Abstract
Hispanic individuals are underrepresented in clinical and research populations and often excluded from clinical trials in the US. Hence, there are few data on the effectiveness of most empirically validated therapies for Hispanic substance users. We conducted a multisite randomized trial comparing the effectiveness of three individual sessions of motivational enhancement therapy (MET) to three individual sessions of counseling as usual (CAU) on treatment retention and frequency of substance use, with all assessment and treatment sessions conducted in Spanish among 405 individuals seeking treatment for any type of current substance use. Treatment exposure was good, with 66% of participants completing all three protocol sessions. Although both interventions resulted in reductions in substance use during the 4-week therapy phase, there were no significant treatment condition by time interactions nor site by treatment condition interactions. Results suggest that the individual treatments delivered in Spanish were both attractive to and effective with this heterogeneous group of Hispanic adults, but the differential effectiveness of MET may be limited to those whose primary substance use problem is alcohol and may be fairly modest in magnitude.
Substance abuse is a significant problem among Hispanic Americans, who represent the largest ethnic minority group in the United States (Amaro, Arevalo, Gonzalez, Szapocznik, & Iguchi, 2006). However, very little is known about what addiction treatments are effective among Hispanic-Americans. People of Hispanic origin may be particularly vulnerable within treatment and treatment systems due to acculturation and other psychosocial stressors, language and cultural barriers, and poverty; moreover, they have been found to have increased rates of alcohol and substance use, HIV infection, as well as higher unemployment rates. Hispanics tend to be less likely to receive mental health and substance abuse treatment services than European and African Americans (Wells, Klap, Koike, & Sherbourne, 2001) and experience disproportionate levels of adverse consequences of substance use (Caetano, 2003).
Hispanics are highly underrepresented in clinical and research samples (Miranda et al., 2005; Wells et al., 2001) where requirements of English literacy systematically exclude many Spanish speaking individuals. The development and evaluation of culturally and linguistically appropriate interventions for this underserved population is a key step in the ultimate goal of providing effective behavioral health services to all Americans (Bernal & Sharron del Rio, 2001; Rogler, Malgady, Constantino, & Blumenthal, 1987). Very few studies have addressed the efficacy or effectiveness on substance use outcomes of well-defined behavioral approaches for adult Hispanic substance users. A recent review (Amaro et al., 2006) identified only two randomized controlled trials of behavioral interventions expressly targeting adult Hispanic substance users, both of which reported very low rates of treatment entry and retention (Burge et al., 1992; Robles et al., 2004).
The Clinical Trials Network (CTN), a large research-provider partnership, was developed by the National Institute on Drug Abuse (NIDA) to improve the quality of drug abuse treatment in the U.S. By virtue of its size and scope, the CTN is also well suited to address ethnic disparities by including larger samples of women, adolescents and racial and ethnic minorities than is feasible in most single-site trials (Carroll et al., 2007). In this report we describe treatment outcomes from a multisite trial of MET versus CAU, delivered in Spanish, among a diverse sample of treatment-seeking Hispanic adult substance users. The present study paralleled the design of an earlier multisite trial conducted in English (Ball et al., 2007), which found overall differential treatment effectiveness favorable to MET over CAU during follow-up, particularly those whose primary substance use problem was alcohol. This study was an independent multisite trial using manuals and materials translated into Spanish expressly for Spanish-speaking drug users and conducted in a different set of sites, and to our knowledge the first multisite trial to evaluate the effectiveness of an empirically validated therapy in a large population of Hispanic-American substance-using adults. Based on findings from the earlier English version protocol (Ball et al., 2007), as well as previous suggestions that motivational interventions may yield stronger effects among ethnic minority populations such as Hispanic Americans (Hettema, Steele, & Miller, 2005), we hypothesized that MET would be more effective than CAU when delivered in community practice settings with diverse groups of Spanish-speaking substance abuse outpatients in terms of reducing the frequency of participants’ substance use (operationalized as days of substance use by week) and enhancing retention (operationalized as days enrolled in the treatment program). We also hypothesized that MET would be more effective in reducing days of alcohol use specifically among participants whose primary substance use problem was alcohol.
Method
This multisite randomized clinical trial was implemented in five outpatient substance abuse treatment programs within the CTN. Sites were located in Miami, FL, New York, NY, Portland, OR, Greenley, CO and Santa Fe, NM (see author notes). Target enrollment of 80 randomized participants was planned for each site. A common study protocol and informed consent procedures were approved by the respective Institutional Review Boards affiliated with each participating university. A Data Safety and Monitoring Board convened by NIDA approved the protocol and reviewed serious adverse events (n=31, none of which were study related).
This Spanish-language protocol was intended to parallel the design and implementation of the earlier English version CTN MET trial (Ball et al., 2007) and thus all aspects of the design and treatment conditions were identical to that study with the following exceptions, which were required for implementing the protocol with a Spanish-speaking population (Suarez-Morales et al., 2007). First, performance sites were selected that offered outpatient substance abuse treatment in Spanish and had at least four bilingual clinicians on staff. To assure adequate Spanish fluency, all study clinicians and research assistants were required to complete a Spanish fluency exercise that involved independent evaluation of audiotapes of their responses to a standardized set of open-ended clinical research questions by a team at the University of Miami. Second, in cases where protocol assessments had not already been translated and validated in Spanish (e.g., the Addiction Severity Index), assessments that had been used in the English version of the protocol were translated into Spanish by a team at the University of Miami, using a translation and back-translation protocol used in several previous trials to protect the integrity of validated assessments through the translation process (Suarez-Morales et al., 2007).
Clinicians at each of the sites were asked to review the translated forms and provide additional regional terminology to ensure the instrument’s accessibility to a broad range of Spanish speakers. A similar procedure was used to translate the consent forms; once they had been approved by the local IRB, the consent forms were sent to the University of Miami for translation and where necessary, reviewed again by the site or local IRB. Recruitment of participants occurred between December 2003 and September 2005 and follow-ups were completed by September 2006.
Participants
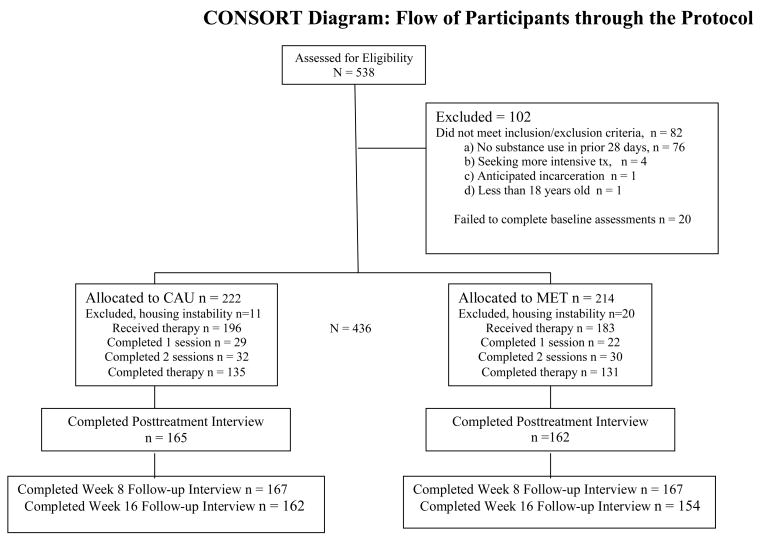
A total of 538 individuals were screened for interest and eligibility, and 102 did not meet inclusion/exclusion criteria. As illustrated in the CONSORT diagram (Figure 1), 436 participants were randomly assigned to one of the two treatment conditions during their first month of treatment at each outpatient program site. Of these, 31 were post randomization exclusions1, yielding an intention to treat sample of 405, of which an additional 26 never attended their first session. Most participants completed all three protocol sessions (266/405, 66%). There were no differences between conditions in the number of sessions completed, indicating a comparable level of exposure to the assigned protocol therapy. Basic demographic and substance use characteristics are provided for the intention to treat sample in Table 1. Alcohol (82% lifetime abuse or dependence) and cocaine (43%) were the most common substance use diagnoses followed by marijuana (29%), or opiates (13%).
Figure 1.
CONSORT diagram of eligibility, enrollment, randomization, treatment, and follow-up rates by condition
Table 1.
Participant characteristics and baseline variables by site
| Site 1 | Site 2 | Site 3 | Site 4 | Site 5 | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean or % |
SD | Mean or % |
SD | Mean or % |
SD | Mean or % |
SD | Mean or % |
SD | Mean or % |
SD | F/X2 | df | p | |
| Female (%) | 4.9 | 3.7 | 17.6 | 3.9 | 27.5 | 11.6 | 35.7 | 4 | .000 | ||||||
| Age | 28.9 | 7.4 | 31.3 | 9.1 | 38.2 | 8.9 | 28.7 | 6.2 | 35.0 | 9.4 | 32.5 | 9.1 | 20.13 | 4, 400 | .000 |
| Years of education | 8.5 | 3.1 | 8.3 | 3.4 | 10.8 | 2.1 | 8.9 | 3.3 | 11.2 | 3.0 | 9.6 | 3.2 | 16.87 | 4, 399 | .000 |
| Employed (% full or pt-time) | 76.8 | 95.1 | 3.5 | 87.0 | 48.8 | 61.5 | 193.9 | 4 | .000 | ||||||
| Marital Status (%) | |||||||||||||||
| Married/Cohabiting | 47.6 | 55.6 | 8.2 | 54.5 | 42.5 | 41.2 | 62.9 | 8 | .000 | ||||||
| Widowed/Separated | 12.2 | 17.3 | 45.9 | 14.3 | 25.0 | 23.2 | |||||||||
| Never Married | 40.2 | 27.2 | 45.9 | 31.2 | 32.5 | 35.6 | |||||||||
| Previous treatment (%) | |||||||||||||||
| Alcohol | 13.4 | 12.3 | 38.8 | 55.8 | 34.2 | 30.7 | 50.3 | 4 | .000 | ||||||
| Drug | 9.8 | 6.2 | 80.0 | 10.4 | 43.0 | 30.4 | 158.2 | 4 | .000 | ||||||
| Court involvement in treatment seeking | |||||||||||||||
| Court Mandated | 0 | 27.2 | 2.4 | 0 | 28.8 | 11.6 | 163.8 | 8 | .000 | ||||||
| Some legal involvement | 89.0 | 40.7 | 43.5 | 96.1 | 27.5 | 59.0 | |||||||||
| No legal issue | 11.0 | 32.1 | 54.1 | 3.9 | 43.8 | 29.4 | |||||||||
| On probation/parole, % | 17.1 | 43.2 | 24.7 | 83.1 | 27.8 | 38.6 | 91.9 | 4 | .000 | ||||||
| # months incarcerated, lifetime, mean | 1.9 | 11.3 | .5 | 1.7 | 29.9 | 37.0 | 3.5 | 9.6 | 5.8 | 18.8 | 8.6 | 22.9 | 31.3 | 4, 399 | .000 |
| Primary substance used (%) | |||||||||||||||
| Alcohol | 90.2 | 84.0 | 17.6 | 72.7 | 37.5 | 60.0 | 223.9 | 20 | .000 | ||||||
| Cocaine | 1.2 | 9.9 | 42.4 | 14.3 | 40.0 | 21.7 | |||||||||
| Marijuana | 2.4 | 6.2 | 12.9 | 2.6 | 18.8 | 8.6 | |||||||||
| Opiates | 1.2 | 0 | 27.1 | 0 | 2.5 | 6.4 | |||||||||
| Benzodiazepines | 0 | 0 | 0 | 0 | 1.3 | .2 | |||||||||
| Methamphetamines | 4.9 | 0 | 0 | 10.4 | 0 | 3.0 | |||||||||
| Mean number of days of substance use past 28 | |||||||||||||||
| Alcohol | 2.0 | 2.2 | 8.2 | 9.1 | 5.0 | 8.3 | 3.2 | 3.9 | 6.1 | 6.5 | 4.9 | 6.9 | 11.2 | 4, 400 | .000 |
| Cocaine | 0.1 | 0.8 | 2.2 | 5.1 | 4.7 | 7.3 | .9 | 4.0 | 4.5 | 7.2 | 2.5 | 5.8 | 12.0 | 4, 400 | .000 |
| Opioid | 0.3 | 3.1 | 0.01 | .11 | 3.2 | 6.5 | 0 | 0 | 0.7 | 3.1 | .9 | 3.8 | 11.4 | 4, 400 | .000 |
| Marijuana | 0.6 | 3.3 | 2.7 | 6.9 | 3.3 | 8.2 | .5 | 2.8 | 3.5 | 7.6 | 2.1 | 6.3 | 4.3 | 4, 400 | .002 |
| Mean years of regular substance abuse | |||||||||||||||
| Primary Substance | 8.1 | 7.7 | 11.9 | 8.7 | 11.4 | 10.0 | 10.2 | 6.8 | 13.8 | 9.4 | 11.1 | 8.8 | 4.7 | 4, 400 | .000 |
| Alcohol | 8.7 | 7.9 | 11.4 | 8.0 | 16.3 | 11.6 | 12.1 | 6.7 | 15.0 | 10.3 | 12.7 | 9.5 | 9.0 | 4, 400 | .000 |
| Cocaine | 0.4 | 1.3 | 2.9 | 6.9 | 9.5 | 8.8 | 1.9 | 3.4 | 5.9 | 7.0 | 4.2 | 7.0 | 28.8 | 4, 400 | .000 |
| Opioid | 0.1 | 0.6 | 0.0 | 0.0 | 8.3 | 11.4 | .1 | 0.3 | 0.5 | 2.0 | 1.8 | 6.2 | 4 | 4, 400 | .000 |
| Marijuana | 0.4 | 1.4 | 2.8 | 7.5 | 9.1 | 10.0 | 2.7 | 5.5 | 4.9 | 6.7 | 4.0 | 7.5 | 18.9 | 4 | .000 |
| Years Living in U.S., mean | 9.1 | 7.5 | 9.4 | 9.2 | 23.9 | 13.3 | 12.6 | 10.3 | 18.2 | 11.8 | 14.7 | 12.1 | 29.6 | 4, 400 | .000 |
| Place of birth, % | |||||||||||||||
| South America | 0 | 0 | 1.2 | 0 | 7.5 | 1.7 | 490.6 | 24 | .000 | ||||||
| Central America | 7.3 | 6.2 | 2.4 | 5.2 | 32.5 | 10.6 | |||||||||
| Mexico | 84.1 | 90.1 | 0 | 72.7 | 2.5 | 49.4 | |||||||||
| Continental US | 6.1 | 3.7 | 32.9 | 22.1 | 15 | 16 | |||||||||
| Cuba | 1.2 | 0 | 1.2 | 0 | 33.8 | 7.2 | |||||||||
| Puerto Rico | 1.2 | 0 | 58.8 | 0 | 7.5 | 14.1 | |||||||||
| Other Caribbean | 0 | 0 | 3.5 | 0 | 1.3 | 1 | |||||||||
| Primary/first language, % | |||||||||||||||
| Spanish | 92.7 | 100 | 74.1 | 98.7 | 100 | 92.8 | 88.4 | 8 | .000 | ||||||
| English | 2.4 | 0 | 25.9 | 1.3 | 0 | 6.2 | |||||||||
| Other | 4.9 | 0 | 0 | 0 | 0 | 1 | |||||||||
Note. N=405.
Analysis of the intention to treat sample (n=405) and the subsamples who initiated treatment (treatment exposed n=379) and completed treatment (n=266) yielded comparable results. Of the 405 appropriately randomized participants, 81% (88% of the treatment-exposed sample) completed the termination (28-day) assessment, 82% (92% of exposed) completed the 1-month follow-up, and 78% (87% of exposed) completed the 3-month follow-up interview. Data on retention in the clinics were available on 97% (392/405) and 96% (388/405) of the randomized sample at the posttreatment and 3-month points, respectively. Most of the randomized (89%) and treatment-exposed (92%) participants were assessed at least once post-treatment. Rates of follow-up varied significantly across the five program sites (e.g., range 67–91%), χ2=18.3, p < .01); however, there were no significant differences between conditions overall or within site.
Therapists and training
Therapists were volunteers drawn from the staff of the participating treatment programs who were: (1) bilingual, (2) willing to be randomized to be trained in and to deliver either CAU or MET, and (3) to have counseling sessions audiotaped. The 24 clinicians were predominantly female (64%), Hispanic (67%) and had a mean of 6 years of counseling experience. Sixty-five percent reported that Spanish was their primary language; 40% had masters degrees, and 60% held bachelors, associates, or high school degrees. With the exception of a centralized, rather than decentralized model of training and the use of fully bilingual expert trainers and supervisors, procedures for therapist training, certification and supervision were identical to that of the Ball et al. (2007) trial.
Procedures
Following baseline assessment, participants were randomly assigned to one of two individual therapy conditions involving three sessions of either CAU or MET using an urn allocation program adapted from several previous multisite clinical trials to provide balance within sites on gender, primary substance used, employment and criminal justice status. The three study therapy sessions in both conditions were delivered within a 28-day time window from the point of randomization. Other aspects of the delivery of CAU and MET were identical to that described in the methods for the completed English trial (Ball et al., 2007).
Assessments and primary outcomes focused on retention in treatment and days of substance use by week, and, other than being delivered in Spanish, were identical to the English version. A total of 1635 urine samples were collected after randomization including 957 during the 29-day treatment window. The urine samples indicated adequate correspondence with participants’ self-reports of recent illicit drug use, with 142/1635 (8.7%) indicating drug use when the participant denied recent use. All protocol treatment sessions were audiotaped, and MET sessions were reviewed and rated by expert MET trainers and clinical supervisors to maintain fidelity. A sample of both MET (n=160) and CAU (n=165) sessions were selected for independent review of adherence and competence. This sample included 87 (33.3%) of the 266 participants who completed all three protocol sessions, as well as at least one session from each therapist so an additional 64 participants who did not complete treatment were represented. This resulted in the following breakdown of rated sessions (116 first sessions, 105 second sessions, and 104 third sessions). Ratings by independent evaluators trained in the use of the adherence/competence rating system validated for the English version, indicated a high level of discriminability between MET and CAU, as well as levels of adherence and competence comparable to those found in the English version (Martino, Ball, Nich, Frankforter, & Carroll, 2008).
The data analytic strategy was also identical to that used in the Ball et al (2007) study. The principal analyses evaluated change in frequency of substance abuse over time. The self-report measure of substance use, derived from the TimeLine Follow-Back method, permitted a longitudinal mixed model analysis of days of any substance use by week, from baseline through 16 continuous weekly data points. Because this analysis consisted of two discrete time phases (4-week period for active study therapy and 12-week period for follow-up assessment), forcing a single linear estimate through 17 data points was not appropriate because changes in slope were expected to accompany the transition between the two time phases. The effects of treatment condition (MET versus CAU), program site (1–5), weeks (0–16 Weeks), and phase (0–4 therapy week versus 5–16 follow-up week periods) and all possible interactions were tested for this outcome with a piecewise hierarchical linear model (see Ball et al., 2007).
Results and Discussion
Table 2 presents primary outcome variables by therapy condition and program site, by phase (treatment versus follow-up). There were no main effects for therapy condition or therapy condition by program site interactions for treatment retention. Overall, participants in both conditions were retained in their outpatient program for an average of about three months. Almost all of the participants (MET = 93%; CAU = 91%) were still enrolled at their program site at the 28-day therapy termination assessment; these rates were approximately halved by the end of the 84-day follow-up period (57% versus 52%).
Table 2.
Primary retention and substance use outcomes by therapy condition, program site, and phase (treatment weeks 1–4, follow-up weeks 5–16)
| Site 1 |
Site 2 |
Site 3 |
Site 4 |
Site 5 |
Total |
Treatment condition |
Site | Condition x site |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | CAU | MET | CAU | MET | CAU | MET | CAU | MET | CAU | MET | CAU | MET | ddf | F | p | F | p | F | p |
| Days enrolled in treatment at CTP through Week 16a | |||||||||||||||||||
| Mean | 113.0 | 105.2 | 68.8 | 75.1 | 72.3 | 84.4 | 88.0 | 102.0 | 70.2 | 83.1 | 83.1 | 90.0 | |||||||
| SD | 25.7 | 35.3 | 37.8 | 36.7 | 44.2 | 66.3 | 38.4 | 25.2 | 45.4 | 45.3 | 42.0 | 46.1 | 378 | 3.09 | .08 | 10.68 | .00 | 0.93 | .44 |
| Effect size | −.25 | .17 | .21 | .43 | .28 | .15 | |||||||||||||
| Percent days abstinent from primary substance used, Weeks 1–4b | |||||||||||||||||||
| Mean | 96.8 | 98.9 | 85.7 | 91.8 | 89.3 | 91.7 | 96.7 | 93.8 | 91.0 | 96.9 | 92.2 | 94.7 | |||||||
| SD | 16.1 | 3.7 | 24.3 | 9.2 | 17.9 | 18.4 | 6.2 | 15.5 | 19.4 | 4.8 | 17.8 | 12.1 | 342 | 2.78 | .09 | 4.21 | .00 | 1.05 | .38 |
| Effect size | 0.16 | 0.33 | 0.13 | −0.24 | 0.41 | 0.16 | |||||||||||||
| Percent days abstinent from primary substance, Weeks 5–16c | |||||||||||||||||||
| Mean | 99.0 | 99.8 | 83.7 | 90.8 | 88.0 | 92.2 | 96.6 | 96.0 | 91.5 | 90.9 | 92.5 | 94.4 | |||||||
| SD | 5.6 | 0.7 | 26.2 | 12.7 | 22.9 | 19.2 | 5.2 | 10.1 | 22.2 | 19.3 | 18.3 | 13.9 | 272 | 1.36 | .24 | 5.98 | .00 | 0.62 | .64 |
| Effect size | 0.19 | 0.34 | 0.19 | −0.06 | −0.02 | 0.08 | |||||||||||||
| Percent positive urine specimens, Weeks 1–4d | |||||||||||||||||||
| Mean | 66.7 | 50.0 | 48.6 | 80.95 | 26.2 | 31.4 | 15.8 | 29.6 | 36.6 | 34.0 | 31.8 | 37.6 | |||||||
| SD | 47.1 | 57.7 | 40.9 | 37.8 | 39.2 | 40.0 | 32.0 | 45.5 | 44.5 | 37.0 | 41.3 | 41.9 | 145 | 0.53 | .46 | 3.21 | .02 | .67 | .61 |
| Effect size | −.32 | 0.82 | .13 | .35 | −.06 | .14 | |||||||||||||
Note. CTP= Community treatment program. Sample sizes differ from 405 due to loss to follow-up for some variables (see Figure 1).
n= 388,
n=352,
n=295,
n=155, as positive urine specimens from primary drug analysis excludes subjects who used alcohol only..
Table 2 also presents the primary substance use outcome variables by therapy condition and program site, by phase. For the hierarchical linear analyses that evaluated the effect of time, phase, site and treatment on days of substance use, there were several effects for time. These included main effects for time ( t (5740) = −3.0, p < .01), phase (treatment versus follow-up, t (5740) = −2.79, p < .01), and the interaction of these two time effects, (t (5740) = 2.64, p < .01), indicating that participants as a group demonstrated reductions in self-reported days of substance use by week, from baseline over time and between the two study phases. There were, however, no significant interactions of treatment condition by time, and no statistically significant interactions of site by time by treatment condition.
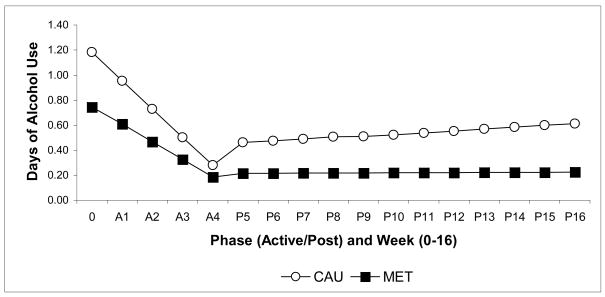
Given the findings in the English version of the protocol which suggested differential effectiveness of MET versus CAU within the subgroup of participants whose primary substance use problem was alcohol (Ball et al., 2007), the principal analyses were repeated for the subgroup of participants whose primary substance used was alcohol (n=243). As shown in Table 3, for the primary alcohol-using subgroup, the ANOVA analyses of the effect of treatment condition on retention was statistically significant. The hierarchical linear analyses indicated a significant interaction of week by phase by treatment condition on days of alcohol use by week (t (3573)=−2.33, p=.02), as well as several other interactions including week (t (3573)=−3.86, p < .01), phase (t (3573)=−2.96, p<.01), and treatment by phase (t (3573)=2.41, p=.02). These effects are illustrated in Figure 2.
Table 3.
Retention by site, subgroup of participants with primary alcohol problems only, N=243
| Site 1 |
Site 2 |
Site 3 |
Site 4 |
Site 5 |
Total |
Condition |
Site |
Condition x site |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | CAU | MET | CAU | MET | CAU | MET | CAU | MET | CAU | MET | CAU | MET | ddf | F | p | F | p | F | p |
| Mean days enrolled in treatment at CTP through week 16a | |||||||||||||||||||
| Mean | 111.7 | 107.5 | 71.4 | 77.6 | 60.0 | 97.0 | 91.2 | 103.2 | 65.6 | 84.1 | 88.7 | 95.2 | 221 | 5.79 | .02 | 10.8 | .00 | 7 | .28 |
| SD | 26.5 | 34.8 | 39.6 | 35.3 | 46.7 | 26.7 | 38.2 | 26.2 | 41.9 | 41.9 | 39.9 | 35.2 | |||||||
| Effect size | −.13 | .16 | .97 | .36 | .43 | .17 | |||||||||||||
Note. CTP=Community treatment program. Sample sizes differ from 243 due to loss to follow-up for some variables
n=231
Figure 2. Estimates from HLM analyses.
Therapy condition by Time (as weeks) by Time (as phase) interaction for days per week of alcohol use across therapy (weeks 0–4) and follow-up (weeks 5–16) phases for the subgroup of 243 participants whose primary substance use problem was alcohol. CAU=Counseling as Usual, MET=Motivational Enhancement Therapy.
In summary, this multisite randomized effectiveness study of MET and CAU, delivered in Spanish, suggested the following: First, retention and substance outcomes were good overall, with high rates of protocol treatment completion and low rates of substance use in both MET and CAU conditions across sites. Second, regarding the primary hypothesis that MET would be more effective in reducing substance use than CAU, for the full sample, there were no significant main effects of treatment condition or treatment by site interactions. Third, there was some support for the secondary hypothesis that within the subgroup of participants whose primary substance use problem was alcohol, there was a significant effect of modest magnitude for treatment condition on days of alcohol use by week. Fourth, there was a high level of variability across sites in participant characteristics and treatment outcomes, underlining the heterogeneity of Hispanic individuals seeking treatment for addiction as well as the systems that serve this population.
Overall, outcomes for this trial paralleled those of the English version in multiple ways: In both trials, MET was not significantly more effective than CAU for the full sample, and there was some evidence of significant treatment differences within the alcohol-using subsample, which was the most prevalent substance use problem in both studies. Thus, this pair of studies adds to the literature suggesting that, within large diverse clinical samples, MET’s effects may be most discernable among those who use alcohol rather than illicit drugs (Miller, Yahne, & Tonigan, 2003). In effectiveness trials such as these, where there is considerable variability in the types and patterns of substance used by participants and in the severity and nature of co-occurring problems, it is possible that such variability makes it more difficult to identify effects that may be detectable among more homogeneous groups.
Second, site variation was considerable in both studies, but particularly so in this version of the protocol where the majority of participants had come to the United States from a variety of different countries and Puerto Rico. Sites differed greatly in terms of primary type of substance used, and demographic characteristics such as criminal justice involvement and employment status. As in the English version of the study, although there were no statistically significant site by treatment interactions for the primary outcomes, main effects for site were consistently significant. Thus, any number of variables (e.g., alcohol versus drug use, level of criminal justice involvement, severity level) could be considered as possible moderators of treatment effects, and interpretation of magnitude of effects across site may be confounded with a number of these variables. Moreover, culturally relevant variables, such as acculturation level, may need to be considered as moderators of treatment outcomes among Hispanic Americans (Hall, 2001; Lau, 2006). It is of note that the overall high rates of treatment retention in both conditions in the present study stand in marked contrast with prior randomized trials of behavioral interventions specifically targeting Hispanic substance users, where rates of treatment exposure and retention and improved outcomes have been low (Burge et al., 1992; Robles et al., 2004).
As with the English version of the protocol, the strengths of this study include recruitment of a comparatively large and diverse sample of outpatients, delivery of treatment by a diverse group of therapists randomized to training condition, and significant attention to therapy fidelity via ongoing supervision as well as independent evaluation of session tapes. In addition, particular care was taken to appropriately adapt the treatment and protocol for use with Spanish-speaking substance users, including careful procedures for translation of assessment instruments, rating forms, and treatment worksheets, assuring language proficiency among study staff, and multiple strategies to support recruitment and retention of a large Spanish-speaking population (Suarez-Morales et al., 2007).
This study has several limitations. First, about 20% of the participants interested in the study were found to be ineligible due to a lack of recent self-reported substance use and 6% of those appropriately randomized did not initiate their assigned study treatment. Second, the time spent by the study therapists in training and supervision was not balanced across conditions, and therapists assigned to deliver MET received regular, observationally-based supervision throughout the trial, while those assigned to deliver CAU participated in standard supervisory procedures at each performance site.
The lack of significant differences in outcome between MET and CAU for the sample as a whole may be viewed in the context of the markedly good retention and treatment outcomes seen here. Once participants initiated therapy, exposure to the protocol treatments was high and comparable across conditions; the majority of participants completed the three sessions offered. Mean days retained in treatment at the sites was over 90, and participants reported high levels of abstinence through the follow-up period. Such high levels of retention have typically been considered not easily achievable among Latino populations. In the context of the heterogeneity of the study population as well as these apparent ceiling effects for retention and outcome, these findings suggest that the individually delivered treatments offered in these sites and delivered in Spanish were both attractive to and effective with this heterogeneous group of Hispanic adults, but suggest the differential effectiveness of MET may be limited to those whose primary substance use problem is alcohol and may be fairly modest in magnitude.
Acknowledgments
This study was funded by the National Institute on Drug Abuse in the form of individual grants to the medicals schools/centers at Yale University (U10 DA13038 awarded to Kathleen Carroll), the University of Miami (U10 DA13720 awarded to José Szapocznik), the University of New Mexico (U10 DA015833 awarded to William R. Miller), Columbia University (U10 DA13035 awarded to Edward V. Nunes), the Oregon Health and Sciences University (U10 DA13036 awarded to Dennis McCarty), and the University of Colorado (U10 DA13716 awarded to Paula Riggs) within the cooperative agreement of the Clinical Trials Network. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIDA.
The authors acknowledge the invaluable support of study coordinators (Lourdes Suarez-Morales, Ani Bisono Ivette Cuzmar, Catherine Dempsey, Jennifer Lima, Lynn Kunkel, Marilyn Macdonald, Jennifer Smith, Jennifer VanLare), program executive directors (Rhonda Bohs, Richard Drandoff, Janet Lerner, Carol Luna-Anderson, John Wilde), expert site supervisors (Manuel Paris, Luis Añez, Paticia Juarez), research assistants (Angie Arrellano, Albert Cabrera, Lisbeth Iglesias-Rios, Laura Hays, Silvia Mestra, Alyson Ortiz, Leonard Pena, Adriana Tobon, Diego Vega, Joanne Weidemann, Theresa Williamson, Diego Vega), tape raters (Tamara Armstrong, Luis Bedegral, Julianna Cossio, Hector Lizcano, Mary Jane Kerr, Ruth Martinez, Marylin Quintero, Darlene Rivera, Michelle Silva, Ivelisse Suarez), CIDI trainers (Dr. Maritza Rubio-Stipec) and study therapists whose names cannot be listed because some institutional review boards considered them human subjects within this study. We add special thanks to Joanne Corvino, Luis Anez and Manuel Paris for the development, training, and coordination of the independent tape rating system and to Melissa Gordon as director of the protocol coordinating center (Advanced Behavioral Health, ABH, Middletown, CT).
Footnotes
These 31 individuals were migrant workers who left the area immediately after being randomized. As suggested by Fergusson (Fergusson, Aaron, Guyatt, & Hebert, 2002), these participants were considered post-randomization exclusions, as the participants would have been excluded prior to randomization if the level of their housing instability was known. Although adequate stability of housing was an inclusion criterion, it is one that is somewhat subjective, and in underserved populations such as this, we sought to err on the side of inclusion rather than exclusion of participants.
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/ccp.
Contributor Information
Kathleen M. Carroll, Yale University School of Medicine
Steve Martino, Yale University School of Medicine.
Samuel A. Ball, Yale University School of Medicine
Charla Nich, Yale University School of Medicine.
Tami Frankforter, Yale University School of Medicine.
Luis M Anez, Yale University School of Medicine.
Manuel Paris, Yale University School of Medicine.
Lourdes Suarez-Morales, University of Miami.
José Szapocznik, University of Miami.
William R. Miller, University of New Mexico
Carmen Rosa, National Institute on Drug Abuse.
Julie Matthews, Advanced Behavioral Health.
Chris Farentinos, Changepoint, Inc..
References
- Amaro H, Arevalo S, Gonzalez G, Szapocznik J, Iguchi MY. Needs and scientific opportunities for research on substance abuse treatments among Hispanic adults. Drug and Alcohol Dependence. 2006;84S:S64–S75. doi: 10.1016/j.drugalcdep.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Ball SA, Martino S, Nich C, Frankforter TL, Van Horn D, Crits-Christoph P, et al. Site matters: Multisite randomized trial of motivational enhancement therapy in community drug abuse clinics. Journal of Consulting & Clinical Psychology. 2007;75(4):556–567. doi: 10.1037/0022-006X.75.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal G, Sharron del Rio MR. Are empirically supported treatment valid for ethnic minorities? Toward an alternative approach for treatment research. Cultural Diversity & Ethnic Minority Psychology. 2001;7:328–342. doi: 10.1037/1099-9809.7.4.328. [DOI] [PubMed] [Google Scholar]
- Burge SK, Amodei N, Elkin B, Catala S, Andrew SR, Lane PA, et al. An evaluation of two primary care interventions for alcohol abuse among Mexican-American patients. Addiction. 1992;12:1705–1716. [PubMed] [Google Scholar]
- Caetano R. Alcohol-related health disparities and treatment related epidemiological findings among Whites, Blacks, and Hispanics in the United States. Alcoholism: Clinical & Experimental Research. 2003;27:1337–1339. doi: 10.1097/01.ALC.0000080342.05229.86. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rosa C, Brown LS, Jr, Daw R, Magruder KM, Beatty L. Addressing ethnic disparities in drug abuse treatment in the Clinical Trials Network. Drug and Alcohol Dependence. 2007;90:101–106. [Google Scholar]
- Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: The intention to treat principle and excluding patients from analysis. British Medical Journal. 2002;325:652–654. doi: 10.1136/bmj.325.7365.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GCN. Psychotherapy research with ethnic minorities: Empirical, ethical, and conceptual issues. Journal of Consulting & Clinical Psychology. 2001;69:502–510. doi: 10.1037//0022-006x.69.3.502. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Lau AS. Making the case for selective and directed cultural adaptations of evidence-based treatments: Examples from parent training. Clinical Psychology: Science and Practice. 2006;13:295–310. [Google Scholar]
- Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. Community program therapist adherence and competence in motivational enhancement therapy. Drug and Alcohol Dependence. 2008;97:37–48. doi: 10.1016/j.drugalcdep.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Yahne CE, Tonigan JS. Motivational interviewing in drug abuse services: A randomized trial. Journal of Consulting and Clinical Psychology. 2003;71(4):754–763. doi: 10.1037/0022-006x.71.4.754. [DOI] [PubMed] [Google Scholar]
- Miranda J, Bernal G, Lau A, Kohn L, Hwang WC, LaFramboise T. State of the science on psychosocial interventions for ethnic minorities. Annual Review of Clinical Psychology. 2005;1:113–142. doi: 10.1146/annurev.clinpsy.1.102803.143822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles RR, Reyes JC, Colon HM, Sahai H, Marrero CA, Matos TD, et al. Effects of combined counseling and case management to reduce HIV risk behaviors among Hispanic drug injectors in Puerto Rico: A randomized controlled study. Journal of Substance Abuse Treatment. 2004;27:145–152. doi: 10.1016/j.jsat.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Rogler LH, Malgady RG, Constantino G, Blumenthal R. What do culturally sensitive mental health services mean? The case of Hispanics. American Psychologist. 1987;42:565–570. doi: 10.1037//0003-066x.42.6.565. [DOI] [PubMed] [Google Scholar]
- Suarez-Morales L, Matthews J, Martino S, Ball SA, Rosa C, Farentinos C, et al. Issues in designing and implementing a Spanish-language multi-site clinical trial. The American Journal on the Addictions. 2007;16(3):206–215. doi: 10.1080/10550490701375707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells K, Klap R, Koike A, Sherbourne C. Ethnic disparities in unmet need for alcoholism, drug abuse, and mental health care. American Journal of Psychiatry. 2001;158:2027–2032. doi: 10.1176/appi.ajp.158.12.2027. [DOI] [PubMed] [Google Scholar]