Abstract
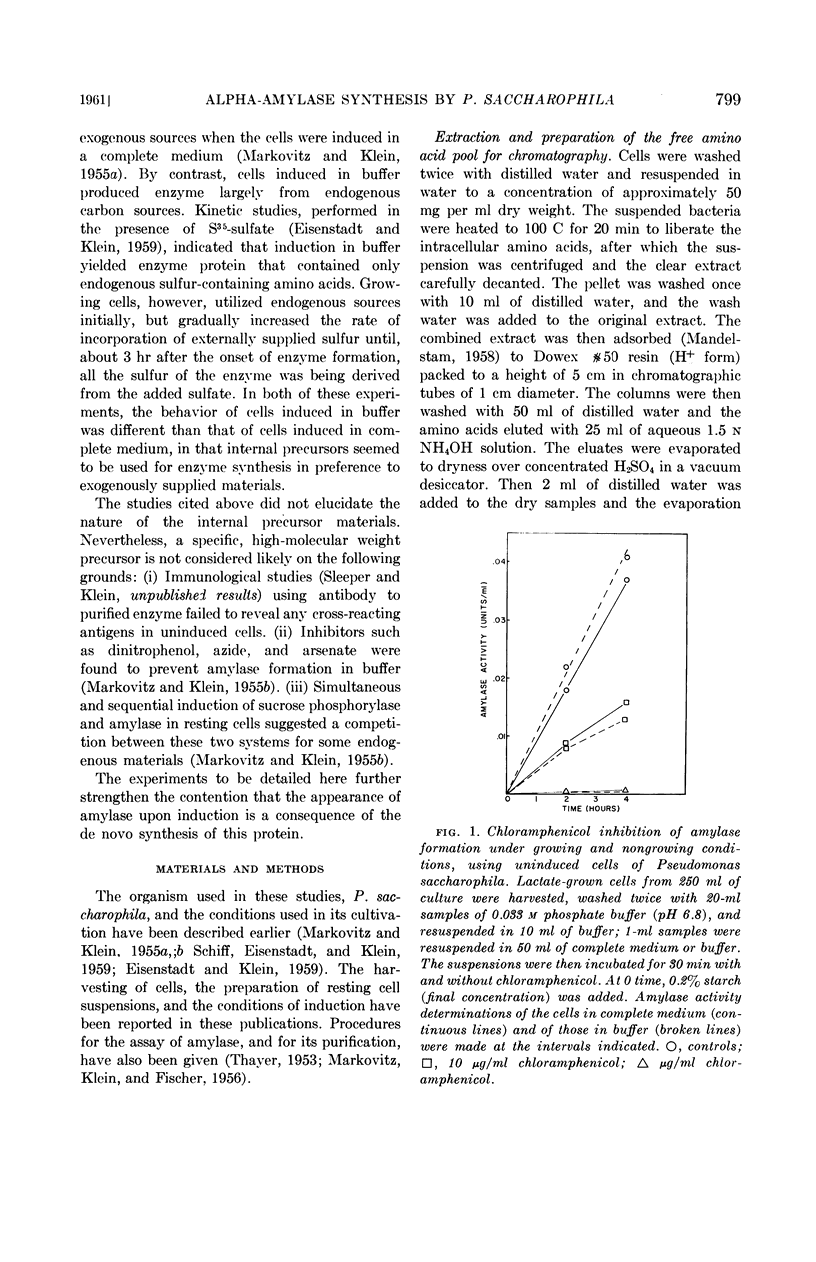
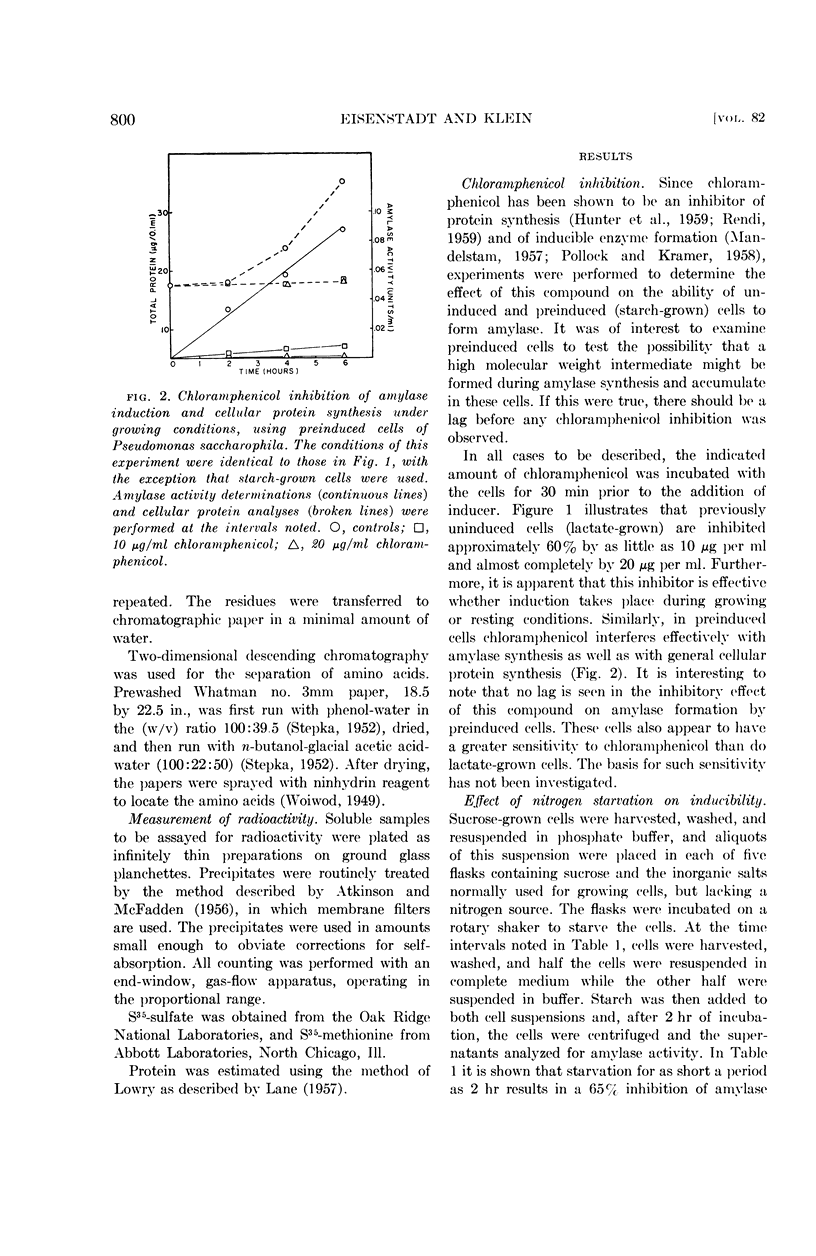
Eisenstadt, Jerome M. (Brandeis University, Waltham, Mass.) and Harold P. Klein. Evidence for the de novo synthesis of the alpha-amylase of Pseudomonas saccharophila. J. Bacteriol. 82:798–807. 1961.—Chloramphenicol at a concentration of 20 μg per ml inhibited the appearance of the inducible α-amylase of Pseudomonas saccharophila. This inhibition was observed when induction was attempted in buffer or in a complete medium. Preinduced cells were also prevented from forming this enzyme under similar conditions. Under all the conditions tested, there was no lag in chloramphenicol inhibition, thus suggesting an absence of any protein precursor in amylase formation.
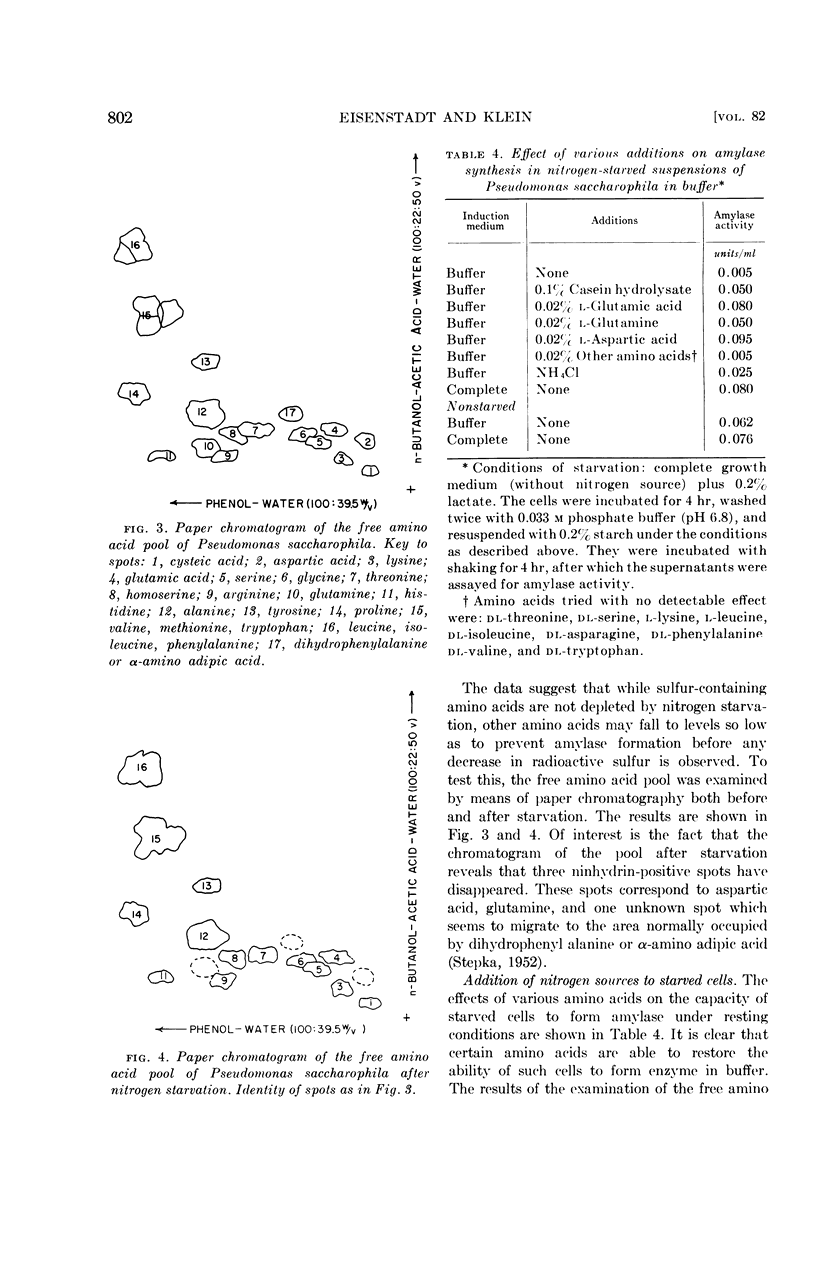
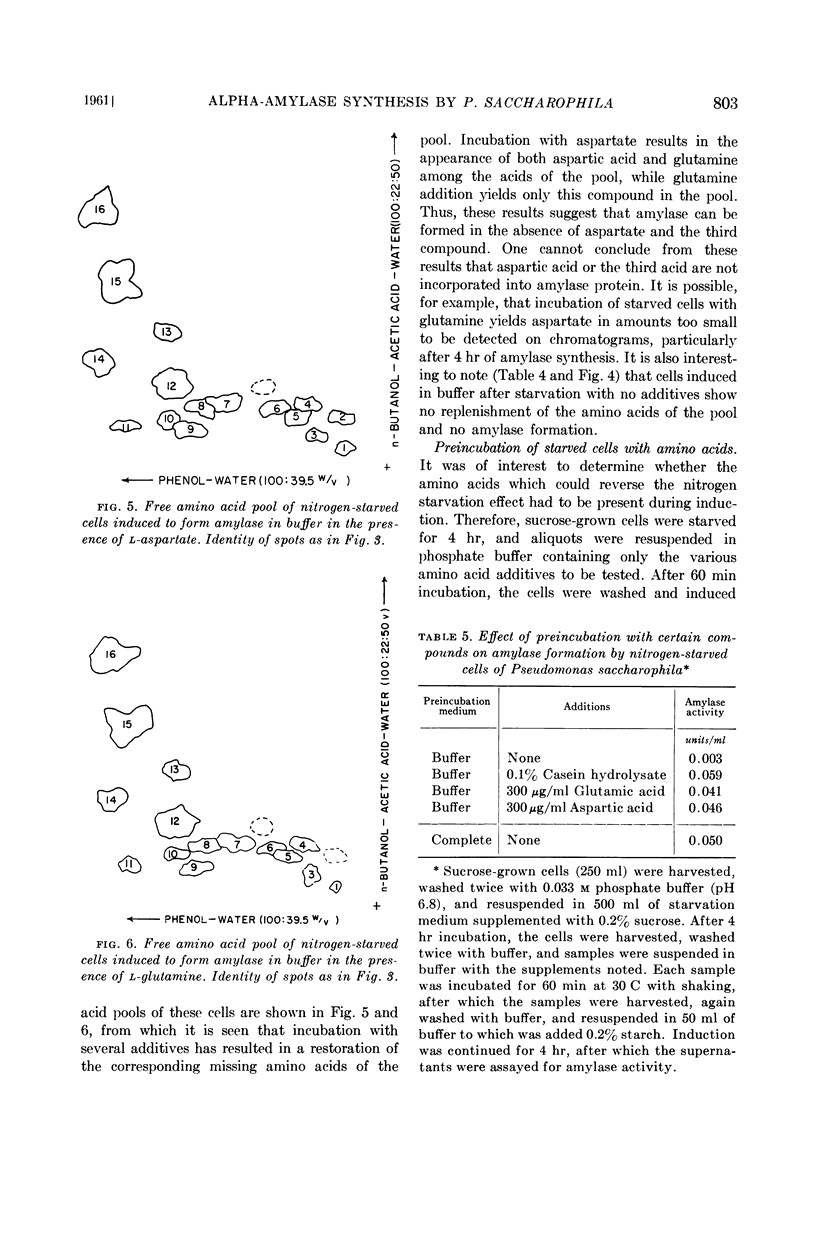
Cells suspended in a complete medium without a nitrogen source lost their capacity to form this enzyme when subsequently induced in buffer. When cells were grown in the presence of radioactive sulfate and then subjected to starvation, the radioactivity of the amino acid pool diminished only slightly. However, examination of the free amino acid pool by paper chromatography showed that the loss of enzyme inducibility was accompanied by the disappearance of glutamine, aspartic acid, and a third, unidentified, compound. Enzyme-forming ability was restored by the addition, to starved cells of casein hydrolysate, glutamate, glutamine, or aspartate. Other amino acids tested were ineffective in this regard.
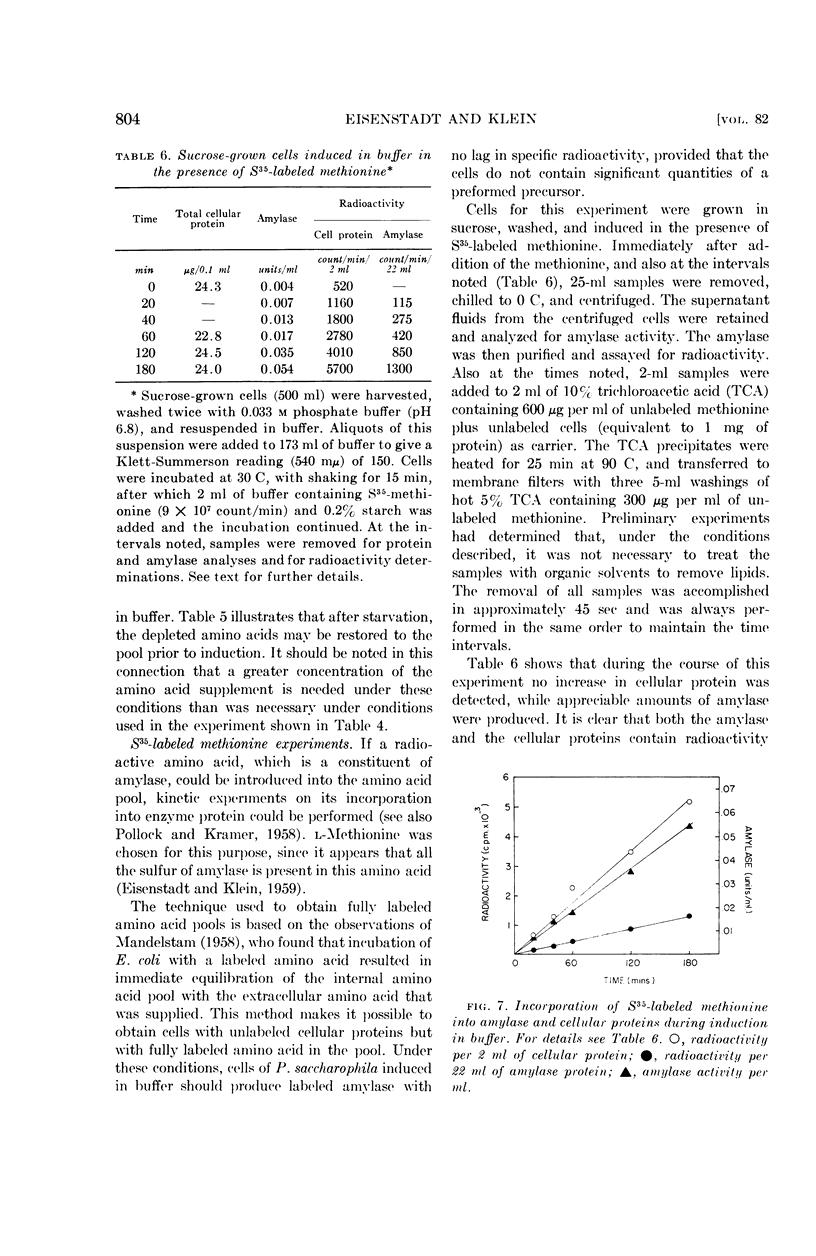
When cells were induced in buffer in the presence of labeled methionine, amylase was formed at a linear rate over a 3-hr period. Furthermore, both the cellular proteins and the extracellular amylase became labeled at a linear rate.
These observations are discussed in relation to the problem of protein turnover, and are interpreted as evidence for the de novo synthesis of α-amylase in this organism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATKINSON D. E., MCFADDEN B. A. Use of membrane filters in the measurement of biological incorporation of radioactive isotopes. J Bacteriol. 1956 Jan;71(1):123–124. doi: 10.1128/jb.71.1.123-124.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENSTADT J. M., KLEIN H. P. Selective formation of alpha-amylase by non-growing cells of Pseudomonas saccharophila. Biochim Biophys Acta. 1960 Oct 21;44:206–208. doi: 10.1016/0006-3002(60)91554-7. [DOI] [PubMed] [Google Scholar]
- EISENSTADT J. M., KLEIN H. P. Sulfur incorporation into the alpha-amylase of Pseudomonas saccharophila. J Bacteriol. 1959 May;77(5):661–666. doi: 10.1128/jb.77.5.661-666.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGNESS D. S., COHN M., MONOD J. Studies on the induced synthesis of beta-galactosidase in Escherichia coli: the kinetics and mechanism of sulfur incorporation. Biochim Biophys Acta. 1955 Jan;16(1):99–116. doi: 10.1016/0006-3002(55)90188-8. [DOI] [PubMed] [Google Scholar]
- HUNTER G. D., BROOKES P., CRATHORN A. R., BUTLER J. A. Intermediate reactions in protein synthesis by the isolated cytoplasmic-membrane fraction of Bacillus megaterium. Biochem J. 1959 Oct;73:369–376. doi: 10.1042/bj0730369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The free amino acids in growing and non-growing populations of Escherichia coli. Biochem J. 1958 May;69(1):103–110. doi: 10.1042/bj0690103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. Turnover of protein in starved bacteria and its relationship to the induced synthesis of enzyme. Nature. 1957 Jun 8;179(4571):1179–1181. doi: 10.1038/1791179a0. [DOI] [PubMed] [Google Scholar]
- MARKOVITZ A., KLEIN H. P., FISCHER E. H. Purification, crystallization, and properties of the alpha-amylase of Pseudomonas saccharophila. Biochim Biophys Acta. 1956 Feb;19(2):267–273. doi: 10.1016/0006-3002(56)90427-9. [DOI] [PubMed] [Google Scholar]
- MARKOVITZ A., KLEIN H. P. On the sources of carbon for the induced biosynthesis of alpha-amylase in Pseudomonas saccharophila. J Bacteriol. 1955 Dec;70(6):649–655. doi: 10.1128/jb.70.6.649-655.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKOVITZ A., KLEIN R. P. Some aspects of the induced biosynthesis of alpha-amylase of Pseudomonas saccharophila. J Bacteriol. 1955 Dec;70(6):641–648. doi: 10.1128/jb.70.6.641-648.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS T., Jr A serum albumin precursor in cytoplasmic particles. J Biol Chem. 1957 Dec;229(2):659–677. [PubMed] [Google Scholar]
- POLLOCK M. R., KRAMER M. Intermediates in the biosynthesis of bacterial penicillinase. Biochem J. 1958 Dec;70(4):665–681. doi: 10.1042/bj0700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENDI R. The effect of chloramphenicol on the incorporation of labeled amino acids into proteins by isolated subcellular fractions from rat liver. Exp Cell Res. 1959 Aug;18:187–189. doi: 10.1016/0014-4827(59)90307-6. [DOI] [PubMed] [Google Scholar]
- ROTMAN B., SPIEGELMAN S. On the origin of the carbon in the induced synthesis beta-galactosidase in Escherichia coli. J Bacteriol. 1954 Oct;68(4):419–429. doi: 10.1128/jb.68.4.419-429.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THAYER P. S. The amylases of pseudomonas saccharophila. J Bacteriol. 1953 Dec;66(6):656–663. doi: 10.1128/jb.66.6.656-663.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]


