing the Homarus americanus calcitonin-like diuretic hormone (CLDH) cDNA
| Primer name | Direction | Sequence |
| Vector-specific primers | ||
| SP6 | Forward | ATTTAGGTGACACTATAG |
| T-Party | Reverse | TTTTTTTTTTTTTTTTTTTV |
| Insert-specific primers | ||
| CLDH F1 | Forward | GTGGTGGACCCAACATCAGTGACG |
| CLDH F2 | Forward | GTTGGAGTTGCTGACGAGATTGG |
| CLDH F3 | Forward | CAGGAGACCATCTTGTTAACTG |
| CLDH F4 | Forward | TCCAGACAACGACGGTCAACCG |
| CLDH R1 | Reverse | CCAATCTCGTCAGCAACTCCAAC |
| CLDH R2 | Reverse | CTAGCTCATTGGCCCTTATGATGGAGTGCC |
Abbreviations: V in T-Party represents A, T or G.
As the cDNA sequenced from clone HA_MX1_53h01 was missing a portion of its putative 5′ coding region, a SMART RACE cDNA Amplification Kit (Clontech/Takeda, Palo Alto, CA, USA) was used to obtain additional sequence information (the starting RNA was isolated as described below). The PCR products obtained using 5′ rapid amplification of cDNA ends (RACE) were cloned into a pCR2.1 TOPO vector using a TOPO TA cloning kit (Invitrogen; catalog no. K4510-20) and sequenced and analyzed as described above.
Nucleotide translation and peptide prediction
Nucleotide translation and peptide prediction were conducted using a previously established protocol (Christie, 2008a; Christie, 2008b; Christie et al., 2008a; Gard et al., 2009; Dickinson et al., 2009; Ma et al., 2009; Stemmler et al., 2010). Specifically, cDNA sequence translation was done using the Translate tool of ExPASy (Swiss Institute of Bioinformatics, Basel, Switzerland; http://www.expasy.ch/tools/dna.html). Signal peptide and signal peptide cleavage prediction were performed via the online program SignalP 3.0, using both the Neural Networks and the Hidden Markov Models algorithms (Center for Biological Sequence Analysis, Technical University of Denmark, Lyngby, Denmark; http://www.cbs.dtu.dk/services/SignalP/) (Bendtsen et al., 2004). Pro-hormone cleavage sites were predicted based on the information presented by Veenstra (Veenstra, 2000). Prediction of the sulfation state of tyrosine residues was done using the online program Sulfinator (Swiss Institute of Bioinformatics, Geneva, Switzerland; http://www.expasy.org/tools/sulfinator/) (Monigatti et al., 2002). Other post-translational modifications (e.g. C-terminal amidation) were predicted by homology to known CLDH isoforms.
RT-PCR tissue profiling
Total RNA was isolated from freshly dissected lobster tissues (eyestalk ganglia, brain, commissural ganglion, CG, heart muscle, midgut proper and posterior midgut caecum) using an SV Total RNA Isolation System (Promega; catalog no. Z3100). For experiments using the eyestalk ganglia, commissural ganglion or the CG, tissues from 2–4 lobsters were pooled to obtain sufficient starting material. For some experiments, the X-organ region of the medulla terminalis of the eyestalk was excised for isolation of X-organ-specific RNA. Likewise, for some experiments, CGs were divided into pacemaker- and motor neuron-specific regions prior to RNA isolation. Each of the tissue types was processed individually and was first manually minced in the RNA lysis buffer with spring scissors and then further homogenized using a QIAshredder spin-column homogenizer (Qiagen, Valencia, CA, USA; catalog no. 79654) prior to proceeding with RNA isolation. RNA concentration was determined using a Nanodrop ND1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA), while RNA quality was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). cDNA was synthesized from the isolated mRNA using a SuperScript™ III First-Strand Synthesis System for RT-PCR (Invitrogen; catalog no. 18080051). PCR was carried out on a DNA Engine thermocycler (Biorad Laboratories, Hercules, CA, USA) using gene-specific primers (Fig. 1) and GoTaq Master Mix (Promega; catalog no. M7138). To confirm the identity of the RT-PCR products, a sample of a given product was cloned into a pCR2.1 TOPO vector using a TOPO TA cloning kit (Invitrogen) and sequenced using vector-specific sequencing primers (Table 2) as described above.
Fig. 1.
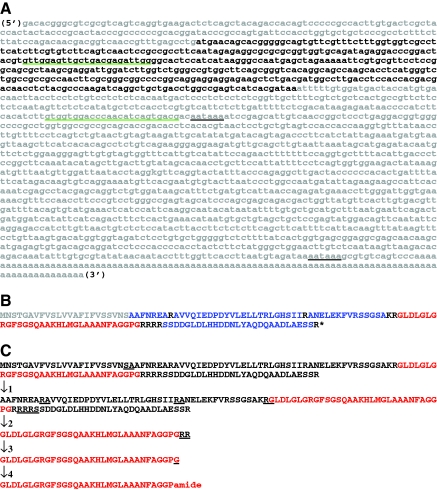
Nucleotide and deduced amino acid sequences of Homarus americanus prepro-calcitonin-like diuretic hormone (Homam-CLDH). (A) Nucleotide sequence of Homam-prepro-CLDH (accession no. GQ290461). The open reading frame of the cDNA, including the stop codon, is shown in bold font, with the two 3′ polyadenylation signal sequences indicated by underline in black. The two primers used for RT-PCR tissue profiling are underlined in green. (B) Deduced amino acid sequence of Homam-prepro-CLDH. The signal peptide is shown in gray, with prohormone convertase cleavage loci shown in black. The encoded CLDH isoform is shown in red, with additional precursor-related peptides shown in blue. The asterisk indicates the position of the stop codon. (C) Putative processing scheme resulting in the production of Homam-CLDH from its precursor protein. The first line of sequence shows the full-length prepro-hormone with the cleavage locus for signal peptidase underlined. The second line of sequence shows the cleaved pro-hormone with the loci for prohormone convertase underlined. The third line of sequence shows the immature CLDH peptide with the site of action for carboxypeptidase underlined. The fourth line of sequence shows the peptide resulting from carboxypeptidase activity with the Gly destined for α-amidation underlined. The final line of sequence shows the putative, mature CLDH after full post-translational processing.
Cardiac physiology
To assess the effects of Homam-CLDH on the neurogenic heart of H. americanus, we recorded the effects of perfusing the peptide through isolated lobster hearts. For these experiments, individual animals were cold-anesthetized by packing them in ice for 30–60 min, after which the posterior dorsal region of the thoracic carapace that lies directly over the heart, together with the underlying cardiac tissue, was removed. The dissected tissue, including the heart, was pinned through the carapace to the bottom of a small Sylgard 184 (KR Anderson, Santa Clara, CA, USA)-lined dish. The dorsal part of the heart remained attached to the carapace, so that the extent to which it was stretched was identical to that in the intact animal. The posterior artery was cannulated with a short piece of polyethylene tubing drawn out to fit the artery, and inserted into the heart. To verify that this perfusion system allowed the perfusate to enter the heart past the valve in the artery, we added low concentrations of fluorescein to the perfusate at the end of some experiments. The heart was continuously perfused with chilled physiological saline at a flow rate of 2.5 ml min–1. In some preparations, it was necessary to clip the ostial muscles so that perfusion fluid could leave the heart, and the heart did not become distended. In all preparations, a second perfusion line directed across the top of the heart helped to maintain temperature, which was monitored continuously and kept between 10 and 12°C. In all experiments, preparations were allowed to stabilize for 1–2 h before the first application of Homam-CLDH (synthesized at the University of Nevada, Reno), which was delivered through the posterior artery cannula for 8 min, after which the perfusion was switched back to control saline. Homam-CLDH was stored at –20°C as a 10–5 mol l–1 solution in deionized water, then diluted in saline to concentrations of 10–11 to 10–8 mol l–1 just before use.
To record heart contractions, three anterior arteries (the median ophthalmic artery and the two lateral antennal arteries) were tied off with 6-0 suture silk and attached to a Grass FT03 force-displacement transducer (Astro-Med, West Warwick, RI, USA) at an angle of approximately 30 degrees, with an initial baseline tension of 2 g. The output of the transducer was amplified via a Brownlee 410 instrumentation amplifier (San Jose, CA, USA), and recorded onto a PC computer using a Micro 1401 data acquisition board and Spike2 version 6 software (Cambridge Electronic Design Limited, Cambridge, UK). Heart rate, contraction amplitude and contraction duration were measured using the built-in functions of Spike2 and a script custom written for this purpose. To calculate percent change, average heartbeat amplitudes, frequencies, and durations at the peak of the peptide effect, 5–6 min after the onset of peptide application, were measured for a 200 s period and compared with those from the 200 s just prior to peptide application. Data were further analyzed and graphed using Prism5 software (GraphPad Software, San Diego, CA, USA).
RESULTS
Identification of the first decapod CLDH using molecular cloning and bioinformatics
During an ongoing H. americanus EST project (Towle and Smith, 2006), a lobster transcript (accession no. EX487346) was serendipitously identified by blastx analysis as having significant homology (Bit score, 63.9; E-value, 6e–09) to a prepro-CLDH from the fruit fly Drosophila melanogaster (accession no. NP_723401). Using a combination of vector- and insert-specific primers (Table 2), we sequenced the clone (HA_MX1_53h01) from which EX487346 was derived. While the resulting transcript appeared to be complete at its 3′ end (i.e. a poly-A tail was identified), a portion of the 5′ sequence appeared to be missing, including a section of the open reading frame (ORF) which putatively encodes the prepro-CLDH. Therefore, we used RACE to extend the clone in the 5′ direction. This combination of methodology led to the cloning of a putative full-length prepro-CLDH cDNA (accession no. GQ290461). As is shown in Fig. 1A, this cDNA (named here Homam-prepro-CLDH) was 2117 base pairs (bp) in length, and consisted of a 187 bp 5′ untranslated region (UTR), a 408 bp ORF (including the stop codon) and a 1522 bp 3′ UTR, which contained two polyadenylation signal sequences located 13 and 1204 bp upstream of a 97 bp poly-A tail.
Translation of the ORF of GQ290461 predicted a 135 amino acid prepro-hormone, named here Homam-prepro-CLDH (Fig. 1B,C). SignalP analysis of this sequence identified the first 23 amino acids as a signal peptide, with cleavage predicted between Ser23 and Ala24 by both the Neural Networks and the Hidden Markov Models algorithms (Fig. 1B,C). Within the remaining pro-hormone, three dibasic and two monobasic prohormone convertase processing sites were identified (Fig. 1B,C). In addition, the C-terminus of the prohormone is capped by an Arg residue. Cleavage at each of these sites, followed by carboxypeptidase activity and α-amidation at the exposed Gly, is predicted to result in the liberation of five peptides from the pro-hormone (listed in their order of appearance in this protein; Fig. 1B,C): AAFNREA, AVVQIEDPDYVLELLTRLGHSII, ANELEKFVRSSGSA, GLDLGLGRGFSGSQAAKH LMGLAAANFAGGPamide and SSDDGLDLHHDDNLYAQDQAADLAESS. Analysis of the second of the predicted peptides by the program Sulfinator suggests that the position 10 Tyr is sulfated (E-value, 8.6), resulting in the putative mature structure AVVQIEDPDY(SO3H)VLELLTRLGHSII for this peptide. The fourth peptide, GLDLGLGRGFSGSQAAKHLMGLAAANFAGG-Pamide, is a putative member of the CLDH family, named here Homam-CLDH; comparisons with other known CLDH/DH31 isoforms revealed that Homam-CLDH is identical to a peptide previously identified from the genome of the red flour beetle T. castaneum, i.e. Trica-DH31 (Li et al., 2007) (Table 1).
RT-PCR tissue profiling identifies the cardiac ganglion as one source of Homam-CLDH
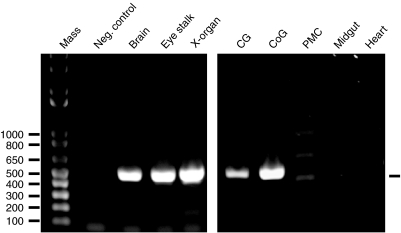
A mixed tissue source (brain, gill, epipodite, branchiostegite, heart, ovary, testis, antennal gland, skeletal muscle and hepatopancreas) was used as the starting material for the generation of the cDNA library from which clone HA_MX1_53h01 was derived. Mixed tissues (brain, heart, midgut and posterior midgut caecum) were likewise the starting material from which GQ290461 was subsequently cloned. Thus, a variety of tissues might be the source of the Homam-prepro-CLDH transcript described above. Using primers designed from the cDNA sequence of GQ290461 (Table 2), we conducted RT-PCR tissue profiling to assess the distribution of the lobster CLDH-encoding message. As Fig. 2 illustrates, a robust band the predicted size of the CLDH PCR product derived from the primer set illustrated in Fig. 1 (496 bp in length) was consistently detected in eyestalk ganglia (including in the X-organ), brain, commissural ganglion (part of the stomatogastric nervous system) and CG, all of which are neural tissues (N=4 independent runs for each tissue). In contrast, no product was detected in either heart muscle or the midgut proper (N=4 independent runs for each tissue; Fig. 2). Three faint bands (Fig. 2) were detected in posterior midgut caecum samples; however, none corresponded to the size of the predicted 496 bp product.
Fig. 2.
RT-PCR profiling of H. americanus calcitonin-like diuretic hormone (Homam-CLDH) transcript in neural, midgut and cardiac tissues of the lobster. Using the gene-specific primer set shown in Fig. 1, RT-PCR tissue profiling was conducted to assess the distribution of the lobster CLDH-encoding message. A robust band of the predicted size of the CLDH PCR product (496 bp in length; base pair ladder shown in lane 1 of the left gel) was consistently detected in brain (lane 3 of the left gel), eyestalk ganglia [including in the X-organ (XO); lanes 4 and 5 of the left gel, respectively], cardiac ganglia (CG; lane 1 of the right gel) and commissural ganglia (CoG; lane 2 of the right gel). In contrast, no product was detected in the midgut proper or heart muscle (lanes 4 and 5 of the right gel, respectively). Three faint bands (Fig. 2) were detected in posterior midgut caecum (PMC; lane 3 of the right gel); however, none corresponded to the size of the predicted 496 bp product. No product was detected in the negative control (Neg. control; lane 1 of the right gel).
To confirm the identity of the detected PCR products, one sample from the CG was subcloned and sequenced. Sequence analysis confirmed that this 496 bp product was identical to the expected sequence predicted from GQ290461 (Fig. 1). In contrast, none of the three bands identified from the posterior midgut caecum were homologous to any portion of the Homam-prepro-CLDH transcript (data not shown).
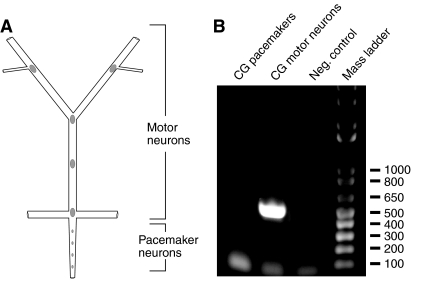
While numerous peptide paracrines/hormones have been localized to the neurons of the lobster eyestalk ganglia (e.g. Ma et al., 2008), brain (e.g. Ma et al., 2008), and commissural ganglion (e.g. Stemmler et al., 2007; Dickinson et al., 2009; Stemmler et al., 2010), none have thus far been detected in the CG somata of H. americanus, or, for that matter, any crustacean species. As the organization of the lobster CG is such that the somata of the two types of neurons present in the ganglion, the pacemaker and the motor neurons, can be readily isolated from one another (Fig. 3A), we conducted RT-PCR profiling on neuron-specific tissue pools (N=3 independent runs for each neuron type) in an attempt to determine in greater detail the origin of the Homam-prepro-CLDH message in the CG. As Fig. 3B shows, a 496 bp PCR product was seen only in amplification of cDNA from the motor neurons, strongly suggesting that these cells, and not the pacemaker neurons, are the source of the CLDH-encoding transcript in the CG.
Fig. 3.
RT-PCR profiling of H. americanus calcitonin-like diuretic hormone (Homam-CLDH) in the pacemaker neuron- and motor neuron-specific sub-regions of the lobster cardiac ganglion (CG). (A) Schematic representation of the lobster CG showing the location of the pacemaker and motor neurons within the ganglion. (B) RT-PCR profiling of CLDH message in the pacemaker (lane 1) and motor neurons (lane 2) of the cardiac ganglion. A robust band of the predicted CLDH PCR product size (496 bp in length; base pair ladder shown in lane 4) was consistently detected only in the motor neurons of the ganglion. No PCR product was dectected in the negative control (lane 3).
Homam-CLDH is a potent modulator of cardiac output
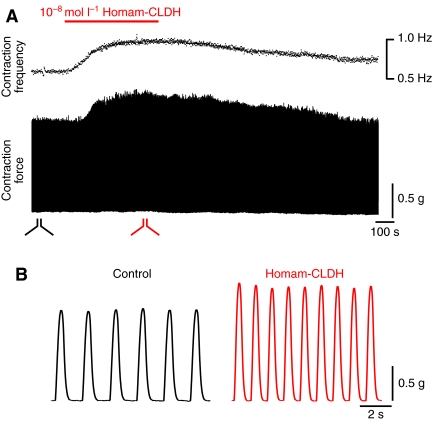
The cardiac neuromuscular system of decapods has been shown to be extensively modulated by a variety of peptide hormones (e.g. Mercier and Russenes, 1992; Wilkens et al., 2005; Cruz-Bermúdez et al., 2006; Cruz-Bermúdez and Marder, 2007; Dickinson et al., 2007; Fort et al., 2007a; Fort et al., 2007b; Christie et al., 2008b; Dickinson et al., 2009). As described earlier, one H. americanus tissue in which CLDH message was detected is the CG, which controls the rhythmic beating of the heart. Thus, we examined the effects of Homam-CLDH on the contractions of the isolated whole heart. Perfusion of the peptide (10–8 mol l–1) through the isolated whole heart increased both the amplitude and frequency of ongoing cardiac contractions (Fig. 4). As seen in Fig. 4A, both parameters increased rapidly, then returned slowly to baseline when the perfusion was returned to control saline. Higher speed recordings in Fig. 4B show more clearly the increases in these two parameters. In addition, it can be seen that contraction duration decreased during peptide perfusion.
Fig. 4.
Homarus americanus calcitonin-like diuretic hormone (Homam-CLDH) increased heartbeat frequency and contraction amplitude, and decreased duration of heart contractions. (A) Contraction frequency is shown graphically with time, on the same slow time scale as the recording of contraction force. Homam-CLDH (10–8 mol l–1) was applied during the time indicated by the bar over the recordings. Both frequency and amplitude increased, then gradually returned to baseline values. (B) Higher speed recordings taken from the regions demarcated in A, showing the increases in amplitude and frequency and the decreased duration of the heartbeats.
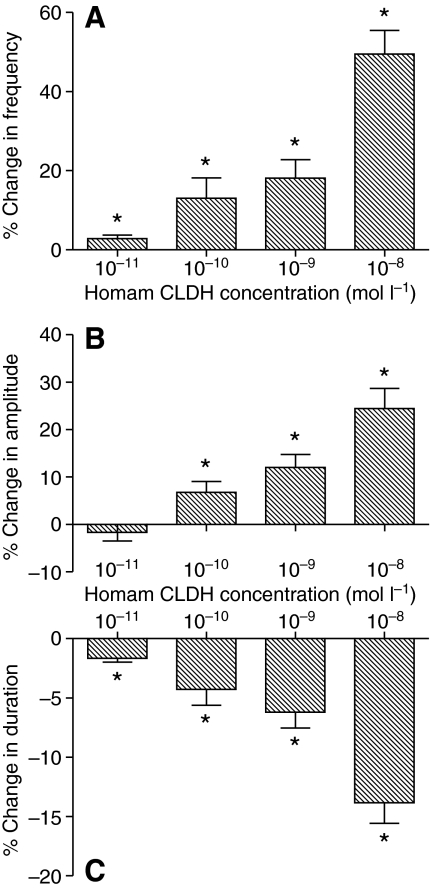
The increases in both contraction amplitude and frequency were dose dependent, as was the decrease in contraction duration, with larger effects seen as the concentration of Homam-CLDH increased. Using the concentrations at which the percent change is significantly different from zero as the definition of threshold, we found that the threshold for the effects of Homam-CLDH on contraction amplitude was between 10–10 and 10–11 mol l–1 (Fig. 5B), while the threshold for the effects on both frequency and duration was less than 10–11 mol l–1 (Fig. 5A,C). Consequently, the duty cycle (duration/period) increased with increasing peptide concentration. The effects on all three parameters at the lowest concentrations were minimal, but they were consistent across preparations, leading us to believe that even these low concentrations can cause reliable, if small, changes in cardiac output.
Fig. 5.
The increases in both contraction amplitude and frequency, as well as the decrease in contraction duration, induced by H. americanus calcitonin-like diuretic hormone (Homam-CLDH) are dose dependent. Pooled data from preparations exposed to concentrations of Homam-CLDH ranging from 10–8 to 10–11 mol l–1 showed that cycle frequency increased (A), contraction amplitude increased (B) and burst duration decreased (C), with larger effects at higher concentrations. Threshold, defined as the concentration at which per cent change is significantly different from zero, was less than 10–10 mol l–1 for all parameters. Specifically, threshold for the increase in contraction amplitude was between 10–10 and 10–11 mol l–1; threshold for both the increase in cycle frequency and the decrease in burst duration was less than 10–11 mol l–1. *Significantly different from zero, one-sample, 2-tailed t-test, P<0.05, N=10. Error bars represent standard errors.
DISCUSSION
Identification of the first decapod calcitonin-like diuretic hormone
In insects, a family of peptides exhibiting sequence homology to the vertebrate calcitonins has been identified and shown to play roles in controlling the physiological processes underlying diuresis in at least some species. These peptides, commonly referred to as calcitonin-like diuretic hormones, are all 31 amino acids in length and are amidated at their C-termini (Table 1). All of the known insect CLDHs exhibit very similar amino acid sequences (Table 1), differing at most by 11 residues from one another. Recently a CLDH was predicted via transcriptomics from the cladoceran crustacean D. pulex (Gard et al., 2009). Like its insect counterparts, this peptide is 31 amino acids in length, is amidated at its C-terminus, and possesses an amino acid sequence similar to the insect CLDHs (Table 1). Though longer than the other family members, a 34 amino acid peptide predicted from the tick I. scapularis, a member of the Chelicerata, appears to be a member of the CLDH family as well (Christie, 2008a) (Table 1).
Here, via molecular cloning and bioinformatics, we have characterized a member of the CLDH family from the American lobster H. americanus, i.e. GLDLGLGRGFSGSQAAKHLMGLAAANFAGGPamide or Homam-CLDH. Like the insect and Daphnia peptides, Homam-CLDH is 31 amino acids in length and amidated at its C-terminus; it is the first CLDH to be identified from a member of the crustacean order Decapoda. Surprisingly, the structure of Homam-CLDH is identical to that of Trica-DH31, previously identified from the red flour beetle T. castaneum (Li et al., 2007). The extreme level of structural conservation of the CLDHs across the Arthropoda, and in particular within the Pancrustacea, suggests limited variation has been tolerated across evolution for this peptide family, and also that the CLDHs, and their receptor(s), play critical, and perhaps conserved, functions in members of this taxon.
While Homam-CLDH is the only family member thus far identified from a decapod, preliminary transcriptome mining has identified a putative CLDH-encoding EST from the penaeid shrimp Marsupenaeus japonicus (accession no. CI997969; A.E.C., unpublished). Translation of this transcript suggests that it encodes a 97 amino acid N-terminal partial prepro-hormone. This deduced protein is very similar in structure to Homam-prepro-CLDH; it possesses a 23 amino acid signal peptide, a similar complement of prohormone convertase cleavage sites, and a nearly identical complement of predicted peptides, including a partial CLDH sequence that is identical to the first 26 amino acids of Homam-CLDH. As the remaining five amino acids are highly conserved within the lobster and many insects (Table 1), it seems likely that the M. japonicus peptide is identical in sequence to that of Homam-CLDH. If this is true, then GLDLGLGRGFSGSQAAKHLMGLAAANFAGGPamide may represent a highly conserved decapod CLDH, as M. japonicus is a member of the basal taxon of the Decapoda (i.e. the Penaeidea), while H. americanus (a member of the infraorder Astacidea) is more derived; several large-scale peptidomic investigations have recently documented broad, and perhaps ubiquitous, conservation of a number of decapod peptides, particularly those that appear to be encoded as a single copy, e.g. the C-type allatostatin pQIRYHQCYFNPISCF (Stemmler et al., 2010), the C-type allatostatin-like peptide SYWKQCAFNAVSCFamide (Dickinson et al., 2009) and the myosuppressin pQDLDHVFLRFamide (Stemmler et al., 2007), as is the case for Homam-CLDH. As more CLDHs are characterized from members of the Decapoda, it will be interesting to see whether this hypothesis is borne out.
The distribution of calcitonin-like diuretic hormone mRNA suggests pleiotropic paracrine/endocrine actions in Homarus americanus
Following our identification of Homam-CLDH, we embarked upon mapping the distribution of the peptide in lobster tissues. Initially, we attempted to do this via immunohistochemistry (data not shown), using an affinity-purified antibody generated against [Cys32]Dippu-DH31; this antibody was used previously to map the distribution of Rhodnius prolixus-DH31 (e.g. Te Brugge et al., 2005; Te Brugge et al., 2008), as well as for immunohistochemistry in Oncopeltus fasciatus (Te Brugge and Orchard, 2008). Rhopr-DH31 is identical in structure to Dippu-DH31 (Te Brugge et al., 2008); R. prolixus and O. fasciatus are both Hemipterans and may contain the same CLDH. However, in the present study, the antibody failed to label any profiles in the H. americanus nervous system [eyestalk ganglia, brain, stomatogastric nervous system (including the paired commissural ganglia) and CG tested] or midgut (midgut proper and posterior midgut caecum tested) (data not shown). While Homam-CLDH and Dippu-DH31 are similar in sequence (Table 1), the failure of the antibody to produce labeling in the lobster was not totally unexpected, as this antibody likewise did not label tissues from the beetle Tenebrio molitor (Holtzhausen and Nicolson, 2007). This is curious in that a peptide identical to Trica-DH31, the T. castaneum/H. americanus peptide, was isolated and identified in T. molitor (D.A.J. and D.A.S., unpublished) using a highly sensitive ELISA assay, which is extremely similar to that employed by Te Brugge and colleagues (Te Brugge et al., 2005); this ELISA used exactly the same antiserum as was used without success for immunohistochemistry in T. molitor and in the present study. The high affinity of this antiserum for the peptide in an ELISA, yet its inability to be used for immunohistochemistry, is paradoxical. Thus, we took an alternative approach to tissue mapping and used RT-PCR profiling to assay lobster tissues for the presence/absence of the peptide-encoding transcript.
Using RT-PCR, Homam-prepro-CLDH was detected in multiple neural tissues, i.e. the eyestalk ganglia (including in the X-organ), the brain, the commissural ganglia and the CG. No transcript was found in either heart muscle or midgut/posterior midgut caecum. The detection of Homam-prepro-CLDH in the brain was expected, as this region of the nervous system is a known source of the peptide in insects (e.g. Te Brugge et al., 2008). Likewise, detection of the transcript in the eyestalk ganglia and the commissural ganglion was not surprising, as both of these regions of the lobster nervous system have been shown to be the location of numerous peptidergic somata (e.g. Stemmler et al., 2007; Ma et al., 2008). In contrast, the finding of Homam-prepro-CLDH message in the CG was surprising; although the cardiac motor pattern is known to be extensively modulated by peptide hormones (e.g. Mercier and Russenes, 1992; Wilkens et al., 2005; Cruz-Bermúdez et al., 2006; Cruz-Bermúdez and Marder, 2007; Dickinson et al., 2007; Fort et al., 2007a; Fort et al., 2007b; Christie et al., 2008b; Dickinson et al., 2009), no peptides have been identified within the ganglion itself. By dividing the CG into pacemaker neuron- and motor neuron-specific regions, we were able to localize the production of the CLDH transcript to the latter cell type. The presence of Homam-CLDH in the CG motor neurons suggests several possible functional roles for the peptide in the cardiac neuromuscular system, including modulation of the heart muscle innervated by the motor neurons and/or feedback modulation of the pacemaker cells by activity in the motor neurons.
As the name diuretic hormone implies, one role of CLDH peptides in at least a subset of insects is in the elimination of water after the ingestion of large quantities of fluid; in other insects, however, CLDHs appear to be minimally involved in diuresis (Coast, 2009). In the species in which CLDHs have been implicated in diuretic control, one component of this suite of diuresis-related events is increased mixing of the hemolymph (Te Brugge et al., 2008). The increased cardiac activity seen in the lobster would result in a similar increase in hemolymph mixing, suggesting the possibility that at least some of the functions of CLDH are conserved between insects and decapods. It would clearly be of interest to determine whether this peptide in H. americanus also alters water and ion transport and activity in the digestive tract. Preliminary data (M.R.B., T.W. and P.S.D., unpublished) suggest that it does not alter activity of the mid/hindgut or of the stomatogastric nervous system, which controls the movement of the foregut musculature.
CLDH is a powerful cardioactive peptide in H. americanus
Like a number of neuropeptides, Homam-CLDH excites the heart by increasing both the frequency and amplitude of heart contractions. The low threshold at which it is active suggests a hormonal effect. Three major endocrine sites are thought to be responsible for releasing the majority of neuroactive peptides that are hormonally delivered in the lobster: the sinus gland, located in the eyestalks, and the pericardial organ, located on the lateral walls of the pericardial chamber that surrounds the heart, both of which are neuroendocrine organs, and the midgut (Cooke and Sullivan, 1982; Christie et al., 2007). Our RT-PCR data suggest that the sinus gland is one potential source of Homam-CLDH in the lobster, as the transcript encoding the peptide is present in the region of the eyestalk containing the X-organ, where the somata that give rise to the sinus gland are located (Cooke and Sullivan, 1982). Our RT-PCR data also suggest that the midgut is not responsible for the hormonal delivery of Homam-CLDH to the heart, since no Homam-prepro-CLDH transcript was found in this tissue. It was unfortunately not possible for us to determine whether the peptide is present in the pericardial organ, as the majority of the cell bodies that project to this neuroendocrine organ are located in the thoracic ganglia, which also contain numerous somata with other functions. As discussed earlier, no antibodies are presently available that recognize Homam-CLDH; thus, immunohistochemical mapping of the peptide in the pericardial organ was not possible.
Surprisingly, we found the transcript encoding Homam-CLDH in the CG itself, which suggests the possibility that it is also released locally to modulate the activity of the heart. While we have not yet applied the peptide at concentrations that might be expected for local release (e.g. 10–7 or 10–6 mol l–1), we would predict, based on the data presented here, that these concentrations would cause even larger increases in heart activity.
A large number of peptides have been shown to modulate the activity of various components of the crustacean cardiac neuromuscular system (e.g. Cruz-Bermúdez and Marder, 2007; Kuramoto and Ebara, 1984; Wilkens et al., 1996). In H. americanus this includes proctolin (Miller and Sullivan, 1981; Sullivan and Miller, 1984; Wilkens et al., 2005; Worden et al., 1995), crustacean cardioactive peptide (CCAP) (Wilkens et al., 1996), a number of FMRFamide-like peptides (Wilkens et al., 2005; Dickinson et al., 2007), two tachykinin-related peptides (Christie et al., 2008b) and the allatostatin C-like peptide SYWKQCAFNAVSCFamide (Dickinson et al., 2009). However, to the best of our knowledge, no peptide has previously been localized to the CG itself. In contrast, there is good evidence for the presence of amines and acetylcholine within the ganglion. For example, acetylcholine (Sullivan and Miller, 1990; Freschi and Livengood, 1989), gamma-amino-butyric acid (GABA) (Delgado et al., 2000) and dopamine (Fort et al., 2004), all of which modulate the activity of the heart, have been found within the ganglion. Both GABA and dopamine, although delivered locally, are nonetheless extrinsic modulators, since they derive from other sites in the nervous system. The neurons delivering dopamine, for example, are located in the commissural ganglia (Fort et al., 2004); fibers containing GABA found in the CG enter the ganglion from the dorsal nerve (Delgado et al., 2000). Acetylcholine, in contrast, is thought to be synthesized and released by neurons within the CG, where it may serve as a locally released transmitter to coordinate the activity of the pacemaker and motor neurons. It has, however, been shown to modulate the heartbeat via effects on both muscarinic and nicotinic receptors (Freschi and Livengood, 1989), and thus could also act as an intrinsic neuromodulator (Katz et al., 1994; Katz, 1998; Morgan et al., 2000).
The synthesis of Homam-CLDH by the motor neurons suggests that this peptide may function as an intrinsic neuromodulator. Interestingly, Morganelli and Sherman (Morganelli and Sherman, 1987) described a variety of different types of synapses in the H. americanus CG. Among these are several types that contain dense-cored vesicles, a characteristic of peptidergic terminals. Moreover, two of the terminal types that contained large numbers of dense-cored vesicles were found largely in the pacemaker region; these terminals did not include clear synaptic vesicles. Morganelli and Sherman suggested that these might be neurosecretory terminals that release modulatory transmitters to alter pacemaker activity. Although Morganelli and Sherman proposed that these terminals derived from extrinsic fibers that contain monoamines, a possibility raised by the identification of Homam-CLDH transcript in the CG motor neurons is that they instead represent a source of intrinsic peptidergic modulation. This possibility is consistent with the fact that axons from at least some of the motor neurons project to the region of the ganglion where the pacemaker cells are located (Cooke and Hartline, 1975).
An alternative possibility is that Homam-CLDH is released from the motor neurons onto the muscle at the periphery, where it could serve to enhance contraction when released. Similar peripheral peptidergic modulation of muscle has been seen in Aplysia californica, where the peptide cotransmitters small cardioactive peptides (SCP) A and B, which are released when the motor neuron fires at high spike frequencies, serve to enhance contraction of the accessory radula closer muscle (Whim and Lloyd, 1989; Whim and Lloyd, 1990). However, although peripheral modulation alone could explain the increased amplitude of heart contractions, it is the pacemaker cells within the CG that determine the heartbeat frequency. Thus, if the major target of CLDH is peripheral, we must postulate that feedback from the periphery is activated or modulated to trigger the observed increases in contraction frequency. That such changes in contraction frequency could result from peripheral modulation and the consequent activation of stretch feedback pathways is strongly supported by a study showing that induced stretch is able to alter contraction frequency in the isopod Ligia pallasii (Sakurai and Wilkens, 2003). Alternatively, it is possible that intrinsically released CLDH has effects different from those recorded here in response to perfusion of the peptide through the whole heart. For example, intrinsically released peptide might modulate only amplitude, while hormonally delivered peptide modulates both frequency and amplitude of contraction. Experiments to determine the sites at which CLDH modulates the heart, by examining the peptide's effects on the isolated CG as well as on isolated cardiac muscle, are ongoing, as are attempts to generate an antibody that recognizes Homam-CLDH and can be utilized to determine the distribution of the peptide itself.
Financial support for this work was provided by NIH Grant Number P20 RR-016463 from the INBRE Program of the National Center for Research Resources (Patricia Hand, PhD, Principal Investigator), through institutional funds provided by MDIBL (to A.E.C.) and by the Nevada Agriculture Experiment Station (Grant NEV000337 to D.A.S). Deposited in PMC for release after 12 months.
- CG
- cardiac ganglion
- CLDH
- calcitonin-like diuretic hormone
- DH31
- diuretic hormone 31
- EST
- expressed sequence tag
- ORF
- open reading frame
- RACE
- rapid amplification of cDNA ends
- RT-PCR
- reverse transcriptase polymerase chain reaction
- UTR
- untranslated region
REFERENCES
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389-3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004). Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340, 783-795 [DOI] [PubMed] [Google Scholar]
- Christie A. E. (2008a). Neuropeptide discovery in Ixodoidea: an in silico investigation using publicly accessible expressed sequence tags. Gen. Comp. Endocrinol. 157, 174-185 [DOI] [PubMed] [Google Scholar]
- Christie A. E. (2008b). In silico analyses of peptide paracrines/hormones in Aphidoidea. Gen. Comp. Endocrinol. 159, 67-79 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Kutz-Naber K. K., Stemmler E. A., Klein A., Messinger D. I., Goiney C. C., Conterato A. J., Bruns E. A., Hsu Y. W., Li L., et al. (2007). Midgut epithelial endocrine cells are a rich source of the neuropeptides APSGFLGMRamide (Cancer borealis tachykinin-related peptide Ia) and GYRKPPFNGSIFamide (Gly1-SIFamide) in the crabs Cancer borealis, Cancer magister and Cancer productus. J. Exp. Biol. 210, 699-714 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Cashman C. R., Brennan H. R., Ma M., Sousa G. L., Li L., Stemmler E. A., Dickinson P. S. (2008a). Identification of putative crustacean neuropeptides using in silico analyses of publicly accessible expressed sequence tags. Gen. Comp. Endocrinol. 156, 246-264 [DOI] [PubMed] [Google Scholar]
- Christie A. E., Cashman C. R., Stevens J. S., Smith C. M., Beale K. M., Stemmler E. A., Greenwood S. J., Towle D. W., Dickinson P. S. (2008b). Identification and cardiotropic actions of brain/gut-derived tachykinin-related peptides (TRPs) from the American lobster Homarus americanus. Peptides 29, 1909-1918 [DOI] [PubMed] [Google Scholar]
- Coast G. M. (2009). Neuroendocrine control of ionic homeostasis in blood-sucking insects. J. Exp. Biol. 212, 378-386 [DOI] [PubMed] [Google Scholar]
- Coast G. M., Webster S. G., Schegg K. M., Tobe S. S., Schooley D. A. (2001). The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J. Exp. Biol. 204, 1795-1804 [DOI] [PubMed] [Google Scholar]
- Coast G. M., Garside C. S., Webster S. G., Schegg K. M., Schooley D. A. (2005). Mosquito natriuretic peptide identified as a calcitonin-like diuretic hormone in Anopheles gambiae (Giles). J. Exp. Biol. 208, 3281-3291 [DOI] [PubMed] [Google Scholar]
- Cooke I. M. (2002). Reliable, responsive pacemaking and pattern generation with minimal cell numbers: the crustacean cardiac ganglion. Biol. Bull. 202, 108-136 [DOI] [PubMed] [Google Scholar]
- Cooke I. M., Hartline D. K. (1975). Neurohormonal alteration of integrative properties of the cardiac ganglion of the lobster Homarus americanus. J. Exp. Biol. 63, 33-52 [DOI] [PubMed] [Google Scholar]
- Cooke I. M., Sullivan R. E. (1982). Hormones and neurosecretion. In The Biology of Crustacea, Vol.3 (ed. Bliss D., Atwood H., and Sandeman D.), pp. 205-290 New York: Academic Press; [Google Scholar]
- Cruz-Bermúdez N. D., Marder E. (2007). Multiple modulators act on the cardiac ganglion of the crab, Cancer borealis. J. Exp. Biol. 210, 2873-2884 [DOI] [PubMed] [Google Scholar]
- Cruz-Bermúdez N. D., Fu Q., Kutz-Naber K. K., Christie A. E., Li L., Marder E. (2006). Mass spectrometric characterization and physiological actions of GAHKNYLRFamide, a novel FMRFamide-like peptide from crabs of the genus Cancer. J. Neurochem. 97, 784-799 [DOI] [PubMed] [Google Scholar]
- Delgado J. Y., Oyola E., Miller M. W. (2000). Localization of GABA- and glutamate-like immunoreactivity in the cardiac ganglion of the lobster Panulirus argus. J. Neurocytol. 29, 605-619 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Stevens J. S., Rus S., Brennan H. R., Goiney C. C., Smith C. M., Li L., Towle D. W., Christie A. E. (2007). Identification and cardiotropic actions of sulfakinin peptides in the American lobster Homarus americanus. J. Exp. Biol. 210, 2278-2289 [DOI] [PubMed] [Google Scholar]
- Dickinson P. S., Wiwatpanit T., Gabranski E. R., Ackerman R. J., Stevens J. S., Cashman C. R., Stemmler E. A., Christie A. E. (2009). Identification of SYWKQCAFNAVSCFamide: a broadly conserved crustacean C-type allatostatin-like peptide with both neuromodulatory and cardioactive properties. J. Exp. Biol. 212, 1140-1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort T. J., Brezina V., Miller M. W. (2004). Modulation of an integrated central pattern generator effector system: dopaminergic regulation of cardiac activity in the blue crab Callinectes sapidus. J. Neurophysiol. 92, 3455-3470 [DOI] [PubMed] [Google Scholar]
- Fort T. J., Brezina V., Miller M. W. (2007a). Regulation of the crab heartbeat by FMRFamide-like peptides: multiple interacting effects on center and periphery. J. Neurophysiol. 98, 2887-2902 [DOI] [PubMed] [Google Scholar]
- Fort T. J., García-Crescioni K., Agricola H. J., Brezina V., Miller M. W. (2007b). Regulation of the crab heartbeat by crustacean cardioactive peptide (CCAP): central and peripheral actions. J. Neurophysiol. 97, 3407-3420 [DOI] [PubMed] [Google Scholar]
- Freschi J. E., Livengood D. R. (1989). Membrane current underlying muscarinic cholinergic excitation of motoneurons in lobster cardiac ganglion. J. Neurophysiol. 62, 984-995 [DOI] [PubMed] [Google Scholar]
- Furuya K., Milchak R. J., Schegg K. M., Zhang J., Tobe S. S., Coast G. M., Schooley D. A. (2000). Cockroach diuretic hormones: characterization of a calcitonin-like peptide in insects. Proc. Natl. Acad. Sci. USA. 97, 6469-6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard A. L., Lenz P. H., Shaw J. R., Christie A. E. (2009). Identification of putative peptide paracrines/hormones in the water flea Daphnia pulex (Crustacea: Branchiopoda; Cladocera) using transcriptomics and immunohistochemistry. Gen. Comp. Endocrinol. 160, 271-287 [DOI] [PubMed] [Google Scholar]
- Holtzhausen W. D., Nicolson S. W. (2007). Beetle diuretic peptides: The response of mealworm (Tenebrio molitor) Malpighian tubules to synthetic peptides, and cross-reactivity studies with a dung beetle (Onthophagus gazella). J. Insect Physiol. 53, 361-369 [DOI] [PubMed] [Google Scholar]
- Kataoka H., Troetschler R. G., Li J. P., Kramer S. J., Carney R. L., Schooley D. A. (1989). Isolation and identification of a diuretic hormone from the tobacco hornworm, Manduca sexta. Proc. Natl. Acad. Sci. USA 86, 2976-2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P. S. (1998). Neuromodulation intrinsic to the central pattern generator for escape swimming in Tritonia. Ann. N. Y. Acad. Sci. 860, 181-188 [DOI] [PubMed] [Google Scholar]
- Katz P. S., Getting P. A., Frost W. N. (1994). Dynamic neuromodulation of synaptic strength intrinsic to a central pattern generator circuit. Nature 367, 729-731 [DOI] [PubMed] [Google Scholar]
- Kuramoto T., Ebara A. (1984). Neurohormonal modulation of the cardiac outflow through the cardioarterial valve in the lobster. J. Exp. Biol. 111, 123-130 [Google Scholar]
- Li B., Predel R., Neupert S., Hauser F., Tanaka Y., Cazzamali G., Williamson M., Arakane Y., Verleyen P., Schoofs L., Schachtner J., Grimmelikhuijzen C. J., Park Y. (2007). Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 18, 113-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Chen R., Sousa G. L., Bors E. K., Kwiatkowski M. A., Goiney C. C., Goy M. F., Christie A. E., Li L. (2008). Mass spectral characterization of peptide transmitters/hormones in the nervous system and neuroendocrine organs of the American lobster Homarus americanus. Gen. Comp. Endocrinol. 156, 395-409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M., Bors E. K., Dickinson E. S., Kwiatkowski M. A., Sousa G. L., Henry R. P., Smith C. M., Towle D. W., Christie A. E., Li L. (2009). Characterization of the Carcinus maenas neuropeptidome by mass spectrometry and functional genomics. Gen. Comp. Endocrinol. 161, 320-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier A. J., Russenes R. T. (1992). Modulation of crayfish hearts by FMRFamide-related peptides. Biol. Bull. 182, 333-340 [DOI] [PubMed] [Google Scholar]
- Miller M. W., Sullivan R. E. (1981). Some effects of proctolin on the cardiac ganglion of the Maine Lobster, Homarus americanus (Milne Edwards). J. Neurobiol. 12, 629-639 [DOI] [PubMed] [Google Scholar]
- Monigatti F., Gasteiger E., Bairoch A., Jung E. (2002). The Sulfinator: predicting tyrosine sulfation sites in protein sequences. Bioinformatics. 18, 769-770 [DOI] [PubMed] [Google Scholar]
- Morgan P. T., Perrins R., Lloyd P. E., Weiss K. R. (2000). Intrinsic and extrinsic modulation of a single central pattern generating circuit. J. Neurophysiol. 84, 1186-1193 [DOI] [PubMed] [Google Scholar]
- Morganelli P. M., Sherman R. G. (1987). Nerve terminals and synapses in the cardiac ganglion of the adult lobster Homarus americanus. J. Morphol. 191, 177-191 [DOI] [PubMed] [Google Scholar]
- Sakurai A., Wilkens J. L. (2003). Tension sensitivity of the heart pacemaker neurons in the isopod crustacean Ligia pallasii. J. Exp. Biol. 206, 105-115 [DOI] [PubMed] [Google Scholar]
- Schooley D. A., Horodyski F. M., Coast G. M. (2005). Hormones controlling homeostasis in insects. In Comprehensive Molecular Insect Science, Vol. 3, Endocrinology (ed. Gilbert L. I., Iatrou K. and Gill S. S.), pp. 493-550 Oxford: Elsevier Pergamon; [Google Scholar]
- Stemmler E. A., Cashman C. R., Messinger D. I., Gardner N. P., Dickinson P. S., Christie A. E. (2007). High-mass-resolution direct-tissue MALDI-FTMS reveals broad conservation of three neuropeptides (APSGFLGMRamide, GYRKPPFNGSIFamide and pQDLDHVFLRFamide) across members of seven decapod crustaean infraorders. Peptides 28, 2104-2115 [DOI] [PubMed] [Google Scholar]
- Stemmler E. A., Bruns E. A., Cashman C. R., Dickinson P. S., Christie A. E. (2010). Molecular and mass spectral identification of the broadly conserved decapod crustacean neuropeptide pQIRYHQCYFNPISCF: the first PISCF-allatostatin (Manduca sexta- or C-type allatostatin) from a non-insect. Gen. Comp. Endocrinol. 165, 1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R. E., Miller M. W. (1984). Dual effects of proctolin on the rhythmic burst activity of the cardiac ganglion. J. Neurobiol. 15, 173-196 [DOI] [PubMed] [Google Scholar]
- Sullivan R. E., Miller M. W. (1990). Cholinergic activation of the lobster cardiac ganglion. J. Neurobiol. 21, 639-650 [DOI] [PubMed] [Google Scholar]
- Te Brugge V. A., Orchard I. (2008). Distribution and activity of a Dippu-DH31-like peptide in the large milkweed bug Oncopeltus fasciatus. Peptides 29, 206-213 [DOI] [PubMed] [Google Scholar]
- Te Brugge V. A., Lombardi V. C., Schooley D. A., Orchard I. (2005). Presence and activity of a Dippu-DH31-like peptide in the blood-feeding bug, Rhodnius prolixus. Peptides 26, 29-42 [DOI] [PubMed] [Google Scholar]
- Te Brugge V. A., Schooley D. A., Orchard I. (2008). Amino acid sequence and biological activity of a calcitonin-like diuretic hormone (DH31) from Rhodnius prolixus. J. Exp. Biol. 211, 382-390 [DOI] [PubMed] [Google Scholar]
- Towle D. W., Smith C. M. (2006). Gene discovery in Carcinus maenas and Homarus americanus via expressed sequence tags. Int. Comp. Biol. 46, 912-918 [DOI] [PubMed] [Google Scholar]
- Veenstra J. A. (2000). Mono-and dibasic proteolytic cleavage sites in insect neuroendocrine peptide precursors. Arch. Insect Biochem. Physiol. 43, 49-63 [DOI] [PubMed] [Google Scholar]
- Whim M. D., Lloyd P. E. (1989). Frequency-dependent release of peptide cotransmitters from identified cholinergic motor neurons in Aplysia Proc. Natl. Acad. Sci. USA 86, 9034-9038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whim M. D., Lloyd P. E. (1990). Neuropeptide cotransmitters released from an identified cholinergic motor neuron modulate neuromuscular efficacy in Aplysia J. Neurosci. 10, 3313-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkens J. L., Kuramoto T., McMahon B. R. (1996). The effects of six pericardial hormones and hypoxia on the semi-isolated heart and sternal arterial valve of the lobster Homarus americanus. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 114, 57-65 [Google Scholar]
- Wilkens J. L., Shinozaki T., Yazawa T., ter Keurs H. E. D. J. (2005). Sites and modes of action of proctolin and FLP F2 on lobster cardiac muscle. J. Exp. Biol. 208, 737-747 [DOI] [PubMed] [Google Scholar]
- Worden M. K., Kravitz E. A., Goy M. F. (1995). Peptide F1, an N-terminally extended analog of FMRFamide, enhances contractile activity in multiple target tissues in lobster. J. Exp. Biol. 198, 97-108 [DOI] [PubMed] [Google Scholar]