Abstract
Objectives:
The present study was undertaken to assess the antibiotic susceptibility patterns of Pseudomonas aeruginosa at a tertiary care hospital in Gujarat, India. Due to significant changes in microbial genetic ecology, as a result of indiscriminate use of anti-microbials, the spread of anti-microbial resistance is now a global problem.
Materials and Methods:
Out of 276 culture positive samples, 56 samples of Pseudomonas aeruginosa were examined and 10 different types of specimen were collected. Microbial sensitivity testing was done using disk diffusion test with Pseudomonas species NCTC 10662, as per CLSI guidelines.
Results:
The highest number of Pseudomonas infections was found in urine, followed by pus and sputum. Pseudomonas species demonstrated marked resistance against monotherapy of penicillins, cephalosporins, fluoroquinolones, tetracyclines and macrolides. Only combination drugs like Ticarcillin + Clavulanic acid, Piperacillin + Tazobactum, Cefoperazone + Sulbactum, Cefotaxime + Sulbactum, Ceftriaxome + Sulbactum and monotherapy of amikacin showed higher sensitivity to Pseudomonas infections; however, the maximum sensitivity was shown by the Carbapenems.
Conclusion:
From the present study, we conclude that urinary tract infection was the most common hospital acquired infection. Also, co-administration of β -lactamase inhibitors markedly expanded the anti-microbial sensitivity of semi-synthetic penicillins and cephalosporins. The aminoglycoside group of antibiotics - amikacin - demonstrated maximum sensitivity against pseudomonas species. Therefore, use of amikacin should be restricted to severe nosocomial infections, in order to avoid rapid emergence of resistant strains. Periodic susceptibility testing should be carried out over a period of two to three years, to detect the resistance trends. Also, a rational strategy on the limited and prudent use of anti-Pseudomonal agents is urgently required.
Keywords: Antimicrobial susceptibility, carbapenem sensitivity, combination antibiotics, disk diffusion technique, Pseudomonas aeruginosa
Introduction
Multiple antibiotic resistance in bacterial populations is a pervasive and growing clinical problem, which is recognized as a threat to public health. Hence, there is a need to conduct area-specific monitoring studies to profile different pathogens responsible for specific infections and their resistance patterns, so as to generate data that would help clinicians to choose the correct empirical treatment.
Pseudomonas aeruginosa (P. aeruginosa) is an epitome of opportunistic nosocomial pathogen, which causes a wide spectrum of infections and leads to substantial morbidity in immuno-compromised patients. Despite therapy, the mortality due to nosocomial pseudomonal pneumonia is approximately 70%.[1] Unfortunately, P. aeruginosa demonstrates resistance to multiple antibiotics, thereby jeopardizing the selection of appropriate treatment.[2] Therefore, the present study was undertaken to find out the antibiotic susceptibility patterns of pathogenic isolates of Pseudomonas aeruginosa from various specimens of hospital acquired infections (HAI).
Materials and Methods
Our study group comprised of samples, which were clinically suspected cases of bacterial infections. The project was undertaken at Rajasthan Hospital, Ahmedabad, India, between January and April 2006.
Five hundred and seventy two non-duplicate isolates were taken (i.e. multiple isolates of the same species from the same patient were excluded). Two hundred and seventy six samples obtained from sputum, endo-tracheal Tract secretion, broncho-alveolar lavage, blood, urine, body tissues, pus, semen, cerebro-spinal fluid (CSF), and body fluids (peritoneal fluid) and costal bronchial Secretions (CBS) reported the presence of bacterial infection.
Identification of all isolates was carried out by a positive reaction to oxidase and production of pyocyanin.[3] Culture examination was carried out using Nutrient agar and MacConkey's medium, followed by inoculation by four flame streak method.
Antibiotic susceptibility was confirmed by disk diffusion technique on Muller-Hinton medium (Becton Dickinson Microbiological Systems, Cockysville, MD), performed according to the Clinical Laboratory Standard Institute (CLSI) guidelines.[3] Paper disks (Hi-media, Mumbai) were impregnated with antibiotics (Sigma Chemical Co., St. Louis, Mo.): Penicillins: ampicillin (10mcg), amoxycillin (20mcg), ticarcillin (75mcg), piperacillin (100mcg); cephalosporins: cephalexin (30 mcg), cefuroxime (30mcg), cefazolin (30mcg), cefotaxime (30mcg), ceftriaxone (30mcg), ceftazidime (30mcg), ceftizoxime (30mcg), cefoperazone (75 mcg), cefpodoxime 10 mcg), cefdinir (5 mcg), cefepime (30 mcg); carbepenems: imipenem (10mcg), meropenem (10 mcg); monobactums: aztreonem (30 mcg); combinations: ampicillin + sulbactum (10/10 mcg), amoxycillin + clavulinic acid (20/10 mcg), piperacillin + tazobactum (100/10 mcg), ticarcillin + clavulinic acid (75/10 mcg), cefoperazone + sulbactum (75/10 mcg), cefotaxime + sulbactum (30/10 mcg), ceftriaxone + sulbactum (30/10 mcg); Aminoglycosides: gentamicin (10 mcg), tobramycin (10 mcg), amikacin (30 mcg), netilmicin (30 mcg); quinolones: ciprofloxacin (5mcg), ofloxacin (5mcg), levofloxacin (5mcg), gatifloxacin (5mcg); tetracyclines: doxycycline (30mcg), minocycline (30mcg); macrolides: azithromycin (15mcg) and miscellaneous: chloramphenicol (30 mcg) respectively. They were incubated overnight at 37°C in 5-10% CO2 enriched environment (candle jar). The diameter of the zone of inhibition was measured and compared to that of standard strain and the results were interpreted as sensitive, intermediate resistant or resistant, based on CLSI guidelines.[4] The category “susceptible” was defined as identification of a strain as susceptible by the disk diffusion method. Quality control strains of Pseudomonas species NCTC-10662 was used to validate the results of the antimicrobial discs.
Susceptibility data were compared by using a Chi-square test with statistical package for the social sciences (SPSS) software for Windows, version 12. Both susceptibility and resistance were calculated as percentages with 95% confidence intervals. The analysis was performed on the cross-tabulated values of the presence of the resistant/intermediate/susceptible isolates, according to the categories of the selected variable. A P value of < 0.05 was considered to be statistically significant.
Results
Of the 572 samples subjected to culture sensitivity, 276 reported presence of bacterial infection, thereby suggesting 48.25% as the occurrence level. The percentage occurrence of Pseudomonas in these 276 samples was only 20.28% (56 samples), of which 62.5% (i.e. 35 samples) and 37.5% (i.e. 21 samples) were reported from males and females respectively. Various specimens studied under the present investigation included urine, pus, sputum, blood, endotracheal secretions (ET), semen, catheter tip (CT), stool, body fluids and body tissues. The age- and gender-wise percentage and frequency of the pathogenic organism (P. aeruginosa) are mentioned in [Table 1].
Table 1.
Age and gender wise percentage and frequency distribution of P. aeruginosa from specipic sites
| Age group (Years) | Total number of cases | Percentage of total case | Specimen site | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine | Pus | Sputum | ET | Semen | CT | Tissue | ||||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | |||
| 0-9 | 1 | 1.78 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10-19 | 0 | 0.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20-29 | 5 | 8.93 | 0 | 1 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30-39 | 8 | 14.28 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 40-49 | 12 | 21.43 | 1 | 0 | 6 | 0 | 0 | 2 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 50-59 | 8 | 14.28 | 1 | 2 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 60-69 | 14 | 25.00 | 4 | 2 | 0 | 1 | 0 | 1 | 4 | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| 70-79 | 4 | 7.14 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 80-more | 4 | 7.14 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 56 | 100 | 6 | 9 | 9 | 6 | 9 | 6 | 7 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
ET = Endo-tracheal tract secretions, CT = Catheter tip, M = Male, F = Female
The majority of specimens from which P. aeruginosa was isolated consisted of urine, pus and sputum [Table 2]. The acid resistant penicillins such as ticarcillin and piperacillin combinations (R=23.21% and 30.36% respectively) (P< 0.001) had significantly greater antibacterial activity against P. aeruginosa, when compared to their respective monotherapies (R=67.86% and 73.21% respectively) [Fig. 1A].
Table 2.
Frequency of specific sites from which P. aeruginosa was isolated
| Specimen | Number of Specimens | % of Total |
|---|---|---|
| Urine | 15 | 26.79 |
| Pus | 15 | 26.79 |
| Sputum | 15 | 26.79 |
| ET | 8 | 14.29 |
| Semen | 1 | 1.78 |
| CT | 1 | 1.78 |
| Tissue | 1 | 1.78 |
| Total | 56 | 100 |
ET: Endo-tracheal tract secretions, CT: Catheter tip
Figure 1a.
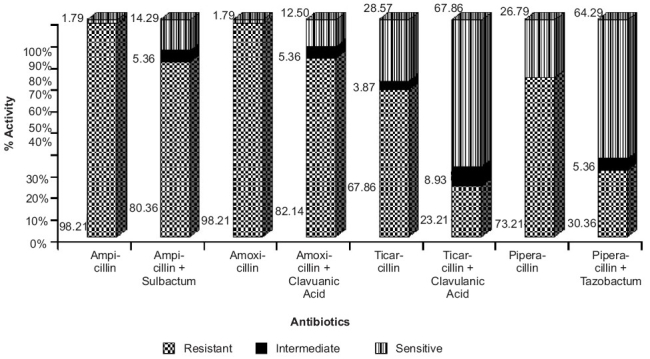
Antibiotic resistance patterns of P. aeruginosa against penicillin group of antibiotics. Ampicillin, amoxicillin, ticarcillin and piperacillin in combination with sulbactum, clavulanic acid and tazobactum demonstrated significantly higher antibacterial activity (P≤0.001, Chi-square test) against P. aeruginosa when compared to the monotherapy of respective antibiotics
A similar scenario was observed for ampicillin and amoxicillin, where the combination therapy with sulbactum and clavulanic acid (P< 0.001) demonstrated significantly higher antibacterial activity against P. aeruginosa, when compared to their respective monotherapies (R=98.21% in both the cases) [Figure 1B]. The organism showed remarkable resistance against cephalosporin group of antibiotics, ranging from 67.86% for ceftazidime to 94.64% for cephalexin [Table 3]. But the extended-spectrum penicillins and the third generation cephalosporins, in combination with sulbactum, tazobactum and clavulanic acid showed a significant decrease in resistance to P. aeruginosa.
Figure 1b.
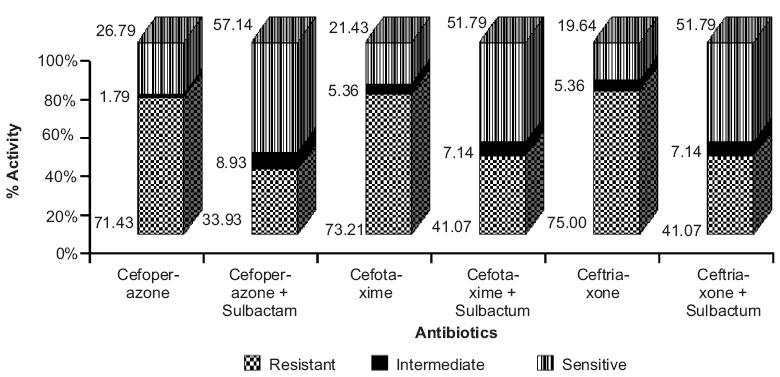
Antibiotic resistance patterns of P. aeruginosa against cephalosporin group of antibiotics. Cefoperazone, cefotaxime and ceftriaxone in combination with sulbactum demonstrated significantly higher antibacterial activity (P≤0.001, Chi-square test) when compared to monotherapy of respective antibiotics
Table 3.
Antibiotic resistance pattern of P. aeruginosa against cephalosporin group of antibiotics
| Cephalosporins | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Cephalexin | 5.36 | 0 | 94.64 |
| Cefazolin | 12.5 | 3.57 | 83.93 |
| Cefuroxime | 7.14 | 0 | 92.86 |
| Cefoperazone | 26.79 | 1.79 | 71.43 |
| Cefpodoxime | 23.21 | 0 | 76.79 |
| Cefdinir | 23.21 | 0 | 76.79 |
| Ceftazidime | 32.14 | 0 | 67.86 |
| Ceftriaxone | 19.64 | 5.36 | 75 |
| Ceftizoxime | 32.21 | 0 | 76.79 |
| Cefotaxime | 21.43 | 5.36 | 73.21 |
| Cefepime | 30.36 | 0 | 69.64 |
Values shown are percentage of total
A notable observation was that piperacillin and ticarcillin combinations demonstrated better antibacterial activity, as compared to cephalosporin combinations (P< 0.001) [Figure 1A and B]. We observed that P. aeruginosa was highly sensitive to the carbapenem group of antibiotics like imipenem (78.57%) and meropenem (69.64%), while aztreonem showed 71.43% resistance (P< 0.001) [Figure 1C]. On the other hand, aminoglycosides, fluoroquinolones, tetracyclines, macrolides and chloramphenicol did not demonstrate statistically significant susceptibility patterns (P>0.05).
Figure 1c.
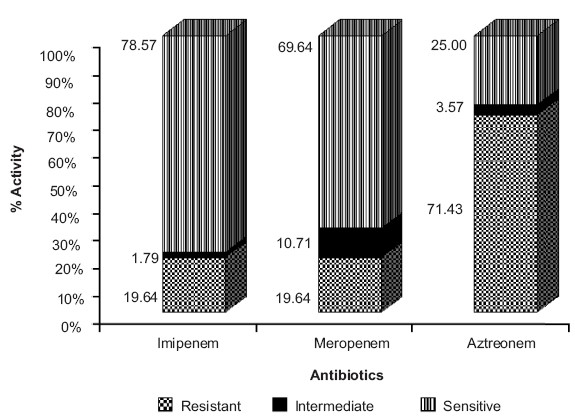
Antibiotic resistance patterns of P. aeruginosa against carbapenem group of antibiotics. Imipenem and meropenem demonstrated significantly higher antibacterial activity (P≤0.001, Chi-square test) when compared to aztreonem
In the present study, sensitivity of P. aeruginosa was in the 32-48% range for aminoglycosides, while for fluoroquinolones, susceptibility was found to be in the 26-37% range [Table 4]. For tetracyclines, macrolides and chloramphenicol, resistance was found to range between 75 and 91% [Table 4]. All antibiotics used as monotherapy in the present study did not demonstrate statistically significant susceptibility patterns (P>0.05), with the exception of imipenem and meropenem.
Table 4.
Antibiotic resistance patterns of P. aeruginosa against different group of antibiotics
| Class of drugs | Sensitive | Intermediate | Resistant |
|---|---|---|---|
| Aminoglycosides | |||
| Gentamicin | 32.14 | 0 | 67.86 |
| Tobramycin | 32.14 | 1.79 | 66.07 |
| Netilmycin | 33.93 | 5.36 | 60.71 |
| Amikacin | 48.21 | 1.79 | 50 |
| Fluoroquinolones | |||
| Ciprofloxacin | 26.79 | 3.57 | 69.64 |
| Ofloxacin | 26.79 | 3.57 | 69.64 |
| Levofloxacin | 35.71 | 1.79 | 62.5 |
| Gatifloxacin | 37.5 | 0 | 62.5 |
| Tetracyclines | |||
| Doxycycline | 8.93 | 0 | 91.07 |
| Minocycline | 10.71 | 1.79 | 87.5 |
| Macrolides | |||
| Azithromycin | 8.93 | 5.36 | 85.71 |
| Miscellaneous | |||
| Chloramphenicol | 22 | 3 | 75 |
Values shown are percentage of total; (n = 56 cases)
Discussion
P. aeruginosa is inherently resistant to many antimicrobial agents, mainly due to the synergy between multi-drug efflux system or a type1 AmpC β-lactamase and low outer membrane permeability.[5–7] The age- and sex-wise distribution of patients diagnosed with infection followed the natural epidemiological pattern.[8] Out of 56 culture positive specimens isolated, 26.78% were urine samples, indicating that urinary tract infection (UTI) is the most common HAI[9] [Table 2]. It is one of the most important causes of morbidity in the general population and is the second most common cause of hospital visits.[10,11] The incidence of UTI is greater in women than men, which may be either due to anatomical predisposition or urolithial mucosal adherence to mucopolysaccharide lining or other host factors.[9,12] Concurrent administration of a β-lactamase inhibitor such as clavulanate or sulbactum markedly expands the spectrum of activity of acid resistant penicillins like ticarcillin and piperacillin. The dose as well as the incidence of toxicity were subsequently reduced with semi-synthetic penicillins like ticarcillin, which makes it the preferred ureidopenicillin against P. aeruginosa infections. Our results are in corroboration with the one reported by other workers,[11] so much so that the overall resistance to various generations of cephalosporins was high on account of the production of extended spectrum β-lactamses (ESBLs) by the bacteria involved.[13]
In our study, notable resistance (19.64%) to P. aeruginosa was observed against carbapenems. The resistance to carbapenems, especially in P. aeruginosa, results from reduced levels of drug accumulation or increased expression of pump efflux.[14,15] The resistance may also be due to the production of metallo-β-lactamases (MBL), which can be chromosomally encoded or plasmid mediated.[16] The carbapenem hydrolyzing enzyme carbapenamase may be class B-metallo β-lactamases or class D-oxacillanases or class A-clavulanic acid inhibitory enzymes.[17]
Among the aminoglycosides, amikacin has the highest sensitivity against P. aeruginosa [Table 4], which is in corroboration with an earlier report published from India.[18] Amikacin was designed as a poor substrate for the enzymes that bring about inactivation by phosphorylation, adenylation or acetylation, but some organisms have developed enzymes that inactivate this agent as well. Amikacin seems to be a promising therapy for Pseudomonal infection. Hence, its use should be restricted to severe nosocomial infections, in order to avoid rapid emergence of resistant strains.[19] The problem of increasing resistance to P. aeruginosa has limited the use of other classes of antibiotics like the fluoroquinolones, tetracyclines, macrolides and chloramphenicol.[20]
In fact, the irrational and inappropriate use of antibiotics is responsible for the development of resistance of Pseudomonas species to antibiotic monotherapy. Hence, there is a need to emphasize the rational use of antimicrobials and strictly adhere to the concept of “reserve drugs” to minimize the misuse of available antimicrobials. In addition, regular antimicrobial susceptibility surveillance is essential for area-wise monitoring of the resistance patterns. An effective national and state level antibiotic policy and draft guidelines should be introduced to preserve the effectiveness of antibiotics and for better patient management.
Hence, based on the observations of present study, we recommend use of either semi-synthetic penicillins like ticarcillin, piperacillin or third generation cephalosporins like cefoperazone, cefotaxime and ceftriaxone along with β-lactamase inhibitors (clavulanate or sulbactum) against Pseudomonas species infections, in similar hospital settings. Further, amikacin should be considered as a reserved drug for the treatment of severe nosocomial infections.
Acknowledgments
The authors wish to thank Dr. Mitesh Patel, M.D. (Microbiology), Assistant Professor, B.J. Medical College and Civil Hospital, Ahmedabad and Dr. Atit Shah, M.D. (Microbiology), Assistant Professor, Smt. NHL Municipal Medical College and V. S. Hospital, Ahmedabad for extending their help and guidance in conducting the microbiological analysis for the present study.
References
- 1.Chastre J, Trouillet JL. Problem pathogens (Pseudomonas aeruginosa and Acinetobacter) Semin Respir Infect. 2000;15:287–98. doi: 10.1053/srin.2000.20944. [DOI] [PubMed] [Google Scholar]
- 2.Obritsch MD, Fish DN, McLaren R, Jung R. National Surveillance of Antimicrobial Resistance in Pseudomonas aeruginosa isolates obtained from Intensive Care Unit Patients from 1993 to 2002. Antimicrob Agents Chem. 2004;48:4606–10. doi: 10.1128/AAC.48.12.4606-4610.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gençer S, Ak O, Benzonana N, Batirel A, Ozer S. Susceptibility pattern and cross resistances of antibiotics against Pseudomonas aeruginosa in a teaching hospital of Turkey. Ann Clin Microbiol Antimicrob. 2002;1:2. doi: 10.1186/1476-0711-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. Approved standard M2 A7 NCCLS. Villanova, PA: 1995. Performance standards for antimicrobial disk susceptibility tests; p. 15. [Google Scholar]
- 5.Das RN, Chandrasekhar TS, Joshi HS, Gurung M, Shrestha N, Shivananda PG. Frequency and susceptibility profile of pathogens causing urinary tract infections at a tertiary care hospital in western Nepal. Singapore Med J. 2006;47:281–5. [PubMed] [Google Scholar]
- 6.Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other non-fermentive gram negative bacteria. Clin Infect Dis. 1998;27:S93–9. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 7.Livermore DM. Of Pseudomonas aeruginosa, porins and Carbapenems. J Antimicrob Chemother. 2001;47:247–50. doi: 10.1093/jac/47.3.247. [DOI] [PubMed] [Google Scholar]
- 8.Livermore DM. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin Infect Dis. 2002;34:634–40. doi: 10.1086/338782. [DOI] [PubMed] [Google Scholar]
- 9.Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotech. 2006;5:1562–5. [Google Scholar]
- 10.Navaneeth BV, Belwadi S, Suganthi N. Urinary pathogens resistant to common antibiotics: A retrospective analysis. Trop Doct. 2002;32:20–2. doi: 10.1177/004947550203200110. [DOI] [PubMed] [Google Scholar]
- 11.Chitnis SV, Chitnis V, Sharma N, Chitnis DS. Current status of drug resistance among gram negative bacilli isolated from admitted cases in a tertiary care centre. J Assoc Physicians India. 2003;51:28–32. [PubMed] [Google Scholar]
- 12.Ronald AR, Pattulo MS. The natural history of urinary infection in adults. Med Clin North Am. 1991;75:299–312. doi: 10.1016/s0025-7125(16)30455-2. [DOI] [PubMed] [Google Scholar]
- 13.Mathur P, Kapil A, Das B, Dhawan B. Prevalence of extended spectrum β-lactamase producing gram negative bacteria in a tertiary care hospital. Indian J Med Res. 2002;115:153–7. [PubMed] [Google Scholar]
- 14.Gupta E, Mohanty S, Sood S, Dhawan B, Das BK, Kapil A. Emerging resistance to Carbapenems in a tertiary care hospital in North India. Indian J Med Res. 2006;124:95–8. [PubMed] [Google Scholar]
- 15.Kurokawa H, Yagi T, Shibata N, Shibayama K, Arakawa Y. Worldwide proliferation of Carbapenem resistant gram negative bacteria. Lancet. 1999;354:955. doi: 10.1016/S0140-6736(05)75707-X. [DOI] [PubMed] [Google Scholar]
- 16.Navneeth BV, Sridaran D, Sahay D, Belwadi MR. A preliminary study on metallo β- lactamase producing Pseudomonas aeruginosa in hospitalized patients. Indian J Med Res. 2002;116:264–7. [PubMed] [Google Scholar]
- 17.Yu YS, Yang Q, Xu XW, Kong HS, Xu GY, Zhong BY. Typing and characterization of Carbapenem resistant Acinobacter calcoaceticus-baumannii complex in a Chinese hospital. J Med Microbiol. 2004;53:653–6. doi: 10.1099/jmm.0.05513-0. [DOI] [PubMed] [Google Scholar]
- 18.Smitha S, Lalitha P, Prajna VN, Srinivasan M. Susceptibility trends of Pseudomonas species from corneal ulcers. Indian J Med Microbiol. 2005;23:168–71. doi: 10.4103/0255-0857.16588. [DOI] [PubMed] [Google Scholar]
- 19.Poole K. Aminoglycosides resistance in Pseudomonas aeruginosa. Antimicrob Agents Chem. 2005;49:479–87. doi: 10.1128/AAC.49.2.479-487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chambers HF. General Principles of antimicrobial therapy. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 11th ed. Mc-Graw Hill: Medical Publishing Division; 2006. pp. 1095–110. [Google Scholar]


