Abstract
OBJECTIVE:
To review clinical aspects of management of tuberculosis (TB) infection and disease in Canadian children in the context of the global TB epidemic and the rising incidence of drug-resistant TB.
DATA SOURCES:
Original and review articles pertinent to: epidemiology of TB globally and in Canada; management of latent TB infection and TB disease in children; diagnostic tests for latent TB infection and TB disease; and management of drug-resistant TB disease. Multiple Medline searches were used including combinations of the MeSH terms ‘Tuberculosis*’ (and its multiple subheadings), ‘Child*’, ‘Drug Resistance’, ‘Mycobacterium tuberculosis*’ and ‘Canada/epidemiology*’. Select relevant textbooks were reviewed.
DATA SELECTION AND EXTRACTION:
The articles were analyzed from the perspective of clinicians managing children in Canada today, and from our experience of managing children with TB in Southern Ontario.
DATA SYNTHESIS:
TB in Canada is largely a disease of the foreign-born and their children, but continues to occur in aboriginal children. Drug resistance is increasing globally and in Canada. Most children with TB disease in Canada are asymptomatic and found through contact tracing. False positive skin tests are frequent where TB prevalence is low.
CONCLUSIONS:
Obtain source case drug sensitivities when treating TB contacts and those with latent TB infection. Obtain cultures before treating TB disease and treat disease with at least four antituberculous drugs while awaiting sensitivities. Use Directly Observed Therapy for TB disease. Confine TB skin testing to children at high risk for TB infection or disease, including contacts of infectious patients and recent immigrants. A team approach and infection control measures including environmental controls are important in managing TB disease.
Keywords: Canada, Drug resistance, Management, Tuberculosis
Abstract
OBJECTIF :
Examiner les aspects cliniques de la prise en charge de la tuberculose (TB)-infection et de la TB-maladie chez les enfants canadiens dans le contexte de l’épidémie mondiale de TB et de l’incidence croissante de TB résistante aux médicaments.
SOURCES DE DONNÉES :
Des articles originaux et des exposés de synthèses portant sur l’épidémiologie de la TB dans le monde et au Canada, la prise en charge de la TB-infection latente et de la TB-maladie chez les enfants, les tests diagnostiques de l’infection latente et de la maladie et la prise en charge de la maladie résistante aux médicaments. Des recherches multiples dans Medline ont été utilisées, y compris les combinaisons suivantes de termes tirés du thésaurus MeSH : « tuberculosi* » (et ses multiples sous-titres), « child* », « drug resistance, Mycobacterium tuberculosis* » et « Canada/epidemiology* ». Des manuels sélectionnés pertinents ont été examinés.
SÉLECTION ET EXTRACTION DE DONNÉES :
Les articles ont été analysés selon la perspective de cliniciens qui soignent des enfants au Canada aujourd’hui et selon notre expérience du traitement d’enfants atteints de TB dans le sud de l’Ontario.
SYNTHÈSE DE DONNÉES :
La TB au Canada est en grande partie une maladie des personnes nées en pays étranger et de leurs enfants, mais elle continue de sévir chez les enfants autochtones. La résistance aux médicaments augmente à l’échelle mondiale et au Canada. La plupart des enfants atteints de TB-maladie au Canada sont asymptomatiques et ont été dépistés par recherche de contacts. Les faux positifs cutanés sont courants dans les régions où la prévalence de TB est faible.
CONCLUSIONS :
Obtenir la réactivité aux médicaments du cas source au moment de traiter les contacts de TB et les personnes atteintes d’une TB-infection latente. Obtenir des cultures avant d’amorcer un traitement pour la TB-maladie et traiter la maladie à l’aide d’au moins quatre antitu-berculeux en attendant les résultats de la réactivité. Utiliser le traitement sous observation directe de la maladie confirmée. Réserver les tests cutanés de TB aux enfants à haut risque de TB-maladie, y compris les contacts des patients infectieux et des immigrants récents. Une démarche multidisciplinaire et des mesures de contrôle de l’infection, y compris des contrôles environnementaux, s’imposent dans la prise en charge de la TB-maladie.
Paediatricians practising in Canada commonly have to decide whether to screen children for infection with tuberculosis (TB). We also may treat children who have been exposed to patients with infectious TB or manage children with suspected or proven TB disease.
This article is meant to supplement recent excellent reviews of the pathogenesis and management of TB in childhood (1–4), including the Canadian Tuberculosis Standards (3), which is freely accessible on the Internet. We hope to discuss childhood TB in Canada in the context of rising drug resistance (5) and rising worldwide prevalence (6), and outline some of the evidence on which our ‘recipes’ for management are based. The accompanying clinical scenarios and quiz (pages 171–172) are based on cases seen in Southern Ontario.
It is estimated that worldwide one in three people alive today are infected with Mycobacterium tuberculosis (6). Most are well and latently infected but are at risk for progression to TB disease. In 1997, the World Health Organization estimated there were eight million new cases of active TB and approximately 1.9 million deaths from TB (6). However, this number, largely based on sputum smear examination, likely excluded many children with active disease (7). Childhood TB is often extrapulmonary and paucibacillary, and many children are unable to produce sputum (4,7).
The incidence of TB in Canada has declined dramatically since the 1930s, especially in Canadian-born individuals (8). TB in Canada is predominantly a disease of foreign-born people and their children (8). Over 75% of active cases are confined to the urban areas of Ontario, Quebec and British Columbia. TB continues to occur in status Indians and Inuit, especially in Manitoba, Saskatchewan and the Northwest Territories. Although rates of TB disease continue to decline, case rates in children younger than five years of age are actually increasing (8).
PATHOGENESIS AND DEFINITIONS
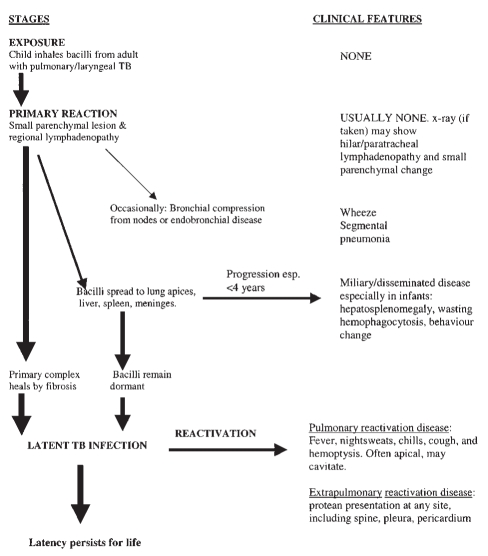
The stages in the pathogenesis of TB in childhood and accompanying clinical features are briefly summarized in Figure 1.
Figure 1).
Stages in the pathogenesis of childhood tuberculosis and related clinical features. esp Especially; TB Tuberculosis
Children typically inhale infectious particles expelled by another person, usually an adult, with active pulmonary disease (4,9). Transplacental transmission leading to true congenital TB is rare (10). Rarely, the skin or gastrointestinal tract may be the site of inoculation.
A primary reaction often occurs, consisting of a small parenchymal lesion and regional lymphadenopathy. Bacilli may now spread widely to the lung apices, liver, spleen, meninges, pleura and bone. These may form the site of reactivation TB; less commonly, meningitis, miliary or otherwise disseminated disease develops within three to six months of the primary infection. This especially occurs in children younger than four years of age (11). Occasionally, the primary complex may enlarge and caseate immediately, or nodes may enlarge and either compress a bronchus or erode through, leading to wheezing and segmental pneumonia or atelectasis (12,13).
In the majority of children, however, the parenchymal and node portions heal by fibrosis and sometimes calcify. Some TB bacilli remain dormant, contained by host defenses, and may reactivate later. This dormant state constitutes latent infection (1).
Most children infected with M tuberculosis have latent infection. Whereas about 10% of infected adults will develop active disease over a lifetime (14), up to 40% of young children may progress to disease following infection, and much of this is extrapulmonary or disseminated (11,14).
Latent TB infection
A child with latent TB infection (LTBI) is said to have a few bacilli sequestered somewhere in the body in a dormant state. We define this practically as a child with a positive tuberculin skin test (TST), no physical findings of disease, and a chest radiograph that is either normal, or only has granulomas or calcification (1,15). A central problem with this approach is the absence of a gold standard for a diagnosis of LTBI; the TST may not identify all those at risk for disease (16).
Because most children acquire TB from adults, risk factors for infection follow the epidemiology of TB disease in the adult population (1,3,17). In Canada this is most commonly related to immigration from a country with a high prevalence of TB (8).
TB disease
TB disease occurs when signs and symptoms or radiographic manifestations caused by M tuberculosis appear. Disease may be pulmonary, extrapulmonary or both.
Risk factors for progression to disease include age, especially younger than four years; immunosuppression, including human immunodeficiency virus (HIV) infection; and diabetes mellitus (1). Recently, the anti-tumour necrosis factor alpha agents infliximab and etanercept have been associated with risk for aggressive and unusual TB disease (18).
Although we distinguish between infection and disease, they are somewhat arbitrary points along a spectrum of increasing bacterial load (15). The higher this load, the greater the need for multiple drugs to prevent resistance and ensure cure (4,15).
CLINICAL PRESENTATION OF TB DISEASE
Most children with TB disease in North America are asymptomatic and are discovered as part of investigation of adult contacts. Typically these children have x-ray abnormalities but appear entirely well, without clinical signs (17).
In contrast, children in resource-poor, high incidence countries, where screening is not widely available, are identified because of significant involvement of almost any organ system (19). This pattern is also seen in Canada, chiefly in immigrant children.
Older children and adolescents are more likely to experience reactivation disease, and often have a classic triad of symptoms: fever, weight loss and night sweats. However, signs are still unusual and may be quite subtle (20).
Miliary disease is much more common in young infants (21) and immunocompromised individuals (22). Miliary refers to diffuse tiny nodules, similar in size to millet seeds, that are seen on x-ray. Hepatosplenomegaly and weight loss are frequent, as is hemophagocytosis. The TST is often negative (21,22).
Children co-infected with HIV and TB have an accelerated progression from infection to TB disease. Co-infected adults often have atypical presentations with extrapulmonary disease, but children with HIV infection usually present with typical childhood disease (23). The skin test is often negative: a search for an infectious adult or adolescent is an important clue to diagnosis (4).
DIAGNOSTIC TESTS
TST
The TST has changed little for almost a century (24). The Mantoux test is the only standardized test that should be used but has many limitations. Purified protein derivative (PPD), an extract of over 200 antigens, some of which are common to M tuberculosis, Mycobacterium bovis and environmental mycobacteria is injected intradermally (25). This elicits a cutaneous delayed hypersensitivity response in sensitized individuals. The result is read at 48 to 72 h. False negative tests occur in up to 10% of children with TB disease (26). False positive tests are common, especially in areas of low prevalence of TB (27). The definition of a positive test is somewhat arbitrary but depends on both the size of the reaction and the risk factors for LTBI. Interpretation of results differs between Canadian and American guidelines (Tables 1,2) (1,28). Practically, an induration of 5 mm or greater is regarded as positive in children exposed to TB disease, while 10 mm or more is considered positive in children born in endemic countries. Although Bacille Calmette-Guerin (BCG) immunization may influence the results, especially for boosted reactions, most children will lose tuberculin reactivity after three years (4,27,29). Because BCG vaccine is frequently used in areas with high rates of transmission of TB, a history of BCG vaccination is generally ignored when administering and interpreting a TST (1,4). The technique for performing and reading the test is as follows.
TABLE 1.
Interpretation of tuberculin skin test (TST) results: American guidelines
| TSTs should be read at 48 to 72 h after placement |
| Induration more than 5 mm |
| Children in close contact with known or suspected contagious cases of tuberculosis disease |
Children suspected to have tuberculosis disease:
|
| Children receiving immunosuppressive therapy or with immunosuppressive conditions, including human immunodeficiency virus infection |
| Induration more than 10 mm |
Children at increased risk of disseminated disease:
|
Children with increased risk of exposure to tuberculosis disease:
|
| Induration less than 15 mm |
| Children four years of age or older without any risk factors |
Data from reference 1
TABLE 2.
Canadian tuberculosis standards: interpretation of tuberculin test
| Tuberculin reaction (induration [mm]) | Setting in which reaction was considered significant (meaning probable tuberculosis infection) |
|---|---|
| 0–4 | Human immunodeficiency virus infection and/or expected risk of tuberculosis infection is high (eg, patient is an immigrant from a country where tuberculosis is endemic, is a household contact, or has an abnormal radiograph). This reaction size is not normally considered significant, but in the presence of immune suppression may be important |
| 5–9 | Human immunodeficiency virus infection |
| Close contact of active contagious case | |
| Abnormal chest radiograph with fibronodular disease | |
| 10 | All others |
Data from reference 28
TST technique:
Introduce 0.1 mL of tuberculin (5 tuberculin units) just under the top layer of skin (intradermally) on the forearm. To ensure the child holds still, a second person may be needed to stabilize the arm of young children. Use a short, disposable, 26-gauge needle, with the needle bevel facing upward. Injections should raise a discrete skin elevation (a wheal) 6 to 10 mm in diameter. Label new vials of tuberculin on opening and discard after one month. Instruct the patient not to rub, scratch, or put a bandage on the test site. The area may be washed and patted dry. To avoid false negative reactions, administer skin tests on the same day as, or at least six weeks after, measles-mumps-rubella vaccine.
Ensure that only a trained health worker reads the test result 48 to 72 h after administration. Measure only the hard, swollen area, known as induration, at its widest point using a flexible ruler. Do not measure any redness. Record the size of the induration in millimeters. If there is no induration, record the result as ‘0 mm’. A reporting form is helpful to ensure the result reaches the patient’s record.
Newer tests for LTBI
Newer tests for LTBI attempt to overcome the problems associated with TSTs, including the need for return visits for reading, and the effect of BCG immunization on test results. Evaluation of these tests is complicated by the lack of a gold standard for LTBI. The QuantiFERON-TB, a whole-blood assay, measures interferon gamma production when PPDs of mycobacteria are incubated with venous blood samples (30). Guidelines for its use and interpretation have recently been published by the Centers for Disease Control and Prevention (31), but the test has not been evaluated in children. Although its performance may be better than the TST, the test still has problems of specificity when M tuberculosis-PPD is used as the antigen (32), and has had disappointing results in populations with a high rate of TB (33). A similar test using early secretory antigenic target (ESAT-6), an antigen specific to M tuberculosis (and not found in BCG), may hold more promise for the future (25). As IFN production is often suppressed in TB disease, both tests are not helpful in the detection of disease caused by M tuberculosis (31).
Chest x-rays
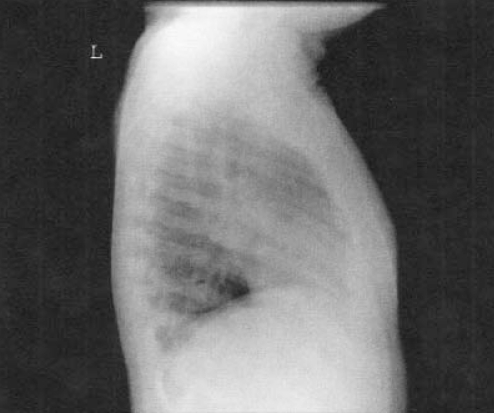
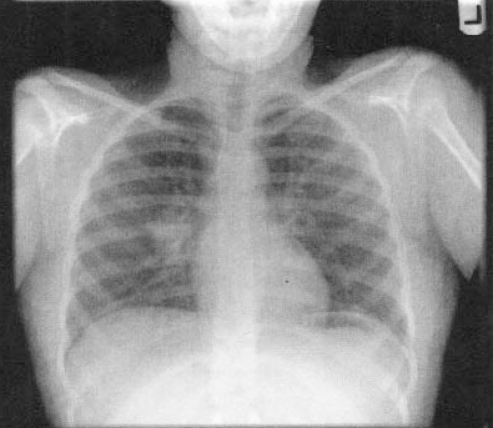
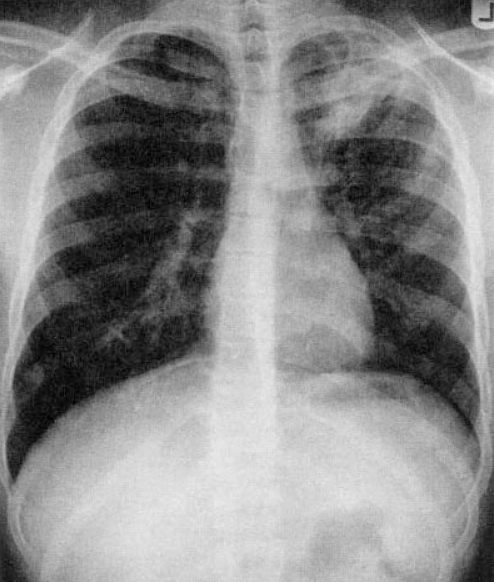
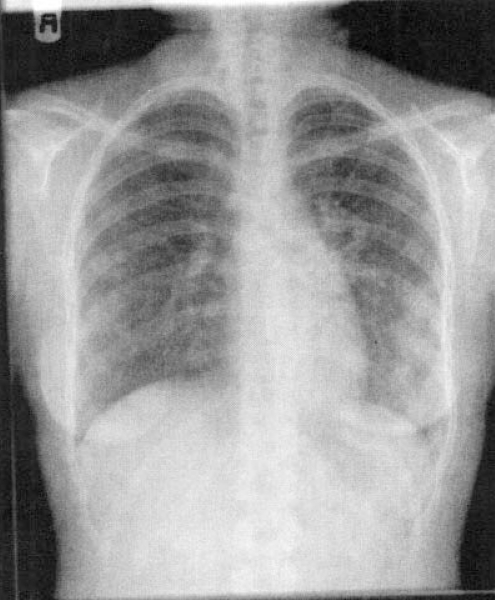
Chest x-rays are important but may be difficult to interpret in a young child. A lateral view is important to evaluate for hilar lymphadenopathy, a hallmark of primary TB (34) (Figures 2,3). Parenchymal lesions may be anywhere in primary disease and are typically, but not always, apical in reactivation disease (35,36) (Figures 4,5).
Figure 2).
Lateral chest x-ray showing marked hilar lymphadenopathy in a child with primary tuberculosis
Figure 3).
Posteroanterior x-ray of the same child as in Figure 2
Figure 4).
Typical cavitary apical disease in an adolescent
Figure 5).
Bilateral infiltrates in an adolescent with smear positive pulmonary tuberculosis. Note that upper lobe disease may be minimal
Magnetic resonance and computed tomography
Magnetic resonance and computed tomography typically show basal cistern inflammation enhancement in central nervous system disease (37). Skeletal TB typically has minimal periosteal reaction on x-ray (38).
Gastric aspirates
Gastric aspirates are used to obtain respiratory specimens in children who are unable to expectorate (usually younger than 10 or 12 years of age). They are probably more effective than bronchoalveolar lavage in mucus culture of children with smear negative TB (39).
Some tips:
During sleep the mucociliary mechanism in their respiratory tract sweeps mucus, which may contain TB bacilli, into the mouth. The material is swallowed and may be a source of organisms, especially if the stomach has not emptied.
Obtain aspirates after a long sleep, at least 6 h, and before the stomach has emptied. Patients should not drink or eat anything overnight to prevent the stomach emptying; avoid exposure to the smell or sight of food, which may encourage gastric emptying. The ideal time is just at the time of waking. Aspirate the stomach contents first, then instill no more than 50 mL of sterile distilled water – the type used for infant feeding is suitable – aspirate back and add the aspirate to the first specimen. As soon as possible, place specimens in a gastric lavage kit for M tuberculosis. There is a special buffer, usually sodium carbonate, in the tube that is essential to prevent rapid death of the organisms.
Sputum induction
Sputum induction using hypertonic saline may be helpful in older children with pulmonary lesions who are not able to expectorate (40). Facilities need to be in place to protect the health worker or respiratory therapist from the droplets generated by this procedure (41).
Cultures
Current culture methods are confirmed positive more rapidly than with older techniques and are often reported positive within two weeks (42). Nucleic acid amplification tests using polymerase chain reaction have great specificity for M tuberculosis (43). Tests such as the Amplified Mycobacterium Direct and polymerase chain reaction, if positive on sputum, are very strong evidence for M tuberculosis infection (44), but may have limited use in children with paucibacillary disease (45). Conversely, a negative result in a specimen that has numerous acid-fast bacilli on direct smear strongly suggests that the infection is with mycobacteria other than TB.
APPROACHES TO TREATMENT
The problem with antimicrobial resistance
There has been a significant increase in the incidence of drug-resistant TB both globally and in Canada (5,46,47). Resistance is chromosomally mediated, and a small proportion of bacilli will be inherently resistant to any one agent. When large bacterial populations are exposed to a single-agent resistance invariably occurs rapidly due to an overgrowth of nonsusceptible bacilli (48). Interruptions in the drug therapy, improper drug prescription and nonadherence to treatment protocols promote drug resistance. Treatment must take into account the possibility of resistance and include at least two drugs, preferably three, to which the isolate is proven or anticipated to be susceptible (48).
Medications
The drugs most commonly used for treating TB are listed in Table 3. These, together with an aminoglycoside such as streptomycin, constitute first line drugs.
TABLE 3.
Drugs used for the treatment of tuberculosis (TB) in children
| Medication | Daily dose | *Intermittent twice weekly dose | Available dosage forms | Adverse reactions |
|---|---|---|---|---|
| Isoniazid (INH) | 10–20 mg/kg† (max 300 mg) | 20–30 mg/kg (max 900 mg) | 10 mg/mL suspension 100 mg tablet 300 mg tablet |
Hepatitis Gastritis Neuropathy (see pyridoxine below) Hypersensitivity |
| Rifampin | 10–20 mg/kg (max 600 mg) | 10–20 mg/kg (max 600 mg) | 10 mg/mL suspension (reconstituted shelf life: 1 month) 150 mg caplet 300 mg caplet |
Vomiting Hepatitis Flu-like illness |
| Pyrazinamide | 15–30 mg/kg (max 2 g) | 50–70 mg/kg (max 4 g) | 500 mg scored tablet | Hepatotoxicity Hyperuricemia Arthralgia |
| Ethambutol | 15 mg/kg‡ | 50 mg/kg | 100 mg tablet 400 mg tablet |
Optic neuritis GI disturbance |
| Pyridoxine (Note: has no anti-TB activity) | 0.1–1 mg/kg/day | 25 and 50 mg tablets | Few. Used to prevent INH neuropathy where diet is pyridoxine deficient (eg, milk- and meat-deficient diets, and in breastfed and lactating patients) |
Intermittent doses are prescribed when Directly Observed Therapy is available. INH and rifampin suspension is usually dispensed in the concentration of 10 mg/mL;
Hepatotoxicity is greater when INH doses are greater than 10 to 15 mg/kg/day;
For ethambutol, 15 mg/kg/day is the preferred dose because there is less risk of optic neuritis. At this dose, ethambutol is bacteriostatic, but it will help to prevent the development of resistance. It is bactericidal at a dose of 25 mg/kg/day. GI Gastrointestinal; max Maximum
Management of contacts
Close contacts of adults with infectious TB are at high risk for infection and for progression to disease. Because it may take up to three months for skin tests to become positive, we provide preventive therapy to young children as soon as they are identified, irrespective of skin test results. Exposed patients require a careful history and physical examination as well as a TST and chest radiograph. It is vital to obtain the sensitivities of the index strain. In the authors’ experiences this may take more than one phone call (for example to the public health department, the physician treating the adult or the central public health laboratory).
If the initial TST is positive (5 mm or more for close contacts) and you have ensured no disease is present, treat the infant or child with a full course of therapy for LTBI, choosing a regimen based on the sensitivities of the contact strain (see below).
If the initial TST is negative, evaluate for clinical disease. If no disease is present, give preventive therapy for all young contacts (typically younger than five years). Isoniazid (INH) is used for INH-sensitive contacts and rifampin for INH-resistant rifampin-sensitive disease. Retest those with an initial negative TST 12 weeks after the last contact. If the TST is still negative, discontinue preventive therapy. If the TST result becomes positive, completely re-evaluate the child for the presence of disease and treat for LTBI if no disease is present.
Treatment of LTBI
Latently infected children are at risk for reactivation TB both soon and in the remote future. Several well-controlled randomized controlled trials have shown INH to be very effective in reducing the progression to disease both over one year and in the long term (49–51). Nine months of treatment is estimated to reduce the risk of this progression by over 80% (1).
If the contact strain is known to be INH-sensitive, use INH 10 mg/kg/day (maximum of 300 mg/day) for nine months. Routine monitoring of blood transaminases is not recommended; however, advise parents that the medicine should be withheld and medical attention sought if anorexia, nausea, vomiting or jaundice occurs. In addition, the patient should be seen by a physician monthly to evaluate for toxicity. Pyridoxine supplementation is given to select children receiving INH (Table 3).
If the source patient is known to have INH resistance, consultation with an infectious diseases expert is advised. In general, provided disease has been excluded, treat with rifampin daily for six months, or twice weekly for nine months. Directly Observed Therapy (DOT) is recommended for all contacts of patients with resistant TB. There is only one randomized controlled trials of rifampin as preventive therapy (52), but in experimental models, rifampin is better at dealing with latent TB organisms than INH (53).
There are no data regarding treatment of LTBI where the source patient has multidrug-resistant (MDR) TB, defined as INH- and rifampin-resistant TB. In this situation it is crucial to exclude disease. We use gastric aspirates or induced sputum and computed tomography if there is any question about the chest x-ray appearance. It may sometimes be preferable to withhold preventive therapy and reserve drugs for treatment of the disease should it develop. If prophylaxis is used, choose two agents to which the source patient’s strain is susceptible (eg, pyrazinamide and ethambutol, or in the older child or adult, pyrazinamide and a quinolone) for six to 12 months. Preventive DOT is mandatory.
The authors also consider BCG immunization for very young infants whose parents have MDR TB and are at high risk for relapse.
If the source patient is unknown, the authors offer treatment for LTBI with INH only. As the prevalence of INH resistance rises this policy may have to be reviewed. A recent decision analysis suggests that rifampin-based regimens may be a better choice for prophylaxis in those emigrating from countries with a high incidence of drug-resistant TB (54).
Treatment of TB disease
A team approach is very helpful in evaluating and treating children with TB disease. The authors’ team includes physicians, one of whom has a specific interest in TB, a clinical nurse practitioner, a clinic nurse, a public health nurse who attends all clinics, a social worker and translator services. In treating disease, the following principles have been found to be helpful.
Ensure patients are well informed and feel like partners in the therapy. Inform patients about reasons for therapy, drug side effects and monitoring. Wherever possible, information should be written and culturally sensitive. Where possible, involve a social worker early in patient assessment. We routinely offer social work contact to all patients. Use translators wherever necessary. Use a telephone-based translation service, if available, when a reliable translator cannot be obtained locally.
Protect yourself and your patients (41). MDR TB has spread from patients to other patients and health care workers (55). Until screened, regard all new adolescent patients and adults accompanying patients as potentially infectious, as well as all children with extensive or cavitary disease. Use TB precautions, including N95 masks. Have an annual TST. Ensure all potentially infectious patients and adults are immediately placed in a negative pressure room (41).
Use DOT for treatment. DOT is the most important advance in the past decade in ensuring cure and preventing resistance (56). Optimally all patients with TB disease should receive DOT. This often depends on the resources of the local health department: paediatricians should advocate DOT where it is unavailable. In children being treated for resistant TB, DOT is mandatory.
Obtain specimens before beginning therapy for TB disease. Because we can no longer predict the sensitivities of the strain we are treating, make every attempt to obtain cultures before starting therapy for TB disease. Gastric aspirates, or sputum if the child is old enough, should be obtained on three separate days from all children with suspected abnormal chest x-rays and submitted for acid-fast bacilli, a rapid amplification test for TB (eg, the Amplified Mycobacterium Direct), cultures and drug susceptibilities. For extrapulmonary TB, obtain the relevant specimens depending on the site of disease: biopsies (eg, lymph nodes, pleura and pleural fluid, and skin), cerebral spinal fluid or early morning urine. Ensure the specimens are suitable for TB culture and are not placed in preservatives. In other instances, the adult isolate is the best available information for guiding therapy. Treatment with an insufficient number of drugs to which the strain is sensitive may result in partly resistant TB being converted into MDR disease.
Unless sensitivities are known begin therapy with at least four drugs. Unless contraindicated, use INH, rifampin, pyrazinamide and ethambutol. When ethambutol is used at a dose of 15 mg/kg/day there is a very small risk of optic neuritis (57). Once sensitivities are obtained, modify therapy.
For fully sensitive TB, discontinue ethambutol, and give INH and rifampin for six months, together with pyrazinamide for the first two months. In sputum-positive cases, pyrazinamide should be continued beyond two months until cultures convert. Meningeal, disseminated and osseous TB require a longer duration of therapy, just as does therapy of disease in the HIV-infected child.
Isolated INH resistance is increasingly common. Use rifampin, pyrazinamide and ethambutol for the duration of treatment. If the patient has extensive disease, consider adding a fourth agent – a fluoroquinolone, an aminoglycoside or capreomycin. Treatment is six to nine months, or six months after culture conversion (whichever is longer) for HIV-seronegative patients.
Management of other resistance patterns includes the use of at least four drugs initially. Wherever possible use two bactericidal drugs to which the isolate is sensitive. Do not use intermittent therapy regimens for resistant disease. Unless the isolate is rifampin-sensitive, it may take 12 to 24 months of therapy to effect a cure: rifampin should not be discontinued because of minor side effects. In general do not treat resistant TB without obtaining expert consultation.
Children receiving aminoglycosides require meticulous attention to monitoring of hearing, drug levels and serum urea and creatinine (tested weekly initially). In the authors’ experience, administration via a long line (eg, a percutaneously inserted central line) is kind to the child and allows weekly blood work to be done without pain. The necessary supports need to be in place for line care. Ensure blood volumes for tests are as small as possible, monitor hemoglobin periodically, and supplement with iron where necessary.
Children receiving ethambutol require monitoring of vision at least monthly, especially when the dose is greater than 15 mg/kg/day. Of second line agents, ethionamide is usually well tolerated by children. Ciprofloxacin has modest efficacy, but an oral suspension is readily available.
Extrapulmonary: meningitis, disseminated (miliary), bone or joint disease
In meningeal, disseminated and skeletal TB, treatment may be prolonged for 12 to 18 months. Regimens are based on the sensitivities of the isolate. Corticosteroids are an important adjunct in the management of TB meningitis (58,59) and endobronchial TB (13,59), and produce at least short term benefit in tuberculous pericarditis (59).
SCREENING FOR LTBI
In North America we have moved from universal screening for TB infection to targeted testing of high risk children (27). The Mantoux test is of unknown sensitivity and specificity for LTBI (15), but there is a potentially high false positive rate depending on the true prevalence of LTBI in the community (Table 4). Even if the test is 95% sensitive and specific, 84% of positive tests will be falsely positive if the true prevalence of LTBI in the population is only 1% (60). In the authors’ clinic, several children are seen who have positive tests with no specific risk factors for infection. The risk/benefit ratio for preventive therapy in this situation is unclear, and much anxiety is unnecessarily generated in the family.
TABLE 4.
Positive predictive value of the tuberculin skin test* related to prevalence of tuberculosis infection in the community
| Tuberculosis prevalence (%) | Positive predictive value (%) |
|---|---|
| 20 | 83 |
| 1 | 16 |
| 0.5 | 9 |
Assume the specificity and sensitivity of the tuberculin skin test is 95%
Routine screening of schoolchildren and infants is not indicated. Energy should be devoted to the testing of children at high risk for LTBI, including:
Contacts of a known case of TB
Persons with suspected disease
Children who have arrived in Canada from countries where TB is endemic
Frequent visitors to endemic countries (recommended ages for testing are at five years and again between 11 and 16 years)
Patients at high risk for progression from TB infection to TB disease but at low risk for infection should be considered for testing. Although most positive tests will be false positive, there are some situations where the treatment or condition may lead to serious or fatal TB disease if LTBI is present. Examples include children who are to undergo solid organ transplants (1) or treatment with anti tumour necrosis factor alpha agents (17), or who are HIV positive (23).
CONCLUSIONS
TB continues to be a major cause of disease and death in the world today (5). This worldwide epidemic inevitably affects Canada, which derives much of its vitality and strength from new immigrants. Although there is a pressing need for better tests for latent infection (15,24), Canadian clinicians should use TSTs to screen children at high risk for infection. Those with positive results should be treated, to both protect them now and to prevent them from becoming the next generation of adults who develop infectious TB. Currently, the management of TB disease must always take into account the possibility of drug resistance. Ultimately, prevention of TB in Canada depends on controlling the disease globally. For both altruistic and selfish reasons we should find ways to assist with this struggle.
Acknowledgments
The authors acknowledge all the members of The Hospital for Sick Children team including Debrah Louch, Robin Salter-Goldie, Dianne Rasmussen, Kirsty Stephenson and Wayne Moore. We are grateful to Dr Murtala Abdurrahman for his review of an early version of the manuscript, and to Dr SE Read and Dr EL Ford-Jones for their encouragement and support.
REFERENCES
- 1.American Academy of Pediatrics Pickering LK.2000Red Book: Report of the Committee on Infectious Diseases 25th ednTuberculosis. Elk Grove Village: American Academy of Pediatrics; 2000593–613. [Google Scholar]
- 2.Nobert E, Chernick V. Tuberculosis: 5. Pediatric disease. CMAJ. 1999;160:1479–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Lemay M, Tapiero B, Chernick V. Pediatric tuberculosis. In: Long R, editor. Canadian Tuberculosis Standards. 5th edn. Ottawa: The Lung Association; 2000. pp. 127–40. < www.hc-sc.gc.ca/pphb-dgspsp/publicat/cts-ncla00/> (Version current at February 25, 2003). [Google Scholar]
- 4.Starke JE. Tuberculosis in infants and children. In: Schlossberg D, editor. Tuberculosis and Non Tuberculous Mycobacterial Infections. 4th edn. Philadelphia: WB Saunders Co; 1999. pp. 303–24. [Google Scholar]
- 5.Pablos-Mendez A, Raviglione MC, Laszlo A, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. N Engl J Med. 1998;338:1641–9. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 6.Donald PR. Childhood tuberculosis: Out of control? Curr Opin Pulm Med. 2002;8:178–82. doi: 10.1097/00063198-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Dye C, Scheele S, Dolin P, et al. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 8.Long R, Njoo H, Hershfield E. Tuberculosis: 3. Epidemiology of the disease in Canada. CMAJ. 1999;160:1185–90. [PMC free article] [PubMed] [Google Scholar]
- 9.Iseman MD, editor. A Clinician’s Guide to Tuberculosis. New York: Lippincott Williams & Wilkins; 2000. Immunity and pathogenesis; pp. 63–96. [Google Scholar]
- 10.Starke JR. Tuberculosis. An old disease but a new threat to the mother, fetus, and neonate. Clin Perinatol. 1997;24:107–27. [PubMed] [Google Scholar]
- 11.Starke JR, Jacobs RF, Jereb J. Resurgence of tuberculosis in children. J Pediatr. 1992;22:839–55. doi: 10.1016/s0022-3476(05)81949-3. [DOI] [PubMed] [Google Scholar]
- 12.Daly JF, Brown DS, Lincoln EM, et al. Endobronchial tuberculosis in children. Dis Chest. 1952;22:380. doi: 10.1378/chest.22.4.380. [DOI] [PubMed] [Google Scholar]
- 13.Kitai IC, Sanders DM, Manungo J. Tuberculosis presenting as corticosteroid responsive wheezing in infancy. Trop Geogr Med. 1989;41:274–6. [PubMed] [Google Scholar]
- 14.Stead WW, To T, Harrison RW, Abraham JH., III Benefit-risk considerations in preventive treatment for tuberculosis in elderly persons. Ann Intern Med. 1987;107:843–5. doi: 10.7326/0003-4819-107-6-843. [DOI] [PubMed] [Google Scholar]
- 15.Jasmer RM, Nahid P, Hopewell PC. Clinical practice. Latent tuberculosis infection. N Engl J Med. 2002;347:1860–6. doi: 10.1056/NEJMcp021045. [DOI] [PubMed] [Google Scholar]
- 16.Khan EA, Starke JR. Diagnosis of tuberculosis in children: Increased need for better methods. Emerg Infect Dis. 1995;1:115–23. doi: 10.3201/eid0104.950402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starke JR, Taylor-Watts KT. Tuberculosis in the pediatric population of Houston, Texas. Pediatrics. 1989;84:28–35. [PubMed] [Google Scholar]
- 18.Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 19.Harries AD, Parry C, Nyongonya Mbewe L, et al. The pattern of tuberculosis in Queen Elizabeth Central Hospital, Blantyre, Malawi: 1986–1995. Int J Tuberc Lung Dis. 1997;1:346–51. [PubMed] [Google Scholar]
- 20.Nemir RL, Krasinski K. Tuberculosis in children and adolescents in the 1980s. Pediatr Infect Dis J. 1988;7:375–9. doi: 10.1097/00006454-198806000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Schaaf HS, Gie RP, Beyers N, Smuts N, Donald PR. Tuberculosis in infants less than 3 months of age. Arch Dis Child. 1993;69:371–4. doi: 10.1136/adc.69.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurkan F, Bosnak M, Dikici B, et al. Miliary tuberculosis in children: A clinical review. Scand J Infect Dis. 1998;30:359–62. doi: 10.1080/00365549850160648. [DOI] [PubMed] [Google Scholar]
- 23.Chan SP, Birnbaum J, Rao M, Steiner P. Clinical manifestation and outcome of tuberculosis in children with acquired immunodeficiency syndrome. Pediatr Infect Dis J. 1996;15:443–7. doi: 10.1097/00006454-199605000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Huebner RE, Schein MF, Bass JB., Jr The tuberculin skin test. Clin Infect Dis. 1993;17:968–75. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 25.Lalvani A, Pathan AA, Durkan H, et al. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–21. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 26.Steginer P, Rao M, Victoria MS, et al. Persistently negative tuberculin reactions: Their presence among children culture positive for M tuberculosis. Am J Dis Child. 1980;134:747–50. doi: 10.1001/archpedi.1980.02130200017007. [DOI] [PubMed] [Google Scholar]
- 27.American Thoracic Society and Centers for Disease Control and Prevention Joint Statement: Targeted tuberculin screening and treatment of latent tuberculosis infection. Am J Respir Crit Care Med. 2000;161:5221–47. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 28.Menzies D, Pourier L. Diagnosis of tuberculosis infection and disease. In: Long R, editor. Canadian Tuberculosis Standards. 5th edn. Ottawa: The Lung Association; 2000. p. 50. [Google Scholar]
- 29.Menzies D. What does tuberculin reactivity after Bacille Calmette-Guerin vaccination tell us? Clin Infect Dis. 2000;31:S71–S74. doi: 10.1086/314075. [DOI] [PubMed] [Google Scholar]
- 30.Mazurek GH, LoBue PA, Daley CL, et al. Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA. 2001;286:1740–7. doi: 10.1001/jama.286.14.1740. [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention Guidelines for using the QuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis infection. MMWR Recomm Rep. 2003;52:15–8. [PubMed] [Google Scholar]
- 32.Brock I, Munk ME, Kok-Jensen A, Andersen P. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis. 2001;5:462–7. [PubMed] [Google Scholar]
- 33.Bellete B, Coberly J, Barnes GL, et al. Evaluation of a whole-blood interferon-gamma release assay for the detection of Mycobacterium tuberculosis infection in 2 study populations. Clin Infect Dis. 2002;34:1449–56. doi: 10.1086/340397. [DOI] [PubMed] [Google Scholar]
- 34.Smuts NA, Beyers N, Gie RP, et al. Value of the lateral chest radiograph in tuberculosis in children. Pediatr Radiol. 1994;24:478–80. doi: 10.1007/BF02015003. [DOI] [PubMed] [Google Scholar]
- 35.Leung AN, Muller NL, Pineda PR, et al. Primary tuberculosis in childhood: Radiographic manifestations. Radiology. 1992;182:87–91. doi: 10.1148/radiology.182.1.1727316. [DOI] [PubMed] [Google Scholar]
- 36.Parisi MT, Jensen MC, Wood BP. Pictorial review of the usual and unusual roentgen manifestations of childhood tuberculosis. Clin Imag. 1994;18:149–54. doi: 10.1016/0899-7071(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 37.Gupta RK, Gupta S, Singh D, Sharma B, Kohli A, Gujral RB. MR imaging and angiography in tuberculous meningitis. Neuroradiology. 1994;36:87–92. doi: 10.1007/BF00588066. [DOI] [PubMed] [Google Scholar]
- 38.Griffith JF, Kumta SM, Leung PC, Cheng JC, Chow LT, Metreweli C. Imaging of musculoskeletal tuberculosis: A new look at an old disease. Clin Orthop. 2002;398:32–9. doi: 10.1097/00003086-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Abadico DL, Steiner P. Gastric lavage is better than bronchoalveolar lavage for isolation of Mycobacterium tuberculosis in childhood tuberculosis. Pediatr Infect Dis J. 1992;11:735–8. doi: 10.1097/00006454-199209000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Shata AMA, Coulter JBS, Parry CM, et al. Sputum induction for the diagnosis of tuberculosis. Arch Dis Child. 1996;74:535–7. doi: 10.1136/adc.74.6.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Menzies D, Conly J. Tuberculosis control in Canadian health care Institutions. In: Long R, editor. Canadian Tuberculosis Standards. 5th edn. Ottawa: The Lung Association; 2000. pp. 207–23. [Google Scholar]
- 42.Laszlo A. Tuberculosis: 7. Laboratory aspects of diagnosis. CMAJ. 1999;160:1725–9. [PMC free article] [PubMed] [Google Scholar]
- 43.Pierre C, Oliver C, Lecossier D, et al. Diagnosis of primary tuberculosis in children by amplification and detection of mycobacterial DNA. Am Rev Resp Dis. 1993;147:420–4. doi: 10.1164/ajrccm/147.2.420. [DOI] [PubMed] [Google Scholar]
- 44.Chedore P, Jamieson FB. Routine use of the Gen-Probe MTD2 amplification test for detection of Mycobacterium tuberculosis in clinical specimens in a large public health mycobacteriology laboratory. Diagn Microbiol Infect Dis. 1999;35:185–91. doi: 10.1016/s0732-8893(99)00086-3. [DOI] [PubMed] [Google Scholar]
- 45.Neu N, Saiman L, San Gabriel P, et al. Diagnosis of pediatric tuberculosis in the modern era. Pediatr Infect Dis J. 1999;18:122–6. doi: 10.1097/00006454-199902000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Remis RS, Jamieson F, Chedore P, Haddad A, Vernich L. Increasing drug resistance of Mycobacterium tuberculosis isolates in Ontario, Canada, 1987–1998. Clin Infect Dis. 2000;31:427–32. doi: 10.1086/313969. [DOI] [PubMed] [Google Scholar]
- 47.Long R. Drug-resistant tuberculosis. CMAJ. 2000;63:425–8. [PMC free article] [PubMed] [Google Scholar]
- 48.Iseman MD, editor. A Clinician’s Guide to Tuberculosis. New York: Lippincott Williams & Wilkins; 2000. Drug-resistant tuberculosis; pp. 323–54. [Google Scholar]
- 49.World Health Organization International Union Against Tuberculosis, Committee on Prophylaxis. Efficacy of various durations of INH preventive therapy for TB: 5 years of follow-up in the IUAT trial. Bull World Health Organ. 1982;60:555–64. [PMC free article] [PubMed] [Google Scholar]
- 50.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis: A general review. Adv Tuberc Res. 1969;17:28–106. [PubMed] [Google Scholar]
- 51.Hsu KHK. Thirty years after isoniazid. JAMA. 1984;251:1283–5. doi: 10.1001/jama.251.10.1283. [DOI] [PubMed] [Google Scholar]
- 52.Hong Kong Chest Service/Tuberculosis Research Centre, Madras/British Medical Research Council A double-blind placebo-controlled clinical trial of three antituberculosis chemoprophylaxis regimens in patients with silicosis in Hong Kong. Am Rev Respir Dis. 1992;145:36–41. doi: 10.1164/ajrccm/145.1.36. [DOI] [PubMed] [Google Scholar]
- 53.Dickinson JM, Mitchison DA. Experimental models to explain the high sterilizing activity of rifampin in the chemotherapy of tuberculosis. Am Rev Respir Dis. 1981;123:367–71. doi: 10.1164/arrd.1981.123.4.367. [DOI] [PubMed] [Google Scholar]
- 54.Khan K, Muennig P, Behta M, Zivin JG. Global drug-resistance patterns and the management of latent tuberculosis infection in immigrants to the United States. N Engl J Med. 2002;347:1850–9. doi: 10.1056/NEJMsa021099. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care facilities, 1994. MMWR. 1994;43:1–32. [PubMed] [Google Scholar]
- 56.Weis SE, Slocum PC, Blais FX, et al. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330:1179–84. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 57.Trebucq A. Should ethambutol be recommended for routine treatment of tuberculosis in children? A review of the literature. Int J Tuberc Lung Dis. 1997;1:12–5. [PubMed] [Google Scholar]
- 58.Girgis NI, Farid Z, Kilpatrick ME, Sultan Y, Mikhail IA. Dexamethasone adjunctive treatment for tuberculous meningitis. Pediatr Infect Dis J. 1991;10:179–83. doi: 10.1097/00006454-199103000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Dooley DP, Carpenter JL, Rademacher S. Adjunctive corticosteroid therapy for tuberculosis: A critical reappraisal of the literature. Clin Infect Dis. 1997;25:872–87. doi: 10.1086/515543. [DOI] [PubMed] [Google Scholar]
- 60.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology: A Basic Science for Clinical Medicine. 2nd edn. Boston: Little Brown and Company; 1991. p. 90. [Google Scholar]