Abstract
Gerber, Dolores F. (University of California, Berkeley) and H. M. S. Watkins. Growth of shigellae in monolayer tissue cultures. J. Bacteriol. 82:815–822. 1961.—The influence of environment on virulence, antigenicity, and antibiotic susceptibility of infectious agents is well recognized. The development of tissue culture monolayer techniques has stimulated new interest in manifestations of these attributes at the cellular level.
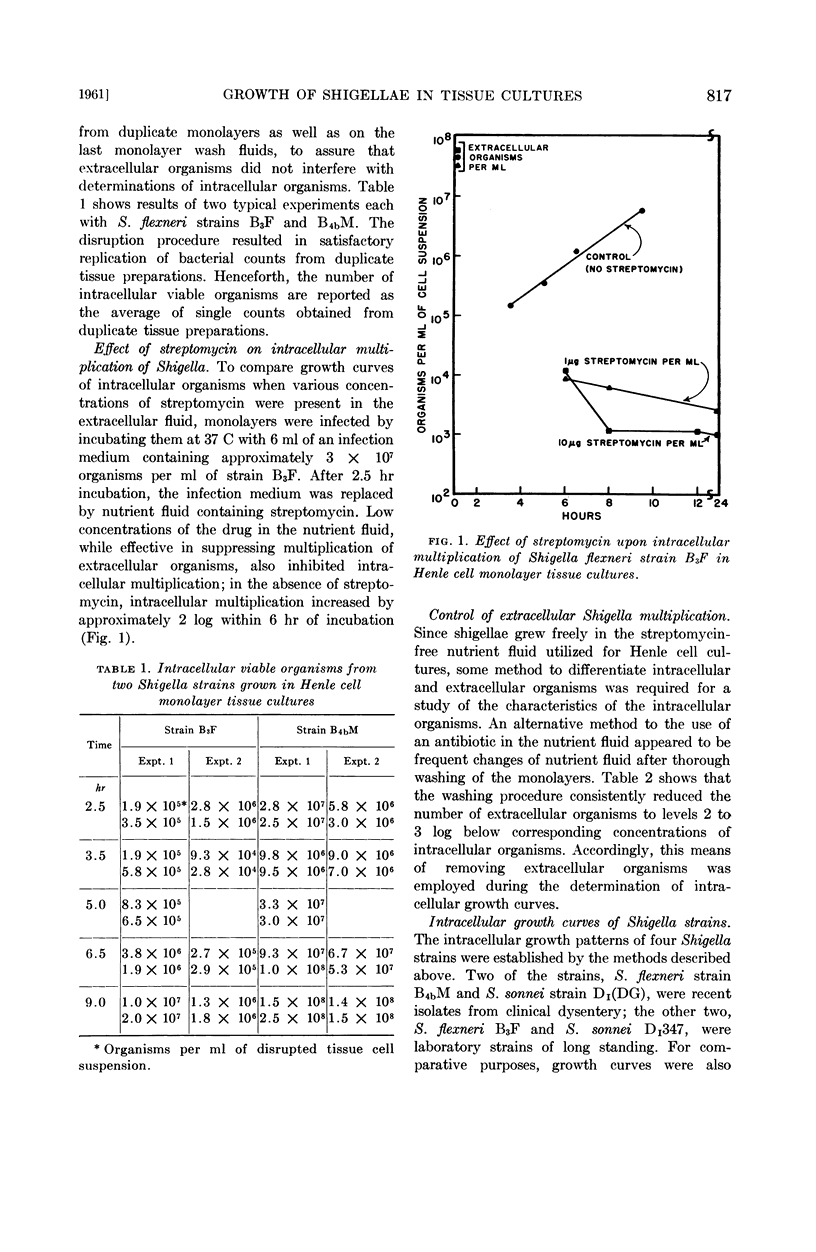
Shigella flexneri and S. sonnei were grown intracellularly in Henle epithelial cell monolayer tissue cultures; uptake of the bacteria was induced by 50% normal horse serum in the infection medium. Since preliminary experiments indicated that streptomycin in the extracellular fluid depressed intracellular multiplication of these organisms, the antibiotic was later omitted from the tissue culture growth fluid. Extracellular multiplication of shigellae was controlled by thorough washing of the infected monolayers and replacement with fresh medium at 2-hr intervals during incubation.
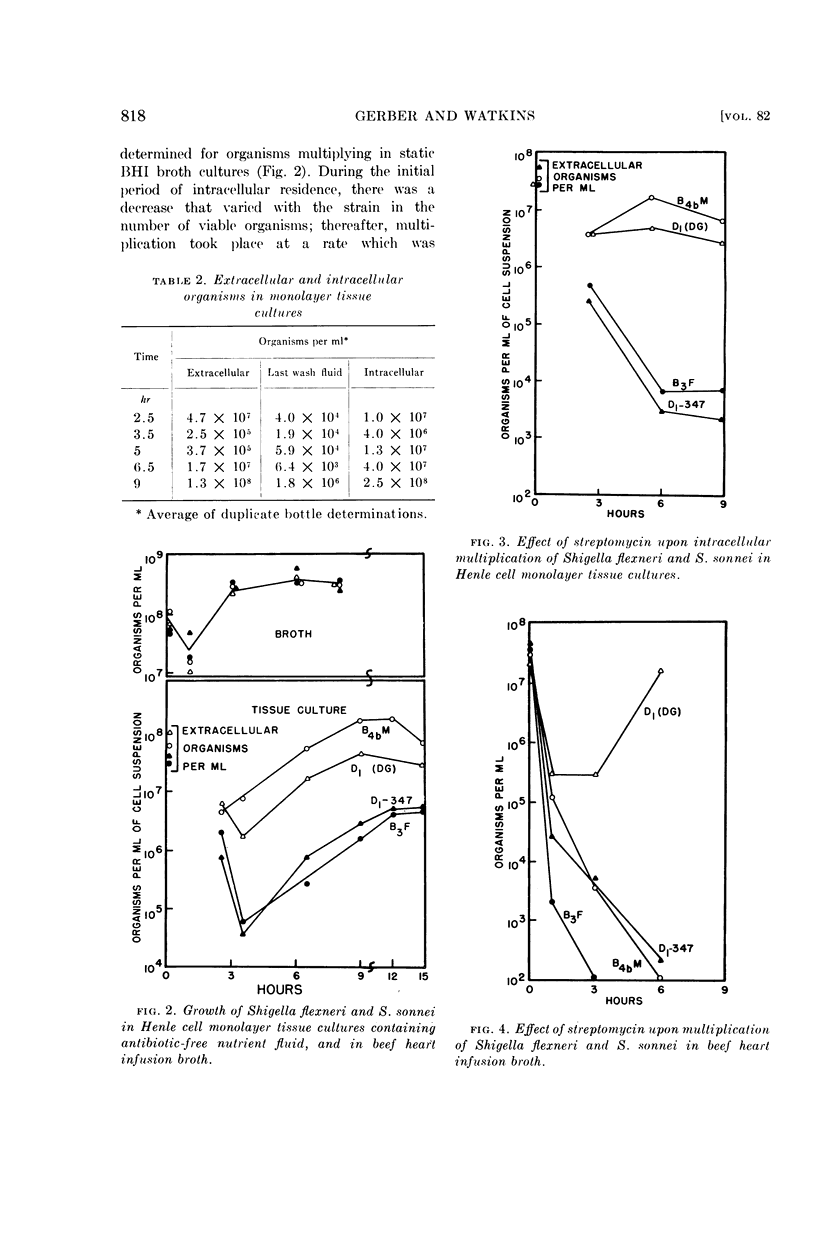
Intracellular growth patterns of two S. flexneri and two S. sonnei strains were established. Differences in the capacity of strains to adapt to the intracellular environment was reflected in the number of organisms subsequently produced, and appeared to be associated with the degree of intracellular resistance to streptomycin. No such variation was seen in cultures grown in broth.
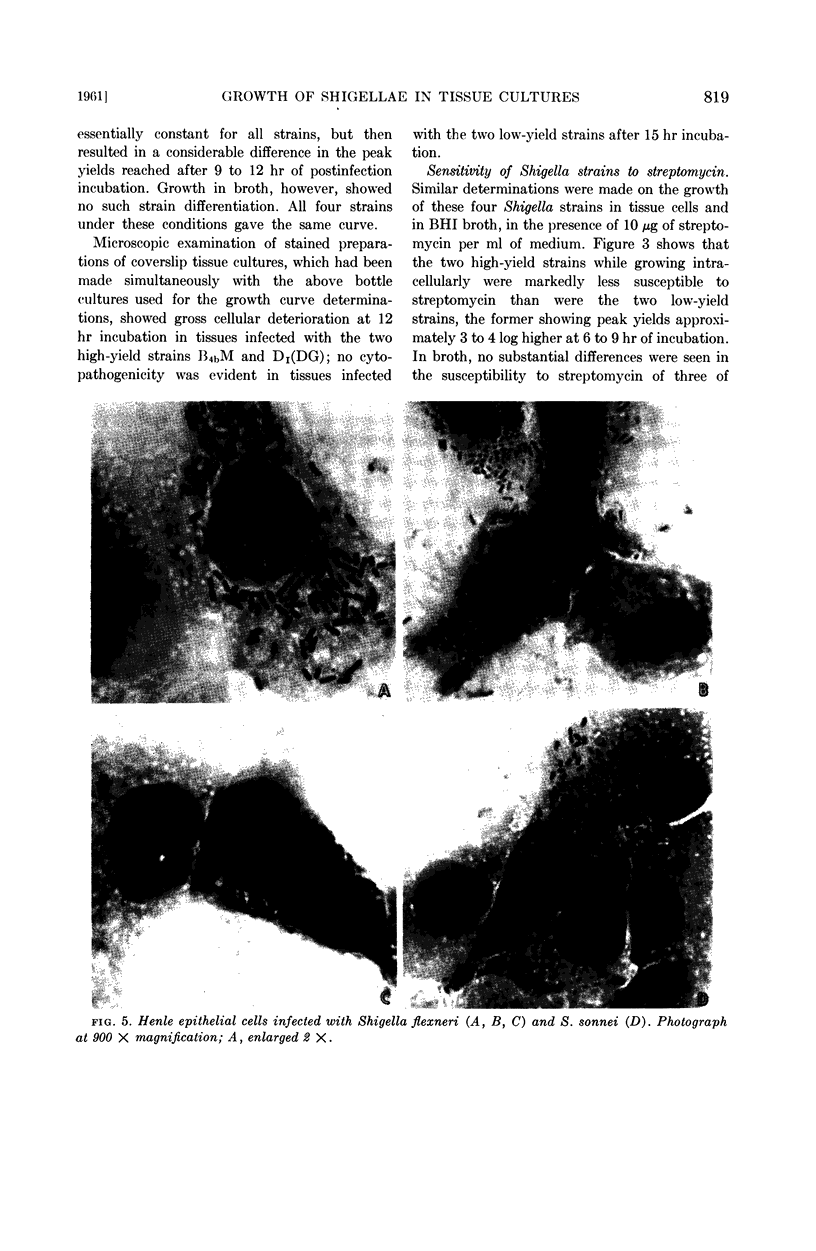
Microscopic examination of stained monolayers showed that intracellular bacterial growth was confined to the cytoplasm, which became filled with organisms prior to cellular disintegration.
Biological implications of the variable response of Shigella strains to the intracellular environment are discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUDE A. I., SIEMIENSKI J. Role of bacterial urease in experimental pyelonephritis. J Bacteriol. 1960 Aug;80:171–179. doi: 10.1128/jb.80.2.171-179.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER M. L., KELLER H. M., WALTERS E. W. Microscopic characteristics of colonies of Shigella flexneri 2a and 2b and their relation to antigenic composition, mouse virulence and immunogenicity. J Immunol. 1957 Mar;78(3):160–171. [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISLER D. M., BEVIS R. E. Use of potassium cyanide in the Giemsa stain. AMA Arch Pathol. 1953 Dec;56(6):647–649. [PubMed] [Google Scholar]
- HENLE G., DEINHARDT F. The establishment of strains of human cells in tissue culture. J Immunol. 1957 Jul;79(1):54–59. [PubMed] [Google Scholar]
- HOLLAND J. J., PICKETT M. J. Intracellular behavior of Brucella variants in chick embryo cells in tissue culture. Proc Soc Exp Biol Med. 1956 Dec;93(3):476–479. doi: 10.3181/00379727-93-22792. [DOI] [PubMed] [Google Scholar]
- MERRIOTT J., SHOEMAKER A., DOWNS C. M. Growth of Pasteurella tularensis in cultured cells. J Infect Dis. 1961 Mar-Apr;108:136–150. doi: 10.1093/infdis/108.2.136. [DOI] [PubMed] [Google Scholar]
- McDERMOTT W. Inapparent infection: relation of latent and dormant infections to microbial persistence. Public Health Rep. 1959 Jun;74(6):485–499. [PMC free article] [PubMed] [Google Scholar]
- McDERMOTT W. Microbial persistence. Yale J Biol Med. 1958 Feb;30(4):257–291. [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Growth characteristics in HeLa cells of the rapidly growing acid fast bacteria, Mycobacterium fortuitum, Mycobacterium phlei, and Mycobacterium smegmatis. J Bacteriol. 1957 Jun;73(6):722–726. doi: 10.1128/jb.73.6.722-726.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Growth characteristics of tubercle bacilli and certain other mycobacteria in HeLa cells. J Exp Med. 1957 Jan 1;105(1):39–48. doi: 10.1084/jem.105.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Nonacid-fast bacteria and HeLa cells: their uptake and subsequent intracellular growth. J Bacteriol. 1959 Jun;77(6):701–714. doi: 10.1128/jb.77.6.701-714.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEPARD C. C. Phagocytosis by HeLa cells and their susceptibility to infection by human tubercle bacilli. Proc Soc Exp Biol Med. 1955 Nov;90(2):392–396. doi: 10.3181/00379727-90-22043. [DOI] [PubMed] [Google Scholar]
- SMADEL J. E. Some aspects of intracellular infections. J Immunol. 1960 Jan;84:1–5. [PubMed] [Google Scholar]
- SMITH H. The use of bacteria grown in vivo for studies on the basis of their pathogenicity. Annu Rev Microbiol. 1958;12:77–102. doi: 10.1146/annurev.mi.12.100158.000453. [DOI] [PubMed] [Google Scholar]
- STINEBRING W. R., KESSEL R. Continuous growth of Brucella abortus in mononuclear phagocytes of rats and guinea pigs. Proc Soc Exp Biol Med. 1959 Jul;101(3):412–415. doi: 10.3181/00379727-101-24962. [DOI] [PubMed] [Google Scholar]
- THAYER J. D., PERRY M. I., FIELD F. W., GARSON W. Failure of penicillin, chloramphenicol, erythromycin, and novobiocin to kill phagocytized gonococci in tissue. Antibiot Annu. 1956:513–517. [PubMed] [Google Scholar]
- WATKINS H. M. Some attributes of virulence in Shigella. Ann N Y Acad Sci. 1960 Nov 21;88:1167–1186. doi: 10.1111/j.1749-6632.1960.tb20107.x. [DOI] [PubMed] [Google Scholar]