Abstract
Orexin neurons in the lateral hypothalamus constitute a critical component in regulation of waking, feeding, and reward-related behaviors. In this study we examined the effects of lipopolysaccharide (LPS) challenge on Fos expression in orexin neurons in rats, to determine changes during sickness in two different behavioral contexts. One cohort of rats was treated with saline or LPS during the daytime, and then tested on an elevated plus maze (EPM) or left in their home cage until sacrifice. Another cohort received LPS or saline shortly before dark onset and was sacrificed 90 minutes into the dark period. The brains were double-stained for Fos and orexin-A immunoreactivity (both cohorts) and for Fos and histidine decarboxylase (dark period cohort). Orexin neurons were strongly activated in context of exploratory behavior (double-labeled for Fos in both medial and lateral portions). LPS challenge prior to maze exposure diminished this activation, most notably among the lateral orexin neurons. In home cage controls, LPS challenge lead to increased Fos expression, most notably in the medial orexin neurons, when compared to saline-injected home cage controls that show little or no Fos during the daytime. In the dark period, Fos expression in both orexin and histaminergic neurons was abundant, which LPS challenge strongly suppressed. These findings are consistent with the hypothesis that the orexin neurons, in conjunction with the histaminergic system, represent a potential target of the neurocircuitry that drives sickness behavior due to peripheral inflammation, likely through functional inhibition of these hypothalamic cell groups.
Keywords: hypocretin, histamine, c-fos, lateral hypothalamus, tuberomammillary nucleus, perifornical area, arousal, fatigue, exploratory behavior, wakefulness
1. Introduction
The perifornical region of the lateral hypothalamus contains a neurochemically distinct group of neurons that produce the peptides orexin-A and -B (also named hypocretin-1 and -2) and give rise to widespread projections throughout the brain (Peyron et al., 1998). The orexin system regulates critical behavioral functions, such as maintenance of waking and vigilance states (Sutcliffe and de Lecea, 2002), and the mediation of food- and other reward-driven motivated behaviors (Harris et al., 2005). Initial studies showed that the peptides provoked, when infused in the lateral ventricles, feeding behavior, hence the terms “orexin”, but soon their critical role in the induction and maintenance of the waking state was discovered as loss of orexins or their receptors leads to narcolepsy and cataplexy, conditions characterized by sudden, inappropriate, onset of sleep during ongoing behavioral activity (Chemelli et al., 1999; Lin et al., 1999). This discovery was soon followed by the identification of a prominent role for the orexin system in facilitating the behavioral responsiveness to fasting (Yamanaka et al., 2003) and towards contexts associated with reward (Harris et al., 2005), demonstrating a facilitating drive of a broader range of motivated and goal-directed behaviors.
Sickness behavior that ensues as a result of infection and inflammation is characterized by diminished arousal and motivation (anhedonia or lethargy), hypersomnolence, and reduced appetite. Because of these changes in behavioral repertoire, it is reasonable to suggest that these emerge as a result of a diminished activity of the hypothalamic orexin system. This raises the possibility that the orexin system in the hypothalamus would be a prime candidate for the neurocircuitry driven by peripheral innate immune activation. To obtain support for this notion, we hypothesized that peripheral inflammation would suppress the expression of the c-fos immediate early gene product Fos in the orexin neurons particularly in the context of both forced and natural behavioral activities, when sickness becomes behaviorally apparent. In the present study, we injected rats with either the pro-inflammatory bacterial cell wall product lipopolysaccharide (LPS) or saline and tested them on the elevated plus maze during the daytime, and injected another group shortly before dark onset to assess the effects in the context of their natural behavioral activity in the dark period, when orexin cells express higher levels of Fos (Estabrooke et al., 2001). The effects of behavioral contexts and inflammatory stimulus on the activity of orexin neurons were assessed by dual staining for Fos protein and orexin-A immunoreactivity. An important mechanism through which orexins induce wakefulness and vigilance comprises their direct excitatory influence on histaminergic neurons in the tuberomammillary region (Yamanaka et al., 2002). To further delineate a role for the arousal-supporting histaminergic system in sickness behavior, as previously tested in the context of maze exploration (Gaykema et al., 2008), we assessed LPS challenge effects on the nocturnal activity of histidine carboxylase-expressing neurons in the same nocturnally tested rats.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats (250 grams body weight, from Taconic Laboratories, Germantown, NY, USA) were housed in pairs and maintained on a 12-hour light-dark cycle (lights on at 7:00 AM, ambient temperature 72 F) with free access to Purina Rat Chow #R001 and water. All animals were handled daily for at least three days to habituate them to the procedures. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals (NIH Publications No. 80-23; revised 1996) and were in accordance with protocols approved by the University of Virginia Animal Care and Use Committee.
2.2. Experimental procedures
Lipopolysaccharide (LPS, Escherichia coli, serotype 0111:B4; Sigma, St Louis, L-2013) was dissolved in sterile pyrogen-free saline (0.9% NaCl) and diluted to a concentration of 0.1mg/ml before use. On the experiment day, rats were injected intraperitoneally (i.p.) with either LPS or saline at a volume of 1 ml/kg (LPS dose of 0.1 mg/kg) and immediately returned to their home cage.
Daytime exploratory task
Twenty rats were used in this experiment, of which eight were transported in their home cages to the behavioral test room adjacent to the colony room between 9 and 10 AM and allowed to acclimate for 30 minutes prior to intraperitoneal injections (LPS or saline, cage mates were staggered 15 minutes apart), after which the animals stayed in their home cage for another 90 minutes, before being removed and placed on the elevated plus maze (constructed of black-painted wood, with two open and two closed 40 × 10 cm arms joined at the 10 × 10 cm center, elevated 40 cm above the floor) to explore during 5 minutes under dim light conditions (approximately 50 lux). The number of open and closed arm entries was recorded as a measure of behavioral activity of each rat. The maze was cleaned with a moist cloth after each test. One hour after the maze exposure the rats were anesthetized (Nembutal, 60 mg/kg i.p.) and sacrificed 10 minutes later by perfusion fixation. The other twelve rats (designated home cage controls) remained in their cages after LPS or saline injections and were anesthetized following the same time span elapsed after LPS/saline injections (155–160 minutes).
Nocturnal behavioral activity
To assess the effect of LPS challenge on the natural active behavior at the start of the dark period, 10 rats were injected with either i.p. LPS or saline 90–110 minutes prior to dark onset, and returned to their home cage. Each pair of cage mates received one LPS and one saline injection spaced 5 minute apart. From the dark onset on, their behavior was scored every 15 minutes as active (grooming, rearing, walking, burying, eating, drinking) or inactive (resting/quite waking or sleeping). A red light was used in the colony room to accommodate observations while each rat had markings on the tail. Three hours following the injections (approximately 90 minutes into the dark period), the rats received Nembutal injections, and when anesthetized, removed from the colony room, and sacrificed by perfusion fixation (cage mates were sacrificed 5 minutes apart).
2.3. Tissue processing
Following anesthesia and perfusion fixation of the rat, parts of the brains containing the hypothalamus were sectioned (50 μm thickness) and collected serially into six-well plates, so that each well contained every sixth section (see Gaykema et al., 2008; Park et al., 2008 for details). One set of sections was reacted sequentially for Fos and orexin A immunoreactivity using a peroxidase staining protocol as described previously (Park et al., 2008). Briefly, sections were incubated for 72h in anti-Fos (Ab5, Oncogene, Cambridge, MA, 1:50,000), overnight in biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, 1:1000), and Vectastain Elite ABC kit (Vector, 1:500). Staining was completed using nickel-enhanced 3,3′-diaminobenzidine (DAB) yielding a black reaction product. Sections were then incubated for 48h in rabbit anti-orexin A (AB3704, Millipore/Chemicon, Temecula, CA, 1:2,000, raised against a synthetic peptide fragment corresponding to the C-terminal portion of orexin-A [aa14-33, identical in bovine, rat, and human]), followed by repeat incubations in secondary antibody and ABC solutions, and staining in regular DAB solution yielding reddish-brown cytoplasmic staining. Prior adsorption of the primary antibody solution in excess control peptide (10 μg/ml C terminal fragment 14-33, Calbiochem/EMD Biosciences, PC362) completely abolished immunostaining for orexin-A. The staining for orexin-A immunoreactivity revealed cell bodies in the posterior lateral, perifornical, and dorsomedial regions of the hypothalamus, many immediately dorsal to the fornix (see Fig. 1I) and fibers with varicosities dispersed throughout the forebrain in a pattern identical to previous descriptions, including prominent extrahypothalamic innervation of the paraventricular thalamus (Peyron et al., 1998). Another series of sections from the dark period experiment was stained for Fos and then histidine decarboxylase (HDC, the histamine-synthesizing enzyme), using guinea pig anti-HDC (H5102, United States Biological Inc., Swampscott, MA, 1:5,000, 2 days), followed by biotinylated goat anti-guinea pig (Jackson ImmunoResearch, 1:1,000), ABC, and regular DAB staining. All sections were mounted, dehydrated in ethanol and histoclear, and coverslipped.
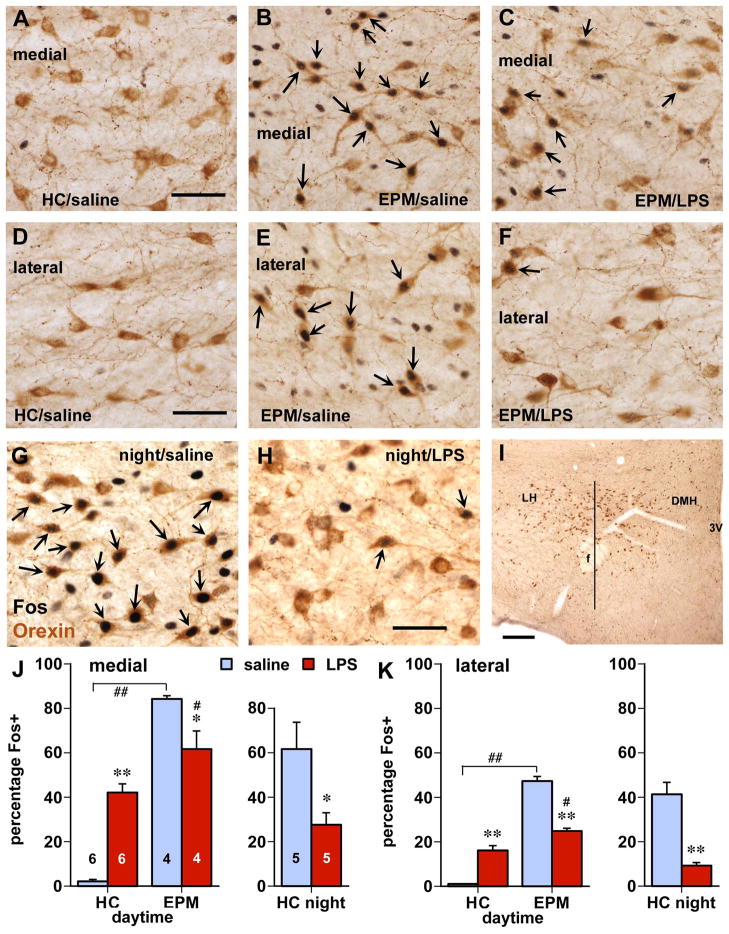
Figure 1.
Behavior-related Fos expression in orexin neurons is inhibited by LPS treatment. Panels A–F depict orexin cells (brown cytoplasmic staining) in medial (A–C) and lateral (D–F) parts of the hypothalamus. Whereas daytime home cage controls (HC) lack Fos in these cells (A,D), elevated plus maze (EPM) testing greatly increased Fos staining (B,E; black nuclear staining, arrows indicate double-labeled neurons). After LPS injection however, Fos expression was reduced in similarly environmentally stimulated, animals (C,F). G,H: Orexin cells express abundant Fos associated which nighttime (natural) wakefulness and behavioral activity in the home cage (G), which is greatly reduced following LPS treatment (H). I: Lower magnification photomicrograph depicting the distribution of orexin neurons in the lateral (LH) and dorsomedial (DMH) regions with superimposed a vertical line through the center of the fornix (f) used to divide the cell population for quantitative analysis. Scale bars in A, D, G: 50 μm; in I: 250 μm. J,K: Quantitative analysis of the influence of involuntary (EPM) and natural environmental (night period) behavioral stimulation on expression of Fos protein in orexin cells and the effect of LPS challenge. Data are expressed in the percentage of orexin cells that were Fos-positive in medial (J) and lateral (K) populations as divided by a vertical line through the fornix (I). Numbers in J indicate group size (also apply to K); * p < 0.05, ** p < 0.0005 LPS vs. saline; # p< ˜5, # p < 0.05; ## p< ˜5: EPM vs. home cage.
2.4. Microscopy
Orexin-stained cells with and without nuclear Fos labeling were counted manually with the guidance of a 10x ocular containing a 10×10 division rectangular grid. The orexin-cell region spanning from the internal capsule to the third ventricle was divided into medial and lateral portions divided by a vertical line through the fornix, and counted separately. Orexin-labeled cell bodies that clearly displayed cell nuclei were counted on four evenly spaced sections through the hypothalamus (300 um apart), and total numbers were then determined by summation of the counts from each hemisphere and all 4 sections. The HDC-labeled neurons in the tuberomammillary region were counted in 4 sections and summated in a total of single and double-labeled cells in each rat. Digital images were captured with a Magnafire digital camera (Optronics, Goleta, CA) and adjusted in brightness and contrast in Adobe Photoshop CS2 (Adobe Systems, Mountain View, CA).
2.5. Analysis
Cell counts and percentages of orexin and HDC-labeled neurons that were Fos-positive (ratio number of double-labeled cells/all orexin cells) were analyzed using a two-way factorial analysis of variance (ANOVA) for the daytime experiment using injection and environment as independent variables, and using one-way ANOVA in the nighttime experiment. If the ANOVA revealed statistically significant main effects or interaction (probability of 0.05 or less), post-hoc Fisher’s protected least significant difference or t-tests were conducted to compare between the individual groups.
3. Results
3.1. Behavioral effects
The saline-treated rats exposed to the elevated plus maze continuously explored all four arms, and remained behaviorally active, e.g., with grooming, upon return in their home cage for at least another 10 minutes. The LPS-treated rats introduced to the EPM moved across the maze arms at a slower gait interspersed with brief moments of immobility. Reduced exploration was reflected in the number of arm entries with an average of 12.5 (+/−1.2) in the saline-treated group and 5.9 (+/−0.7) in the LPS-injected rats (main effect of injection: F(1,6) = 23.7, p < 0.01). During the light period, home cage rats were mostly sleeping or quietly resting irrespective of LPS treatment. After dark onset, all saline-injected rats were behaviorally active (e.g., moving around, rearing, grooming, eating) at each 15 min-interval observation, whereas all LPS-injected rats were either quietly awake while laying down or asleep.
3.2. Fos expression: environment and LPS effects
In the rats assessed during the daytime, the test conditions and injections had large effects on Fos expression in the orexin cell population (Fig. 1; treatment x test interaction F(1,16) = 31.9 and 109.0 for medial and lateral cell groups, p < 0.0001). Whereas only sparse hypothalamic Fos staining was present in the saline-treated home cage rats (Fig. 1A,D), EPM exposure strongly increased Fos expression (p < 0.0001) in the both the medial (Fig. 1B, from 2% to 84% Fos-positive, and lateral groups of orexin neurons (Fig. 1E, from 1% to 47% Fos-positive; also see bar graphs in Fig. 1J,K depicting percentages of orexin neurons that were Fos-positive). Among the EPM-exposed rats, LPS treatment reduced Fos expression in orexin neurons (down to 62% in medial (Fig. 1C,p = 0.035) and to 25% in lateral populations (Fig. 1F, p = 0.0001). In the home cage groups, LPS treatment increased Fos expression (to 40% in medial and 16% in lateral orexin neurons). Interestingly, among the LPS-treated rats the difference in Fos expression between the home cage and EPM conditions was greatly diminished. There were no significant differences in the total number of orexin neurons between the experimental groups. Although not quantified, behavioral test-associated Fos expression also occurred among neurons not stained for orexin-A, which was subsequently reduced in the rats that received LPS challenge (see, e.g., Fig. A–C).
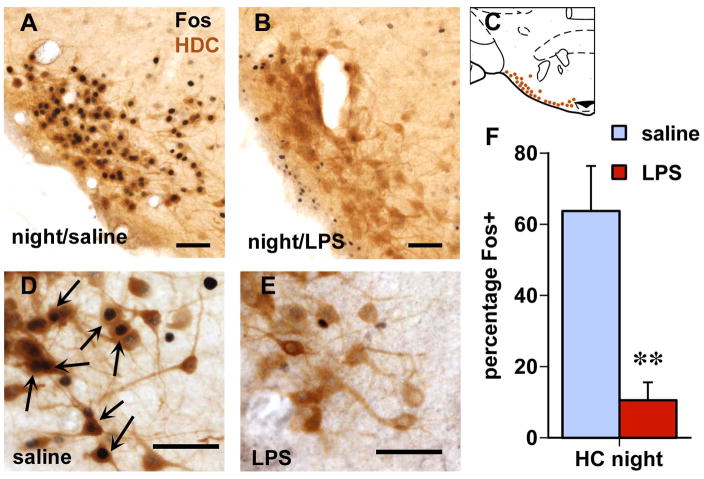
In rats sacrificed in the early part of the dark period, there was strong Fos staining in the orexin cells as 62% in the medial portion and 42% in the lateral portion were double-labeled after saline injection (Fig. 1G). Following LPS challenge, the proportion of orexin cells that were Fos-positive was much lower (Fig. 1H, also see right bar graphs in Fig. 1J,K), ranging from 9% in the lateral (p = 0.0004) to 28% in the medial portions (p = 0.028). Among the HDC-immunoreactive (histaminergic) neurons in the tuberomammillary hypothalamus, the proportion double-labeled for Fos decreased from 64% in the saline-treated rats to 11% in the LPS-treated ones (Fig. 2; p = 0.004). The total numbers of orexin and HDC-positive neurons were not different between the groups.
Figure 2.
Histaminergic neurons (brown staining for histidine decarboxylase (HDC) immunoreactivity show abundant staining for Fos (black cell nuclei) when rats were sacrificed 2 h into the dark period (A, with larger magnification in D; arrows point at double-labeled cells), which is strongly suppressed in LPS-injected rats (B,E). Scale bars: 50 μm. Brain diagram in C (modified after Paxinos and Watson, 1998) depicts the location of HDC-ir neurons in the tuberomammillary nucleus at the base of the posterior hypothalamus. Bar graph in F depicts the percentage of HDC neurons that are Fos-positive in each group (average and SEM; **, n = 5/group; p < 0.005 compared to saline).
4. Discussion
The present study demonstrates that the environment stimulus- and behavior-associated activation of orexin neurons (as measured by Fos expression) was greatly reduced following systemic challenge LPS, a potent pro-inflammatory stimulus. The manifestation of sickness behavior, e.g., reduced mobility and exploration in the EPM and cessation of behavioral activity in the dark period in the home cage environment, coincides with decreased Fos expression in orexin neurons consistent with similar effects of LPS reported on Fos expression in orexin neurons associated with fasting (Becskei et al., 2008) and with consumption of highly palatable food (Park et al., 2008). Although the correlation between behavioral and neuroanatomical parameters does not provide evidence for a causative link, it suggests that the functional inhibition of the orexin system in response to peripheral inflammation could in part underlie decreased behavioral activity that is characteristic for sickness, including decreased locomotion, alertness, and drop in food intake due to reduced meal frequency (Langhans et al., 1989) as orexins constitute an important central link to behavioral arousal to, e.g., metabolic cues (Yamanaka et al., 2003). Although the effects of LPS challenge was not limited to the orexin neuron population but also seemed to reduce lateral hypothalamic/perifornical Fos expression in other neurons, the effects seen in the orexin cells could be significant for sickness behavior. This line of thinking finds support in previous studies showing that daytime infusion of orexin into the brain induces behavioral arousal in conscious animals (Yamanaka et al., 2002), whereas treatment with orexin receptor antagonists increases sleepiness and decreases alertness (Brisbare-Roche et al., 2007). Consistent with this view, the onset of orexin neuron discharge herald waking from paradoxical (REM) sleep (Lee et al., 2005), and orexin cells are moderately active during eating and grooming and maximally active during exploratory behavior (Mileykovskly et al., 2005).
Tuberomammillary histaminergic neurons constitute an important target for orexin neurons to mediate behavioral arousal is as histamine antagonism prevents the waking and arousing effects of orexin (Yamanaka et al., 2002). In the present study, the dark period-associated activation of histaminergic neurons was also strongly reduced following LPS challenge, which is in line with isuch challenge’s general inhibitory effect on histaminergic neurons in a variety of environmental stimuli (Gaykema et al., 2008). Thus, a combined functional brake on both the orexin and histaminergic systems could contribute to the suppressed behavioral response to environmental stimuli in sickness.
Several studies provide compelling evidence for a medial-lateral differentiation in functional properties, such that medial-most orexin neurons are implicated in promoting alertness and vigilance, as well as homeostatic and autonomic regulation, whereas the lateral-most orexin neurons play a particularly distinct facilitating role in reward processing and behavioral responses to reward-associated contexts (Harris et al., 2005, 2007), possibly via their connections with the dopaminergic neurons in the ventral tegmental area (Fadel and Deutch, 2002). Consistent with this dichotomy, our findings indicate differences in responses between medial and lateral populations of orexin cells. The medially situated orexin cells consistently express the highest levels of Fos expression, and are more prominently responsive to LPS challenge itself when rats remained in the home cage at daytime. The lateral orexin neurons, on the other hand, are most strongly inhibited by LPS challenge under environmentally stimulating conditions (EPM, nighttime), which may then contribute to the LPS-driven inhibition of behavior via suppression of reward-related drive or motivation. The significant LPS-induced reduction in EPM- and night-associated Fos expression in the medial orexin neurons suggests a suppressive influence on the orexin system’s behavioral arousal-promoting function as well. In the light of these interpretations, the LPS-induced increase in Fos expression in daytime home cage animals seems paradoxical, but rats may experience visceral discomfort that could lead to some degree of arousal, autonomic readjustment, and temporary disruption of deep sleep.
In conclusion, the present study indicates that, along with the histaminergic system, orexin neurons are significantly inhibited (with Fos staining as a measure for activation) by immune challenge in the context of environmental stimuli that normally would excite them. It is possible that the reduced levels of excitation among the orexin and histaminergic neurons, despite the behavioral context, represent one of the contributing mechanisms that lead to diminished behavioral arousal that is characteristic in sickness. Because these neurons contribute to regulation of most, if not all, aspects of active behavior drastically altered by sickness, it is reasonable to suggest that the orexin system, in conjunction with the histaminergic, represents a critical target for the neurocircuitry that drives sickness behavior. This exploratory investigation into a possible role for the orexin system provide a blueprint for further studies on the involvement of the orexin system and the sources and mechanisms of its afferent modulation in acute sickness models.
Acknowledgments
This work was supported by NIH grant R01 MH068834. We greatly appreciate the technical assistance of Mary E. Tyler, Gregory Thacker, and Meghan Jones.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becskei C, Riediger T, Hernadfavley N, Arsenijvic D, Lutz TA, Langhans W. Inhibitory effects of lipopolysaccharide on hypothalamic nuclei implicated in the control of food intake. Brain Behav Immun. 2008;22:56–64. doi: 10.1016/j.bbi.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roche C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qui C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nature Medicine. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neurosci. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Gaykema RPA, Park SM, McKibbin CR, Goehler LE. Lipopolysaccharide suppresses activation of the tuberomammillary histaminergic system concomitant with behavior: a novel target of immune-sensory pathways. Neurosci. 2008;152:273–282. doi: 10.1016/j.neuroscience.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183:43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans W, Harlacher R, Scharrer E. Verapamil and indomethacin attenuate endotoxin-induced anorexia. Physiol Behav. 1989;46:535–539. doi: 10.1016/0031-9384(89)90032-2. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6717–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Mileykovskly BY, Klyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SM, Gaykema RPA, Goehler LE. How does immune challenge inhibit ingestion of palatable food? Evidence that systemic lipopolysaccharide treatment modulates key nodal points of feeding neurocircuitry. Brain Behav Immun. 2008;22:1160–1172. doi: 10.1016/j.bbi.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliff JG, de Lecea L. The hypocretins: setting the arousal threshold. Nature Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Tsujino N, Funahashi H, Honda K, Guan JI, Wang QP, Tominaga M, Goto K, Shiioda S, Sakurai T. Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Comm. 2002;290:1237–1245. doi: 10.1006/bbrc.2001.6318. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Beuckmann CT, Willie JT, Hara J, Tsujino N, Mieda M, Tominaga M, Yagami K, Sugiyama F, Goto K, Yanagisawa M, Sakurai T. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]