Abstract
Optical coherence tomography (OCT) is a catheter-based imaging technology with powerful resolution capable of identifying vulnerable plaques and guiding coronary intervention. However, a significant limitation of intravascular OCT imaging is its attenuation by blood. We propose that the use of an oxygen-carrying blood substitute could potentially optimize OCT image quality. Surgical isolation of the descending thoracic aorta of six rabbits is performed, followed by intravascular OCT imaging of the abdominal aorta. Perfluorodecalin (PFD) is oxygenated using a bubble-through technique with 100% oxygen. OCT imaging is performed and compared using three different flushing modalities: PFD; saline; and blood. OCT imaging of the rabbit abdominal aorta is successful in all of the subjects. In each of the six studied subjects, flushing with PFD consistently provides dramatically better imaging of the vessel wall tissue structures. OCT image quality is highly dependent on the ability of the flushing modality to remove blood from the imaging field. From this proof-of-concept study, we demonstrate that endovascular flushing with an oxygen-carrying blood substitute (PFD) is optically superior to saline flushing for intravascular imaging.
Keywords: optical coherence tomography, oxygen-carrying blood substitute, perfluorocarbon, intravascular imaging
1 Introduction
More than 13 million individuals suffer from coronary artery disease in the United States. Of these, 1.1 million patients present with acute myocardial infarction every year,1 most of which are caused by the rupture of small lipid-filled plaques in the coronary arteries; typically those plaques are referred to as thin-capped, fibroatheromas (TCFA). Clinically, we have been unable to identify the culprit plaques before rupture, and intervention has always focused on management only after rupture has produced an acute coronary event.2 Optical coherence tomography (OCT) is a catheter-based imaging technology that provides micron-scale resolution that could potentially help delineate these culprit lesions.
OCT is the laser light equivalent of ultrasound imaging, measuring the intensity of backscattered infrared light rather than sound waves. This technology has been studied extensively with respect to intravascular imaging of coronary artery plaques.3–6 However, a significant limitation of intravascular imaging with OCT is its attenuation by blood. Hemoglobin and red blood cells (RBCs) attenuate light from the OCT catheter through absorption and scattering of light, respectively.7–9 Several techniques have been developed to overcome the limitation of light attenuation. One proposed technique is to modify the plasma to match the index of refraction to that of red blood cells to reduce the scattering of light by blood.10 A common practice, however, has been the application of saline flushes11 plus balloon occlusion.12 To create a temporary bloodless imaging environment, gentle balloon inflation proximal to the image zone is used in addition to vessel flushing with saline infusion. Although this practice has been used successfully in humans, there is a potential for ischemia, given the removal of oxygen-carrying hemoglobin. Normal saline has no oxygen-carrying capacity and could possibly cause tissue ischemia in sensitive myocardial cells already susceptible to decreased perfusion due to vessel atherosclerosis. Another potential complication of using saline flushes includes possible fluid overload and pulmonary edema. These possible complications limit the imaging time needed to adequately evaluate the coronary anatomy, and most importantly, overall image quality may be suboptimal.
The use of an oxygen-carrying blood substitute could potentially optimize OCT image quality as well as provide for extending imaging time without compromising sensitive myocardial cell perfusion. In a prior study, OCT imaging of murine right ventricle walls was substantially improved by isovolumic blood replacement with a hemoglobin-based blood substitute.13 Our study compares the use of an oxygen-carrying perfluorocarbon, perfluordecalin (PFD), to saline flushing during OCT imaging.
2 Methods
2.1 Animal Protocol
This protocol was approved by the University of California, Irvine Institutional Animal Care and Use Committee (IACUC). All animals were treated in accordance with federal and state regulatory guidelines. Six male New Zealand white rabbits weighing 3.9±0.4 kg were anesthetized with a mixture of ketamine HCl and xylazine. The animals were intubated and mechanically ventilated during the surgical and imaging procedure. On completion of the experiment, the subjects were euthanized with an intravenous injection of Eutha-6 (1.0 to 2.0 cc) administered through the marginal ear vein.
2.2 Surgical Procedure
A median sternotomy incision was made using a combination of surgical blade and heavy surgical scissors. The pericardium was then incised to expose the heart and major vessels, specifically the descending thoracic aorta. Isolation of the thoracic aorta was performed by blunt dissection. Arteriotomy was then performed distal to the left subclavian artery but proximal to the diaphragm. A 6-F catheter was introduced into the aorta at this point and advanced 2 cm distally toward the diaphragm (Fig. 1). The 3-F OCT imaging probe was then introduced through the lumen of the catheter distally to image the abdominal aorta.
Fig. 1.
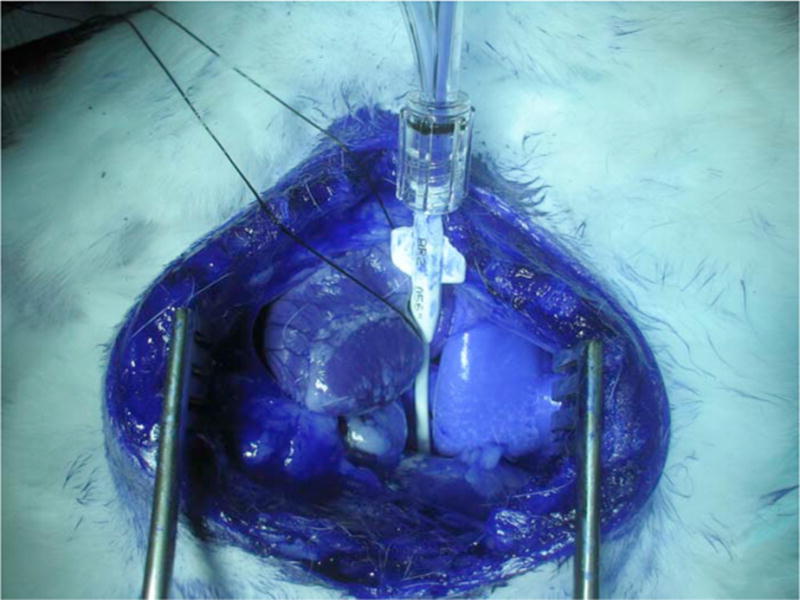
Photograph of the surgical procedure.
2.3 Optical Coherence Tomography Imaging
The objective of the study was the imaging of vessel wall anatomy on a microscopic level using three different flushing modalities: 1. PFD (C10F18, Sigma-Aldrich, Saint Louis, Missouri), which has a significant oxygen-carrying capacity (49 mg O2/100 ml); 2. normal saline; and 3. blood. The PFD was oxygenated using a bubble-through technique with 100% oxygen. OCT imaging of the rabbit abdominal aorta was done during the different flushing modalities. The thoracic aorta proximal to the introduction of the guiding catheter was occluded using a temporary 3-0 silk surgical suture that was released after imaging. A total of 10 cc of flush for each modality was used and injected through the guiding catheter over a 5-s period of time.
2.4 Optical Coherence Tomography Instrumentation
OCT is an interferometric technique based on a broadband light source and coherent cross correlation detection of light. The principles of OCT have been previously described. The time domain OCT system prototype in this study utilized a Michelson interferometer with a low coherence source to measure light reflected from turbid structures. The OCT system used in this study contains a superluminescent diode source that delivers an output power of 10 mW at a central wavelength of 1310 nm with a full width at half maximum (FWHM) of 75 nm. In our system, lateral resolution and axial resolution are 10 μm and focal length is 8 mm.
Flexible fiber optic OCT probes were constructed from single-mode optical fibers. The bare-ended fiber was attached to a 0.7-mm-diam gradient index (GRIN) lens and a 0.7-mm-diam prism. The probe was placed in fluorinated ethylene propylene tubing for added fiber support and to seal the optical compartments from the surrounding blood. The single-mode fiber delivers the incident beam to the distal imaging apparatus and transmits the reflected signal back. A linear motor was used to drive the coated flexible fiber optic distally and proximally along the length of the probe within the sheath, moving the GRIN lens and prism imaging components within the sheath to obtain linear images along the long axis of the abdominal aorta vessel. Axial scans were obtained every 10 μm along the length of the probe during 16-mm-long scan sweeps. The axial line scanning rate is 500 Hz, and the modulation frequency of the phase modulator is 500 kHz. A 16-mm scan is obtained in 3.2 sec.
3 Results
A total of six live rabbits was used in this study. OCT imaging of the rabbit abdominal aorta was successful in all of the subjects. The time span of clear imaging with the OCT catheter averaged 5.0 sec during intra-aorta injections of flush. The efficiency of OCT imaging in providing a clear view of the vessel wall structure was influenced by the ability of the flushing modality to remove blood from the imaging field. In this study, normal rabbits with no significant atherosclerotic plaques were studied. The rabbits were similar in size and abdominal aorta vessels were similar in diameter. Thus, the distance between the vessel wall and the OCT probe was consistent with each subject animal.
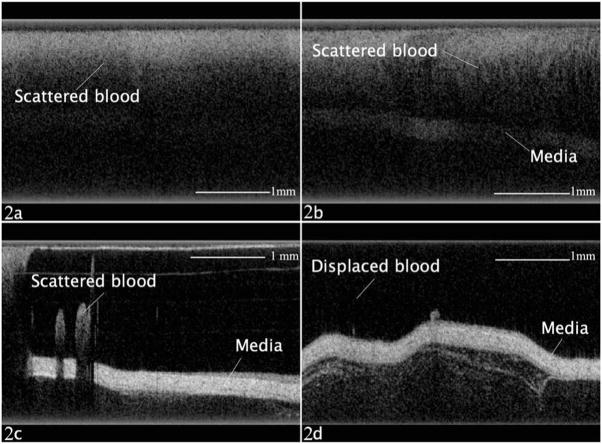
In each of the six studied subjects, PFD flushing consistently provided dramatically better imaging of the vessel wall tissue structures when compared to the other two flushing modalities. In Fig. 2, images abstracted from the videos of each flushing modality are presented. Figure 2(a) shows OCT imaging with no flushing and blood present in the imaging window. There is significant image degradation with no clear identification of vessel wall anatomy. Figure 2(b) shows an OCT image with saline flushing and after proximal vessel occlusion. There is some delineation of vessel wall anatomy; however, the residual scattered blood degrades the image quality. In Figs. 2(c) and 2(d), PFD flush is utilized after proximal vessel occlusion with significant improvement in the delineation of vessel wall anatomy. Figure 2(c) is obtained immediately after the start of PFD flushing, and Fig. 2(d) toward the end of the flushing period. Figure 2(c) represents the imaging border between a significantly degraded image and clear imaging of vessel anatomy at the beginning of PFD flushing.
Fig. 2.
(a) OCT imaging with no flushing and blood present in the imaging window. (b) OCT image with saline flushing and after proximal vessel occlusion. (c) and (d) OCT imaging with PFD flushing after proximal vessel occlusion.
4 Discussion
Intravascular imaging with OCT has vast potential to resolve atherosclerotic lesions at the microscopic level and reveal important characteristics that may be prone to sudden rupture. However, one major limitation is its attenuation by blood, specifically absorption by hemoglobin and scattering by RBCs. From this study, we have demonstrated the proof of concept that endovascular flushing with an oxygen-carrying blood substitute PFD is optically superior to saline flushing for intravascular imaging. The viscosity of PFD is 5.10 mPa s (dynamic) or 2.61 mm2/s (kinematic) at 25°C. The refractive index of PFD is 1.313 nD20. The physical properties of perfluorocarbons, specifically their immiscibility in blood, allows for superior displacement of blood from the imaging window. With saline, a significant amount of mixing occurs rather than complete displacement of blood. Therefore, there may still be a substantial amount of absorption and scattering of light. Because PFC is not hemoglobin based and does not absorb significantly in the 1310-nm wavelength region, the potential for attenuation from absorption and scattering is improved once blood is displaced. A recent study by Prati et al.,14 demonstrated the effectiveness of nonocclusive intra-coronary OCT imaging with iodinated contrast media flushing. Both nonionic contrast and perfluorocarbons have higher viscosity than blood and therefore effectively displace blood from the image zone.
Another potential benefit of using an oxygen-carrying blood substitute such as PFD is the increase in imaging time without compromising myocardial tissue oxygenation. Cleman, Jaffee, and Wohlgelernter15 infused oxygenated Fluosol DA 20%, a perfluorochemical oxygen transport fluid, at the distal tip of the balloon catheter during percutaneous transluminal coronary angioplasty (PTCA) of 20 human patients. They reported profound regional left ventricular dysfunction with a greater than 90% decrease in regional contraction by echocardiogram when PTCA was performed with Ringer’s lactate solution or nonoxygenated Fluosol DA 20%. In contrast, regional contraction during transcatheter infusion of oxygenated Fluosol DA 20% remained at normal levels throughout balloon inflation.15
The optical properties and ability of perfluorocarbons to oxygenate should enable a broad range of imaging procedures by allowing longer imaging intervals and improved image quality. As more broadband laser light sources are developed, axial resolution capabilities will improve, and scattering from interfering blood may limit further advances in the imaging resolution with saline compared to perfluorocarbon flushing. The second-generation Fourier domain OCT systems provide rapid sampling rates, enabling longer vessel segment imaging without the need for balloon occlusion. We would expect the advantages of flushing with perfluorocarbons to be even greater in the nonocclusive setting. With faster acquisition, substantial optical information can be obtained from OCT images, including functional optical coherence tomography, polarization sensitive tomography, and second-harmonic-generation OCT. All of these imaging modalities extract additional information about the biological properties of the vasculature, but require additional acquisition time. Thus, the ability to oxygenate with perfluorocarbons will likely become even more important as this field progresses.
In 1989, The United States Food and Drug Administration (FDA) approved Fluosol, an emulsified perfluorocarbon, for perfusion of ischemic tissue in the setting of PTCA.16 However, Fluosol production was discontinued due to lack of commercial success. Perfluorocarbons such as PFD are water insoluble and require emulsification. To date, there is no FDA-approved emulsified PFC available, although different PFC formulations are in development.17
In conclusion, we demonstrate that flushing during endovascular OCT imaging with an oxygenated perfluorocarbon blood substitute such as PFD dramatically improves optical imaging conditions when compared to the traditional method of flushing with saline. Because of its oxygen carrying capacity, immiscibility resulting in effective clearance of blood, and optical transparency at near-infrared wavelengths, the use of blood substitute has potential to improve intravascular optical imaging applications.
Acknowledgments
We acknowledge a grant to Brenner: NIH 1R01CA 124967, California TRDRP 16RT-0082, AF-9550-04-1-0101; also grant to Narula: NIH/NHLRI R01-1FL-078681.
Contributor Information
Khiet C. Hoang, University of California, Irvine Department of Medicine, Division of Cardiology, 101 The City Drive, Building 53, Room 100, Route 81, Orange, California 92868
Ahmad Edris, University of California, Irvine Department of Medicine, Division of Cardiology, 101 The City Drive, Building 53, Room 100, Route 81, Orange, California 92868.
Jianping Su, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
David S. Mukai, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612
Sari Mahon, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Artiom D. Petrov, University of California, Irvine Department of Medicine, Division of Cardiology, 101 The City Drive, Building 53, Room 100, Route 81, Orange, California 92868
Morton Kern, University of California, Irvine Department of Medicine, Division of Cardiology, 101 The City Drive, Building 53, Room 100, Route 81, Orange, California 92868.
Chowdhury Ashan, Nevada Heart and Vascular Center, 5380 South Rainbow Boulevard, Las Vegas, Nevada 89118.
Zhongping Chen, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Bruce J. Tromberg, University of California, Irvine Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612
Jagat Narula, University of California, Irvine Department of Medicine, Division of Cardiology, 101 The City Drive, Building 53, Room 100, Route 81, Orange, California 92868.
Matthew Brenner, Email: mbrenner@uci.edu, University of California Irvine Medical Center, Pulmonary and Critical Care Division, Building 53, Room 119, 101 The City Drive, Orange, California 92868.
References
- 1.American Heart Association. 2001 Heart and Stroke Statistical Update. American Heart Association; Dallas, TX: 2001. [Google Scholar]
- 2.Narula J, Finn A, DeMaria A. Picking plaques that pop. J Am Coll Cardiol. 2005;45:1970–1973. doi: 10.1016/j.jacc.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 3.Brezinski ME, Tearney GJ, Bouma BE, Izatt JA, Hee MR, Swanson EA, Southern JF, Fujimoto JG. Optical coherence tomography for optical biopsy: properties and demonstration of vascular pathology. Circulation. 1996;93:1206–1213. doi: 10.1161/01.cir.93.6.1206. [DOI] [PubMed] [Google Scholar]
- 4.Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Kang DH, Halpern EF, Tearney GJ. Characterization of human atherosclerosis by OCT. Circulation. 2002;106:1640–1645. doi: 10.1161/01.cir.0000029927.92825.f6. [DOI] [PubMed] [Google Scholar]
- 5.Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB, Choi KB, Shishkov M, Schlendorf K, Pomerantsev E, Houser SL, Aretz HT, Tearney GJ. Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol. 2002;39:604–609. doi: 10.1016/s0735-1097(01)01799-5. [DOI] [PubMed] [Google Scholar]
- 6.Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, Bouma BE. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–1555. doi: 10.1161/01.CIR.0000159354.43778.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Twersky V. Absorption and multiple scattering by biological suspensions. J Opt Soc Am A. 1970;60:1084–1093. doi: 10.1364/josa.60.001084. [DOI] [PubMed] [Google Scholar]
- 8.Stenke J, Sheppard A. Role of light scattering in whole blood oximetry. Trans Biomed End. 1986;33:294–301. doi: 10.1109/tbme.1986.325713. [DOI] [PubMed] [Google Scholar]
- 9.Roggan A, Freibel M, Dorschel K. Optical properties of circulating human blood in the wavelength range 400–2500 nm. J Biomed Opt. 1999;4(1):36–46. doi: 10.1117/1.429919. [DOI] [PubMed] [Google Scholar]
- 10.Brezinski M, Saunders K, Jesser C, Li X, Fujimoto J. Index matching to improve OCT imaging through blood. Circulation. 2001;103:1999–2003. doi: 10.1161/01.cir.103.15.1999. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Sandoval L, Bouma B, Tearney G, Jang I. Optical coherence tomography as a tool for percutaneous coronary interventions. Catheter Cardiovasc Intervent. 2005;65:492–496. doi: 10.1002/ccd.20340. [DOI] [PubMed] [Google Scholar]
- 12.Kawase Y, Hoshino K, Yoneyama R, MacGregor J, Hajiar RJ, Jang IK, Hayase M. In vivo volumetric analysis of coronary stent using optical coherence tomography with a novel balloon occusion-flushing catheter: a comparison with intravascular ultrasound. Ultrasound Med Biol. 2005;31(10):1343–1349. doi: 10.1016/j.ultrasmedbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Villard J, Feldman M, Kim J, Milner T, Freeman G. Use of a blood substitute to determine instantaneous murine right ventricular thickening with optical coherence tomography. Circulation. 2002;105:1843–1849. doi: 10.1161/01.cir.0000014418.99708.86. [DOI] [PubMed] [Google Scholar]
- 14.Prati1 F, Cera1 M, Ramazzotti1 V, Imola F, Giudice R, Albertucci M. Safety and feasibility of a new non-occlusive technique for facilitated intracoronary optical coherence tomography (OCT) acquisition in various clinical and anatomical scenarios. EuroIntervent. 2007;3:365–370. doi: 10.4244/eijv3i3a66. [DOI] [PubMed] [Google Scholar]
- 15.Cleman M, Jaffee C, Wohlgelernter D. Prevention of ischemia during percutaneous transluminal coronary angioplasty by transcatheter infusion of oxygenated Fluosol DA 20% Circulation. 1986;74:555–562. doi: 10.1161/01.cir.74.3.555. [DOI] [PubMed] [Google Scholar]
- 16.Kerin DM. Role of the perfluorocarbon Fluosol-DA in the coronary angioplasty. Am J Med Sci. 1994;307(3):218–221. doi: 10.1097/00000441-199403000-00009. [DOI] [PubMed] [Google Scholar]
- 17. [accessed on 8 July 2008]; See http://www.sanguine-corp.com/products.htm.