Abstract
Aims
We tested the hypothesis that, in heart failure with normal ejection fraction (HFNEF), diastolic dysfunction is accentuated at increasing heart rates, and this contributes to impaired frequency-dependent augmentation of cardiac output.
Methods and results
In 17 patients with HFNEF (median age 69 years, 13 female) and seven age-matched control patients, systolic and diastolic function was analysed by pressure–volume loops at baseline heart rate and during atrial pacing to 100 and 120 min−1. At baseline, relaxation was prolonged and end-diastolic left ventricular stiffness was higher in HFNEF, whereas all parameters of systolic function were not different from control patients. This resulted in smaller end-diastolic volumes, higher end-diastolic pressure, and a lower stroke volume and cardiac index in HFNEF vs. control patients. During pacing, frequency-dependent upregulation of contractility indices (+dP/dtmax and Ees) occurred similarly in HFNEF and control patients, but frequency-dependent acceleration of relaxation (dP/dtmin) was blunted in HFNEF. In HFNEF, end-diastolic volume and stroke volume decreased with higher heart rates while both remained unchanged in control patients.
Conclusion
In HFNEF, frequency-dependent upregulation of cardiac output is blunted. This results from progressive volume unloading of the left ventricle due to limited relaxation reserve in combination with increased LV passive stiffness, despite preserved force–frequency relation.
Keywords: Diastolic function, Heart failure, Pressure volume loops, Force–frequency relation
Introduction
Nearly, half of patients presenting with heart failure have normal ejection fraction (HFNEF).1,2 Recent data show that the mortality of patients with HFNEF is comparable to patients with systolic heart failure (SHF).1,2 In addition, it has been speculated that HFNEF and SHF are different stages of the same disease.3 Moreover, mostly men and relatively young patients have been studied in the few available invasive studies,4–6 whereas the majority of patients with HFNEF in epidemiological studies are elderly females.1
A characteristic feature of SHF is an altered force–frequency relation with blunted frequency potentiation of contractility resulting in the absence of frequency-dependent upregulation of cardiac output.7 Little is known thus far about the impact of atrial pacing and the force–frequency relation in patients with HFNEF.
Accordingly, the present study was performed to test the hypothesis that in HFNEF frequency-dependent upregulation of cardiac function is blunted through mechanisms different from those which have been described in SHF. We specially focused on a cohort of HFNEF patients that resembles the typical age and gender characteristics observed in large-scale, epidemiological studies.
Methods
Patient recruitment
Patients with suspected diastolic heart failure
Between January 2005 and April 2008, patients suffering from symptoms of heart failure undergoing cardiac catherization at the Department of Cardiology and Pneumology at the University of Göttingen were screened for eligibility to this study. If other reasons for dyspnoea [e.g. abnormal pulmonary function on spirometry, valvular disease greater first degree, impaired systolic function (ejection fraction <50%)] were excluded, diastolic heart failure was suspected and patients were offered to undergo an invasive study to confirm the diagnosis of diastolic heart failure. Of note, the presence or absence of diastolic dysfunction by echocardiography was not an inclusion criteria. A total of 19 patients were studied.
One patient had hypertrophic obstructive cardiomyopathy and was excluded from further analysis. Significant coronary artery disease (stenosis >50%) was excluded in all but one patient. In this one patient who complained of angina and dyspnoea on exertion, angioplasty of the circumflex artery resolved the angina, but the dyspnoea remained and therefore pressure–volume loop analysis was performed in a second procedure.
Therefore, 18 patients were analysed for this study, and all but one patient (n = 17) fulfilled the recent criteria for the invasive diagnosis of diastolic heart failure.8 All patients were extensively informed about the procedure and all potential complications and gave written informed consent.
Control patients
Six patients who were referred to the Department of Cardiology and Pneumology of the Charité Campus Benjamin Franklin in Berlin because of atypical chest pain served as controls. Data on some of the control patients have been published previously.4 In addition, one patient in Göttingen, in whom diastolic heart failure was invasively excluded, was also part of the control group. Significant coronary artery disease was excluded in all of the control patients.
Invasive haemodynamic assessment by conductance technique
The conductance procedure has previously been described in detail.4 In brief, the seven French conductance catheter (CD Leycom, Zoetermeer, The Netherlands) was placed into the left ventricle. This catheter contains seven segments, each segment provides a separate conductance signal and a pressure sensor. After calibration including analysis of the conductance of the blood (rho), analysis of the so-called slope factor α (calibration by thermodilution) and parallel conductance (Vc) of the surrounding tissue (e.g. myocardium, pericardium) by transient infusion of hypertonic saline solution (10%), this conductance signal can be transverted into a volume signal. The total LV volume signal was then calculated from only those segments that were completely placed within the left ventricle.
To assess pressure–volume relationships, preload was reduced by use of a vena cava occlusion balloon that was placed in the right atrium and drawn back into the inferior vena cava to acutely lower venous return at each heart rate to obtain left ventricular elastance (Ees) and left ventricular stiffness constant β. Analysis of the pressure–volume loops was performed with custom software as described previously,9 τ was determined by the Glantz method.10 Relaxation time (Trelax) was defined as the time from dP/dtmin to minimal left ventricular pressure and relaxation was considered incomplete, if the ratio of Trelax to τ was less than 3.5.5,11 Pacing was performed by a temporal pacing lead in the right atrium.
Spiroergometry
Spiroergometry was performed by standard technique on a bicycle ergometer (ZAN 600, nSpire Health GmbH, Oberthulba, Germany). Symptom-limited cardiopulmonary exercise testing on a bicycle ergometer started at a workload of 20 W with a stepwise 20 W increment every 2 min. Peak VO2 was defined as the maximum value of the last three 10 s averages.
Echocardiography
Echocardiographic assessment was performed as previously described,12 according to the Guidelines of the American Society of Echocardiography.13 Left ventricular mass was estimated by the Devereux formula.14 Left ventricular mass index (LVMI) was defined as the ratio of left ventricular mass to body surface area. Left ventricular hypertrophy was defined as a LVMI > 95 g/m2 in women and a LVMI > 115 g/m2 in men. For the determination of the E/Ea ratio, the mean of septal and lateral mitral annulus tissue velocities8 was used. Diastolic dysfunction was classified in accordance with a large epidemiological study.15
Laboratory measurements
N-terminal pro brain natriuretic peptide (NT-proBNP) was analysed by a commercially available ELISA (Elecsys, Roche Diagnostics).
Statistics
Data were analysed using SPSS 15.0 software (SPSS, Chicago, IL, USA) and by SigmaStat 3.5 (Systat Software, Erkrath, Germany). Unless stated otherwise, metric data are expressed as median (25th–75th percentile). Differences between groups of patients were assessed by a Mann–Whitney U test (for unpaired metric data) or χ2 test (for categorical data). For the analysis of pacing effects, an ANOVA for repeated measurements was used with a Tukey's post hoc test. If the compound symmetry assumption was not fulfilled, a Greenhouse–Geisser correction was used. A two-tailed P < 0.05 was defined as statistical significant.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read the manuscript and agree to it as written.
Results
Patient characteristics
Table 1 shows baseline characteristics of the patients with HFNEF and the control patients. HFNEF and controls were of similar age, but more patients in the HFNEF group were female. Furthermore, HFNEF patients had more often hypertension and were more obese than controls. All HFNEF patients had NYHA II or III heart failure symptoms and significantly higher NT-proBNP values when compared with controls. Exercise capacity as assessed by spiroergometry was largely reduced in HFNEF patients. Patients with HFNEF showed typical echocardiographic signs of left ventricular hypertrophy (increased left ventricular wall diameters and LVMI), diastolic dysfunction and left atrial enlargement (decreased E/A ratio, increased E/Ea ratio, dilated left atrium). Left ventricular hypertrophy was present in 13 patients with HFNEF and in one of the control patients (P = 0.005). At Doppler echocardiography, two patients in the HFNEF group had first-degree diastolic dysfunction, the other 15 had second-degree diastolic dysfunction.
Table 1.
Baseline characteristics
| HFNEF (n = 17) | Controls (n = 7) | P-value | |
|---|---|---|---|
| Demographic data | |||
| Age (years) | 69 (63–74) | 65 (62–68) | 0.226 |
| Female/male (n) | 13/4 | 2/5 | 0.028 |
| Body mass index (kg/m2) | 29.4 (26.8–32.3) | 26.3 (24.8–26.6) | 0.016 |
| Hypertension (%) | 94.1 | 57.1 | 0.027 |
| Diabetes (%) | 11.8 | 14.3 | 0.865 |
| CAD (%) | 5.9 | 0 | 0.512 |
| Symptoms | |||
| NYHA II (%) | 52.9 | 0 | <0.001 |
| NYHA III (%) | 47.1 | 0 | <0.001 |
| NT-proBNP (pg/mL) | 254 (128.5–499.8) | 41.5 (22–55) | 0.001 |
| Medication | |||
| ACE-inhibition/AT1-antagonist (%) | 76.5 | 42.9 | 0.112 |
| Beta-blockers (%) | 70.6 | 28.6 | 0.058 |
| Calcium channel blockers (%) | 41.2 | 14.3 | 0.204 |
| Diuretics (%) | 70.6 | 14.3 | 0.012 |
| Echocardiography | |||
| IVS (mm) | 12.0 (11.0–14.0) | 10.0 (8.0–10.0) | 0.001 |
| LVPW (mm) | 11.0 (10.0–12.0) | 10.0 (8.0–11.0) | 0.075 |
| LVEDD (mm) | 49.0 (47.0–50.8) | 47.0 (46–49) | 0.383 |
| Left atrial diameter (mm) | 43 (41–48) | 38 (35–40) | 0.011 |
| LVMI (g/m2) | 120 (104–140) | 83 (67–94) | 0.008 |
| E/A ratio | 0.95 (0.86–1.20) | 1.23 (1.05–1.65) | 0.055 |
| EDCT (ms) | 253 (191–303) | 177 (160–190) | 0.011 |
| IVRT (ms) | 85 (73–107) | 91 (86–92) | 0.783 |
| E/Ea (septal) ratio | 13.9 (10.0–22.5) | 7.7 (6.5–9.6) | 0.005 |
| E/Ea (lateral) ratio | 11.5 (8.0–16.0) | 6.8 (4.7–7.9) | 0.001 |
| Spiroergometry (only in HFNEF) | |||
| Maximal performance (W) | 80 (70–100) | ||
| Peak VO2 (mL/kg/min) | 12.5 (10.4–15.0) | ||
| VE/VCO2 slope | 33.5 (30.2–38.9) | ||
CAD, coronary artery disease; NYHA, New York Heart Association; ACE, angiotensin converting enzyme; AT, angiotensin; IVS, interventricular septum diameter; LVPW, left ventricular posterior wall diameter; LVEDD, left ventricular enddiastolic diameter; LVMI, left ventricular mass index; E, transmitral early left ventricular diastolic filling velocity; A, transmitral late left ventricular diastolic filling velocity; EDCT, E deceleration time; IVRT, isovolumetric relaxation time; Ea, mitral annular early diastolic tissue Doppler velocity; VO2, oxygen uptake; VE, ventilatory exchange; VCO2, carbon dioxide output.
Invasive haemodynamic data
Figure 1 shows typical pressure–volume loop recordings in a patient with normal diastolic function (left panel) and in a HFNEF patient. In HFNEF, the diastolic pressure–volume relation (red line) was shifted upwards and to the left, so the left ventricle in these patients is operating with higher pressures at smaller volumes near end-diastole.
Figure 1.
Typical pressure volume loops during transient preload reduction by vena cava occlusion balloon. Left: 49-year-old female with normal left ventricular function (control). Right: 64-year-old female patient with HFNEF. The red line indicates the end-diastolic pressure–volume relation. The pressure–volume relation was shifted upwards and to the left in the HFNEF patient.
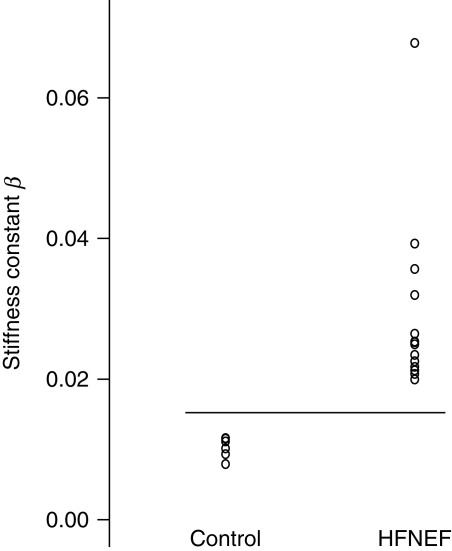
Table 2 summarizes haemodynamic parameters at baseline. Indices of systolic function did not differ between HFNEF patients and controls. End-diastolic volume and end-diastolic volume index were reduced and end-diastolic pressure increased in HFNEF; HFNEF patients had impaired active relaxation and passive diastolic function parameters. The left ventricular stiffness constant β [an estimate of the passive elastic properties (distensibility) of the ventricle16] was significantly higher in HFNEF (0.0245) when compared with controls (0.0107, P < 0.001) with no overlap between groups (Figure 2).
Table 2.
Haemodynamic data at baseline heart rate
| HFNEF (n = 17) | Controls (n = 7) | P-value | |
|---|---|---|---|
| Systolic function | |||
| End-systolic pressure (mmHg) | 133 (114–164) | 118 (111–130) | 0.172 |
| End-systolic volume (mL) | 27 (24–41) | 64 (30–74) | 0.181 |
| End-systolic volume index (mL/m2) | 21 (13–24) | 35 (18–38) | 0.054 |
| Ejection fraction (%) | 71 (63–76) | 66 (63–81) | 0.974 |
| End-systolic elastance (Ees) (mmHg/mL) | 1.79 (1.19–3.04) | 1.40 (1.26–1.65) | 0.312 |
| dP/dtmax (mmHg/s) | 1444 (1244–1748) | 1562 (1549–1866) | 0.153 |
| Diastolic function | |||
| End-diastolic pressure (mmHg) | 16.8 (13.1–20.2) | 6.3 (4.9–8.6) | <0.001 |
| End-diastolic volume (mL) | 108 (92–127) | 158 (114–182) | 0.019 |
| End-diastolic volume index (mL/m2) | 61 (51–68) | 76 (72–91) | 0.009 |
| τ (ms) | 55.3 (49.8–63.8) | 41.8 (36.3–42.6) | <0.001 |
| dP/dtmin (mmHg/s) | −1643 (−1924–−1364) | −1857 (−2084–−1577) | 0.325 |
| Stiffness constant β | 0.0245 (0.0203–0.0315) | 0.0107 (0.0089–0.0120) | <0.001 |
| Global cardiac function | |||
| Heart rate (b.p.m.) | 66 (60–68) | 72 (67–90) | 0.114 |
| Cardiac index (mL/min/m2) | 2812 (2570–2997) | 3980 (3066–4638) | 0.007 |
| Stroke volume index (mL/m2) | 44 (38–46) | 61 (43–63) | 0.061 |
Figure 2.
Left ventricular stiffness constant β in control patients and in patients with HFNEF. Stiffness was higher in all the HFNEF patients with no overlap to controls.
Haemodynamic effects of temporal atrial pacing
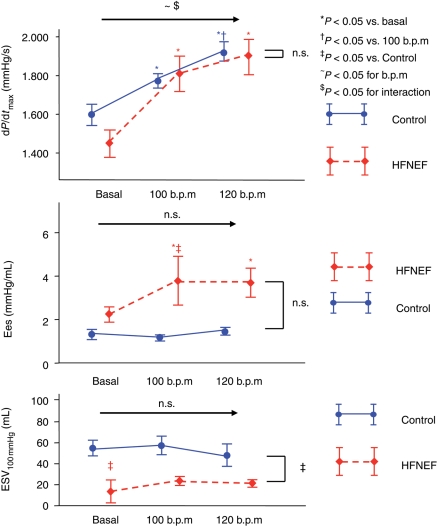
Temporal atrial pacing was performed at rates of 100 and 120 b.p.m. Parameters of systolic function at baseline and with pacing are shown in Figure 3 and Table 3. There was a comparable significant increase in dP/dtmax in HFNEF and control patients, indicating a preserved frequency potentiation of contractility in HFNEF. End-systolic elastance (Ees) increased with pacing in the HFNEF patients, but not in the control patients. End-systolic volume at 100 mmHg (ESV100 mmHg) did not change in both groups with pacing, but was smaller in HFNEF at any heart rate.
Figure 3.
Parameters of contractility (dP/dtmax, end-systolic elastance and end-systolic volume at 100 mmHg) at baseline and with pacing at 100 and 120 b.p.m. (mean, SEM). Data are shown in red for the HFNEF and in blue for the control patients. Results of the multiple measurements ANOVA are shown above the horizontal arrow (for the effects of beats per minute and interaction) and on the right side of the figure (for comparison between HFNEF and controls).
Table 3.
Haemodynamic indices at 100 and 120 b.p.m.
| HFNEF (n = 17) |
Controls (n = 7) |
|||
|---|---|---|---|---|
| 100/min | 120/min | 100/min | 120/min | |
| Systolic function | ||||
| End-systolic pressure (mmHg) | 134 (113–152) | 119 (109–136)† | 121 (106–124) | 128 (101–145) |
| Ejection fraction (%) | 62 (58–76) | 66 (54–73) | 73 (68–77) | 67 (66–78) |
| Diastolic function | ||||
| End-diastolic pressure (mmHg) | 7.6 (6.0–16.7)*‡ | 8.3 (5.6–17.7)*‡ | 4.6 (3.6–6.0) | 4.5 (4.0–6.0) |
*P < 0.05 vs. baseline.
†P < 0.05 vs. 100/min.
‡P < 0.05 vs. control.
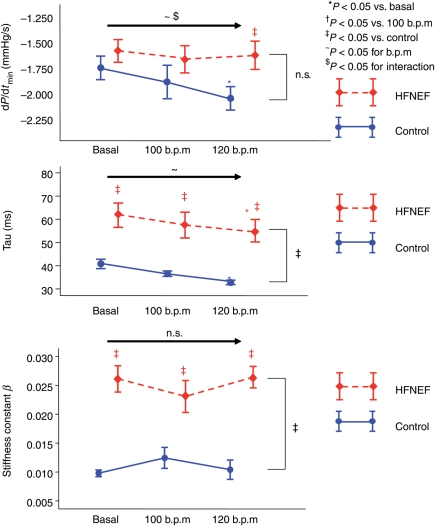
Parameters of diastolic function and their change with pacing are shown in Figure 4 and Table 3. With pacing, active relaxation (τ, dP/dtmin) became faster in both groups, however, even with the highest heart rate, relaxation parameters were still abnormal in the HFNEF group and different between HFNEF and control. The results were similar in the groups with and without beta-blocker therapy. The stiffness constant β as a parameter of passive diastolic properties did not change in either group, but β was ≈2.5 times higher in HFNEF patients at all frequencies.
Figure 4.
Parameters of active relaxation (dP/dtmin, τ) and passive stiffness (stiffness constant β) at baseline and with pacing at 100 and 120 b.p.m. (mean, SEM). Data are shown in red for the HFNEF and in blue for the control patients. Results of the multiple measurements ANOVA are shown above the horizontal arrow (for the effects of beats per minute and interaction) and on the right side of the figure (for comparison between HFNEF and controls).
Relaxation was considered incomplete according to our definition (Trelax < 3.5τ) and was prominent in the HFNEF group, while it was complete in nearly all controls. These data and data on relaxation time and time in systole/diastole are shown in Table 4. With pacing, the systolic and diastolic time intervals were similarly reduced in both groups, and the ratio of systole/diastole increased with pacing in both groups. Relaxation became more incomplete with pacing in the HFNEF group, which was due to a largely shortened relaxation time while τ decreased only slightly. In the controls, relaxation time and τ decreased similarly, and Trelax/τ remained unchanged.
Table 4.
Time in systole, diastole, and relaxation time in HFNEF patients
| HFNEF (n = 17) |
Controls (n = 7) |
|||||
|---|---|---|---|---|---|---|
| Basal | 100/min | 120/min | Basal | 100/min | 120/min | |
| Tsys (ms) | 420 (385–453)‡ | 343 (332–370)* | 312 (297–336)*†‡ | 354 (332–374) | 332 (308–334)* | 299 (261–313)*† |
| Tdiast (ms) | 508 (450–568)‡ | 246 (230–264)* | 193 (180–206)*†‡ | 476 (362–515) | 265 (243–293)* | 191 (184–227)*† |
| Tsys (%) | 45.7 (43.1–46.8) | 58.4 (56.0–61.4)* | 62.6 (59.1–63.8)*† | 42.7 (40.5–48.2) | S.7 (51.1–58.1)* | 61.5 (53.5–63.4)*† |
| Trelax (ms) | 166 (146–190) | 153 (143–165)* | 120 (74–145)*† | 152 (122–161) | 136 (110–142) | 118 (112–135) |
| τ (ms) | 55 (50–64)‡ | 50 (45–66)‡ | 51 (45–55)*‡ | 42 (36–43) | 38 (36–40)* | 34 (31–36)*† |
| Trelax/τ | 3.08 (2.48–3.40) | 2.98 (2.14–3.36)‡ | 2.00 (1.57–3.00)*†‡ | 3.63 (3.30–4.21) | 3.71 (2.89–4.03) | 3.53 (3.29–4.12) |
Tsys, time in systole; Tdiast, time in diastole; Tsys (%), relative portion of systole; Trelax, relaxation time; Trelax/τ, ratio relaxation time to τ (isovolumetric time constant of relaxation).
*P < 0.05 vs. basal.
†P < 0.05 vs. 100/min.
‡P < 0.05 vs. controls.
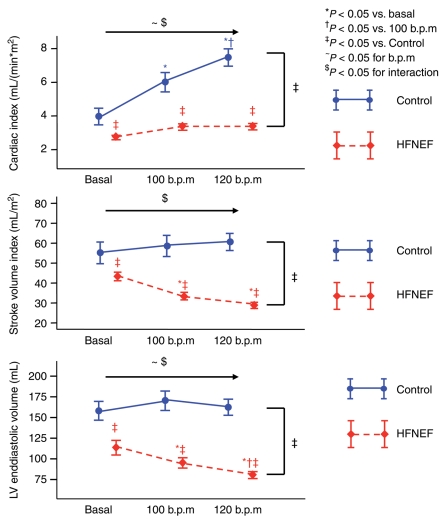
Figure 5 shows the changes in cardiac index, stroke volume index, and left ventricular end-diastolic volume with pacing. Control patients maintained stroke volume and end-diastolic volume at higher heart rates, which led to an increase in cardiac output. In HFNEF, stroke volume and end-diastolic volume decreased and the decrease in stroke volume outbalanced the increase in heart rate, leading to only a slight increase in cardiac output at 100 b.p.m. and no further increase at 120 b.p.m.
Figure 5.
Cardiac index, stroke volume index, and left ventricular end-diastolic volume at comparable baseline heart rate and at pacing rates of 100 and 120 b.p.m. All data are shown as mean ± SEM. Data are shown in red for the HFNEF and in blue for the control patients. Results of the multiple measurements ANOVA are shown above the horizontal arrow (for the effects of beats per minute and interaction) and on the right side of the figure (for comparison between HFNEF and controls).
Discussion
The present study shows that frequency-dependent upregulation of cardiac output is blunted in HFNEF similar to recent observations in SHF. However, underlying mechanisms are completely different, because in HFNEF,
Frequency-dependent upregulation of contractility parameters is preserved, despite a decrease in preload;
Frequency-dependent upregulation of relaxation parameters is significantly blunted;
A progressive decrease of end-diastolic volume at higher heart rates results in reduction of stroke volume and blunting of frequency-dependent upregulation of cardiac output.
This study indicates that mechanisms of dysfunction at least regarding frequency-dependent regulation of cardiac performance are different in SHF and HFNEF. The absolute value of dP/dtmax and its increase with pacing is comparable to normal control patients in our study as well as to control patients in other studies.7 Also, end-systolic elastance is higher and ESV100 mmHg is smaller, indicating a rather increased contractility in HFNEF. From these findings, we conclude that HFNEF patients do not have clinically significant systolic dysfunction as cause of their symptoms. Preserved systolic function in HFNEF was previously described by others.4–6
In contrast to virtually no difference in global systolic function, we could clearly demonstrate abnormal relaxation and compliance in HFNEF patients when compared with controls at baseline. At resting heart rates, Tau was prolonged, and dP/dtmin tended to be lower in HFNEF. With pacing, dP/dtmin and Tau significantly declined in controls, compatible with a frequency-dependent upregulation of relaxation processes. In contrast, dP/dtmin did not decline with higher heart rates in HFNEF and was significantly slower at 120 b.p.m. when compared with controls; Tau declined also in HFNEF with higher pacing rates, but its absolute value remained significantly higher than in controls and above the normal value of 48 ms at each pacing rate. These data indicate that HFNEF patients show an impaired relaxation–frequency relation. We also demonstrate alterations in the passive properties of the left ventricle, e.g. in ventricular stiffness in HFNEF, which agree with previously published data in younger male patients by Zile et al.5
However, all of these patients were asymptomatic during rest, but experienced symptoms only during physical activity. A reduced exercise capacity can be easily explained on the basis of a blunted increase in cardiac output at higher heart rates in HFNEF, but dyspnoea is more difficult to explain from our data. While the subjective symptoms of dyspnoea may result from multifactorial causes, impaired ventricular diastolic filling resulting in pulmonary congestion is a commonly used explanation. We could clearly demonstrate a heart rate dependent potentiation of relaxation abnormalities in HFNEF, but left ventricular end-diastolic pressure (LVEDP) decreased in our patients at higher pacing rates. While this observation is understandable from a haemodynamic point of view (reduced volume filling will result in reduced filling pressure at the end of diastole), it may not adequately reflect the situation under more physiological conditions. In fact, in a recent study,4 handgrip exercise was associated with an increase in LVEDP in HFNEF patients. In summary, impaired filling of the ventricle during exercise may result in reduced cardiac output and increased pulmonary congestion, and these pathologies will be accelerated at higher heart rates in these patients.
Epidemiological studies suggest that the majority of patients with HFNEF are women of older age.1,15,17,18 Comparing these characteristics to other invasive studies in HFNEF, patients in former invasive studies were much younger (between 58 and 66 years4–6) and the percentage of women was relatively low in most of these studies. Moreover, 88% of our patients had moderate or severe diastolic dysfunction, consistent with epidemiological data.15 This underscores that we have, for the first time, invasively assessed a typical patient population with symptomatic and advanced HFNEF.
Role of incomplete relaxation
Incomplete relaxation in HFNEF has been evaluated in animal models19,20 and also in humans.5 Zile et al.5 found incomplete relaxation to be present in all patients with HFNEF they examined and we also observed a high prevalence of incomplete relaxation. Interestingly, despite incomplete relaxation in most patients, LVEDP decreased with higher pacing rates in HFNEF, a finding consistent with a recent invasive human study.4 In contrast, Hay et al.,21 using a computer model, assumed that incomplete relaxation leads to an increase in LVEDP at higher heart rates. This difference might be due to changes in parameters not perfectly resembling the real human situation in this computer-based model, e.g. end-diastolic volume at 120 b.p.m.
With higher heart rates, our diastole was shortened and incomplete relaxation worsened in HFNEF patients. Given the fact that other indices of diastolic function did not alter or changed only marginally, we conclude that pre-existing diastolic dysfunction together with a more incomplete relaxation at higher heart rates may promote heart failure symptoms in HFNEF.
Rate-dependent changes of pump function
Previous studies analysing left ventricular volumes at increasing heart rates (similar to the range in our study) showed conflicting results in healths22–26 with decreases or no change in stolic volume during pacing. An explanation for these conflicting results might be related to differences in the age of the control patients: a significant rate-dependent decrease in left ventricular volumes was observed in studies with younger control subjects (median age 35,23 37,24 5022 years, respectively), whereas in our (median age 65 years) and other studies with older controls (5125 and 6026 years), only minor changes were observed. Further studies in HFNEF should incorporate a second independent measure of stroke volume with pacing (e.g. thermodilution).
The role of heart rate in HFNEF is controversial. Patients with HFNEF do, to a significant portion, have chronotropic incompetence.27,28 Therefore, pacing may improve exercise intolerance in HFNEF. In contrast, there is also evidence that a reduction in heart rate is beneficial.29 In our study, HFNEF patients were unable to adequately increase cardiac output at higher heart rates. This finding is consistent with a recent echocardiographic study in patients with diastolic dysfunction.30 The relevance of blunting of the heart rate dependent increase in cardiac output may depend on the severity of the disease: Westermann et al.,4 who studied a cohort of relatively young patients in Stage I diastolic dysfunction, did not observe a difference in cardiac output at rest between control and HFNEF patients, and observed an increase in cardiac output with pacing in HFNEF, which was smaller than the increase in the control group. Therefore, one might speculate that blunting of frequency-depending upregulation of cardiac output in HFNEF may progress with the severity of the disease.
Recruitment of patients
It has been previously shown that in a carefully selected cohort of patients with heart failure and normal ejection fraction, objective evidence of diastolic dysfunction may not always be necessary.31 However, in the Echo substudy of the CHARM trial in HFNEF, 33% of patients had no evidence of diastolic dysfunction, and moderate or severe diastolic dysfunction was found in only 44% of the patients.32 We therefore believe that in clinical trials, objective evidence of diastolic dysfunction, as proposed in a recent consensus paper,8 should be obtained.
Limitations
Atrial pacing may not completely mimic the effects of exercise on heart rate, myocardial function and global haemodynamics since exercise-dependent heart rate increases may be associated with alterations in sympathetic drive and peripheral vascular resistance. For example, in the recent study by Westermann et al.,4 pacing was associated with a decline, but handgrip exercise with an increase in LVEDP. Thus, future invasive studies should focus on cardiac haemodynamics with exercise in patients with HFNEF.
The definition of incomplete relaxation in humans is not well standardized. We used a method previously described by others,5 but this method is still a matter of debate.
Conclusion
Patients with HFNEF have preserved global systolic function and a preserved frequency-dependent upregulation of contractility, but impaired diastolic function and an impaired relaxation–frequency relation. Our data clearly indicate that increased LV stiffness and impaired relaxation in HFNEF underlie substantial alterations in global haemodynamics that are further aggravated by tachycardia and may explain the symptoms of these patients: dyspnoea on exertion and exercise intolerance.
Funding
This work was supported by grants from the German Federal Ministry of Education and Research [German Heart Failure Network, TP 7 (FK/01 GI0205) and clinical trial program ALDO-DHF (FKZ 01KG0506)], and the Deutsche Forschungsgemeinschaft (TR-SFB-19). Funding to pay the Open Access publication charges for this article was provided by the University of Göttingen.
Conflict of interest: none declared.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 3.De Keulenaer DW, Brutsaert DL. Systolic and diastolic heart failure: different phenotypes of the same disease? Eur J Heart Fail. 2007;9:136–143. doi: 10.1016/j.ejheart.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Westermann D, Kasner M, Steendijk P, Riad A, Spillmann F, Weitmann K, Hoffmann W, Poller W, Schultheiß HP, Pauschinger M, Tschöpe C. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation. 2008;117:2051–2060. doi: 10.1161/CIRCULATIONAHA.107.716886. [DOI] [PubMed] [Google Scholar]
- 5.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004. 350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 6.Yamanaka T, Onishi K, Tanabe M, Dohi K, Funabiki-Yamanaka K, Fujimoto N, Kurita T, Tanigawa T, Kitamura T, Ito M, Nobori T, Nakano T. Force- and relaxation–frequency relations in patients with diastolic heart failure. Am Heart J. 2006;152:966.e1–966.e7. doi: 10.1016/j.ahj.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Hasenfuss G, Holubarsch C, Hermann HP, Astheimer K, Pieske B, Just H. Influence of the force–frequency relationship on haemodynamics and left ventricular function in patients with non-failing hearts and in patients with dilated cardiomyopathy. Eur Heart J. 1994;15:164–170. doi: 10.1093/oxfordjournals.eurheartj.a060471. [DOI] [PubMed] [Google Scholar]
- 8.Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 9.Steendijk P, Tulner SA, Schreuder JJ, Bax JJ, van Erven L, van der Wall EE, Dion RA, Schalij MJ, Baan J. Quantification of left ventricular mechanical dyssynchrony by conductance catheter in heart failure patients. Am J Physiol Heart Circ Physiol. 2004;286:H723–H730. doi: 10.1152/ajpheart.00555.2003. [DOI] [PubMed] [Google Scholar]
- 10.Leeuwenburgh BPJ, Steendijk P, Helbing WA, Baan J. Indexes of diastolic RV function: load dependence and changes after chronic RV pressure overload in lambs. Am J Physiol Heart Circ Physiol. 2002;282:H1350–H1358. doi: 10.1152/ajpheart.00782.2001. [DOI] [PubMed] [Google Scholar]
- 11.Weisfeldt ML, Weiss JL, Frederiksen JT, Yin FCP. Quantification of incomplete left ventricular relaxation: relationship to the time constant for isovolumic pressure fall. Eur Heart J. 1980;1(Suppl. A):119–129. doi: 10.1093/eurheartj/1.suppl_1.119. [DOI] [PubMed] [Google Scholar]
- 12.Wachter R, Lüers C, Kleta S, Griebel K, Herrmann-Lingen CH, Binder L, Janicke N, Wetzel D, Kochen MM, Pieske B. Impact of diabetes on diastolic function in patients with arterial hypertension. Eur J Heart Failure. 2007;9:469–476. doi: 10.1016/j.ejheart.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC, Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: a report of the American College of Cardiology/American Heart Association Task force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography) 2003 American College of Cardiology Web site. Available at: www.acc.org/clinical/guidelines/echo/index.pdf . [Google Scholar]
- 14.Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 15.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. J Am Med Assoc. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 16.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure. I. Diagnosis, prognosis, measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 17.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98:2282–2289. doi: 10.1161/01.cir.98.21.2282. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW, Gardin JM, Gottdiener JS, Arnold AM, Boineau R, Aurigemma GP, Marino E, Lyles M, Cushman M, Enright P For the Cardiovascular Health Study Group. Importance of heart failure with preserved systolic function in patients>or=65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 19.Yellin EL, Hori M, Yoran C, Sonnenblick EH, Gabbay S, Frater RWM. Left ventricular relaxation in the filling and nonfilling intact canine heart. Am J Physiol. 1986;250:H620–H629. doi: 10.1152/ajpheart.1986.250.4.H620. [DOI] [PubMed] [Google Scholar]
- 20.Blaustein AS, Gaasch WH. Myocardial relaxation. III. Reoxygenation mechanics in the intact dog heart. Circ Res. 1981;49:633–639. doi: 10.1161/01.res.49.3.633. [DOI] [PubMed] [Google Scholar]
- 21.Hay I, Rich J, Ferber P, Burkhoff D, Maurer MS. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol. 2005;288:H1203–H1208. doi: 10.1152/ajpheart.00681.2004. [DOI] [PubMed] [Google Scholar]
- 22.Dehmer GJ, Firth BG, Nicod P, Lewis SE, Hillis LD. Alterations in left ventricular volumes and ejection fraction during atrial pacing in patients with coronary artery disease: Assessment with radionuclide ventriculography. Am Heart J. 1983;106:114–124. doi: 10.1016/0002-8703(83)90448-9. [DOI] [PubMed] [Google Scholar]
- 23.Hung J, Kelly DT, Hutton BF, Uther JB, Baird DK. Influence of heart rate and atrial transport on left ventricular volume and function: relation to hemodynamic changes produced by supraventricular arrhythmia. Am J Cardiol. 1981;48:632–638. doi: 10.1016/0002-9149(81)90140-5. [DOI] [PubMed] [Google Scholar]
- 24.Liu CP, Ting CT, Lawrence W, Maughan WL, Chang MS, Kass DA. Diminished contractile response to increased heart rate in intact human left ventricular hypertrophy. Systolic versus diastolic determinants. Circulation. 1993;88:1893–1906. doi: 10.1161/01.cir.88.4.1893. [DOI] [PubMed] [Google Scholar]
- 25.Erbel R, Schweizer P, Krebs W, Langen HJ, Meyer J, Effert S. Effects of heart rate changes on left ventricular volume and ejection fraction: a 2-dimensional echocardiographic study. Am J Cardiol. 1984;53:590–597. doi: 10.1016/0002-9149(84)90036-5. [DOI] [PubMed] [Google Scholar]
- 26.Aroesty JM, McKay RG, Heller GV, Royal HD, Als AV, Grossman W. Simultaneous assessment of left ventricular systolic and diastolic dysfunction during pacing-induced ischemia. Circulation. 1985;71:889–900. doi: 10.1161/01.cir.71.5.889. [DOI] [PubMed] [Google Scholar]
- 27.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114:2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker PH, Joo KC, Stewart KP, Fray B, Morre B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26:86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Bergström A, Andersson B, Edner M, Nylander E, Persson H, Dahlström U. Effect of carvedilol on diastolic function in patients with diastolic heart failure and preserved systolic function. Results of the Swedish Doppler-echocardiographic study (SWEDIC) Eur J Heart Fail. 2004;6:453–461. doi: 10.1016/j.ejheart.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Sohn DW, Kim HK, Park JS, Chang HJ, Kim YJ, Zo ZH, Oh BH, Park YB, Choi YS. Hemodynamic effects of tachycardia in patients with relaxation abnormality: abnormal stroke volume response as an overlooked mechanism of dyspnea associated with tachycardia in diastolic heart failure. J Am Soc Echocardiogr. 2007;20:171–176. doi: 10.1016/j.echo.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation. 2001;104:779–782. doi: 10.1161/hc3201.094226. [DOI] [PubMed] [Google Scholar]
- 32.Persson H, Lonn E, Edner M, Baruch L, Lang CC, Morton JJ, Ostergren J, McKelvie RS Investigators of the CHARM echocardiographic Substudy-CHARMES. J Am Coll Cardiol. 2007;49:687–694. doi: 10.1016/j.jacc.2006.08.062. [DOI] [PubMed] [Google Scholar]