Abstract
The salivary pellicle plays an important role in oral physiology, yet noninvasive in situ characterization and mapping of this layer remains elusive. The goal of this study is to develop an optical approach for the real-time, noninvasive mapping and characterization of salivary pellicles using optical coherence tomography (OCT) and optical coherence microscopy (OCM). The long-term goals are to improve diagnostic capabilities in the oral cavity, gain a better understanding of physiological and pathological processes related to the oral hard tissues, and monitor treatment responses. A salivary pellicle is incubated on small enamel cubes using human whole saliva. OCT and OCM imaging occurs at 0, 10, 30, 60 min, and 24 h. For some imaging, spherical gold nanoparticles (15 nm) are added to determine whether this would increase the optical signal from the pellicle. Multiphoton microscopy (MPM) provides the baseline information. In the saliva-incubated samples, a surface signal from the developing pellicle is visible in OCT images. Pellicle “islands” form, which increase in complexity over time until they merge to form a continuous layer over the enamel surface. Noninvasive, in situ time-based pellicle formation on the enamel surface is visualized and characterized using optical imaging.
Keywords: salivary pellicle, optical coherence tomography, optical coherence microscopy, gold nanoparticle, imaging, plaque
1 Introduction
Selective adsorption of specific salivary biopolymers on the enamel surface leads to the formation of an acquired salivary pellicle.1 The adsorbed protein layer can be considered a dynamic biofilm that regulates and modulates interactions at the interface between the tooth surface and oral cavity.1–5 The pellicle serves as a lubricant, protecting the dentition against abrasion and attrition.1 In addition, the salivary pellicle may protect against demineralization of dental hard tissues caused by acid attack.1–5 The adsorbed pellicular phosphoproteins and mucins reduce enamel surface solubility, acting as a perm-selective membrane and regulating not only the diffusion of calcium and phosphate ions, but also the acidic agents.1–5 In addition to its protective functions of lubricating, inhibiting demineralization, and working as a diffusion barrier against chemical attacks of the enamel, the pellicle can act as a reservoir of agents promoting remineralization. The maturation age of the pellicle, when it can effectively inhibit erosive activity, remains unclear.
Oral biofilm contains a multitude of factors important to oral microbial ecology and tissue surface properties. It is directly implicated in the etiology of oral soft tissue pathologies, including periodontal disease, providing a binding site for pathogenic bacteria.6,7 The morphology of the oral biofilm layer appears to affect bacterial binding to the tooth surface.6,7
Current characterizations of the pellicle regarding its composition, architecture, and chemistry are based on in vitro or ex vivo studies. Noninvasive in situ characterization and mapping of the salivary pellicle remains elusive. The effects of specific events in the oral cavity such as eating, oral hygiene, preventive, restorative, and pharmacological agents on in vivo salivary pellicle presence, structure, and properties remain, to a large extent, unknown. The primary obstacle has been the inability to noninvasively characterize in vivo the presence, structure, and dynamics of the acquired salivary pellicle. Yet such capability is crucial to a better understanding of oral pathogenesis, prevention, and therapeutics.
Optical coherence tomography (OCT) is a high-resolution optical technique that permits minimally invasive imaging of near-surface abnormalities in complex tissues. Its principles are described in the literature.8–13 Crosssectional images of tissues are constructed in real time, at near histologic resolution (approximately 3 to 10 μm with our current technology). This permits in vivo noninvasive imaging of the macroscopic characteristics of surface and subsurface tissues. Two-dimensional images may be combined to generate 3-D images that can be sectioned and manipulated in many ways. In vivo OCT images are acquired in seconds or less using a hand-held probe, so they can easily be used in the clinical setting. Higher resolution in vivo OCT imaging is possible by using optical coherence microscopy (OCM).14,15 OCT or OCM can be combined with in vivo multiphoton microscopy (MPM), generating high-resolution imaging of specific tissue components and fluorescence using many wavelengths of light.16
The purpose of this study was to investigate the use of multimodality noninvasive imaging to visualize, characterize, and quantify the acquired salivary pellicle. The development of such a capability will, in the long term, serve to improve diagnostic, preventive, and interventional capabilities in the oral cavity, and to provide a better understanding of physiological and pathological processes related to oral hard tissues.
2 Materials and Methods
2.1 Tooth Preparation
15 clinically and radiographically caries-free human molars and anterior teeth with sound smooth surfaces were used. They had been newly extracted due to severe periodontitis. These samples were cleaned and polished with pumice slurry and a cotton wheel to remove organic contaminants. 30 enamel blocks were produced from the smooth surfaces of each tooth using a water-cooled diamond disk (DynaFlex®, NTI, Germany) connected to a low speed microengine (Rite torque V, Rite Dent, Miami, Florida) (Table 1). To remove any fine debris or bacterial remnants, the enamel blocks were sterilized with 50% ethanol for 30 min, cleaned with running distilled water for 20 min, and then put in an ultrasound cleaner for 30 min.
Table 1.
Sample preparation. Total number of tooth samples in the study: n = 30.
| Goal | Incubation media | Incubation time (min) | Number of samples | ||||
|---|---|---|---|---|---|---|---|
| Imaging time-based salivary | Saliva only | 0 | 10 | 30 | 60 | 24 h | 15 |
| pellicle growth | Distilled water only (control) | 15 | |||||
Each sample was fixed in a silicone mold (Vinyl Polysiloxane Kit, Defend®, Mydent International, Hauppauge, New York) to ensure a stable position during incubation and imaging (Fig. 1) and marked with two sharp oil ink points to ensure accurate reproducible multiple imaging at the same location.
Fig. 1.

Sample preparation. Teeth set in silicone mold for OCT imaging. Two oil ink markers indicate the area for imaging.
2.2 Collection of Experimental Saliva
One healthy donor (35-year-old male) with good systemic and oral health (devoid of any medication or special dietary habits, salivary gland diseases, caries lesions, periodontal pathology, and salivary dysfunctions) was used as a source for the salivary samples to eliminate variation in the samples. The human subject gave written informed consent prior to participation in this study, which was approved by the University of California, Irvine IRB (approval 2002–2805). To ensure an equilibrated oral environment, the donor refrained from eating, drinking, and brushing at least 2 h prior to the study. Also, the donor refrained from using fluoridated dentifrices and mouthwash at least 12 h prior to sample collection. The donor was permitted to engage in regular oral hygiene procedures (brushing and flossing) immediately after breakfast on the experimental day.
Parotid and submandibular/sublingual saliva was collected by means of an ice-chilled sterile centrifuge tube (15 ml) using light stimulation with 2% citric acid after chewing on a piece of paraffin (3M Company, Minneapolis, Minnesota). Collection always occurred in the morning between 9 to 10 AM to minimize the effects of diurnal changes in saliva composition. After collection, a few milliliters of whole saliva were discarded to minimize the amount of oral debris.
Collected saliva was clarified by centrifugation at 1000 g × for 20 min to remove cellular debris. The clear supernatant was mixed with 0.02% sodium azide to prevent bacterial growth and stored at 4 °C in a cold room prior to the experiment (Fig. 2). All saliva samples were used on the day of collection.
Fig. 2.

Saliva preparation. Clarified saliva after centrifugation. In this study, 1000-g ×20-min centrifugation was performed and the resulting clear supernatant (red arrow) was collected using careful pipetting to prevent disturbance in the precipitate (yellow arrow). (Color online only.)
2.3 Pellicle Formation
Experimental pellicles were formed by time-based incubation (0, 10, 30, 60, 120 min, and 24 h) of the tooth samples with 5 ml of the saliva. Each specimen was incubated with its own aliquot of saliva at 37.5 °C to avoid interaction between the tooth samples. The time points for incubation were selected based on the literature and the determinative role that pellicle age plays in defining the in vivo dentition’s response to acid attack. After incubation, excessive saliva and loosely bound proteins were removed from the tooth surface by a brief, careful 10-s rinsing with distilled water to avoid damaging the developing salivary pellicle.
Tooth samples for the control group were incubated under the same conditions, but in distilled water rather than saliva. During incubation, whether in saliva or distilled water, all samples were stirred gently at 10-min intervals.
2.4 Imaging
In addition to conventional OCT, multimodality imaging OCM connected to multiphoton microscopy (MPM) was used to improve resolution and imaging specificity. Fluorescein and Coolblue (Coolblue®, Pfizer, New York) dyes were used as optical contrast agents during fluorescence imaging with MPM. Each dye was mixed with saliva at a 1:100 volume ratio. Second harmonic generation (SHG) and two photon excited fluorescence (TPEF) images were acquired for comparison with OCM images.
To ensure accurate relocalization during sequential reimaging events, the silicone and acrylic molds were stably fixed on the adjustable imaging stage using a holder, and the visible imaging aiming beam scanned in a line between the two oil ink marker points.
During MPM and OCM imaging, all of the samples were protected with a thin clear imaging glass cover with border sealing using silicone materials to reduce the effects of dehydration. In addition, 100-μm-thickness polyethylene film with a 3×3-mm window was seated under the imaging site to prevent direct contact between the salivary pellicle and the imaging glass (Fig. 3).
Fig. 3.
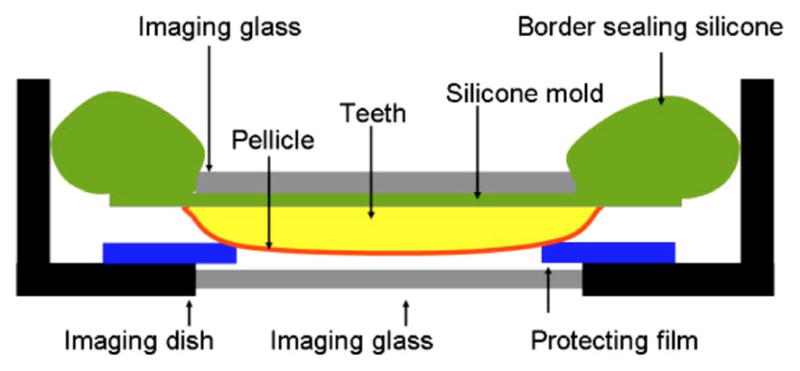
Imaging dish specifically designed for salivary pellicle imaging. To protect against dehydration and mechanical irritation of the developing salivary pellicle, the imaging dish border was sealed using silicone (top), and the window border was protected by 100-μm-thick plastic film. The gap between the imaging glass and the salivary pellicle assures an intact and unchanged salivary pellicle throughout imaging.
3 Results
3.1 Time-Based Salivary Pellicle Development
3.1.1 Optical coherence tomography images
Over time, the saliva-incubated samples showed a gradual increase in surface signal in the top view of the 3 D reconstructed OCT images [Fig. 4(b)]. This implies surface pellicle development in the samples incubated in saliva over 24 h. In contrast, the samples incubated in distilled water did not show a change in surface signal over time [Fig 4(a)].
Fig. 4.
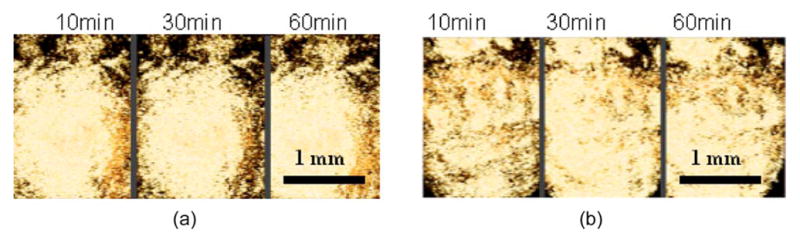
Time-based 2-D OCT images extracted from 3-D reconstructed images. (a) Top view of the enamel surface incubated in distilled water for 10, 30, and 60 min showing no change over time. (b) Top view of the enamel surface incubated in saliva for 10, 30, and 60 min showing development of the salivary pellicle.
3.1.2 Optical coherence microscopy
OCM images were superimposed with reflectance and secondharmonic images from microscopy. Fluorescein and Coolblue (Coolblue®, Pfizer, New York) were used as dye for the fluorescent images. After 120 min of incubation, the samples incubated in saliva showed a clearly distinguishable layer averaging 20 μm in thickness, [Figs. 5(c) and 5(d)]. The thickness was subject to intersample and intrasample variations. The samples incubated in distilled water did not demonstrate any distinct layer between the tooth and the water [Figs. 5(a) and 5(b)].
Fig. 5.
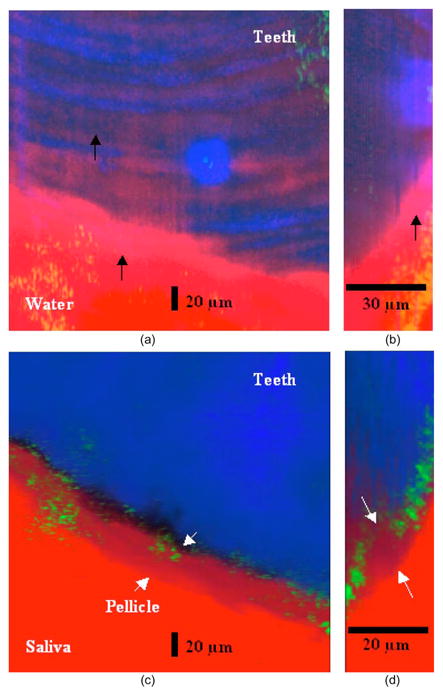
OCM images of salivary pellicle. OCM images (green signal) were superimposed with fluorescent (red signal) and secondharmonic (blue signal) microscopic images. (a) Top view of 3-D reconstructed OCM image of distilled water-incubated sample. After 120-min incubation, there is no distinctive layer between teeth and water. The light-pink-colored signal is related to fluorescence from the imaging glass (black arrows). (b) Optical section of 3-D image, providing a lateral view of (a). (c) Top view of 3-D reconstructed OCM image of saliva-incubated sample. A distinctive pellicle layer between tooth and saliva is clearly visible (white arrows). (d) Optical section of 3-D image, providing a lateral view of (c). The pellicle with an average thickness of 20 μm is clearly visible on the tooth surface (white arrows). (Color online only.)
3.1.3 Multiphoton microscopy
After 120 min of incubation in saliva, a layer exceeding 10 μm in thickness was visible at the interface between tooth and saliva [Figs. 6(d)–6(f)]. Enamel rod structures, which were visible prior to incubation with saliva, became progressively covered by the developing overlying pellicle layer. In control samples incubated in distilled water [Figs. 6(a)–6(c)], enamel rod structures remained visible throughout the study.
Fig. 6.
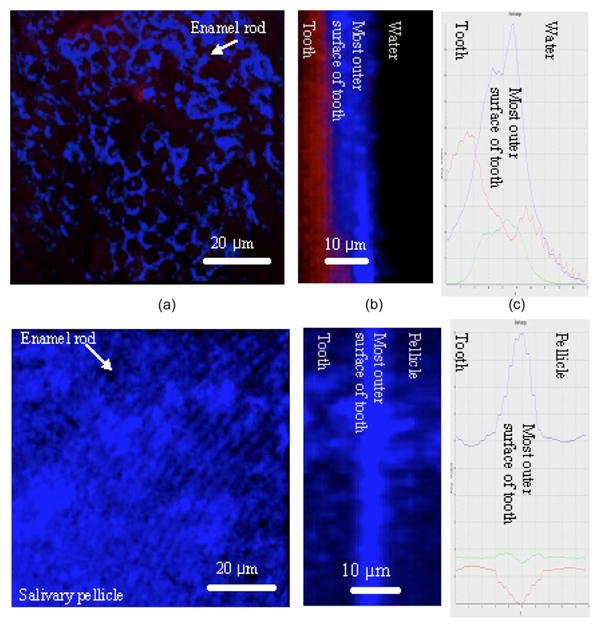
MPM images of salivary pellicle. (a) Tooth incubated in distilled water for 120 min. Enamel rods are clearly visible in this 3-D reconstructed top view image. (b) Optical section of 3-D image, providing a lateral view of (a) and confirming the absence of pellicle. (c) Isoline plot of (b). The outermost zone of enamel shows high signal intensity. (d) Tooth incubated in saliva for 120 min. Due to the development of an overlying pellicle layer, the enamel rods are mostly obstructed in this top view image. (e) Optical section of 3-D image, providing a lateral view of (d). The pellicle layer covers the outer tooth surface. (f) Isoline plot of (e). The new signal on the outside tooth surface, which is not seen in the samples incubated in water (c) implies the existence of a salivary pellicle.
3.1.4 Growth and development of pellicle
Over time, the developing pellicle layers showed maturation of the initial globular-like pellicle “seeds.” Images showed pellicle island presence and growth (Figs. 7 and 8). The early globular pellicle islands varied in size and appeared to be randomly distributed on the sample surfaces (Fig. 7). Over time, the number and size of these islands gradually increased [Figs. 8(a) through 8(d)]. Eventually, a pellicle layer covered the entire sample surface, once the islands had fused [Fig. 8(d)].
Fig. 7.

Growth and development of pellicle. OCM images after 120-min incubation in saliva. Globular growth of pellicle is clearly visible. (a) Top view of 3-D reconstructed image. Fluorescein in dentinal tubule (white arrows) and pellicle (yellow arrows) shows red signal. (b) Optically sectioned lateral view of 3-D reconstructed image. Pellicle islands are also visible in later view (yellow circle). (Color online only.)
Fig. 8.
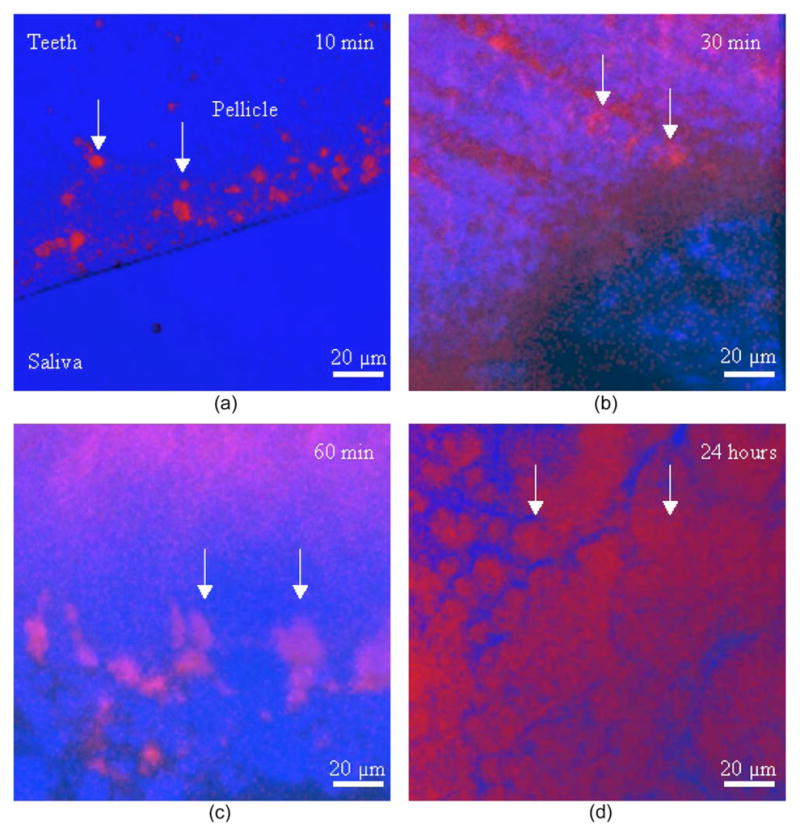
Progressive growth and development of pellicle (white arrows) on one tooth sample. Top view of 3-D reconstructed MPM images at progressive saliva incubation time points. (a) 10-min incubation. (b) 30-min incubation. (c) 60-min incubation. (d) 24-h incubation. Blue signal originates from tooth and saliva; pink and red signals originate from the salivary pellicle. Over time, the number and diameter of pellicle islands gradually increased. (Color online only.)
4 Discussion
Saliva secretion is a complex process, with most saliva by volume being secreted by the major salivary glands, and the remaining amount coming from the minor glands. Saliva contains a multitude of factors important to oral microbial ecology, biofilm formation, and health. Loss of salivation may result in rampant tooth decay.1,2 This point becomes increasingly important given the high cariogenicity of the present day Western diet, not only due to the high amount of fermentable carbohydrates, but also because food intake occurs with high frequency throughout the day. Pellicle formation is a dynamic process that is continuously influenced by adsorption-desorption processes, structural modification of adsorbed molecules by microbial or host enzymes, and intermolecular complexion with other macromolecules.1,2
The salivary pellicle serves to protect dental enamel against hostile conditions that include abrupt changes in pH and temperature, combined with high repetitive mechanical loading during mastication. It mitigates against a wide range of conditions, including enamel erosion, abrasion, and decay, while concurrently mediating a host of biological responses to permit adhesion of microbial and host cells1,17
Although there is general agreement regarding the importance of the salivary pellicle, many unknowns remain, including definition, formation, thickness, and maturation of the pellicle. One commonly used definition describes the pellicle as a “bacteria-free, proteinaceous” dynamic film.18 Sometimes it is identified as a multilayered film that is formed initially by the selective adsorption of salivary molecules to oral surfaces followed by homo- or heterotypic complexion of these molecules with other molecules in the ambient saliva.18 During this study, multilayered pellicles were occasionally seen in MPM images [Fig. 8(b)], but most of the samples showed one blunt pellicle layer [Figs. 5(c), 5(d), 6(b), 8(a), 8(c), and 8(d)]. These multilayered pellicles disappeared with pellicle maturation (Fig. 8), so that perhaps these layers may represent an early stage in the maturation process of the pellicle.
The reported time for salivary pellicle formation varies enormously, Widely divergent estimates for time to maturation of the oral biofilm are reported from in vitro studies, ranging from a few minutes to several days.2 This factor is important because the in vivo pellicle is periodically removed or altered by mechanical or chemical influences such as tooth brushing or chemical rinses. It has been suggested that only mature, several-days-old salivary pellicles can effectively inhibit the erosive attack of acidic agents.4 However, in another study, no significant differences were identified in enamel protective function between 24-h and 7-day-old salivary pellicles formed in situ.5 According to Amaechi et al.19 erosive destruction of the enamel surface is considerably reduced by the presence of 60-min-old salivary pellicle. Indeed, according to recent findings,5 enamel protection by salivary pellicle formed in situ over periods ranging from 2 to 24 h is independent of pellicle age. In this study, OCT showed a significant signal increase, implying the formation of salivary pellicle over the tooth surface after approximately 30 min of incubation in saliva [Fig. 4(b)].
Little is known regarding variations of in vivo salivary pellicle thickness between individuals, upper versus lower arches, and specific locations on each tooth. Yet this factor may considerably influence the sites and severity of dental erosion, as well as de- and remineralization, outright decay, and other hard and soft tissue pathologies. Reports of oral biofilm thickness from in vitro studies range from 100 to 200 nm to 0.06 to 0.09 mm.6,7,20,21 These discrepancies may in part be due to varying rates and amounts of salivary protein and glycoprotein adsorption to the enamel surface. Data from some studies suggest that oral biofilm composition may well be site-dependent. Factors may include the nature of the underlying tissue (smooth surface versus fissure versus periodontal sulcus), proximity to salivary duct openings, differing redox potentials, or nutrient availability. To date, mapping oral biofilm in vivo to evaluate effects of specific factors on biofilm presence and characteristics has not been possible due to lack of a noninvasive diagnostic modality. However, data from ex vivo studies have been weakened by the very real potential for damage or alteration of the salivary pellicle during sample preparation for imaging.
The morphology of the pellicle layer has mainly been visualized using scanning and transmission electron microscopy techniques. Typically, sample preparation involves fixing and dehydrating, which may affect the morphology of the pellicle in comparison to its normally hydrated state. Although atomic force microscopy (AFM) can reduce the required imaging time, there is always the possibility of dehydration during imaging.22 In this study, potential modifiers of the salivary pellicle such as dehydration and mechanical damage were avoided by use of a specially designed incubating stage and imaging dish [Figs. 1(a), 1(b), and 3]. An average of 10 to 20 μm thickness of salivary pellicle was observed after 120 min of incubation [Figs. 5(c), 5(d), and 6(b), 10(c), and 10(d)].
The initial adsorption of discrete salivary proteins onto the enamel surface may result from electrostatic interactions through the hydrophilic regions, leaving the more hydrophobic parts of the salivary protein molecules exposed at the surface.23 Enhanced adhesion to the initial protein layer has been mentioned as the cause of the globular surface morphology of acquired salivary pellicle, with globule diameters ranging from 80 to 120 nm after 2 h of incubation.21 In this study, globular pellicle formation was also identified (Figs. 7, 8, and 9). The number and diameter of globules increased gradually until they eventually fused (Fig. 8). This observation may explain the large range of globule sizes and pellicle thicknesses described by previous researches. Moreover, size and distribution of globules may be affected by underlying hard tissue surface characteristics and by the chemical composition of the saliva in a specific location.
5 Conclusion
This study demonstrates the potential of using a noninvasive optical approach for precise in situ imaging of the human salivary pellicle. While this study is performed ex vivo, rapid innovation in probe development should permit in vivo in situ imaging of salivary pellicle in the near future.
Acknowledgments
We gratefully acknowledge the support of GlaxoSmithKline Pharmaceuticals, NIH (LAMMP) RR01192, and Military Photomedicine Program, AFSOR Grant No. FA9550-08-1-00384.
Contributor Information
Jae Ho Baek, Ulsan University Hospital, Department of Orthodontics, 290-3 Jeonha dong, Dong gu, Ulsan, 682-714, Korea and University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Tatiana Krasieva, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Shuo Tang, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Yehchan Ahn, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Chang Soo Kim, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Diana Vu, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Zhongping Chen, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
Petra Wilder-Smith, University of California, Irvine, Beckman Laser Institute, 1002 Health Sciences Road East, Irvine, California 92612.
References
- 1.Hannig M, Fiebiger M, Güntzer M, Döbert A, Zimehl R, Nekrashevych Y. Protective effect of the in situ formed short-term salivary pellicle. Arch Oral Biol. 2004;49:903–910. doi: 10.1016/j.archoralbio.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Nieuw Amerongen AV, Oderkerk CH, Driessen AA. Role of mucins from human whole saliva in the protection of tooth enamel against demineralization in vitro. Caries Res. 1987;21:297–309. doi: 10.1159/000261033. [DOI] [PubMed] [Google Scholar]
- 3.Hannig C, Wasser M, Becker K, Hannig M, Huber K, Attin T. Influence of different restorative materials on lysozyme and amylase activity of the salivary pellicle in situ. J Biomed Mater Res Part A. 2006;78A:755–761. doi: 10.1002/jbm.a.30758. [DOI] [PubMed] [Google Scholar]
- 4.Zahradnik RT, Moreno EC, Burke EJ. Effect of salivary pellicle on enamel subsurface demineralization in vitro. J Dent Res. 1976;55:664–670. doi: 10.1177/00220345760550042101. [DOI] [PubMed] [Google Scholar]
- 5.Hannig M, Hess NJ, Hoth-Hannig W, de Vrese M. Influence of salivary pellicle formation time on enamel demineralization-an in situ pilot study. Clin Oral Investig. 2003;7:158–161. doi: 10.1007/s00784-003-0219-2. [DOI] [PubMed] [Google Scholar]
- 6.Schilling KM, Bowe WH. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for streptococcus mutans. Infect Immun. 1992;60:284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong K, Mailloux L, Herzberg MC. Salivary film expresses a complex, macromolecular binding site for streptococcus sanguis. J Biol Chem. 2000;275:8970–8974. doi: 10.1074/jbc.275.12.8970. [DOI] [PubMed] [Google Scholar]
- 8.Izatt JA, Kobayashi K, Sivak MV, et al. Optical coherence tomography for biodiagnostics. Opt Photonics News. 1997;8:41–47. [Google Scholar]
- 9.Ding Z, Ren H, Zhao Y, Nelson JS, Chen Z. High-resolution optical coherence tomography over a large depth range with an axicon lens. Opt Lett. 2002;27:243–245. doi: 10.1364/ol.27.000243. [DOI] [PubMed] [Google Scholar]
- 10.Huang DSE, Lin CP, Schuman JS, et al. Optical coherence tomography. Science. 1991;254:1178–1191. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujimoto JGHM, Izatt JA, Boppart SA, et al. Biomedical imaging using optical coherent tomography. Proc SPIE. 1999;3749:402. [Google Scholar]
- 12.Bouma BTG, Boppart SA, Hee MR, et al. High-resolution optical coherence tomographic imaging using a mode-locked Ti:Al/sub 2/O/sub 3/laser source. Opt Lett. 1995;20:1486–1488. doi: 10.1364/ol.20.001486. [DOI] [PubMed] [Google Scholar]
- 13.Boppart SA. Optical coherence tomography: technology and applications for neuroimaging. Psychophysiology. 2003;40:529–541. doi: 10.1111/1469-8986.00055. [DOI] [PubMed] [Google Scholar]
- 14.Vokes DE, Jackson R, Guo S, Perez JA, Su J, Ridgway JM, Armstrong WB, Chen Z, Wong BJ. Optical coherence tomography-enhanced microlaryngoscopy: preliminary report of a noncontact optical coherence tomography system integrated with a surgical microscope. Ann Otol Rhinol Laryngol. 2008;117(7):538–547. doi: 10.1177/000348940811700713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chelliyil RG, Ralston TS, Marks DL, Boppart SA. High-speed processing architecture for spectral-domain optical coherence microscopy. J Biomed Opt. 2008;13(4):44013. doi: 10.1117/1.2960018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumen C, Mempel TR, Mazo IB, von Andrian UH. Intravital microscopy: visualizing immunity in context. Immunity. 2004;21(3):315–329. doi: 10.1016/j.immuni.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Carlén A, Eliasson L, Aronsson G, Birkhed D. Human minor and major gland saliva proteins and ability to mediate Actinomyces naeslundii adherence. Arch Oral Biol. 2004;49(3):177–181. doi: 10.1016/j.archoralbio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Leung KP, Crowe TD, Abercrombie JJ, Molina CM, Bradshaw CJ, Jensen CL, Luo Q, Thompson GA. Control of oral biofilm formation by an antimicrobial decapeptide. J Dent Res. 2005;84:1172–1177. doi: 10.1177/154405910508401215. [DOI] [PubMed] [Google Scholar]
- 19.Amaechi BT, Higham SM, Edgar WM, Milosevic A. Thickness of acquired salivary pellicle as a determinant of the sites of dental erosion. J Dent Res. 1999;78(12):1821–1828. doi: 10.1177/00220345990780120901. [DOI] [PubMed] [Google Scholar]
- 20.Lendenmann U, Grogan J, Oppenheim FG. Saliva and dental pellicle—A Review. Adv Dent Res. 2000;14:22–28. doi: 10.1177/08959374000140010301. [DOI] [PubMed] [Google Scholar]
- 21.Dickinson ME, Mann AB. Nanoscale characteristics of salivary pellicle. Mater Res Soc Symp Proc. 2005;841:231–236. [Google Scholar]
- 22.Arvidsson A, Löfgren CD, Christersson CE, Glantz PO, Wennerberg A. Characterization of structures in salivary secretion film formation. An experimental study with atomic force microscopy. Biofouling. 2004;20(3):181–188. doi: 10.1080/08927010400001790. [DOI] [PubMed] [Google Scholar]
- 23.Lindh L. On the adsorption behaviour of saliva and purified salivary proteins at solid/liquid interfaces. Swed Dent J Suppl. 2002;152:1–57. [PubMed] [Google Scholar]


