Abstract
Aims
To examine a relationship between alterations of structure and function of the arterial wall in response to glucose-lowering therapy in type 2 diabetes mellitus (DM) after a 1-year follow-up (FU).
Methods and results
In DM (n = 22) and in healthy controls (n = 17), coronary artery calcification (CAC) was assessed with electron beam tomography and carotid intima–media thickness (IMT) with ultrasound, whereas coronary function was determined with positron emission tomography-measured myocardial blood flow (MBF) at rest, during cold pressor testing (CPT), and during adenosine stimulation at baseline and after FU. The decrease in plasma glucose in DM after a mean FU of 14 ± 1.9 months correlated with a lower progression of CAC and carotid IMT (r = 0.48, P ≤ 0.036 and r = 0.46, P ≤ 0.055) and with an improvement in endothelium-related ΔMBF to CPT and to adenosine (r = 0.46, P ≤ 0.038 and r = 0.36, P ≤ 0.056). After adjusting for metabolic parameters by multivariate analysis, the increases in ΔMBF to CPT after glucose-lowering treatment remained a statistically significant independent predictor of the progression of CAC (P ≤ 0.001 by one-way analysis of variance).
Conclusion
In DM, glucose-lowering treatment may beneficially affect structure and function of the vascular wall, whereas the observed improvement in endothelium-related coronary artery function may also mediate direct preventive effects on the progression of CAC.
Keywords: Cardiovascular disease prevention, Carotid IMT, Coronary artery calcification, Coronary circulation, Endothelium, Diabetes mellitus, Positron emission tomography
Introduction
Type 2 diabetes mellitus (DM) is accompanied by the early development of accelerated coronary artery disease (CAD) that accounts for excess morbidity and mortality in these patients.1 According to the American Diabetes Association, there is a continuous increase in the prevalence of DM and, therefore, poses a considerable public health concern.1 Subclinical measures of CAD such as coronary circulatory dysfunction,2,3 increased carotid intima–media thickness (IMT),4 a surrogate marker for subintimal CAD process, and coronary artery calcification (CAC)5 have all been shown to independently predict the development of CAD and future cardiovascular events.2,5 Diabetes mellitus induces structural and functional alterations of the arterial wall, which are associated with pro-atherosclerotic and pro-thrombotic effects, and provide an important mechanistic link between early stages of vascular disease and adverse cardiovascular outcome in DM.2
As we have shown previously,6 a progressive worsening of coronary artery function parallels the increasing severity of insulin resistance states and of carbohydrate intolerance, which can be reversed or be ameliorated by glycaemic control,7 which in turn may slow the development of diabetic vasculopathy.8 The effects of glucose-lowering on both, structure and function of the arterial wall, however, have not been evaluated concurrently in patients with DM. Apart from direct adverse effects of cardiovascular risk factors on the arterial wall, functional abnormalities of the coronary circulation, associated with a loss of endothelium-derived and atheroprotective nitric oxide, have been widely realized to initiate and promote atherosclerotic disease.2 Therapy-induced improvements of endothelium-dependent coronary artery function might therefore delay the development of structural changes of the arterial wall.
Herein, we intended to evaluate (i) the effects of glucose-lowering treatment on CAC, increased carotid IMT, and coronary artery function in patients with DM, and (ii) a possible association between an improvement in coronary artery function and the rate of progression of structural alterations of the arterial wall.
Methods
Study population
The study population consisted of 17 healthy controls [CON; 9 male, 8 female; mean [standard deviation (SD)] age 39 ± 13 years] and 22 asymptomatic type 2 diabetes mellitus patients (DM; 10 male, 12 female; mean age 53 ± 7 years) (Tables 1 and 2). Inclusion criteria were, at the time of the initial screening visit, the presence of DM diagnosed by standard criteria. Exclusion criteria included a history of coronary or peripheral vascular disease, prior myocardial infarction, hypercholesterolaemia (total plasma cholesterol ≥240 mg/dL and LDL plasma cholesterol ≥155 mg/dL), chronically elevated blood pressure (≥140/90 mmHg) measured on at least two occasions, current or prior smoking, a family history of premature CAD, and any endocrine, hepatic, renal, or inflammatory disease. The presence of DM was established by standard clinical criteria and confirmed at the time of the screening visit by fasting plasma glucose concentrations of >126 mg/dL on at least two occasions and elevated HbA1c concentrations.7 All study participants were normal on physical examination and had normal electrocardiograms at rest. The patients reported a mean duration of their diabetes of 25 ± 16 months (range 3–48). These DM were not on anti-diabetic treatment or any other medications before study recruitment and at baseline examinations. Before study inclusion, DM patients tried to control diabetes by diet, weight loss, and physical activity. Twenty out of these 22 DM as well as 17 CON were part of a previous investigation assessing the short-term effect of euglycaemic control on coronary artery function.7
Table 1.
Demographics and laboratory findings in normal controls, the whole group of type 2 diabetic patients, and diabetic responders
| Controls |
Diabetes |
Diabetes responders |
||||
|---|---|---|---|---|---|---|
| Baseline | 1-year follow-up | Baseline | 1-year follow-up | Baseline | 1-year follow-up | |
| Number, n | 17 | 17 | 22 | 22 | 15 | 15 |
| Age (years) | 39 ± 13 | — | 53 ± 7* | — | 53 ± 6* | — |
| Body mass index (kg/m2) | 26.8 ± 3.17 | 26.8 ± 3.34 | 28.9 ± 3.64 | 29.1 ± 3.60 | 29.3 ± 3.3* | 29.9 ± 3.27 |
| HbA1c (%) | 5.5 ± 0.2 | 5.3 ± 0.5 | 9.83 ± 2.7* | 8.48 ± 1.91† | 10.6 ± 2.9* | 8.4 ± 2.07† |
| Fasting plasma concentrations | ||||||
| Glucose (mg/dL) | 84 ± 14 | 85 ± 11 | 205 ± 72* | 160 ± 44† | 231 ± 65* | 158 ± 42† |
| Lactic acid (mg/dL) | 13.9 ± 4.1 | 13.0 ± 3.8 | 16.3 ± 3.7 | 16.5 ± 3.8 | 16.0 ± 3.8 | 17.1 ± 3.8 |
| Free fatty acids (mmol/L) | 0.59 ± 0.26 | 0.64 ± 0.27 | 0.77 ± 0.34 | 0.63 ± 0.23 | 0.80 ± 0.40 | 0.59 ± 0.19 |
| Insulin (µU/mL) | 8.36 ± 4.34 | 8.77 ± 6.82 | 10.9 ± 11.0 | 13.7 ± 9.5 | 11.6 ± 13.1 | 15.2 ± 10.6 |
| HOMA | 1.77 ± 1.11 | 2.00 ± 1.82 | 5.42 ± 7.07* | 5.08 ± 3.17 | 6.36 ± 8.42* | 5.47 ± 3.47 |
| Total cholesterol (mg/dL) | 184 ± 28 | 183 ± 24 | 208 ± 53 | 205 ± 41 | 223 ± 54* | 212 ± 36 |
| LDL cholesterol (mg/dL) | 114 ± 23 | 113 ± 20 | 117 ± 36 | 119 ± 33 | 128 ± 33 | 123 ± 30 |
| HDL cholesterol (mg/dL) | 44 ± 11 | 47 ± 9 | 45 ± 11 | 45 ± 10 | 48 ± 10 | 45 ± 9 |
| Triglycerides (mg/dL) | 123 ± 96 | 97 ± 42 | 202 ± 126* | 208 ± 95 | 191 ± 122* | 220 ± 102 |
HbA1c, haemoglobin A1c; HOMA, homeostatic model assessment; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
*P < 0.05 vs. controls.
†P < 0.05 vs. baseline in diabetes.
Table 2.
Haemodynamic data and myocardial blood flows in normal controls, the whole group of type 2 diabetic patients, and diabetic responders
| Controls |
Diabetes |
Diabetes responders |
||||
|---|---|---|---|---|---|---|
| Baseline | 1-year follow-up | Baseline | 1-year follow-up | Baseline | 1-year follow-up | |
| Numbers | 17 | 17 | 22 | 22 | 15 | 15 |
| CCS (mean, SD) | 4.25 ± 9 | 6.74 ± 19 | 35.28 ± 68* | 59.88 ± 102† | 42.7 ± 81* | 65.0 ± 121 |
| CCS (median, IQR) | 0 (0–0.93) | 0 (0–1.40) | 3.72 (0–27.1)* | 5.12 (0–76.9)† | 3.25 (0–27.1)* | 4.4 (0–39.0) |
| IMT (mm) | 0.68 ± 0.11 | 0.70 ± 0.11 | 0.83 ± 0.19* | 0.86 ± 0.18† | 0.82 ± 0.20* | 0.84 ± 0.19 |
| Haemodynamics | ||||||
| Rest | ||||||
| Heart rate (b.p.m.) | 68 ± 9 | 69 ± 8 | 71 ± 11 | 73 ± 14 | 74 ± 11 | 76 ± 14 |
| SBP (mmHg) | 120 ± 18 | 116 ± 15 | 139 ± 21 | 133 ± 21 | 139 ± 20* | 133 ± 20 |
| RPP (mmHg/min) | 8061 ± 1443 | 7952 ± 1107 | 9917 ± 2162 | 9828 ± 2827 | 10 345 ± 2139* | 10 297 ± 2683 |
| MBF (mL/min/g) | 0.65 ± 0.14 | 0.67 ± 0.12 | 0.77 ± 0.22 | 0.78 ± 0.17 | 0.81 ± 0.22* | 0.81 ± 0.15 |
| Adenosine-stimulated hyperaemia | ||||||
| Heart rate (b.p.m.) | 97 ± 19 | 97 ± 16 | 95 ± 15 | 94 ± 14 | 99 ± 14 | 98 ± 14 |
| SBP (mmHg) | 119 ± 13 | 115 ± 13 | 134 ± 22 | 131 ± 18 | 134 ± 20* | 132 ± 20 |
| RPP (mmHg/min) | 11 579 ± 2395 | 11 063 ± 1748 | 12 951 ± 3433 | 12 442 ± 2956 | 13 413 ± 3442 | 13 015 ± 3109 |
| MBF (mL/min/g) | 2.02 ± 0.44 | 2.01 ± 0.39 | 1.85 ± 0.33 | 1.97 ± 0.54 | 1.86 ± 0.40 | 2.15 ± 0.45† |
| ΔMBF (mL/min/g) | 1.37 ± 0.41 | 1.33 ± 0.37 | 1.08 ± 0.31* | 1.19 ± 0.51 | 1.05 ± 0.35* | 1.34 ± 0.44† |
| MFR | 2.97 ± 0.77 | 3.01 ± 0.67 | 2.55 ± 0.67* | 2.59 ± 0.76 | 2.38 ± 0.46* | 2.72 ± 0.71† |
| Cold pressor testing | ||||||
| Heart rate (b.p.m.) | 76 ± 10 | 76 ± 10 | 78 ± 13 | 79 ± 17 | 81 ± 13 | 83 ± 17 |
| SBP (mmHg) | 139 ± 23 | 142 ± 24 | 164 ± 28 | 162 ± 20 | 166 ± 30* | 163 ± 19 |
| RPP (mmHg/min) | 10 525 ± 2000 | 10 758 ± 2208 | 12 767 ± 2998 | 12 822 ± 3140 | 13 515 ± 3215* | 13 522 ± 3024 |
| MBF (mL/min/g) | 0.90 ± 0.21 | 0.94 ± 0.21 | 0.86 ± 0.24 | 0.97 ± 0.26 | 0.87 ± 0.21 | 1.04 ± 0.25† |
| ΔRPP (mmHg/min) | 2464 ± 1540 | 2805 ± 1465 | 2849 ± 1850 | 2995 ± 1482 | 3169 ± 2123 | 3225 ± 1740 |
| ΔMBF (mL/min/g) | 0.25 ± 0.12 | 0.27 ± 0.16 | 0.09 ± 0.09* | 0.19 ± 0.17† | 0.07 ± 0.07* | 0.23 ± 0.17† |
CCS, coronary artery calcium score; SD, standard deviation; IQR, inter-quartile range; IMT, intima–media thickness; SBP, systolic blood pressure; RPP, rate–pressure product (heart rate × systolic blood pressure, b.p.m. × mmHg); MBF, myocardial blood flow.
*P < 0.05 vs. controls baseline.
†P < 0.05 vs. baseline in diabetes.
Study protocol
Diabetic patients considered eligible to participate in the study did meet with the physician and the diabetes nurse 2 weeks before start of glucose-lowering treatment with glyburide and/or metformin. Subsequently, after the baseline measurements of CAC using electron beam tomography (EBT), carotid IMT with high-resolution vascular ultrasound and myocardial blood flows (MBFs) at rest and during vasomotor stress with N-13 ammonia positron emission tomography (PET) were obtained in DM, and glucose-lowering treatment with glyburide was started. Treatment was commenced with oral glyburide at a dose of 10 mg/day. If, after 10 days, fasting glucose concentrations remained >126 mg/dL, the dose was increased to 10 mg twice a day (total of 20 mg/day). If, after an additional 20 days, no euglycaemic control (plasma glucose ≤126 mg/dL) was achieved, metformin at a dose between 500 and 1000 mg (maximal dose of 1000 mg/day) was added to the glyburide. A medium dose of glyburide and/or metformin was applied as it yields ∼80% of the glucose-lowering effect and, the same time, minimizes potential side effects such as hypoglycaemic events. Patients and immediate family members were instructed to contact one of the investigators in instances of hypoglycaemia or other adverse reactions. The diabetic patients visited the hospital outpatient clinic after start of the glucose-lowering treatment at 4-month intervals. The follow-up (FU) measurements of CAC, carotid IMT, MBF and blood chemistry function test were performed after the outpatient visit after at least 12 months. Positron emission tomography flow measurements and blood chemistry function test at baseline and FU were performed in the morning at fasting condition, whereas measurements of CAC and carotid IMT took place on the same day in the afternoon. A subgroup of diabetic responders was defined as those DM who did demonstrate a decrease in plasma glucose after the FU when compared with baseline.
Evaluation of coronary artery calcium
Coronary artery calcification was determined with EBT (GE Imatron, Milwaukee, WI, USA). Two prospective cardiac-gated (at 60% R–R interval) scans were acquired using 3 mm collimation CVS mode. Utilizing the Accu-Image workstation, areas of CAC were considered to be present, if hyperattenuating lesions above a threshold of 130 Hounsfield units (HU) with an area of three or more contiguous pixels (at least 1 mm2) were identified.9 The size of the lesion was automatically calculated, and the CAC was scored using the Agatston algorithm.9 The coronary artery calcium score (CCS) was assessed across all lesions within the left main, left anterior descending, left circumflex, and right coronary artery, and the sum of all lesion scores yielded the total CCS. An abnormal CAC was defined as a CCS >75th percentile of age-related CAC. Based on the total CCS, study participants were assigned into groups of minor, moderate, or severe CAC with CCS of ≤100, >100 and <400, and ≥400 U, respectively.9
Determination of carotid intima–media thickness
Using a high-resolution B-mode ultrasound machine (ATL Ultramark IV, Bothell, WA, USA), images of the common carotid artery were acquired with a 10 MHz linear array transducer. Carotid IMT was assessed using an automated computerized edge detection algorithm. Measurements of the right and left common carotid arteries were performed between the intima–lumen and media–adventitia interfaces along a 1 cm length just distal to the carotid bulb. This approach standardizes the location and distance over which carotid IMT is determined and ascertains that the same portion of the arterial wall is measured in each image and across individual persons.10 The reported IMT (in mm) for each study participant represented the average of 10 measurements (5 measurements from the right and 5 from the left common carotid artery). Measurements were obtained by an experienced vascular sonographer (Y.L.) who was blinded to the clinical and laboratory profile of the study participants.
Assessment of coronary artery function with positron emission tomography
Myocardial blood flow was measured in mL/min/g with N-13 ammonia, serial PET image acquisition, and a two-compartment tracer kinetic model as described previously.7 Re-orientated N-13 ammonia perfusion images were also evaluated visually and semi-quantitatively for rest and stress regional perfusion abnormalities. Myocardial blood flow was measured at rest, during sympathetic stimulation with cold pressor testing (CPT), reflecting predominantly endothelium-dependent vasomotion, and during adenosine-stimulated hyperaemia (140 µg/kg/min adenosine), reflecting predominantly endothelium-independent vascular smooth muscle relaxation.7 The increase in MBF from rest to CPT or to adenosine was defined as ΔMBF (mL/min/g). The myocardial flow reserve (MFR) was calculated as the ratio of adenosine MBF over rest MBF. Heart rate, blood pressure, and a 12-lead electrocardiogram were recorded continuously during each MBF measurement. The rate–pressure product (RPP), which is a product of the mean heart rate and systolic blood pressure (SBP) during the first 2 min of image acquisition, served as an index of cardiac work.
Statistical analysis
The statistical analysis was performed using SPSS software 11.0 (SPSS Inc.). Data are presented as mean (SD) for quantitative and absolute frequencies for qualitative variables. The appropriate Wilcoxon rank test for independent or paired samples was used. A comparison of CPT-induced ΔMBF or adenosine-stimulated hyperaemic MBF or its change from rest ΔMBF between the different groups was performed by one-way analysis of variance (ANOVA), followed by Scheffe's multiple comparison test. According to previous investigations,10 an abnormal increase in carotid IMT was defined as ≥0.8 mm. Since CCSs followed a marked skewed distribution, the CCSs were also logarithmically transformed (log-CCS). Correlations between selected variables were estimated by the Spearman correlation coefficients (r). Multivariable analysis was performed with the logistic regression model adjusting for the following predictors of the difference in Δlog-CCS after glucose-lowering treatment: differences in body mass index (BMI), plasma glucose, homeostasis model assessment (HOMA), triglycerides, free fatty acids, lipids, and SBP. Statistical significance was assumed if the null hypothesis could be rejected at the P ≤ 0.05 level. Based on an SD of 7.55 of CCS measurements with EBT, and a minimum clinically relevant difference in repeat CCS measurements of 15.8, α = 0.05, and a power (1 − β) of 0.8, the number of patients necessary for repeat long-term studies was calculated to be at least 10 individuals.11 Similarly, using an SD of 0.18 of ΔMBF to vasomotor stress, and a minimum clinically relevant difference in ΔMBF of 0.20 in flow response, α = 0.05, and a power (1 − β) of 0.8, a minimum sample size of 10 individuals was estimated to be needed for repeat long-term PET flow studies.12
Results
Baseline studies
Blood chemistry
Plasma glucose levels, HOMA as index for insulin resistance, and triglycerides were significantly higher in DM than in CON (Table 1). Total plasma cholesterol, LDL plasma cholesterol, free fatty acid, and insulin levels tended to be higher in DM than in the CON, whereas HDL plasma cholesterol was similar.
Coronary artery calcification and carotid intima–media thickness
For the entire study population, the prevalence of CACs was 56% (22/39). In CON, CAC was identified in 47% (8/17), which was non-significantly lower to that of 64% (14/22) of CAC in DM. As given in Table 2, CCS in DM was observed to be significantly higher than in CON. The average CCS of 39 ± 67 in the study population was relatively low. Given the CCS for each patient, 11 DM had minor (≤100), three had moderate (>100 to ≤400), and none had severe CCS (>400), whereas 8 CON presented minor CCS. Further, only four in DM and one in CON had abnormal increased CCS, when it was related to the age of the individual study participant (>75th percentile). In the current study population, no correlation between log-CCS and the age of the study participants was observed (r = 0.33, P = 0.13). As regards the carotid IMT (Table 2), it was abnormally increased in DM than in CON. When an abnormal increase in carotid IMT was defined as ≥0.8 mm according to previous investigations,10 it was found in 11 of 22 (50%) DM and in 3 of 17 (18%) CON.
Positron emission tomography measurements of myocardial blood flow
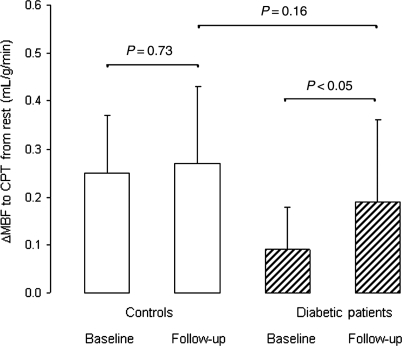
N-13 ammonia PET rest–stress perfusion images displayed a homogeneous radiotracer distribution of the left ventricle arguing against the presence of advanced and flow-limiting coronary lesions. Further, eight DM had blood pressures ≥140/90 mmHg at the day of the PET study. At rest, heart rate, SBP, and RPPs, however, did not differ significantly between CON and DM (Table 2). Sympathetic stimulation with CPT raised heart rate and SBP in both groups (Table 2). Despite similar RPP at rest and during CPT in the two study groups, CPT was associated with a significantly diminished MBF increase (ΔMBF) in DM than in CON (Table 2). Thus, despite comparable increases in cardiac work with CPT, the increase in endothelium-related coronary flow was significantly less in DM than in CON (Table 2 and Figure 1). Further, the adenosine-stimulated hyperaemic MBFs were associated with a significant increase in heart rates, whereas a minor decrease in SBP was observed (Table 2). Hyperaemic MBFs during adenosine stimulation were lower in DM than in CON, but it did not reach statistically significance. When the change in hyperaemic MBF from rest (ΔMBF) and the MFR were evaluated, the ΔMBF during adenosine stimulation and the MFR in DM were significantly less than in CON.
Figure 1.
Endothelium-related change in myocardial blood flow (ΔMBF) to sympathetic stimulation with cold pressor testing in controls and patients with type 2 diabetes mellitus at baseline and follow-up.
Follow-up studies after glucose lowering
Clinical characteristics
Type 2 diabetes mellitus patients were treated with glyburide or glyburide/metformin (mean daily dose of glyburide and metformin: 18 ± 4 and 941 ± 153 mg, respectively) for at least 12 months with a mean FU period of 14 ± 1.9 months (range: 12–17), which did not differ significantly from the mean FU of 15 ± 1.7 months (range: 12–18) in CON (P = 0.43). Eleven DM were on glyburide treatment only and further 11 DM were on added therapy with metformin. Only 6 out of 22 (30%) of DM had achieved euglycaemic control (plasma glucose ≤126 mg/dL) after the FU. At FU, plasma glucose and HbA1c concentrations were significantly lower than at baseline (Table 1), whereas insulin, HOMA, triglycerides, free fatty acids, lipids, and SBP had not changed significantly.
Coronary artery calcification and carotid intima–media thickness
Possible changes in structural alterations of the arterial wall after the 1-year FU were evaluated. After the FU, the CCS in DM was significantly but mildly increased, not observed in CON (Table 2). Out of 22 DM, 2 patients had new vascular segments of calcium depositions, whereas in 10 patients, calcium lesions were identified to be more intense at FU than at baseline investigation. In CON with mild CAC, at FU, no new vascular calcium depositions were noted, and in two individuals, the CCS had mildly increased. In both groups of all DM and diabetic responders, the CCS had non-significantly increased (Table 2). Further, the progression in CCS from baseline to FU (ΔCCS) was comparable between both groups (24.6 ± 43 vs. 22.2 ± 47; P = 0.87).
As denoted in Table 2, carotid IMT had mildly but significantly increased in DM, whereas no significant alteration was noted in CON (Table 1). In the subgroup of diabetic responders, the carotid IMT tended to be mildly higher at FU than at baseline (Table 2). Further, the increase in carotid IMT baseline to FU (ΔIMT) tended to be higher in the whole group of DM than in the subgroup of diabetic responders (0.014 ± 0.03 vs. 0.003 ± 0.02 mm2, P = 0.78).
Positron emission tomography flow measurements
At FU, N-13 ammonia PET rest–stress perfusion images were normal in all study participants. The endothelium-related increase in MBF during CPT (ΔMBF) in DM had significantly improved after the FU, but still tended to be less than that in CON (Table 2 and Figure 1). In CON, ΔMBF to CPT remained comparable between baseline and FU studies. The group comparison of the increase in ΔMBF to CPT in DM after the FU period was significantly different from CON (P < 0.0001 by ANOVA). The adenosine-stimulated MBF increases, and its change of MBF from rest (ΔMBF) and the MFR in DM at FU tended to be higher than at baseline (Table 2). In the group of diabetic responders (n = 15), hyperaemic MBF during adenosine, ΔMBF to adenosine stimulation, and MFR were significantly increased at FU than at baseline (Table 2). In CON, hyperaemic MBF during adenosine, ΔMBF to adenosine stimulation, and MFR were not altered between baseline and FU studies. The group comparison of an increase in hyperaemic MBF during adenosine, ΔMBF to adenosine stimulation, and MFR in diabetic responders after the FU period was significantly different from CON (P < 0.0001 by ANOVA).
Comparison of changes in plasma glucose and structural and functional alterations of the artery
Alterations in fasting plasma glucose concentrations from baseline to FU were expressed as the difference in glucose concentrations at baseline and FU. Changes in glucose concentrations in DM were compared with those of CCS, carotid IMT, and the improvement in the flow response to CPT and adenosine stimulation. These changes were defined as the difference in log-CCS, carotid IMT, and ΔMBFs between FU and baseline (e.g. difference Δlog-CCS = log-CCS baseline − log-CCS FU).
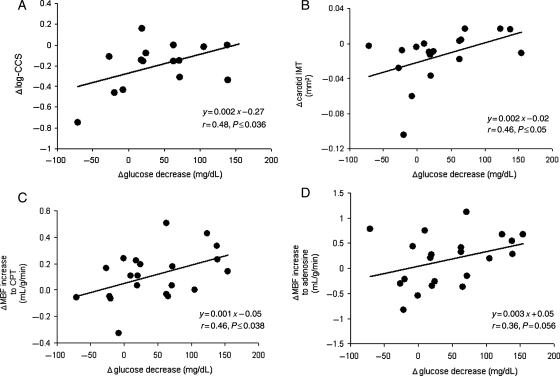
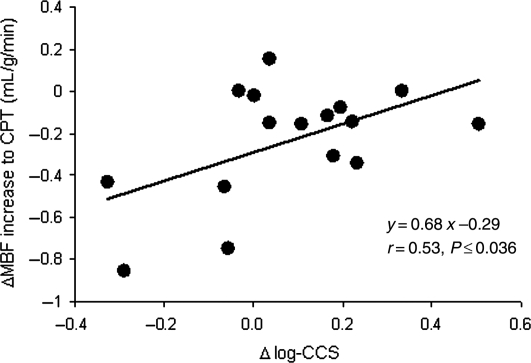
As regards the structural alterations of the arterial wall, log-CCS as well as carotid IMT only correlated with the decrease in plasma glucose concentrations, respectively (r = 0.48, P ≤ 0.036 and r = 0.46, P ≤ 0.05) (Figure 2A and B). Further, the decrease in plasma glucose concentration correlated significantly with an improvement in the endothelium-related flow response to cold (r = 0.46, P ≤ 0.038; Figure 2C) and also with the change in hyperaemic flow from rest during adenosine stimulation (r = 0.36, P ≤ 0.05; Figure 2D). We also evaluated the relationship between glucose-lowering-induced alterations in structure and function of the arterial wall in DM. We did not observe correlations between changes in carotid IMT and MBF responses to CPT, ΔMBF to adenosine, during adenosine stimulation, MFR, respectively (r = 0.003, P = 0.99; r = 0.07, P = 0.76; r = 0.11, P = 0.67; and r = 0.02, P = 0.93). Conversely, the alterations in CCS correlated significantly with the endothelium-related increase in coronary flow to CPT (r = 0.53, P < 0.036) (Figure 3). Thus, the better the improvement in endothelium-dependent coronary artery function, the lower the progression of CCS. After adjusting for metabolic parameters by multivariable analysis (Table 3), the increase in endothelium-related ΔMBF to CPT after glucose-lowering treatment, apart from alterations in SBP and triglycerides, remained statistically significant independent predictors of the progression of CCS in DM. The alterations in CCS after the 1-year FU, however, did not correlate with changes in ΔMBF to adenosine, during adenosine stimulation, and MFR, respectively (r = 0.38, P = 0.15; r = 0.29, P = 0.28; and r = 0.41, P = 0.12).
Figure 2.
(A) Correlation between the differences in Δlog-CCS (coronary calcium score) and in Δglucose decrease between baseline and follow-up (negative values on the y-ordinate are indicative for a progression of coronary artery calcification). (B) Correlation between the differences in ΔIMT and in Δglucose decrease between baseline and follow-up (negative values on the y-ordinate are indicative for a progression of carotid intima–media thickness). (C) Correlation between the differences in ΔMBF to cold pressor testing and in Δglucose decrease between baseline and follow-up. (D) Correlation between the differences in ΔMBF to adenosine stimulation and in Δglucose decrease between baseline and follow-up.
Figure 3.
Correlation between the differences in ΔMBF to cold pressor testing and in Δlog-CCS (coronary calcium score) between baseline and follow-up (negative values on the x-ordinate are indicative for a progression of coronary artery calcification).
Table 3.
Determinants of alterations in log-CCS after glucose-lowering treatment
| Differences in | Difference in log-CCS |
|||
|---|---|---|---|---|
| Univariate analysis |
Multivariable analysis |
|||
| Coefficient β (95% CI) | P-value | Coefficient β (95% CI) | P-value | |
| ΔMBF to CPT (mL/g/min) | 0.526 (0.050 to 1.305) | 0.036 | 0.756 (0.472 to 0.590) | 0.001 |
| BMI (kg/m2) | 0.229 (−0.047 to 0.112) | 0.393 | — | — |
| SBP (mmHg) | 0.159 (−0.006 to 0.011) | 0.556 | 0.576 (0.004 to 0.006) | 0.001 |
| Glucose (mg/dL) | 0.576 (0.000 to 0.004) | 0.036 | — | — |
| Insulin (µU/mL) | −0.195 (−0.017 to 0.010) | 0.544 | — | — |
| HOMA | −0.345 (−0.019 to 0.006) | 0.298 | — | — |
| Total cholesterol (mg/dL) | 0.117 (−0.003 to 0.004) | 0.667 | — | — |
| LDL cholesterol (mg/dL) | 0.230 (−0.003 to 0.006) | 0.410 | — | — |
| HDL cholesterol (mg/dL) | 0.278 (−0.011 to 0.034) | 0.298 | — | — |
| Triglycerides (mg/dL) | −0.292 (−0.003 to 0.001) | 0.290 | 0.105 (0.000 to 0.000) | 0.032 |
| Free fatty acids (mg/dL) | 0.396 (−0.464 to 1.509) | 0.257 | — | — |
P-values by analysis of variance; CCS, coronary artery calcium score; MBF, myocardial blood flow; HOMA, homeostatic model assessment; SBP, systolic blood pressure; LDL cholesterol, low-density lipoprotein cholesterol; HDL cholesterol, high-density lipoprotein cholesterol.
Discussion
The results of the current study provide several novel observations. At first, glucose-lowering therapy in DM beneficially affected structural alterations of the vascular arterial wall. The decrease in plasma glucose levels after a 1-year FU was associated with a lower progression of CAC and carotid IMT, respectively. These findings suggest direct adverse effect of elevated plasma glucose levels on diabetes-related structural alterations of the vascular wall. Glucose-lowering treatment in DM had also beneficially altered coronary artery function as evidenced by a close correlation between reductions in plasma glucose levels and an improvement in coronary artery function. In particular, the improvement in endothelium-related coronary artery function due to glucose-lowering treatment in DM was independently associated with a slowed progression of CAC. This observation is first of its kind to denote indeed that an improvement in endothelium-dependent coronary artery function in DM may mediate, at least in part, direct preventive effects on the progression of diabetic vasculopathy, in addition to that derived from glucose-lowering treatment.
Baseline examination of structure and function of the arterial wall
As expected, hyperglycaemia in DM had not only induced early structural alterations of the arterial wall, as denoted by an abnormal increase in CAC and subintimal thickening of the carotid artery, but also resulted in an abnormal functioning of the coronary circulation. Structural abnormalities of the arterial wall, therefore, were accompanied by diabetes-related early functional abnormalities of the coronary circulation.9 Such abnormalities in coronary artery function may not only accompany but they may also precede atherosclerotic disease-related structural alterations of the arterial wall as also observed in the current and previous investigations.9 Increases in carotid IMT4,13 in the current DM population were determined as a surrogate marker for subintimal CAD process. This appears important as a negative EBT coronary calcium study does not necessarily exclude the presence of atherosclerotic plaque burden but implies a very low likelihood of significant luminal coronary artery obstruction.14 EBT measurements of CAC compare well with those with multi-detector slice computed tomography (MDCT) and are highly reproducible.11 Thus, both modalities allow an accurate and non-invasive quantification of calcified areas of >2 mm2 and the resulting calcium scores can be related to the extent and severity of CAD, while it may also improve the cardiovascular risk prediction.5 When EBT or MDCT identify CAC, it confirms the presence of coronary atherosclerotic plaque disease. Notably, the greater the amount of CAC, the greater is the likelihood of the presence of obstructive CAD.14 Nevertheless, there is neither a one-to-one relationship between the amount of CAC and obstructive CAD nor is it cite-specific. Although EBT- or MDCT-measured CAC identifies anatomical disease, it does not assess the downstream effect on myocardial perfusion. Normal stress and rest N-13 ammonia PET perfusion images in the current study population, however, argued against the presence of haemodynamically significant obstructive coronary artery lesions. Interestingly, a few study participants of healthy CON had also some minor CAC. The reason for this is unknown but may be related to age-dependent process of atherosclerotic disease, unknown genetic factors, and/or overweight CON with insulin resistance.
Effects of glucose-lowering therapy
One year glucose-lowering treatment with glyburide or glyburide in concert with metformin in DM beneficially affected structural alterations of the arterial wall. As it was observed, decreases in plasma glucose levels were correlated with a reduced progression of CAC and carotid IMT, respectively. The observed close association between the decrease in plasma glucose levels and the slowed progression of vascular structural alterations indicates that glucose-lowering treatment had directly prevented a further progression of structural alterations of the arterial wall. Possible mechanisms underlying this beneficial effect of glucose-lowering treatment on diabetic vasculopathy may imply a reduction of a continued accumulation of long-lived advanced glycation end products in the arterial wall, their interactions with advanced glycation end products receptors, a reduced proliferation of vascular smooth muscle cells and fibrous tissue, decreases in vascular inflammation and oxidative stress burden, and as yet unknown variables.15 The decreases in plasma glucose concentrations in DM, however, were not only correlated with a slowed progression of structural alterations of the arterial wall, but also with an improvement in endothelium-related coronary artery function, that is consistent with own previous observations,7 and also with an increase in total integrated coronary vasodilatory capacity. Possible mechanism underlying the improvement in coronary artery dysfunction may comprise reductions in hypergylcaemia-stimulated NADPH-oxidase with the formation of reactive oxygen species and also an inactivation of protein kinase C associated with an increase in the bioavailability of endothelium-derived and atheroprotective nitric oxide.1 Further, insulin secretagogues such as sulphonylureas or glyburide raise basal and postprandial insulin secretion,7 which most likely has added to the improvement in coronary artery function through insulin-stimulated release of endothelium-derived derived nitric oxide. Conversely, metformin lowers plasma glucose concentrations predominantly through its insulin-sensitizing properties.7 Thus, the insulin-raising effect of glyburide and, at the same time, the metformin-mediated decrease in insulin resistance may have directly enhanced the bioavailability of endothelium-derived nitric oxide associated with an improvement in coronary artery function. Notably, after adjusting for alterations in insulin-resistance, glucose, and lipids by multivariable analysis, the improvement of endothelium-related myocardial flow increases to CPT remained an independent predictor for the slowed progression of CAC in DM. This observation provides some first direct in vivo evidence that an improvement in endothelium-dependent coronary artery function in DM may indeed mediate, at least in part, direct preventive effects on the progression of CAC. The causes for the mechanistic link between the observed improvement in coronary endothelial function and the slowed progression of CAC in DM is certainly multifactorial but may be predominantly related to an enhanced flow-mediated and, thus, endothelium-dependent release of nitric oxide of the coronary circulation, implying numerous antithrombotic and anti-atherosclerotic effects.2
Interestingly, the current investigation revealed a certain dissociation of the responsiveness of the structure of the arterial wall and of the function of the coronary artery to glucose-lowering treatment in DM. The reasons for this remain uncertain but the mild progression of structural atherosclerotic disease is most likely related to suboptimal control of elevated glucose plasma and, as yet unravelled, other pathophysiological mechanism within the diabetic arterial wall. This consideration is substantiated by recent pooled analysis of five intravascular ultrasound trials with a total of 2237 coronary risk individuals evaluating the effect of DM on progression of coronary atherosclerotic disease.15 Despite the common application of standard preventive medical therapy, DM was associated with a greater coronary atherosclerotic burden and accelerated disease progression than in those cardiovascular risk individual but without DM. The latter and current observations may highlight the need for additional preventive medical therapy strategies in DM, apart from euglycaemic control, such as reductions in inflammation and oxidative stress burden within the arterial wall aiming to prevent or halt the progression of diabetic vasculopathy and thereby to improve the cardiovascular outcome.15 Further, it is also possible that coronary artery function, reflecting more ongoing processes that affect the functional status of the arterial wall,2 responded more rapidly to glucose-lowering treatment, whereas not so for diabetes-induced structural alterations of the arterial wall. As abnormalities in structure and function of the arterial wall are considered as independent predictors of future cardiovascular events in DM,2 an improvement in coronary artery structure and, in particular, of its function, apart from euglycaemic control, and/or behavioural interventions to weight, diet, and physical activity, could emerge as a new specific targets for primary preventive medical intervention to improve the cardiovascular outcome that should be further tested.
Limitations
There are limitations to be considered in interpreting these data. In the current investigation, 50% of DM had a combined therapy of anti-diabetic medication. Thus, it remains uncertain to what extent glyburide-mediated insulin increase and metformin-stimulated insulin sensitivity had affected structure and function of the arterial wall. For the baseline study, it can also be observed that DM and CON were not perfectly matched for the age. As atherosclerotic disease is also in part an age-dependent process, we cannot exclude entirely that the age difference between both groups at baseline studies may have mildly contributed to the observed significant alterations in structure and function of the arterial wall in DM when compared with CON. Further, in view of the relatively small sample size of the study population, the current clinical investigation does certainly not allow to draw definite conclusions but may stimulate further large-scale clinical trials with sufficient long-term FUs to better define the role of non-invasively determined subclinical markers of CAD to successfully guide primary and secondary preventive medical intervention of the CAD process.13
Conclusions
In DM, glucose-lowering treatment may beneficially affect structure and function of the vascular arterial wall, whereas the observed improvement in endothelium-related coronary artery function may also mediate direct preventive effects on the progression of epicardial structural disease.
Funding
This work was supported by National Heart, Lung and Blood Institute (NIH), Bethesda, Maryland [grant number: HL 33177].
Conflict of interest: none declared.
Acknowledgements
The authors thank Larry Pang, Richard Eugene, and Jennie Kusnadi for assisting in the PET studies, Nagichettiar Satyamurthy and staff for 13N-ammonia, and Mary Smith for administrative assistance.
References
- 1.Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H. Prevention Conference VI: diabetes and cardiovascular disease. Writing Group II: pathogenesis of atherosclerosis in diabetes. Circulation. 2002;105:e138–e143. doi: 10.1161/01.cir.0000013954.65303.c5. [DOI] [PubMed] [Google Scholar]
- 2.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 3.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. Eur Heart J. 2009;30:1837–1843. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 4.Juonala M, Kahonen M, Laitinen T, Hutri-Kahonen N, Jokinen E, Taittonen L, Pietikainen M, Helenius H, Viikari JS, Raitakari OT. Effect of age and sex on carotid intima-media thickness, elasticity and brachial endothelial function in healthy adults: the cardiovascular risk in Young Finns Study. Eur Heart J. 2008;29:1198–1206. doi: 10.1093/eurheartj/ehm556. [DOI] [PubMed] [Google Scholar]
- 5.Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, Lipkin D, Lahiri A. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713–721. doi: 10.1093/eurheartj/ehi808. [DOI] [PubMed] [Google Scholar]
- 6.Prior JO, Quinones MJ, Hernandez-Pampaloni M, Facta AD, Schindler TH, Sayre JW, Hsueh WA, Schelbert HR. Coronary circulatory dysfunction in insulin resistance, impaired glucose tolerance, and type 2 diabetes mellitus. Circulation. 2005;111:2291–2298. doi: 10.1161/01.CIR.0000164232.62768.51. [DOI] [PubMed] [Google Scholar]
- 7.Schindler TH, Facta AD, Prior JO, Cadenas J, Hsueh WA, Quinones MJ, Schelbert HR. Improvement in coronary vascular dysfunction produced with euglycaemic control in patients with type 2 diabetes. Heart. 2007;93:345–349. doi: 10.1136/hrt.2006.094128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nissen SE, Nicholls SJ, Wolski K, Nesto R, Kupfer S, Perez A, Jure H, De Larochelliere R, Staniloae CS, Mavromatis K, Saw J, Hu B, Lincoff AM, Tuzcu EM. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008;299:1561–1573. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- 9.Schindler TH, Facta AD, Prior JO, Cadenas J, Zhang XL, Li Y, Sayre J, Goldin J, Schelbert HR. Structural alterations of the coronary arterial wall are associated with myocardial flow heterogeneity in type 2 diabetes mellitus. Eur J Nucl Med Mol Imaging. 2009;36:219–229. doi: 10.1007/s00259-008-0885-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodis HN, Mack WJ, Zheng L, Li Y, Torres M, Sevilla D, Stewart Y, Hollen B, Garcia K, Alaupovic P, Buchanan TA. Effect of peroxisome proliferator-activated receptor gamma agonist treatment on subclinical atherosclerosis in patients with insulin-requiring type 2 diabetes. Diabetes Care. 2006;29:1545–1553. doi: 10.2337/dc05-2462. [DOI] [PubMed] [Google Scholar]
- 11.Detrano RC, Anderson M, Nelson J, Wong ND, Carr JJ, McNitt-Gray M, Bild DE. Coronary calcium measurements: effect of CT scanner type and calcium measure on rescan reproducibility—MESA study. Radiology. 2005;236:477–484. doi: 10.1148/radiol.2362040513. [DOI] [PubMed] [Google Scholar]
- 12.Schindler TH, Campisi R, Dorsey D, Prior JO, Olschewski M, Sayre J, Schelbert HR. Effect of hormone replacement therapy on vasomotor function of the coronary microcirculation in post-menopausal women with medically treated cardiovascular risk factors. Eur Heart J. 2009;30:978–986. doi: 10.1093/eurheartj/ehp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simon A, Chironi G, Levenson J. Comparative performance of subclinical atherosclerosis tests in predicting coronary heart disease in asymptomatic individuals. Eur Heart J. 2007;28:2967–2971. doi: 10.1093/eurheartj/ehm487. [DOI] [PubMed] [Google Scholar]
- 14.Schmermund A, Baumgart D, Sack S, Mohlenkamp S, Gronemeyer D, Seibel R, Erbel R. Assessment of coronary calcification by electron-beam computed tomography in symptomatic patients with normal, abnormal or equivocal exercise stress test. Eur Heart J. 2000;21:1674–1682. doi: 10.1053/euhj.2000.2183. [DOI] [PubMed] [Google Scholar]
- 15.Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52:255–262. doi: 10.1016/j.jacc.2008.03.051. [DOI] [PubMed] [Google Scholar]