Abstract
Campbell, J. N. (American Meat Institute Foundation, Chicago, Ill.), James B. Evans Jerome J. Perry, and C. F. Niven, Jr. An extracellular material elaborated by Micrococcus sodonensis. J. Bacteriol. 82:828–837. 1961.—When actively growing in a yeast extract-tryptone broth, Micrococcus sodonensis produced rather large quantities of an extracellular material. On a dry weight basis, this material consisted of approximately 80% deoxyribonucleic acid (DNA), 6% ribonucleic acid, 13% protein, and a small quantity of a cell pigment which contained an associated iron porphyrinlike compound.
The extracellular material was not produced in other complex or synthetic media. Removal of cells from the appropriate growth medium and suspension in distilled water resulted in the prevention of formation of the material if it had not already begun, or its immediate cessation in those cells which had been actively producing the material.
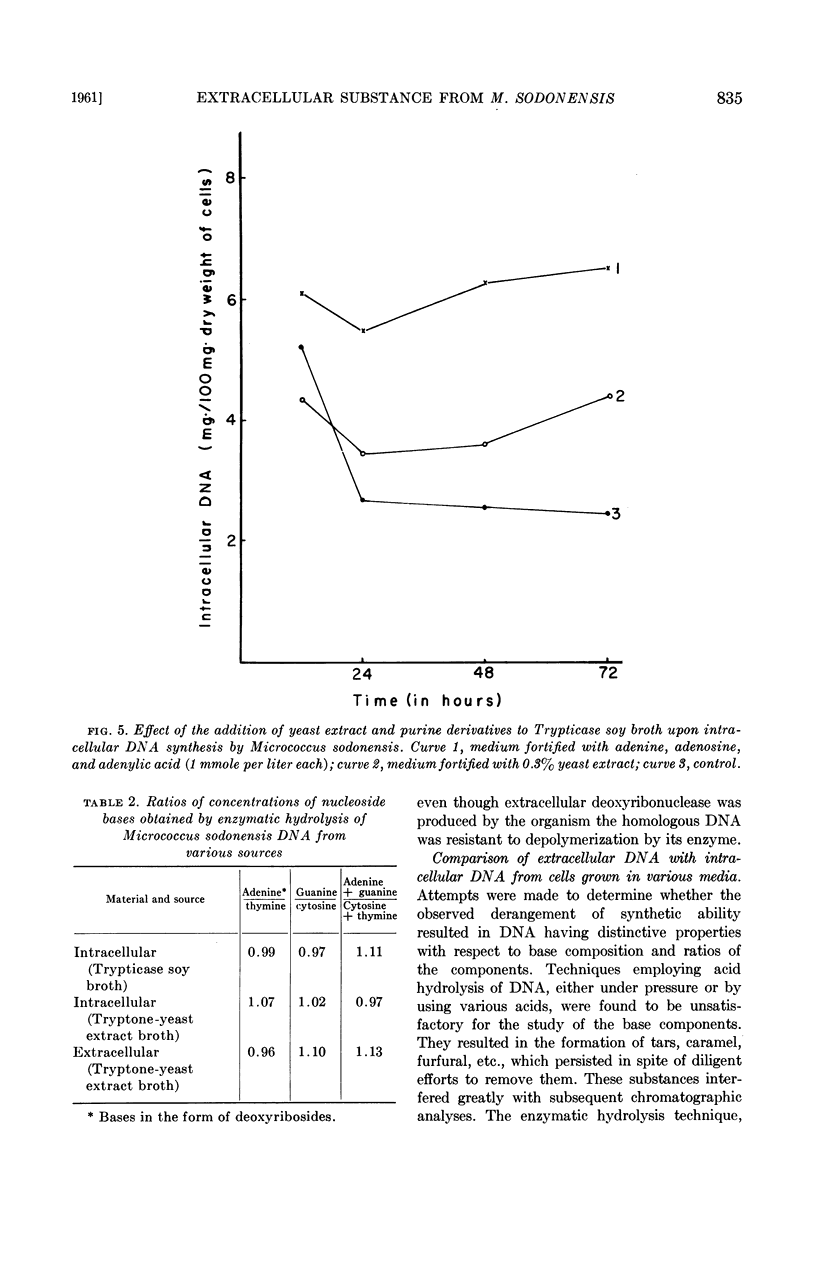
Cells producing the extracellular material exhibited an increasingly higher concentration of intracellular DNA as incubation proceeded, whereas cells growing under conditions not supporting such production showed a decline in intracellular DNA as the culture aged. A similar increase of intracellular DNA, but not release of extracellular material, could be effected by addition of yeast extract or high levels of purine compounds to a medium which did not support the production of extracellular material.
The base composition and ratios of both extracellular and intracellular DNA produced under all conditions tested were compared and found to be similar or identical, and to correspond to the classical Watson-Crick structure for DNA.
Full text
PDF

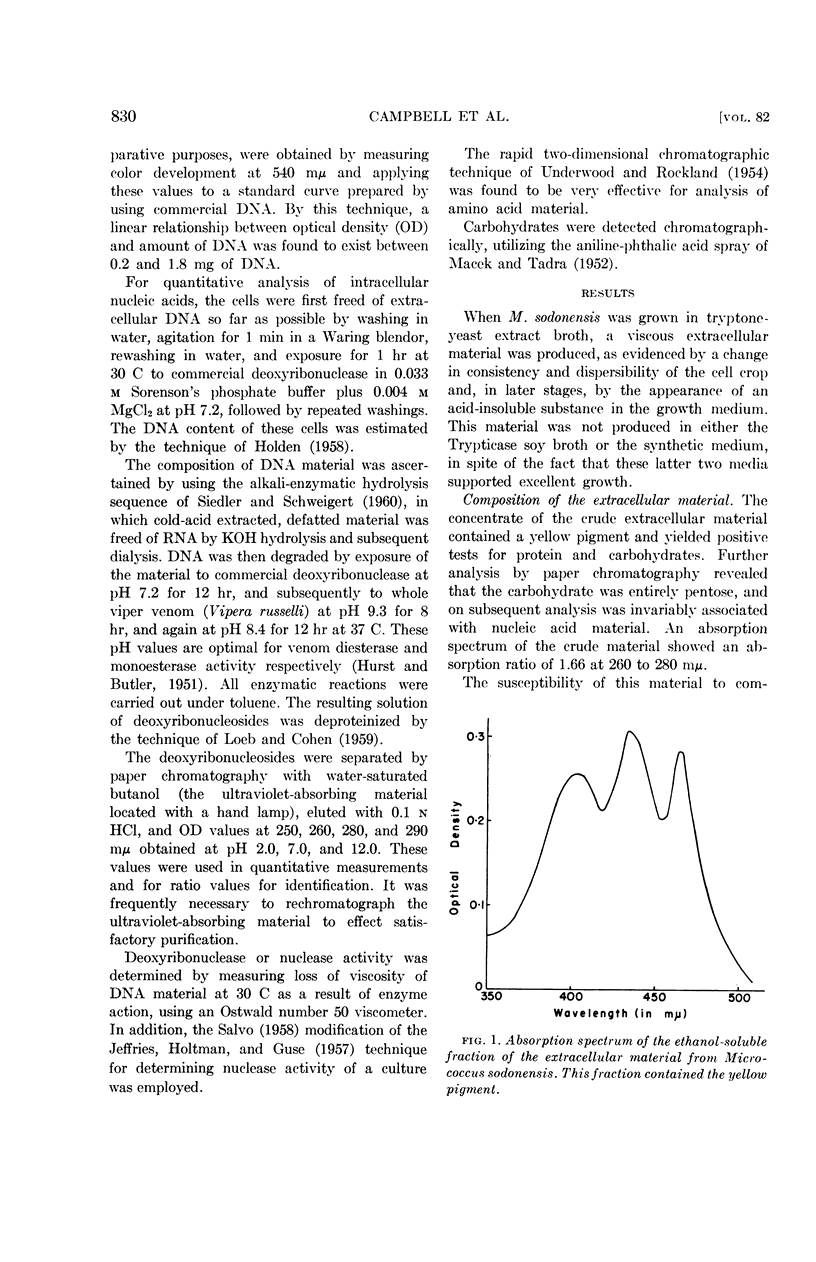

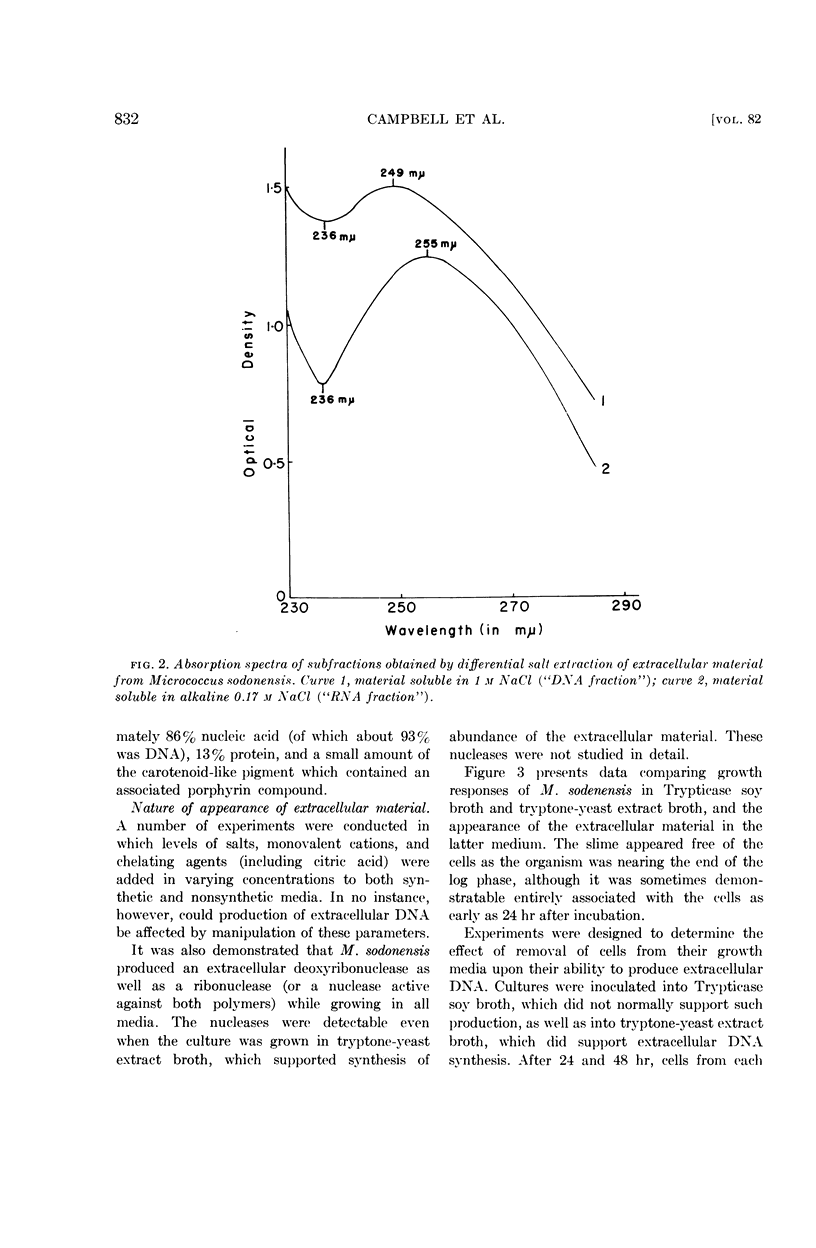
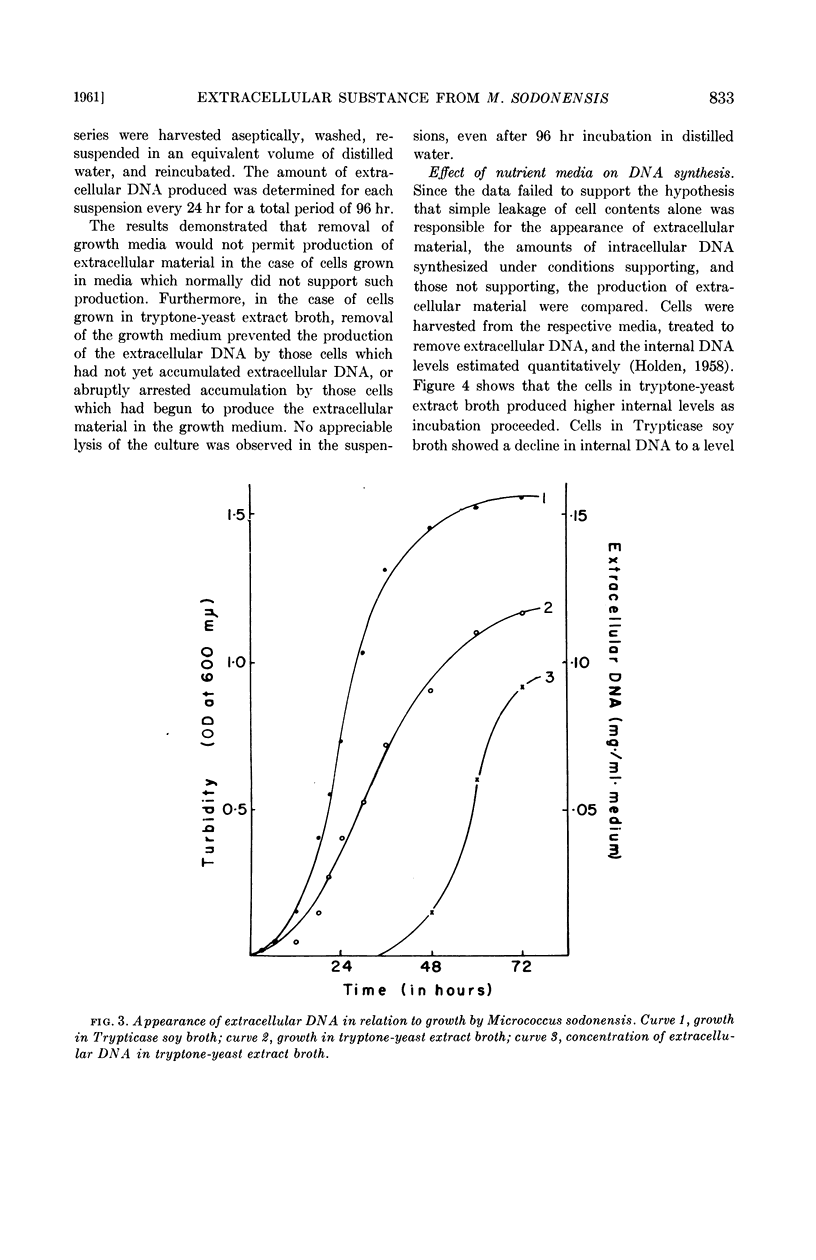
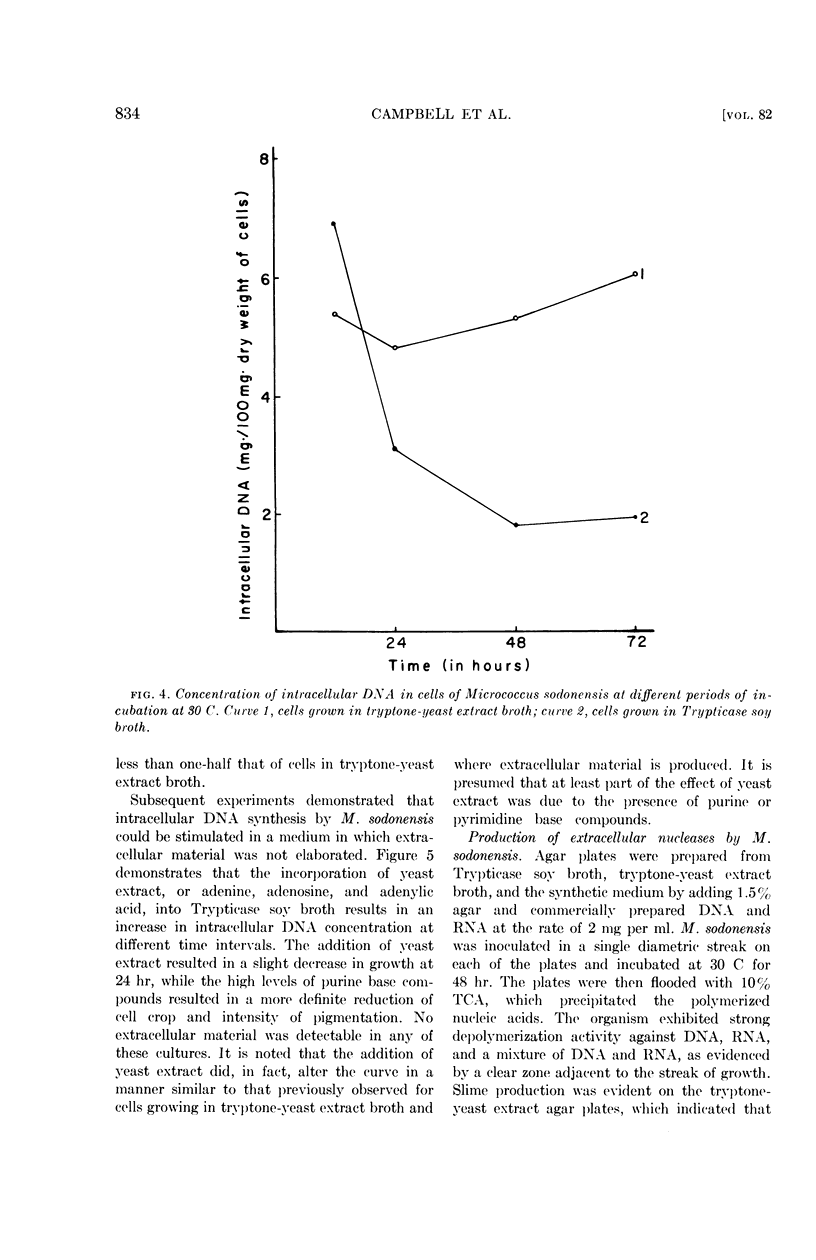


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AARONSON S. Biotin assay with a coccus, Micrococcus sodonensis, nov. sp. J Bacteriol. 1955 Jan;69(1):67–69. doi: 10.1128/jb.69.1.67-69.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W., CUNNINGHAM L. S. Studies of extracellular and intracellular bacterial deoxyribonucleic acids. J Gen Microbiol. 1958 Dec;19(3):522–539. doi: 10.1099/00221287-19-3-522. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W. Extracellular deoxyribonucleic acid of bacteria and a deoxyribonuclease inhibitor. Science. 1956 Sep 7;124(3219):441–442. doi: 10.1126/science.124.3219.441. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W. Transformation of Neisseria meningitidis by deoxyribonucleates from cells and from culture slime. J Bacteriol. 1960 Apr;79:579–590. doi: 10.1128/jb.79.4.579-590.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W. [Interspecific transformation of Neisseria by culture slime containing deoxyribonucleate]. Science. 1960 Feb 26;131(3400):608–610. doi: 10.1126/science.131.3400.608-a. [DOI] [PubMed] [Google Scholar]
- DEIBEL R. H., EVANS J. B. Modified benzidine test for the detection of cytochrome-containing respiratory systems in microorganisms. J Bacteriol. 1960 Mar;79:356–360. doi: 10.1128/jb.79.3.356-360.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLDEN J. T. Degradation of intracellular nucleic acid and leakage of fragments by Lactobacillus arabinosus. Biochim Biophys Acta. 1958 Sep;29(3):667–668. doi: 10.1016/0006-3002(58)90041-6. [DOI] [PubMed] [Google Scholar]
- HURST R. O., BUTLER G. C. The chromatographic separation of phosphatases in snake venoms. J Biol Chem. 1951 Nov;193(1):91–96. [PubMed] [Google Scholar]
- JACKSON F. L., LAWTON V. D. A cytochrome of the b group from Micrococcus lysodeikticus. Biochim Biophys Acta. 1959 Sep;35:76–84. doi: 10.1016/0006-3002(59)90336-1. [DOI] [PubMed] [Google Scholar]
- JEFFRIES C. D., HOLTMAN D. F., GUSE D. G. Rapid method for determining the activity of microorganisms on nucleic acids. J Bacteriol. 1957 Apr;73(4):590–591. doi: 10.1128/jb.73.4.590-591.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOEB M. R., COHEN S. S. The origin of purine and pyrimidine deoxyribose in Escherichia coli systems. J Biol Chem. 1959 Feb;234(2):360–363. [PubMed] [Google Scholar]
- PERRY J. J., EVANS J. B. Oxidative metabolism of lactate and acetate by Micrococcus sodonensis. J Bacteriol. 1960 Jan;79:113–118. doi: 10.1128/jb.79.1.113-118.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEDLER A. J., SCHWEIGERT B. S. Influence of ribonucleotides on the utilization of deoxyribonucleotides by Lactobacillus acidophilus. J Bacteriol. 1959 Apr;77(4):514–515. doi: 10.1128/jb.77.4.514-515.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITHIES W. R., GIBBONS N. E. The deoxyribose nucleic acid slime layer of some halophilic bacteria. Can J Microbiol. 1955 Oct;1(8):614–621. doi: 10.1139/m55-074. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I., GIBBONS N. E. Effect of salt concentration on the extracellular nucleic acids of Micrococcus halodenitrificans. Can J Microbiol. 1957 Aug;3(5):687–694. doi: 10.1139/m57-076. [DOI] [PubMed] [Google Scholar]
- VOLKIN E., COHN W. E. Estimation of nucleic acids. Methods Biochem Anal. 1954;1:287–305. doi: 10.1002/9780470110171.ch11. [DOI] [PubMed] [Google Scholar]



