Abstract
It has been proposed that patients with schizophrenia and some of their relatives suffer from reduced neurocognitive efficiency, increasing their sensitivity to experimental task demands. The present study evaluated such a possibility during performance of a working memory task by schizophrenia patients and their co-twins along with a healthy control sample.
Electrophysiological data were obtained from sets of 9 twin pairs (monozygotic and dizygotic pairs collapsed) discordant for a diagnosis of schizophrenia and from 9 matched, healthy control twin pairs, during administration of a variable-load spatial working memory task. Event-related potentials (ERPs) were measured immediately after memory set onset and during a delay period. For correctly performed trials, slow-wave ERP activity measured during the late stimulus encoding and delay periods exhibited a significant Diagnostic Group-by-Memory Load interaction, with schizophrenia patients showing a differentially strong load effect. Patients’ co-twins displayed an intermediate level of load sensitivity while healthy controls showed no significant load effect. These results support an inefficiency model of neurocognitive dysfunction in schizophrenia, a pattern that appears to be related to the pathogenesis and inheritance of the disorder. Furthermore, this inefficiency appeared during the late stimulus encoding stage of working memory functioning, possibly reflecting disruptions in stimulus representation consolidation.
Keywords: electrophysiology, ERP, performance, hyperactivation, CNV, P3
1. INTRODUCTION
Impaired spatial working memory (WM) has been observed consistently among schizophrenia patients (Park and Holzman, 1992; reviewed, Piskulic et al., 2007), and to a lesser extent, among individuals diagnosed with other schizophrenia spectrum disorders (Park et al., 1995; Saperstein et al., 2006) and among non-schizophrenic relatives of patients (Glahn et al., 2003), suggesting that it may serve as an endophenotypic indicator of genetic liability to the disorder (Gottesman and Gould, 2003).
Functional neuroimaging of the affected WM system has generated findings that are generally consistent with the hypothesis that the neurocognitive mechanisms supporting successful WM performance operate with less efficiency in schizophrenia patients (Callicott et al., 2003; Manoach, 2003; Tan et al., 2005; van Snellenberg et al., 2006; Schlösser et al., 2008) and their first-degree relatives (Thermenos et al., 2004; Karlsgodt et al., 2007). For instance, studying a particularly large sample of schizophrenia patients, Potkin and colleagues (2009) demonstrated that regions of prefrontal cortex are hyper-responsive to increases in WM load, including under circumstances in which patient and control groups are matched on task performance. Here neural inefficiency is operationalized as a relatively increased, task-dependent brain response engaged in the service of achieving a constant behavioral criterion.
Published reports are not unanimous in support of this inefficiency hypothesis, though. At least two studies (Tan et al., 2005; Schlösser et al., 2008) demonstrate that physiological evidence of inefficiency may be restricted to only a subset of cortical regions associated with WM performance. Furthermore, a number of earlier reports describe results that may be better summarized by a simple hypoactivation model of abnormal neural activity in patients (reviewed, Barch, 2005). A final source of ambiguity involves the nature of relationship between neural activity and the associated hemodynamic response, and the manner in which neural inefficiency may be reflected in observed cerebrovascular activity (e.g., Rypma et al., 2006; Mangia et al., 2008).
Electrophysiology has the potential to provide a complementary test of the inefficiency hypothesis, given its direct demonstration large scale neural activity (Birbaumer et al., 1990; Nunez and Srinivasan, 2006), and its reflection of both event-related phasic responses (e.g., to stimulus onset) and more tonic activity corresponding to periods of information maintenance over a delay period (e.g., Muller and Knight, 2002). One event-related potential (ERP) in particular, the contingent negative variation (CNV; Walter et al., 1964), has been shown to index neural activity associated with information maintenance over a several-second delay period. Specifically, CNV amplitude during a delay period decreases - or becomes more positive - with increasing memory load (Roth et al., 1975; McEvoy et al., 1998). Although CNV amplitude has been measured at least three times previously in schizophrenia patients performing WM tasks (Klein et al., 1996; Low et al., 2000; Cameron et al., 2003), none of these experimental protocols manipulated WM demand in a parametric manner, which is necessary to permit characterization of WM system efficiency; instead, they convolved engagement of additional cognitive processes with increases in task difficulty.
Alternatively, if information maintenance demands are varied parametrically, load-dependent decreases in CNV amplitude should reflect the WM memory system’s level of efficiency, as evidenced by a greater physiological response associated with accurate task performance in less efficient subjects. Stated differently, less efficient subjects would be expected to incur a relatively greater depletion of resources in order to reach the same level of performance as more efficient subjects (Kahneman, 1973; Hockey, 1997). Formally, this prediction parallels the inefficiency argument as delineated in the functional magnetic resonance imaging (fMRI) literature (e.g., Callicott et al., 2003; Manoach, 2003; van Snellenberg et al., 2006), and discussed above with respect to the demonstration by Potkin and colleagues (2009) that schizophrenia patients display greater prefrontal cortex activation than controls do when behavioral performance is matched between groups. The translation of this model to electrophysiology, however, allows the hypothesis that schizophrenia patients suffer from decreased efficiency of neurocognitive WM mechanisms to be tested using a direct measure of ensemble neural activity (Birbaumer et al., 1990; Nunez and Srinivasan, 2006).
With respect to more phasic ERP activity, the visually-evoked P1 and N1 components reflect relatively early perceptual encoding demands (Handy and Mangun, 2000). Moreover, P3 amplitude has been shown to be associated with the allocation of cognitive resources to support less perceptually-bound processes (Sirevaag et al., 1989), such as working memory encoding and maintenance processes (Jeon and Polich, 2003), possibly including representation consolidation (Vogel and Luck, 2002) and subsequent updating (Donchin and Coles, 1988). Even without assuming that these ERP components reflect entirely discrete, independent processing stages (e.g., Smulders et al., 1995), the P1, N1, P3, and CNV taken collectively provide temporal resolution adequate to parse the observed physiological response into cognitively meaningful components, potentially isolating the information processing stage at which inefficiency or other abnormalities begin to emerge.
In light of evidence that WM deficits in schizophrenia patients may be attributable largely to degraded encoding of perceptual information into durable mental representations that can be maintained over a delay (Lee and Park, 2005) - or more specifically, the representation consolidation phase of stimulus encoding - it might be expected that evidence of neurocognitive inefficiency in patients would arise during the consolidation stage of task performance (Fuller et al., 2005), coinciding with the development of the P3 (Vogel and Luck, 2002).
We predicted that schizophrenia patients and to an intermediate extent, their non-schizophrenic co-twins, would exhibit increased load sensitivity relative to healthy control twin pairs during performance of a spatial WM task, beginning relatively late in the stimulus encoding process as reflected by the P3 and extending through the development and resolution of the delay-period-specific CNV. Although auditory P3 amplitude reductions (especially over frontal electrodes) are a well replicated finding among patients and their relatives (e.g., Bramon et al., 2005, Sumich et al., 2008), visually induced P3 amplitude findings are less readily available (one example is Groom et al., 2008). Nevertheless, these findings, in addition to the aforementioned behavioral and BOLD results showing patients’ relatives performing similar to patients, but at a less severe level, suggests that patients’ co-twins should show a P3 abnormality similar to, but less in extant than, patients’ abnormalities.
2. METHODS
2.1. Sample ascertainment and assessment
Participants were recruited from the cohort of same-sex twin pairs born in Finland between 1940 and 1957 and in which one co-twin received a DSM-III-R diagnosis of schizophrenia or schizoaffective disorder (not predominantly affective type) and the other did not meet criteria for any psychotic disorder. Diagnoses were confirmed using the Structured Clinical Interview for DSM-III-R Disorders (Spitzer et al., 1979). Patients also were rated using the Scale for the Assessment of Positive Symptoms (Andreasen, 1984a) and the Scale for the Assessment of Negative Symptoms (Andreasen, 1984b). Mood and anxiety disorders were diagnosed in a minority of patients, co-twins, and controls, but the prevalence of non-psychotic disorders did not differ among the three groups. None of the study participants displayed evidence of neurological abnormality other than those potentially associated with schizophrenia vulnerability and illness in patients and their co-twins. No participant had an estimated total IQ score at or below 70. Additional subject identification, recruitment, and assessment details are provided elsewhere (Cannon et al., 1998; Kaprio and Koskenvuo, 2002).
Twenty-nine of these discordant pairs (selected to maintain the distributions of demographic variables present in the larger, overall sample) participated, although upon inspection of the raw EEG data, it became apparent that technical factors had introduced significant noise to a substantial portion of subjects’ data sets. Session notes indicted that large-scale subject and hardware movement (including loss of contact between subject and sensors and possibly between leads and amplifiers) associated with construction nearby the laboratory accounted for the majority of contamination. Additionally, although the testing apparatus are located in a shielded room and have an extensive history of collecting clean data, artifacts consistent with episodes of electromagnetic interference were apparent. These particular instances of interference are difficult to account for definitively; however, they are evenly distributed across diagnostic groups and task conditions.
Since only intact twin pairs were included, patients and their non-schizophrenic co-twins were eliminated from the study sample at equivalent rates. Another 4 discordant pairs were excluded because their behavioral performance fell below chance, leaving 9 discordant pairs. After screening for any psychotic disorder, Cluster A diagnosis (Personality Disorders Examination; Loranger et al., 1985), or history of psychosis-related treatment or work disability in any first-degree relatives, 9 demographically-matched, control twin pairs were recruited from the same database. The data contamination discussed above did not affect the discordant and control pairs differentially (16 discordant sets and 14 control sets eliminated).
All patients except one had long-standing treatment with conventional neuroleptics, with daily chloropromazine equivalents ranging as high as 800 mg (mean +/− 1 s.d. = 243+/− 170 mg). The patient not on neuroleptic medication at the time of testing did have a history of exposure to conventional neuroleptics, but discontinued their use at least two years prior to the test date. None of the patients’ co-twins had any history of neuroleptic exposure.
Participants were included if their data sets yielded at least 14 correct, non-artifact-contaminated EEG epochs per memory set size (the minimum number of trials required to ensure stability of relevant averaged ERP waveforms, established using a partially overlapping set of EEG data). Number of epochs included in analyses did not differ significantly between groups. Resulting sample sizes (9 discordant pairs and 9 control pairs) were not large enough for separate consideration of monozygotic and dizygotic twins; therefore, the twin pair types were collapsed. After a complete description of the study was provided, written informed consent was obtained. The study protocol was approved by the ethical committee of the Helsinki and Uusimaa Hospital District, and the Institutional Review Boards of the University of California, Los Angeles, and the University of Pennsylvania.
2.2 Behavioral task
The task consisted of 144 delayed match-to-sample trials in which participants were shown a memory set of 1, 3, 5, or 7 equiprobable locations arrayed around a central fixation point (36 trials at each memory set size); after a 3000 ms delay in which only the fixation point was visible, participants were asked to judge whether a single probe dot matched the location of any of the stimuli in the memory set as fast and as accurately as possible, and indicate responses on a fiber optic button box using the index finger of their dominant hand. Each trial was preceded by a 50 ms warning tone, followed by a 950 ms preparation and visual fixation interval. Memory set exposure lasted for 2000 ms to allow for possible slower stimulus encoding and responding by patients. Additionally, the probe stimulus remained on the screen for 3000 ms, during which performance accuracy (match/non-match choice) and reaction time (RT) were recorded. Among all trials, 50% were true positives and 50% were true negatives. Excluding instructions and self-paced breaks, administration lasted approximately 24 minutes. The same pseudo-random trial sequence was used for all subjects, and self-paced breaks were provided as needed. Prior to administration of the task, participants completed a series of practice trials during which performance feedback was provided; additionally, subjects had performed the same task (with different trial order) on the preceding day. The internal validity of the task was determined previously in a substantially larger sample of schizophrenia patients, their non-schizophrenic co-twins, and healthy twin pairs (Glahn et al., 2003).
2.3. EEG recording and signal conditioning
EEG recordings were obtained in a magnetically-shielded room (Euroshield, Ltd.) while subjects sat with their heads placed inside a whole-head EEG/MEG instrument (Elekta Neuromag, Ltd.). The EEG signal was recorded from an array of 66 equally-spaced Ag/AgCl electrodes referenced to the nose (Virtanen et al., 1996). Signals were digitized at 500 Hz, with the band-pass filter set at 0.01 and 160 Hz, with a 12 dB/oct roll-off. A notch filter set at 48 and 52 Hz (symmetrical 48 dB/oct roll-off) was also applied as part of post-processing, to account for 50 Hz line noise. Bipolar vertical and horizontal electro-oculograms (EOG) were recorded with a bandpass filter of 0.5–30 Hz (12 dB/oct roll-off).
Continuous data were subjected to an independent components analysis with Bell and Sejnowski’s “Infomax” algorithm using EEGLAB (Delorme and Makeig, 2004) and implemented in MATLAB (The Mathworks, Inc., Natick, MA, USA). On average, 16.1% (S.E.M.=1.3%), 14.0% (S.E.M.=1.9%), and 14.3% (S.E.M.=1.9%) of extracted artifact components were removed from data obtained in controls, co-twins, and patients, respectively, with no significant difference between the groups, F(2,35) = 0.55, P = 0.58. Non-cerebral artifacts were identified and removed according to standardized criteria (available upon request), by technicians blind to diagnostic status. Examples of such criteria included: possible artifactual activity in an independent component must correspond temporally to artifactual activity visible in the raw data in order to be removed; also, discontinuous artifactual activity must have an amplitude at least three times as large as background (e.g., pre-stimulus) activity in order to be removed. Post-processing of the EEG data included offline low-pass filtering (zero-phase shift) set at 70 Hz, using Neuroscan Edit 4.3.1 (Compumedics Neuroscan, El Paso, TX, USA) The EEG was then epoched and a baseline correction was applied using the mean amplitude over the 200 ms preceding the onset of the memory set. Epochs were sorted for accuracy and only correct trials were retained.
2.4. ERP scoring
Peak amplitude and latency values for each ERP were determined with an automated algorithm and confirmed by visual inspection, using the following time windows: P1 (70–130 ms), N1 (P1-210 ms), P2 (N1-340 ms), N2 (P2-460 ms), and P3 (N2-700 ms). Visual inspection confirmed that these intervals adequately captured each subject’s averaged component peaks in each experimental condition. Measurements were made at 3 electrode sites chosen to correspond as closely as possible to Fz, Cz, and Pz, in the International 10–20 System (Jasper, 1958). The sites are denoted as Fz’, Cz’, and Pz’ to indicate their approximate location. For each of the components specified above, amplitudes were calculated from peak-to-peak measures.
For slow-wave ERP derivation, epochs were subjected to an offline low-pass filter set at 30 Hz with a 12 dB/oct roll-off. Amplitudes were determined at the same 3 electrode sites as the earlier components, and represented mean voltage values over the following time windows: initial CNV or iCNV (1000–2000 ms), and terminal CNV or tCNV (4000–5000 ms). References to overall CNV amplitude indicate iCNV and tCNV amplitudes collapsed.
2.5. Statistical analyses
Data analysis employed the general linear mixed model ANOVA with repeated measures in SAS, Version 8 (SAS Institute, Cary, NC). Twin pair membership was coded and entered as a random variable, and the Satterthwaite correction was used to estimate degrees of freedom, accounting for correlation among members of the same family. Diagnostic risk group was modeled as a fixed effect predictor (3 levels: schizophrenia patients, patients’ co-twins, and control twins), as was memory set size (4 levels: 1, 3, 5, or 7 locations) which was repeated within subjects. In this manner, average group differences and Group x Set Size interactions were tested separately for the following variables: behavioral accuracy (% correct), RT, and P1, N1, and P3 amplitude and latency. Consistent with the literature on visually evoked ERPs, P1, N1, and P3 were measured at Pz’ (e.g., Muller and Knight, 2002), and CNV was measured at Cz’ (e.g., McEvoy et al., 1998); these locations represented the midline sites of maximal amplitude for each of the respective components. Several hypotheses were modeled as contrast statements within the relevant mixed model ANOVAs; specific contrasts are discussed in the RESULTS section below. The CNV was tested in the manner described for the earlier ERPs but with two adjustments. Additionally, subcomponents of the waveform were modeled as a repeated measure (2 levels: iCNV and tCNV) for statistical examination of CNV amplitude (e.g., Chiu et al., 2004).
A set of second-order analyses also was carried out, including measurement of bivariate Pearson correlations between behavioral and electrophysiological measures, and SANS and SAPS global symptom ratings and extent of antipsychotic medication exposure. No significant relationships were observed between any of the ERP amplitudes and medication exposure or between ERP amplitudes and symptom scale scores.
3. RESULTS
3.1. Behavioral measures
With respect to performance accuracy, the main effects of group and set size were significant - F(2,45)=3.75, P=0.03, and F(3,105)=50.90, P<0.01, respectively - but the Group x Set Size interaction was not, F(6,105)=1.32, P=0.25. To determine if the expected relationship between performance and set size was present, planned contrast analyses revealed a significant linear trend between decreasing accuracy and increasing memory set size, F(1,107) = 126.38, P<0.01. The same pattern of results held for RT: the main effects of group and set size were significant, F(2,38)=9.09, P<0.01, and F(3,106)=49.65, P<0.01, respectively, but the Group x Set Size interaction was not, F(6,105)=0.33, P=0.92. The present accuracy and RT findings are consistent with results of prior administration of the present task to a larger sample of twins discordant for schizophrenia (Glahn et al., 2003). For both accuracy and RT, results of pair-wise comparisons are denoted in Table 1. Also denoted in Table 1 are scores from the entire sample (i.e., including subjects excluded due to technical problems), which did not differ from the scores of the sub-sample of subjects whose electrophysiological data were included in the present analyses.
Table 1. Demographic and Behavioral Performance Data.
For % Correct and RT data, values in first line reflect sample included in ERP analyses and values in second line reflect full sample. Patient - co-twin pairs and control pairs did not differ in age (patient - co-twins: mean of 48.0 years, control twins: mean of 48.7 years; F(1, 35) = 0.07, P = 0.80), gender distribution, handedness, or race/ethnicity (all White, non-Hispanic).
|
Schizophrenia Patients (n=9) Mean (S.E.M.) |
Patients’ Co-twins (n=9) Mean (S.E.M.) |
Control Twins (n=18) Mean (S.E.M.) |
|
|---|---|---|---|
| Descriptive Variable | |||
| Age (years) | 47.97 (1.85) | 47.97 (1.85) | 48.67 (1.02) |
| Gender | 4 female | 4 female | 8 female |
| Handedness | 9 right-handed | 9 right-handed | 18 right-handed |
| SAPS score | 2.8 (0.4) | ||
| SANS score | 4.4 (1.2) | ||
| Task Condition | |||
| % Correct - 1 location | 92.8 (2.3) 94.6 |
96.1 (1.3) 95.9 |
96.6 (1.1) 97.3 |
| % Correct - 3 locations * | 78.7 (3.5) 78.8 |
91.3 (1.9) 91.2 |
83.6 (2.8) 83.7 |
| % Correct - 5 locations | 72.3 (3.9) 72.2 |
76.4 (3.1) 77.7 |
76.4 (3.4) 76.4 |
| % Correct - 7 locations | 69.5 (4.6) 65.0 |
79.8 (3.8) 78.0 |
73.6 (3.7) 65.5 |
| RT (ms) - 1 location §* | 1001 (64) 999 |
733 (36) 737 |
767 (30) 793 |
| RT (ms) - 3 locations * | 1405 (134) 1401 |
1070 (90) 1066 |
1116 (43) 1157 |
| RT (ms) - 5 locations | 1473 (134) 1479 |
1253 (100) 1243 |
1243 (40) 1237 |
| RT (ms) - 7 locations | 1374 (141) 1430 |
1310 (113) 1314 |
1313 (41) 1375 |
Patients different from controls, P < 0.05
Patients different from their cotwins, P < 0.05
3.2. ERP measures
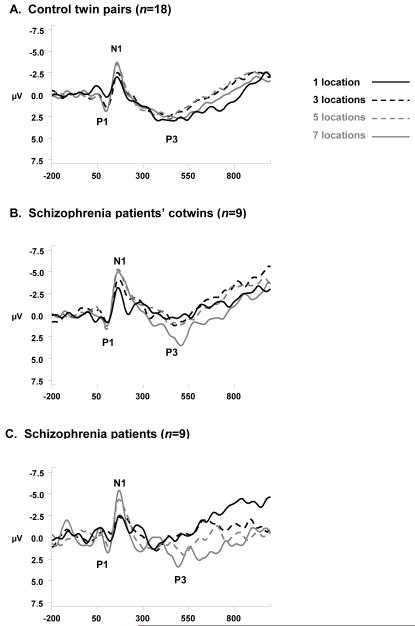
P1 and N1 amplitudes (Figure 1) showed a significant effect of set size, F(3,115)=4.56, P<.01, and F(3,116)=13.18, P<0.01, respectively. Modeled as a planned contrast, a significant linear fit indicated that increases in memory set size are associated with concomitant augmentation of P1 amplitude, F(1,115)=10.98, P<0.01 and N1 amplitude, F(1,115)=12.23, P<0.01. Diagnostic group did not show a main effect on P1amplitude, F(2,28)=0.01, P=0.99, or on N1 amplitude, F(2,29)=1.10, P=0.35; similarly, Group did not interact with Set Size to affect either of these amplitudes, (P1: F(6,115)=0.21, P=0.97; N1: F(6,115)=0.43, P=0.86).
Figure 1. ERP Waveforms to Memory Array Onset at Pz’.
ERP waveforms at Pz’ elicited by memory set stimulus onset, displayed at each memory load level for controls (A), for patients’ cotwins (B), and schizophrenia patients (C). Waveforms were low-pass filtered at 16 Hz (24 dB/oct roll-off) for display purposes only.
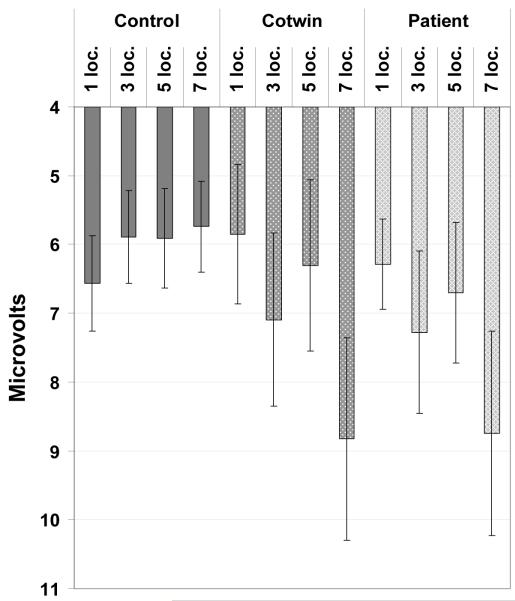
For P3 amplitude (Figure 2), the fixed effect of memory set size was significant at a trend level, F(3,115)=2.66, P=0.05, but the fixed effect of group, F(2,28)=0.63, P=0.54, and the Group x Set Size interaction were not statistically significant, F(6,115)=1.65, P=0.14. On the basis of a priori hypotheses, a planned contrast modeling P3 amplitude effects was carried out in an exploratory manner; these results should be interpreted cautiously given the absence of a significant interaction effect. The prediction that patients and their co-twins would account disproportionately for the overall increase in P3 amplitude at higher memory loads (modeled as one planned contrast) was supported, F(1,115)=7.38, P<0.01, albeit with tenuous justification for the contrast given the lack of a significant interaction. It is worth noting that whereas patients and their co-twins do appear at a group level to have very similar P3 amplitudes (Figure 2), the significant contrast modeled co-twins at an intermediate level.
Figure 2. P3 Amplitude to Memory Array Onset at Pz’.
P3 component mean amplitude at Pz’ to memory array onset (error bars representing ± 1 S. E. M.).
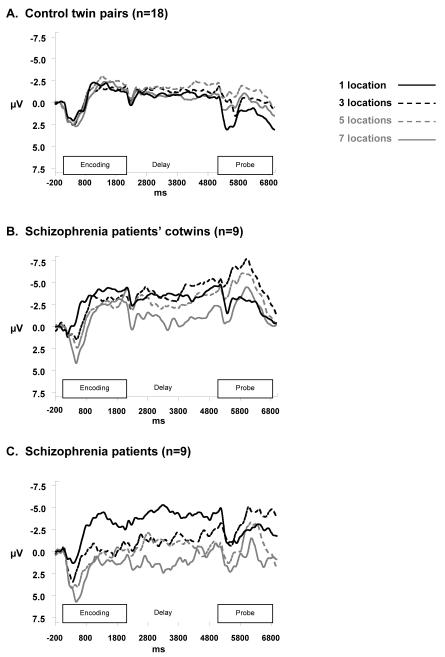
With respect to the CNV component, the ANOVA resulted in significant effects of diagnostic group, F(2,29)=9.14, P<0.01, memory set size, F(3,246)=4.18, P<0.01, and an interaction between group and set size, F(6,246)=2.45, P=0.03. The CNV (Figure 3) exhibited the enhanced effect of load in schizophrenia patients as compared to controls, with patients’ co-twins appearing to show an intermediate effect. Looking at Figure 3, this memory load effect emerges in the period 200 to 800 ms after memory array onset and appears to remain stable throughout the remainder of the trial, suggesting that diagnostic group effects are isolated to encoding period activity.
Figure 3. CNV Waveforms over Entire Trial at Cz’.
CNV waveforms at Cz’ during memory set encoding period (0 – 2000 ms) and delay period (2000 – 5000 ms), displayed across memory load levels for controls (A), for patients’ cotwins (B), and schizophrenia patients (C). Waveforms were low-pass filtered at 16 Hz and smoothed with a weighted, 15-point moving average for display purposes only.
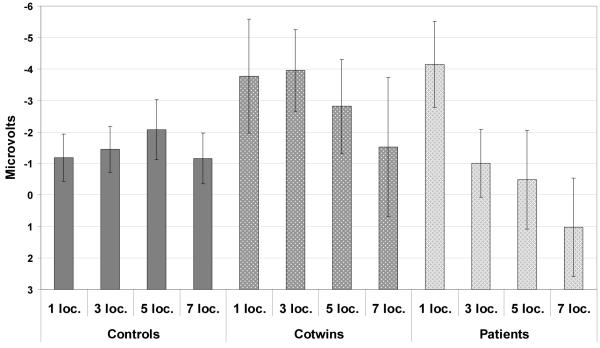
A planned contrast predicting that patients and their co-twins would show decreased CNV amplitude as compared to controls, irrespective of memory set size, was only marginally significant, F(1,16)=3.26, P=0.09; however, the contrast modeling a larger decrease in CNV amplitude with increasing memory set size among only patients and their co-twins was statistically significant, F(1,246)=9.22, P<0.01 (see Figure 4). Neither CNV subcomponent, F(1,246)=0.09, P=0.76, nor any of its interactions was significant (Subcomponent x Group: F[2,246]=1.29, P=0.28; Subcomponent x Load: F[3,246]=0.63, P=0.60; Subcomponent x Group x Load: F[6,246]=0.15, P=0.99).
Figure 4. CNV Amplitude over Entire Trial at Cz’.
Mean CNV amplitude at Cz’ (error bars representing ± 1 S. E. M.).
4. DISCUSSION
The principal finding of this study is that schizophrenia patients, and to an apparently intermediate extent, their non-psychotic co-twins, displayed an increased sensitivity to information maintenance demands when successfully performing a spatial working memory task as indexed by scalp-recordings of brain activity. This increased sensitivity emerged late in the stimulus encoding stage of a delayed match-to-sample task and persisted throughout the delay period, with relatively decreased CNV amplitude, until a correct behavioral response was provided. Based upon models of cognitive demand (Kahneman, 1973; Hockey, 1997), we infer the increased activation associated with behavioral success, to reflect greater allocation of cognitive resources to overcome decreased neurocognitive efficiency among affected twin pairs.
Although these results should be considered carefully, in light of the restricted samples included, they appear to provide evidence of a pattern of neural activity that parallel the findings from fMRI studies and offer converging support for the inefficiency hypothesis of schizophrenia-related WM deficits (Callicott et al., 2003; Manoach, 2003). Additionally, the current research indicates that significant inefficiency begins to emerge by the late stimulus encoding - perhaps stimulus representation consolidation (Vogel and Luck, 2002) - stage of active information maintenance. Moreover, this inefficiency exists in vulnerable, non-psychotic individuals, excluding the possibility that it results from medication exposure or symptom expression, and supporting the contention that it is instead associated with a causal factor shared by patients and their co-twins (either genetic or common environmental).
4.1. Study strengths and limitations
In highlighting the importance of the late encoding stage of information maintenance, the present results do not preclude the possibility that earlier abnormalities in visual stimulus encoding (e.g., Doniger et al., 2002; Butler et al., 2007; Sumich et al., 2008) contribute to “downstream” behavioral deficits. Given the relatively small sample size and long stimulus exposure period, the current protocol is not optimal for identifying such earlier perceptual differences. The presence of undetected, early perceptual deficits, however, would not negate the significance of the substantial increase in load sensitivity among patients and controls later in the stimulus encoding stage.
Several other study design issues also bear consideration. For instance, an independent component analysis-based artifact removal protocol was implemented in order to remove as much non-cerebral artifact as possible, while leaving EEG signals unaltered (Jung et al., 2000). Another signal processing-related consideration involves the use of a mean amplitude measure for CNV, computed over a relatively long time window, to ensure that the most stable value possible was used to describe the component that was most central to our analyses. From a statistical standpoint, none of the tests used assume equal variances between groups, and the inclusion of a minimum number of accurate, artifact-free epochs per condition criterion, further add to inferential power.
Finally, the exclusion of subjects performing below chance at two or more memory loads and perhaps, more importantly, the inclusion of only correct trials removed any uncertainty inherent in consideration of cognitive processes leading to incorrect responses. Isolating accurate performance in this manner removes influence of differences in performance achieved, a potential moderator of physiological effects (Van Snellenberg et al., 2006). Of course, the extent to which this subject selection procedure creates a relatively less representative patient sample must also be considered; on the other hand, any skewing towards better performance among patients and their co-twins would bias against our finding the significant group and Group-by-Load effects we report above. We are therefore confident that despite the somewhat small sample size, measures taken both prospectively and retrospectively place us on firm ground to draw our present conclusions.
Our focus on trials performed correctly also precludes examination of neural activity related to inaccurate performance. The current interpretive framework (Kahneman, 1973; Hockey, 1997) would predict that when patients and their co-twins exhaust the auxiliary neurocognitive resources used to compensate for inefficiency among more primary information maintenance mechanisms - due for instance to affected individuals having overall decreased attentional resources (e.g., Granholm et al., 1997) - they reach a functional capacity limit. For patients’ co-twins, the auxiliary resources are adequate to facilitate reaching (or possibly even surpassing) a given behavioral criterion (see also, Waldo et al, 1988, Davenport et al., 2006, or Sumich et al., 2008). Patients, however, fail to protect behavioral performance. In focusing on electrophysiological activity in participants working below or at capacity (i.e., examining only correct trials), however, the present study eschews the capacity limitations question and instead focuses on examining the neural response per unit increase in cognitive demand. Under these circumstances, we find patients and vulnerable individuals exhibit significant deficits in neurocognitive efficiency, as indicated by their larger changes in physiological indices of stimulus encoding and maintenance.
One noteworthy alternative interpretation of our findings is that the increase in late encoding and delay period activity seen in patients and their co-twins does not in itself indicate neural inefficiency, but instead reflects enhanced activation related to improved performance associated with schizophrenia vulnerability. In this case, patients’ co-twins’ behavioral performance, which is non-significantly better than controls’ performance, may reflect this enhancement; whereas, patients themselves may experience neurocognitive impairment secondary to the function of the WM network that prevents them from taking advantage of this enhancement, and even degrading their performance below that of controls (similar to Haenschel et al., 2007). Future research testing candidate impairments incidental to, but potentially influencing, the core WM system will be necessary to rule out this possibility.
4.2. Conclusions
The present results provide a unique electrophysiological perspective to the evolving debate on the nature of cognitive deficits in schizophrenia vulnerability and diagnosis. Specifically, they provide evidence that patients and their non-schizophrenic co-twins suffer from a loss of neurocognitive efficiency that becomes apparent relatively late in perceptual encoding and early in the maintenance of spatial information, possibly during stimulus representation consolidation. Reports published to date have suggested that such an abnormality may exist, but none have provided such direct, temporally-specific evidence. Furthermore, patients’ co-twins appear capable of allocating additional cognitive resources in a manner that successfully compensates for this inefficiency, preventing overt expression of behavioral deficits. The schizophrenia patients themselves appear unable to compensate for these impairments to the extent that they are prevented from finding behavioral expression.
4.3. Future directions
First and foremost, the present findings await replication in a larger, more diverse sample of patients and their non-schizophrenic relatives in order to speak to the generalizability of this electrophysiological inefficiency effect. Also, additional study will be necessary to determine specifically what heritable factors contribute to this vulnerability-related inefficiency. Promising candidates include genetic polymorphisms (e.g, Gasperoni et al., 2003, and Bertolino et al., 2004) influencing cortical dopamine regulation, a factor critical to both the encoding of stimuli into mental representations supported by neural assemblies, and the efficient differentiation of electrophysiological signal from background noise in processes functioning to maintain such representations (Winterer and Weinberger, 2004).
Supplementary Material
ACKNOWLEDGMENT
The authors wish to thank Ulla Mustonen, Pirjo Käki, and Eila Voipio for their contributions to subject recruitment and evaluation, Antti Tanskanen for his contributions to the register searches, Kauko Heikkilä for his contributions to data management of the Finnish Twin Cohort Study, Theo van Erp for statistical consultation, several anonymous reviewers for helpful comments, and the Finnish twins for their participation in the study.
Funding for this study was provided by NIMH grants R01 MH052857 and T32 MH014584, from NIMH fellowship F31 MH068121, from Academy of Finland Research Grant 53294, from Academy of Finland Center of Excellence in Complex Disease Genetics, and from gifts to the UCLA Foundation from the Staglin Music Festival for Mental Health. These funding sources had no further role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the paper for publication.
Footnotes
Previous Presentation
Portions of this work were presented at the meeting of the Society for Psychophysiology Research in Vancouver, BC, October 25 – 29, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andreasen NC. The Scale for the Assessment of Positive Symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS) The University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Barch DM. The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology. 2005;1:321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Barch DM, Smith E. The cognitive neuroscience of working memory: Relevance to CNTRICS and schizophrenia. Biological Psychiatry. doi: 10.1016/j.biopsych.2008.03.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V. Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. American Journal of Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Elbert T, Canavan AG, Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiological Reviews. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, Sham PC, Frangou S, Murray RM. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27:960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: More than up or down. American Journal of Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cameron AM, Geffen GM, Kavanagh DJ, Wright MJ, McGrath JJ, Geffen LB. Event-related potential correlates of impaired visuospatial working memory in schizophrenia. Psychophysiology. 2003;40:702–715. doi: 10.1111/1469-8986.00071. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Kaprio J, Lönnqvist J, Huttunen M, Koskenvuo M. The genetic epidemiology of schizophrenia in a Finnish twin cohort: A population-based modeling study. Archives of General Psychiatry. 1998;55:67–74. doi: 10.1001/archpsyc.55.1.67. [DOI] [PubMed] [Google Scholar]
- Chiu P, Ambady N, Deldin P. Contingent negative variation to emotional in- and out-group stimuli differentiates high- and low-prejudiced individuals. Journal of Cognitive Neuroscience. 2004;16:1830–1839. doi: 10.1162/0898929042947946. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Sponheim SR, Stanwyck JJ. Neural anomalies during visual search in schizophrenia patients and unaffected siblings of schizophrenia patients. Schizophrenia Research. 2006;82:15–26. doi: 10.1016/j.schres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Archives of General Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. Working memory consolidation is abnormally slow in schizophrenia. Journal of Abnormal Psychology. 2005;114:279–290. doi: 10.1037/0021-843X.114.2.279. [DOI] [PubMed] [Google Scholar]
- Gasperoni TL, Ekelund J, Huttunen M, Palmer CG, Tuulio-Henriksson A, Lönnqvist J, et al. Genetic linkage and association between chromosome 1q and working memory function in schizophrenia. American Journal of Medical Genetics. 2003;116B:8–16. doi: 10.1002/ajmg.b.10757. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Therman S, Manninen M, Huttunen M, Kaprio J, Lönnqvist J, Cannon TD. Spatial working memory as an endophenotype for schizophrenia. Biological Psychiatry. 2003;53:624–626. doi: 10.1016/s0006-3223(02)01641-4. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Granholm E, Morris SK, Sarkin AJ, Asarnow RF, Jeste DV. Pupillary responses index overload of working memory resources in schizophrenia. Journal of Abnormal Psychology. 1997;106:458–467. doi: 10.1037//0021-843x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Groom MJ, Bates AT, Jackson GM, Calton TG, Liddle PF, Hollis C. Event-related potentials in adolescents with schizophrenia and their siblings: A comparison with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2008;63:784–792. doi: 10.1016/j.biopsych.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DEJ. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia. Archives of General Psychiatry. 2007;64:1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Handy TC, Mangun GR. Attention and spatial selection: Electrophysiological evidence for modulation by perceptual load. Perception and Psychophysics. 2000;62:175–186. doi: 10.3758/bf03212070. [DOI] [PubMed] [Google Scholar]
- Hockey GR. Compensatory control in the regulation of human performance under stress and high workload: A cognitive-energetical framework. Biological Psychology. 1997;45:73–93. doi: 10.1016/s0301-0511(96)05223-4. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Report of the Committee on Methods of Clinical Examination in Electroencephalography. Electroencephalography and Clinical Neurophysiology. 1958;10:370–371. [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and Effort. Prentice Hall; New Jersey: 1973. [Google Scholar]
- Kaprio J, Koskenvuo M. Genetic and environmental factors in complex diseases: The older Finnish Twin Cohort. Twin Research. 2002;5:358–365. doi: 10.1375/136905202320906093. [DOI] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TG, Therman S, Huttunen M, Manninen M. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Klein C, Rockstroh B, Cohen R, Berg P. Contingent negative variation (CNV) and determinants of the post-imperative negative variation (PINV) in schizophrenic patients and healthy controls. Schizophrenia Research. 1996;21:97–110. doi: 10.1016/0920-9964(96)00028-x. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. Journal of Abnormal Psychology. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- Loranger AW, Sussman VL, Oldham JM, Russakoff LM. Personality disorder examination: A structured interview for making diagnosis of DSM-III-R personality disorders. Cornell Medical College; White Plains, NY: 1985. [Google Scholar]
- Low A, Rockstroh B, Harsch S, Berg P, Cohen R. Event-related potentials in a working-memory task in schizophrenics and controls. Schizophrenia Research. 2000;46:175–186. doi: 10.1016/s0920-9964(00)00012-8. [DOI] [PubMed] [Google Scholar]
- Mangia S, Giove F, Tkáč I, Logothetis NK, Henry P-G, Olman CA, Maraviglia B, DiSalle F, Uğurbil K. Metabolic and hemodynamic events after changes in neuronal activity: Current hypotheses, theoretical predictions and in vivo NMR experimental findings. Journal of Cerebral Blood Flow and Metabolism. 2008:1–23. doi: 10.1038/jcbfm.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: Reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- McEvoy LK, Smith ME, Gevins A. Dynamic cortical networks of verbal and spatial working memory: Effects of memory load and task practice. Cerebral Cortex. 1998;8:563–574. doi: 10.1093/cercor/8.7.563. [DOI] [PubMed] [Google Scholar]
- Muller NG, Knight RT. Age-related changes in fronto-parietal networks during spatial memory: An ERP study. Brain Research: Cognitive Brain Research. 2002;13:221–234. doi: 10.1016/s0926-6410(01)00119-7. [DOI] [PubMed] [Google Scholar]
- Nunez P, Srinivasan R. Electric fields of the brain. The neurophysics of EEG. 2nd ed. Oxford University Press; New York: 2006. [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Archives of General Psychiatry. 1992;49:975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Archives of General Psychiatry. 1995;52:821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Piskulic D, Olver JS, Norman TR, Maruff P. Behavioral studies of spatial working memory dysfunction in schizophrenia: A quantitative literature review. Psychiatry Research. 2007;150:111–121. doi: 10.1016/j.psychres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Brown GG, McCarthy G, Greve DN, Glover GH, Manoach DS, Belger A, Diaz M, Wible CG, Ford JM, Mathalon DH, Gollub R. Working memory and DLPFC inefficiency in schizophrenia: The FBIRN study. Schizophrenia Bulletin. 2009;35:19–31. doi: 10.1093/schbul/sbn162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth WT, Kopell BS, Tinklenberg JR, Darley CF, Sikora R, Vesecky TB. The contingent negative variation during a memory retrieval task. Electroencephalography and Clinical Neurophysiology. 1975;38:171–174. doi: 10.1016/0013-4694(75)90226-6. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Prabhakaran V, Bly BM, Kimberg DY, Biswal B, D’Esposito M. Neural correlates of cognitive efficiency. Neuroimage. 2006;33:969–979. doi: 10.1016/j.neuroimage.2006.05.065. [DOI] [PubMed] [Google Scholar]
- Schlösser RGM, Koch K, Wagner G, Nenadic I, Roebel M, Schachtzabel C. Inefficient executive cognitive control in schizophrenia preceded by altered functional activation during information encoding: An fMRI study. Neuropsychologia. 2008;46:336–347. doi: 10.1016/j.neuropsychologia.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Sirevaag EJ, Kramer AF, Coles MG, Donchin E. Resource reciprocity: An event-related brain potentials analysis. Acta Psycholica (Amsterdam) 1989;70:77–97. doi: 10.1016/0001-6918(89)90061-9. [DOI] [PubMed] [Google Scholar]
- Smulders FTY, Kok A, Kenemans JL, Bashore TR. The temporal selectivity of additive factor effects on the reaction process revealed in ERP component latencies. Acta Psychologica. 1995;90:97–109. doi: 10.1016/0001-6918(95)00032-p. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Williams JB. Research diagnostic criteria. Archives of General Psychiatry. 1979;36:1381–1383. doi: 10.1001/archpsyc.1979.01780120111013. [DOI] [PubMed] [Google Scholar]
- Sumich A, Kumari V, Dodd P, Ettinger U, Hughes C, Zachariah E, Sharma T. N100 and P300 amplitude to Go and No-Go variants of the auditory oddball in siblings discordant for schizophrenia. Schizophrenia Research. 2008;98:265–277. doi: 10.1016/j.schres.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Tan HY, Choo WC, Fones CS, Chee MW. The effect of working memory performance on functional MRI in schizophrenia. Schizophrenia Research. 2005;74:179–194. doi: 10.1016/j.schres.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Thermenos HW, Seidman LJ, Breiter H, Goldstein JM, Goodman JM, Poldrack R. Functional magnetic resonance imaging during auditory verbal working memory in nonpsychotic relatives of persons with schizophrenia: A pilot study. Biological Psychiatry. 2004;55:490–500. doi: 10.1016/j.biopsych.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Van Snellenberg JX, Torres IJ, Thornton AE. Functional neuroimaging of working memory in schizophrenia: Task performance as a moderating variable. Neuropsychology. 2006;20:497–510. doi: 10.1037/0894-4105.20.5.497. [DOI] [PubMed] [Google Scholar]
- Virtanen J, Rinne T, Ilmoniemi RJ, Näätänen R. MEG-compatible multichannel EEG electrode array. Electroencephalography and Clinical Neurophysiology. 1996;99:568–580. doi: 10.1016/s0013-4694(96)96575-x. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Luck SJ. Delayed working memory consolidation during the attentional blink. Psychonomic Bulletin and Review. 2002;9:739–743. doi: 10.3758/bf03196329. [DOI] [PubMed] [Google Scholar]
- Waldo MC, Adler LE, Freedman R. Defects in auditory sensory gating and their apparent compensation in relatives of schizophrenics. Schizophrenia Research. 1988;1:19–24. doi: 10.1016/0920-9964(88)90035-7. [DOI] [PubMed] [Google Scholar]
- Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent negative variation: An electric sign of sensorimotor association and expectancy in the human brain. Nature. 1964;203:380–384. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in Neurosciences. 2004;27:683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.