Abstract
The coefficient of friction (COF) of articular cartilage is thought to increase with osteoarthritis (OA) progression, and this increase may occur due to a decrease in lubricin concentration. The objectives of this study were to measure the COF of guinea pig tibiofemoral joints with different stages of OA, and to establish relationships between COF, lubricin concentrations in synovial fluid, and degradation status using the Hartley guinea pig model. Both hind limbs from 24 animals were harvested: seven 3-month-old (no OA), seven 12-month-old (mild OA), and ten that were euthanized at 12-months of age after undergoing unilateral anterior cruciate ligament (ACL) transection at 3-months of age (moderate OA). Contralateral knees served as age matched controls. COFs of the tibiofemoral joints were measured using a pendulum apparatus. Synovial fluid lavages were analyzed to determine the concentration and integrity of lubricin using ELISA and western blot, and the overall articular cartilage status was evaluated by histology. The results showed that the mean COF in the ACL-deficient knees was significantly greater than that of the 3-month knees (p<0.01) and the 12-month knees (p<0.01). Lubricin concentrations in the ACL-deficient knees were significantly lower than that of the 3-month knees (p<0.01) and 12-month knees (p<0.01). No significant differences in COF or lubricin concentration were found between the 3-month and the 12-month knees. Histology verified the extent of cartilage damage in each group.
Conclusion
COF values increased and lubricin levels decreased with cartilage damage following ACL transection.
Keywords: ACL, injury, cartilage, osteoarthritis, friction, lubrication
INTRODUCTION
The mechanisms responsible for maintaining the low-friction environment of the articular surfaces of synovial joints are a topic of continued interest. Proposed mechanisms include hydrodynamic lubrication,(1) boundary lubrication,(2) elastic deformation,(3) and fluid pressurization.(4) Also important are the effects of acute joint trauma and altered biomechanics on the frictional properties of the articular surface and subsequent degeneration. Lubricin, a glycoprotein thought to be a boundary lubricant in synovial fluid and articular cartilage,(5,6) is deficient in aspirates of acute post-traumatic knee effusions, which is likely due to inflammatory destruction.(7) In the absence of lubricin, the articular surface is vulnerable to frictional damage, as evidenced by the accelerated progression of joint degeneration in individuals with camptodactyly arthropathy coxa vara pericarditis syndrome, a disease linked to a defect in the gene encoding the lubricin protein,(8) and by the rapidly progressive joint degeneration observed in the lubricin knockout mouse.(5)
Prior knee injury is a known risk factor for OA development.(9) Altered biomechanics, such as changes in joint alignment, ligamentous laxity, or deficient proprioception, may all lead to OA after injury.(10) Alterations in surface friction, possibly due to inflammatory destruction of synovial fluid lubricants and altered cartilage tissue properties, may also contribute to the onset of disease. (11) Although OA is classified as a non-inflammatory arthritis, inflammation likely plays a role in post-traumatic disease. Acute joint trauma, such as an anterior cruciate ligament (ACL) tear, stimulates the synoviocytes and inflammatory cells to release cytokines (i.e. MMPs, IL-1, IL-6 and TNF-α), which in turn may alter chondrocyte metabolism and impair the lubricating mechanisms that protect the joint from wear.(11) Acute inflammation following injury, as well as ongoing low-grade intra-articular inflammation persisting after the acute phase has resolved, may possibly be responsible for these changes. Understanding the changes in the frictional properties of the cartilage and the role of inflammation could lead to novel means to suppress joint degeneration.
The Hartley guinea pig is an animal model frequently used to study OA progression.(12,13) At 3 months of age, these animals are skeletally mature, with no clinically detectable cartilage degeneration. The animals uniformly develop mild OA by 12 months of age. The model has also been used to study arthritis progression following ACL transection, (13) and the effects of intervention.(14) The Hartley guinea pig model was selected for this study in order to examine the progression of natural OA and post-traumatic OA.
The objectives of this study were to measure the COF of the tibiofemoral joints of the Hartley guinea pig knee with no histological evidence of degeneration (3-month animals), mild degeneration (12-month animals, no surgical intervention), and moderate osteochondral degeneration (12-month animals with ACL-transection at 3-months of age), and to correlate changes in the COFs with the concentrations of lubricin in the synovial fluid aspirated from these joints. The hypotheses were: 1) joint COFs will increase with joint degeneration; 2) lubricin concentrations in the synovial fluid will decrease with joint degeneration, and 3) there is a significant negative correlation between the COF and lubricin concentrations.
MATERIALS AND METHODS
Specimens
After the study received IACUC approval, twenty-four 3-month-old male Hartley guinea pigs were obtained. Seven animals were euthanized at 3 months of age (Group 1), seven animals were housed until they reached 12 months of age and then euthanized (Group 2), and ten 3-month old animals underwent surgical ACL transection in the right leg and were euthanized at 12 months of age (Group 3; Right knee). The contralateral (ACL-intact) knee of the ACL-transected animals served as an additional control (Group 3; Left knee). After euthanasia, both hind limbs were harvested, dissected down to the joint capsule, and stored at −80°C.
ACL Transection Procedure
The surgical animals were anesthetized with an intraperitoneal injection of ketamine (40mg/Kg) and medetomidine (0.5mg/Kg). The right knee was shaved and prepped with betadine. Animals were placed in the prone position, and a 1.5cm midline incision was made over the anterior knee. The skin was mobilized to expose the patellar tendon. An incision through the joint capsule was made immediately lateral to the patellar tendon. The patella was then everted, and the ACL was incised with the knee in a flexed position. Complete sectioning of the ACL was confirmed by manually testing anterior laxity. The joint capsule, fascia and skin were closed in layers using interrupted 4-0 Vicryl sutures. Post-operative analgesia was maintained using buprenorphine hydrochloride (0.05mg/Kg SQ for a minimum of 3 days). Animals were allowed to bear weight on the limbs as tolerated. Equal weight bearing between limbs was generally noted within three weeks of surgery.
Pendulum Apparatus
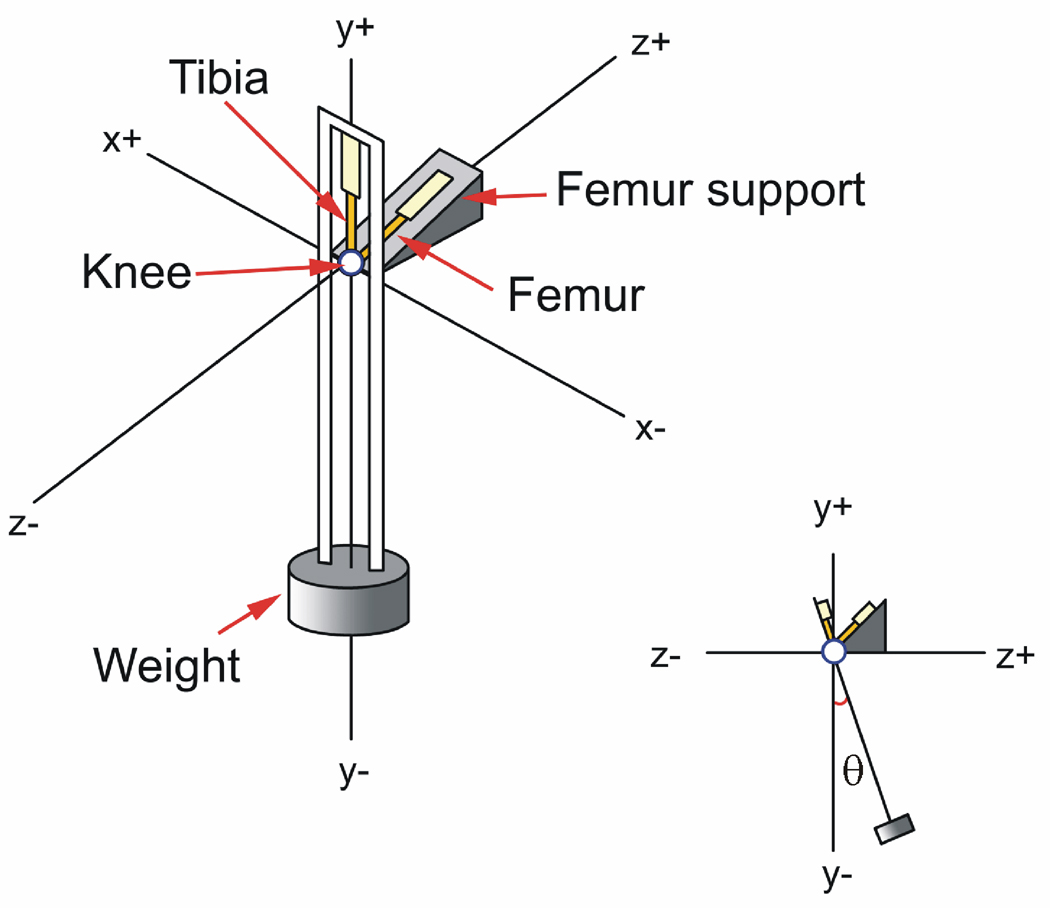
A pendulum system, in which the tibiofemoral joint served as the fulcrum, was used to measure the COF, ex vivo (Fig. 1).(15) The pendulum swung the tibia relative to a fixed femur at 1 Hz. A compressive load of 300g (approximately 0.5 times body weight) was maintained across the joint. The Optotrak System (Northern Digital Inc., Waterloo, Ontario), which measures 3-D rigid body motion accurate to 0.1mm and 0.1°, was used to record the motion of the tibia with respect to the femur. Three infrared light-emitting diodes were mounted on both the fixed femur/base and on the tibia/pendulum, and were used to define the rigid bodies and measure rotational motion about the knee: flexion-extension (F-E), internal-external (I-E), and varus-valgus (V-V).(16) The rotations were referenced to an anatomic coordinate system which was established using the Optotrak digitizer prior to performing the pendulum tests on each knee.(15) The rotations that occurred outside the sagittal plane were also recorded.
Fig. 1.
The COFs were determined using a pendulum system where the guinea pig knee served as the fulcrum. [Used with permission (15)].
Pendulum Test Protocol
Each leg was thawed to room temperature, and the distal tibia and proximal femur were potted in rigid plastic tubing to facilitate mounting within the pendulum. The femur was secured 45° off the horizontal in the fixed femoral support, and the tibia was positioned at 135° of knee flexion when the pendulum was at rest. This angle was chosen based on observations of the weight bearing resting angle of the tibiofemoral joint in live guinea pigs.(15) Motion was initiated by rotating the tibial pendulum 17° (range 15°to 20°) about the F-E axis (θ) relative to its equilibrium position and releasing it via a gated mechanism (Fig. 1).
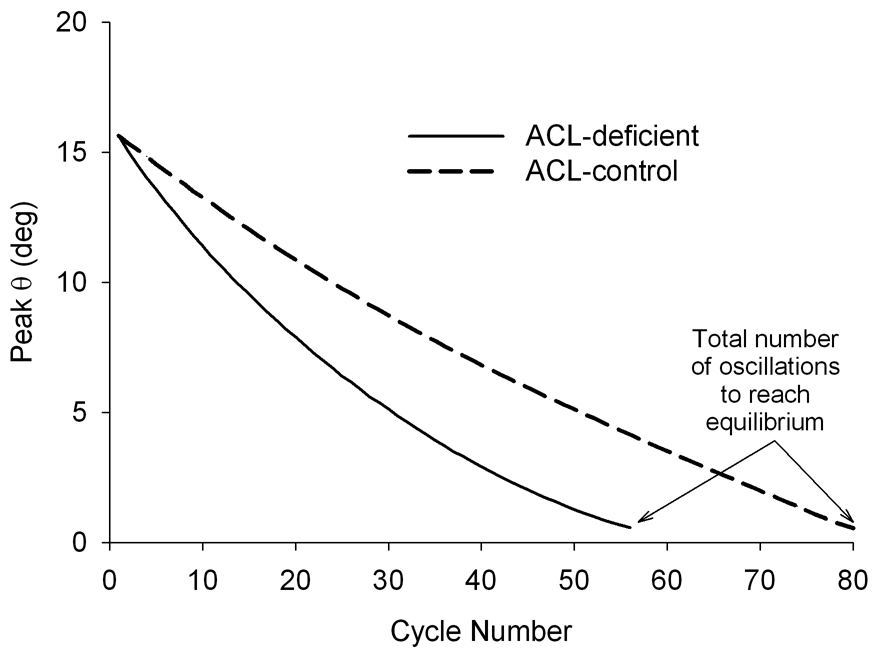
The COF was calculated using Stanton’s Equation, COF = ΔθL/(4r), where Δθ was the average change in the peak F-E rotation of the pendulum per cycle (Fig. 2), L was the distance between the pendulum center of gravity and the center of the F-E axis (L = 0.22 m), and r is the radius of the femoral condyle (r = 0.002 m).(15) The equation is based upon the assumption that peak rotation amplitude decays linearly with time.(17–19) Both hind limbs of each animal were tested.
Fig. 2.
The typical decay responses for both the ACL-transected and contralateral control knees of a Group 3 animal. The decay was tracked until the peak flexion angle dropped below ±1°.
ELISA/Western Blot
Each leg was removed from the pendulum and 100μL of isotonic saline was injected intra-articularly. The knee was manually cycled through flexion and extension ten times to distribute the fluid. The fluid was aspirated and frozen at −80°C prior to assaying for lubricin.
A sandwich ELISA using peanut agglutinin (PNA) and anti-lubricin Mab S6.89 (20) was utilized. High binding 96 well plates were coated overnight with PNA in 50mM sodium bicarbonate buffer, pH 9.5 at a final concentration of 10µg/ml. The following day, serial dilutions of purified human lubricin and aspirated synovial fluid were incubated on the PNA-coated plates for 60 minutes. The plate was subsequently washed with phosphate buffer saline (PBS) + 0.1% Tween 20. S6.89 was subsequently added at a 1:10,000 dilution and incubated for 60 minutes, followed by washing with PBS − 0.1% Tween 20. Goat anti-mouse IgG-alkaline phosphatase was added to the plate at 1:1,000 dilution and incubated for 60 minutes. Finally, 4-methylumbelliferyl phosphate was added and the fluorescence was measured using 465nm and 550nm as emission and excitation wavelengths, respectively (Fusion: Packard Bioscience, Waltham, MA).
Electrophoresis was performed on pre-cast SDS-PAGE 4–15% gels (BioRad, Hercules CA) under reducing conditions. High molecular weight standards were simultaneously performed with synovial fluid lavages from different animal groups. Western transfer to nitrocellulose was carried out under semi-dry conditions at 20 volts for 40 minutes. The blot was blocked overnight at 4°C with 2% (w/v) BSA in phosphate buffer saline. Probing was performed by incubation with Mab S6.89 at 1:10,000 dilution followed by incubation with anti-mouse IgG-HRP at 1:5,000 dilution for 60 minutes. Immunopositive bands were detected using a chemiluminescent substrate in a darkroom on BioMax film (Kodak). Since the high molecular weight (full length) lubricin has tribologic properties, the SDS-PAGE and Western blot was limited to that region.
Histology
The joints were thawed, dissected, and the soft tissues were removed to expose the cartilage plates. The distal femur and proximal tibia were amputated and immersed in 10% formalin for at least 72 hours. The specimens were then decalcified in Richman-Gelfand-Hill solution and bisected in the sagittal plane. They were processed in a Tissue-Tek VIP 1000 tissue processor (Model#4617, Miles, Elkhart, IN) and embedded in a single block of Paraplast X-tra (Fisher, Santa Clara, CA) with the bisection surface on the cutting face of the block. Blocks were trimmed to expose tissue using a rotary microtome (Model#2030, Reichart-Jung, Austria). Slices were taken from the mid-sagittal plane within each condyle. The slices were sectioned 6µm thick, mounted on slides, and stained with safranin-O/fast green.(21)
The severity of cartilage damage of each joint was assessed using the modified Mankin grading system.(22) For each limb, mid-sagittal sections were evaluated from the medial and lateral condyles of the distal femur and proximal tibia. Three independent observers scored each section, and the scores for the medial and lateral tibial and femoral condyles were averaged within each joint. All scoring was done in a random order and in a blinded fashion.
Statistical Analyses
Analyses of variance for a split plot design were utilized to compare the COFs and lubricin concentrations between groups (Groups 1, 2, and 3) and between legs (left vs right). F-tests were used to examine simple effects. The correlation coefficient was calculated to establish the relationship between lubricin concentration and COFs.
RESULTS
Coefficients of Friction
There was a nearly linear decrease in the peak F-E rotation as a function of cycle number for both knees of all animals (Fig. 2). The mean (±standard deviation) total numbers of oscillations to reach equilibrium (Fig. 2) were: Group 1 Right [91 (±19)], Left [93 (±16)]; Group 2 Right [84 (±24)], Left [75 (±16)]; Group 3 Injured [56 (±13)], Control [71 (±9)]. The primary motion was about the F-E axis, and we assumed that the out of plane rotations were negligible in order to calculate the COF. The mean maximal out of plane rotations were: Group 1 I-E [2.0 (±1.2)°], V-V [4.1 (±2.5)°]; Group 2 I-E [2.6 (±1.9)°], V-V [4.1 (±2.1)°]; Group 3-ACL injured I-E [1.9 (±1.1)°], V-V [3.3 (±2.2)°]; and Group 3-Contralateral control I-E [1.7 (±1.1)°], V-V [4.1 (±2.0)°].
The mean COF in the ACL-deficient knees (Group 3; moderate degeneration) was significantly greater than that of the contralateral uninjured knees (Group 3; p = 0.01) (Fig. 3a). The COF increased by 21.7%. Similarly, the COF of the ACL-deficient knee was 25.7% and 37.3% greater than those from the Group 2 (mild degeneration; p<0.001) and the Group 1 (no degeneration; p<0.001) guinea pig knees, respectively. No significant differences were found between the right and left knees of Group 1 (p=0.84) or Group 2 (p=0.57) (Fig. 3a).
Fig. 3.
A) The mean COF value was increased in the ACL-deficient knee when compared to those of the other knees. B) Mean lubricin concentration was reduced in the ACL transected limb of the ACL-deficient knee compared to those of the other knees. The error bars represent one standard deviation.
Lubricin Concentrations in Synovial Fluid
The mean lubricin concentration in the synovial fluid of the ACL-deficient knees (Group 3) was significantly less than that of the contralateral uninjured knees (p<0.001) (Fig. 3b). Lubricin concentrations decreased by 73%. Lubricin concentrations in the ACL-deficient knees were 73% and 75.1% less than those from the Group 2 (p<0.001) and Group 1 (p<0.001) knees, respectively. No significant difference was found between the right and left knees of the Group 2 animals (p=0.62). However, there was a significant difference (p=0.04) in lubricin concentrations between the right and left knees of the Group 1 animals (Fig. 3b). Although significant, the difference was relatively small (16%) compared to that of the operated animals (73%).
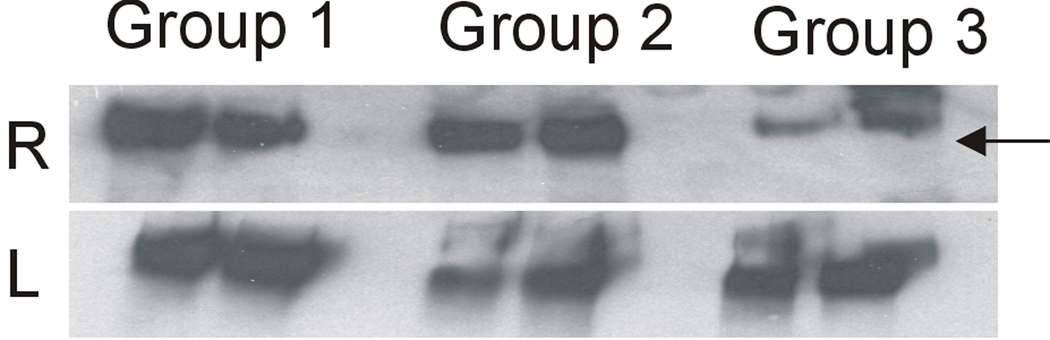
Western blot analysis of synovial fluid lavages from the ACL-transected joints exhibited decreased staining intensity when compared to those from 3- and 12-month-old animals (Fig. 4). The synovial fluid lavages from the contra-lateral joints of Group 3 showed lubricin staining comparable to the synovial fluid lavages from 3- (Group 1) and 12-month (Group 2) animals.
Fig. 4.
Western blot analysis of lubricin in synovial fluid lavages (2 animals/group) of the 3-month, 12-month, and 12-month operated animals (R) and the contralateral knees (L).
Correlation Analyses
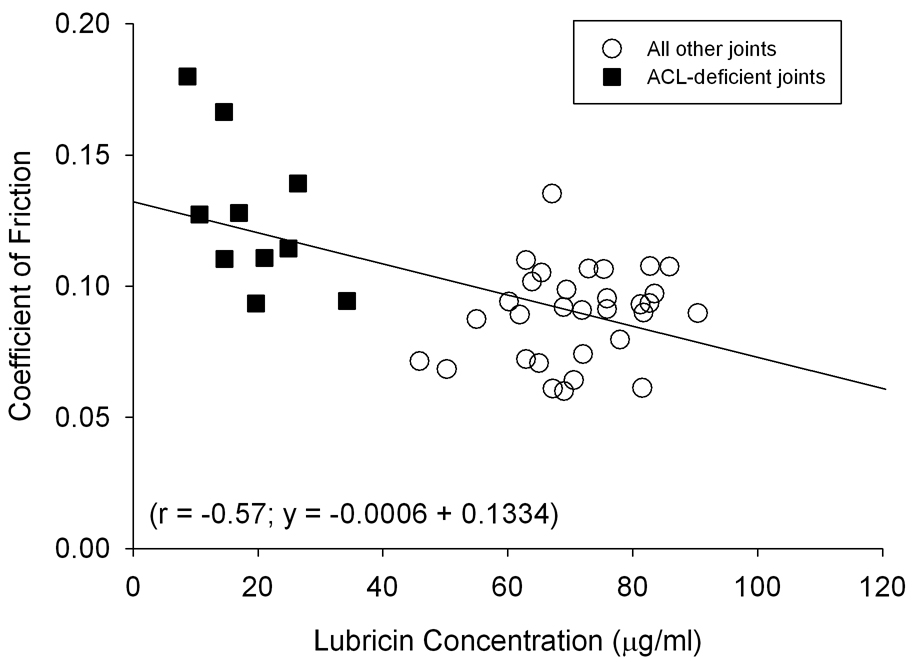
Correlation analysis of COF and lubricin levels was performed for all animals (n=41) for which lubricin levels were obtained (r=−0.57; r2=0.32) (Fig. 5). When performed using data from the ACL-deficient animals only (n=10), the correlations were slightly greater (r=−0.59; r2=0.35).
Fig. 5.
Lubricin concentrations were inversely proportional to the COF (p<0.01).
Light Microscopy
Histological analysis confirmed that there was minimal evidence of joint degeneration in the Group 1 animals, mild degeneration in the Group 2 animals, and moderate degeneration in the ACL-deficient knee of the Group 3 animals (Fig. 6). The mean (±standard deviation) Mankin scores for these groups were 1.6 (±1.2), 2.8 (±2.0) and 5.7 (±3.2), respectively. The mean Mankin score for the contralateral uninjured knee of the Group 3 animals was 3.0 (±2.2).
Fig. 6.
Safranin O/Fast Green staining indicates that the cartilage damage is more severe in the Group 3 ACL transected knee (A) when compared to the Group 3 contralateral ACL-intact knee (B), or the Group 1 (3-month) ACL-intact knee (C).
DISCUSSION
The results of this study show an increase in the COF in the later stages of OA following ACL transection in the Hartley guinea pig knee. No significant increases in the COF values were seen in the mild, naturally occurring OA of the 12-month-old animals. It should be noted that a significant difference in lubricin content was observed between the right and left legs of the 3-month animals, though this difference was only a fraction of that seen between the knees of the Group 3 animals (Fig. 3b). These data were normally distributed and given that this finding is on the border of statistical significance (p=0.04), it may be spurious.
Our results suggest that increased whole joint friction did not occur until later in OA, or that it increases in post-traumatic OA (Group 3) via a mechanism different from that of natural OA (Group 2). Previous studies have shown normal lubricating ability in synovial fluid from human joints with OA.(7) In contrast, decreased lubricating ability of synovial fluid has been reported after a number of inflammatory and traumatic insults including rheumatoid arthritis,(11) post-traumatic knee effusions,(7) meniscectomy,(23) and after ACL transection.(24) Increased whole joint friction has also been observed when lubricin is reduced: after enzymatic degradation of the superficial layer of the articular surface in guinea pig knee joints,(15) in the lubricin knockout mouse,(25) after ACL transection,(24) and in antigen-induced arthritis in rat knee joints.(11) Our results show a similar decrease in whole joint lubrication and increased friction that is present long after the acute stage of the injury. The mechanisms responsible for the loss of lubricin is not known but may be due to decrease expression of lubricin by the synoviocytes or superficial zone chondrocytes, the loss of those cells, and/or an increased catabolism of lubricin. Lubricin concentrations have been shown to be reduced by inflammatory cytokines (i.e. IL-1β),(26) and through the catabolism mediated by Cathepsin-B.(11) The elevated diarthrodial joint friction may possibly be due to loss of superficial zone chondrocytes which produce lubricin. However, this latter possibility must be reconciled with the observation that SF from patients with advanced idiopathic OA demonstrates near normal lubricating activity in vitro.(7)
Interestingly, a decrease in the amount of cartilage damage following ACL-transection has been observed in rabbits receiving intra-articular hyaluronic acid and dipalmitoyl phosphatidylcholine injections.(27) This effect may be secondary to the anti-inflammatory action of hyaluronic acid, which has been found to downregulate gene expression of inflammatory cytokines in synoviocytes.(28) Some also argue that hyaluronic acid plays a lubricating role in synovial joints.(29) Whether hyaluronic acid acts directly or indirectly on joint lubrication and the inflammatory destruction of lubricin and cartilage matrix influence, the possibility that OA progression may be slowed by enhancing joint lubrication emphasizes the value in understanding the timing and mechanisms of frictional damage.
Pendulum methods provide an efficient approach to study joint mechanics and whole joint friction. We used Stanton’s equation for calculating the COF, which is the most frequently cited method.(15) For this analysis, we assumed linear decay, which was the dominant mode of decay when observing graphs of displacement versus cycle number (Fig. 2). The small curvilinear component may reflect nonlinear modes of friction including fluid film and cartilage deformation mechanisms, and damping by stretch on the joint capsule and ligaments.(15,30) When the curvilinear component is small, the error in the COF prediction is low.(30) The dominant mode of lubrication cannot be inferred from the damping curve since the COF values represent a sum of the contributions of multiple mechanisms of lubrication.(1,2,15,31,32) Although retaining the capsule and ligaments introduces an additional source of damping, the advantage of this method is that the joint capsule, synovial fluid, and joint alignment are not violated. Systems studying the frictional properties of cartilage using disarticulated joints or cartilage plugs have the disadvantage that the lubrication mechanism may not be preserved. Aerodynamic drag and drag from the wire attachments of the diodes were assumed to be negligible as demonstrated in a previously published study using the same pendulum apparatus.(15)
Of note, the COF calculations were based only on rotations about the F-E axis of the joint. This was justified by the small average magnitude of maximal displacement in the V-V and I-E rotation planes. A concern regarding ACL transection is that the joint would become unstable, and additional energy losses due to out of plane rotations could falsely elevate the COF data for these limbs. Given that the ranges of V-V and I-E rotations were similar in all groups, the concern is minimized. In the range of motion of our pendulum (135°±18), the knee is always in flexion and under a 300g compressive load. This is approximately half the weight of a 3-month old guinea pig and is well within the range of loads normally experienced by the joint.(15)
This study was limited by the absence of data from animals acutely following ACL injury and at a time point when cartilage damage is still moderate in severity following the trauma. To obtain synovial fluid from the guinea pig knee, a 100µl lavage was required. When aspirated approximately 50µl was retrieved. We assumed that the dilution was consistent across knees. The amount of fluid appeared not to be dependent on the animals group assignment, and there was no evidence of joint swelling suggesting that differences in dilution were not present.
Our study found increased COF and decreased synovial fluid lubricin concentrations in animals with OA 9 months following ACL transection. No increase in COF, or decreases in lubricin concentration were observed in animals with mild, naturally occurring OA. OA progression after ACL injury may progress by persistent deficient joint lubrication, a mechanism unique from that seen in natural OA progression. Another possibility is that lubrication does not become deficient until the later stages of OA. Further studies are underway to address these questions. Our data suggest that increased friction may contribute to the progression of cartilage damage following trauma. Compensating for deficient joint lubrication may provide a means to prevent or slow OA following injury. Potential applications for these therapies would include treatment for acute joint injury as well post-operative treatment.
ACKNOWLEDGEMENTS
The project was funded by the National Institutes of Health (AR049199; AR050180; AR047910; AR047910S1), and RIH Orthopaedic Foundation. The authors acknowledge the assistance of Nigel Gomez, Scott McAllister, and Gary Badger (University of Vermont). The authors thank Dr. Thomas Schmid (Rush University, Chicago IL) for providing the Mab S6.89.
REFERENCES
- 1.Jones ES. Joint Lubrication. Lancet. 1934;1:1426–1427. [Google Scholar]
- 2.Charnley J. The lubrication of animal joints; Symposium on Biomechanics Institution of Mechanical Engineers; 1959. pp. 12–19. [Google Scholar]
- 3.Dowson D, Jin Z-M. Micro-elastohydrodynamic lubrication of synovial joints. Engin Med. 1986;15:63–65. doi: 10.1243/emed_jour_1986_015_019_02. [DOI] [PubMed] [Google Scholar]
- 4.Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22:565–570. doi: 10.1016/j.orthres.2003.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhee DK, Marcelino J, Baker M, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–631. doi: 10.1172/JCI200522263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schumacher BL, Block JA, Schmid TM, et al. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophysics. 1994;311:142–152. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 7.Jay GD, Elsaid KA, Zack J, et al. Lubricating ability of aspirated synovial fluid from emergency department patients with knee joint synovitis. J Rheumatol. 2004;31:557–564. [PubMed] [Google Scholar]
- 8.Marcelino J, Carpten JD, Suwairi WM, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23:319–322. doi: 10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 9.Buckwalter JA. Sports, joint injury, and posttraumatic osteoarthritis. J Orthop Sports Phys Ther. 2003;33:578–588. doi: 10.2519/jospt.2003.33.10.578. [DOI] [PubMed] [Google Scholar]
- 10.Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehab. 2006;85:S2–S11. doi: 10.1097/01.phm.0000245568.69434.1a. [DOI] [PubMed] [Google Scholar]
- 11.Elsaid KA, Chichester CO. Review: Collagen markers in early arthritic disease. Clin Chim Acta. 2006;365:68–77. doi: 10.1016/j.cca.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Bendele AM, Hulman JF. Spontaneous cartilage degeneration in guinea pigs. Arthritis Rheum. 1988;31:561–565. doi: 10.1002/art.1780310416. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez PA, Harlan PM, Chavarria AE. Induction of osteoarthritis in guinea pigs by transection of the anterior cruciate ligament: radiographic and histopathological changes. Inflammation Res. 1995;44:s129–s130. doi: 10.1007/BF01778296. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz ER, Leveille CR, Oh WH. Experimentally-induced osteoarthritis in guinea pigs: Effects of surgical procedure and dietary intake of Vitamin C. Lab Anim Sci. 1981;31:683–687. [PubMed] [Google Scholar]
- 15.Teeple E, Fleming BC, Mechrefe AP, et al. Frictional Properties of Hartley Guinea Pig Knees With and Without Proteolytic Disruption of the Articular Surfaces. Osteoarthritis Cartilage. 2007;15:309–315. doi: 10.1016/j.joca.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three dimensional motions: Application to the knee. J Biomech Engin. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 17.Stanton TE. Boundary Lubrication in Engineering Practice. Engineer. 1923;135:678–680. [Google Scholar]
- 18.Tanaka E, Tatsunori I, Dalla-Bona DA, et al. The effect of experimental cartilage damage and impairment and restoration of synovial lubrication on friction in the temporomandibular joint. J Orofac Pain. 2005;19:331–336. [PubMed] [Google Scholar]
- 19.Mori S, Naito M, Moriyama S, et al. Highly viscous sodium hyaluronate and joint lubrication. Int Orthop. 2002;26:116–121. doi: 10.1007/s00264-002-0330-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su JL, Schumacher BL, Lindley KM, et al. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20:149–157. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg. 1971;53A:69–82. [PubMed] [Google Scholar]
- 22.van der Sluijs JA, Geesink RGT, van der linden AJ, et al. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10:59–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]
- 23.Young AA, McLennan S, Smith MM, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8:R41. doi: 10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsaid KA, Jay GD, Warman ML, et al. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1632–1633. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 25.Jay GD, Carpten JD, Rhee DK, et al. Analysis of the frictional characteristics of CACP knockout mice joints with the modified stanton pendulum technique. Trans Orthop Res Soc. 2003;28:0136. [Google Scholar]
- 26.Flannery CR, Hughes CE, Schumacher BL, et al. Articular cartilage superficial zone protein (SZP) is homologous to megakaryocyte stimulating factor precursor and Is a multifunctional proteoglycan with potential growth-promoting, cytoprotective, and lubricating properties in cartilage metabolism. Biochem Biophys Res Com. 1999;254:535–541. doi: 10.1006/bbrc.1998.0104. [DOI] [PubMed] [Google Scholar]
- 27.Kawano T, Miura H, Mawatari T, et al. Mechanical effects of the intraarticular administration of high molecular weight hyaluronic acid plus phospholipid on synovial joint lubrication and prevention of articular cartilage degeneration in experimental osteoarthritis. Arthritis Rheum. 2003;48:1923–1929. doi: 10.1002/art.11172. [DOI] [PubMed] [Google Scholar]
- 28.Wang CT, Lin YT, Chiang BL, et al. High molecular weight hyaluronic acid down-regulates the gene expression of osteoarthritis-associated cytokines and enzymes in fibroblast-like synoviocytes from patients with early osteoarthritis. Osteoarthritis Cartilage. 2006;14:1237–1247. doi: 10.1016/j.joca.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Antonacci JM, Schmidt TA, Serventi LA, et al. Effects of joint injury on synovial fluid and boundary lubrication of cartilage. Trans Orthop Res Soc. 2007;53:156. [Google Scholar]
- 30.Crisco JJ, Blume J, Teeple E, et al. Assuming Exponential Decay by incorporating Viscous Damping Improves the Prediction of the Coefficient of Friction in Pendulum Tests of Whole Articular Joints. J Engin Med. 2007;221:325–333. doi: 10.1243/09544119JEIM248. [DOI] [PubMed] [Google Scholar]
- 31.Linn FC. Lubrication of Animal Joints II: The Mechanism. Journal of Biomechanics. 1968;1:193–205. doi: 10.1016/0021-9290(68)90004-3. [DOI] [PubMed] [Google Scholar]
- 32.Unsworth A, Dowson D, Wright V. The Frictional Behavior of Synovial Joints – Part 1: Natural Joints. J Lubr Technol. 1975:369–376. [Google Scholar]