Abstract
Stillbirth is a major obstetric complication, with 3.2 million stillbirths worldwide and 26,000 stillbirths in the United States every year. The Eunice Kennedy Shriver National Institute of Child Health and Human Development held a workshop from October 22–24, 2007, to review the pathophysiology of conditions underlying stillbirth to define causes of death. The optimal classification system would identify the pathophysiologic entity initiating the chain of events that irreversibly led to death. Because the integrity of the classification is based on available pathologic, clinical, and diagnostic data, experts emphasized that a complete stillbirth workup should be performed. Experts developed evidence-based characteristics of maternal, fetal, and placental conditions to attribute a condition as a cause of stillbirth. These conditions include infection, maternal medical conditions, antiphospholipid syndrome, heritable thrombophilias, red cell alloimmunization, platelet alloimmunization, congenital malformations, chromosomal abnormalities including confined placental mosaicism, fetomaternal hemorrhage, placental and umbilical cord abnormalities including vasa previa and placental abruption, complications of multifetal gestation, and uterine complications. In all cases, owing to lack of sufficient knowledge about disease states and normal development, there will be a degree of uncertainty regarding whether a specific condition was indeed the cause of death.
Stillbirth is a major obstetric complication. There are at least 3.2 million stillbirths worldwide1 and 26,000 stillbirths in the United States2 every year. Assignment of a probable cause of death is important to develop interventions for stillbirth prevention. From October 22–24, 2007, the Eunice Kennedy Shriver National Institute of Child Health and Human Development convened a workshop of international experts to summarize the issues surrounding defining cause of death for stillbirth. There are currently at least 32 classification systems of stillbirth, many of which have been developed for different purposes. These have differing categories for classifying causes, numerous definitions for relevant conditions, and varying levels of complexity. As a result, no single system is accepted universally. To make progress in this field, it would be advantageous to have a single international system for classifying stillbirths that not only lists and defines potential causes of stillbirth but also estimates the degree of certainty with which the loss can be ascribed to these factors.
After discussing the merits of existing systems, the workshop participants agreed that a valuable classification system for research would identify the pathophysiologic entity initiating the chain of events that irreversibly led to death based on pathologic, clinical, and diagnostic data. There was consensus among experts that the criteria to be used to categorize a particular condition as a cause of stillbirth should consider the following principles: 1) there is epidemiologic data demonstrating an excess of stillbirth associated with the condition, 2) there is biologic plausibility that the condition causes stillbirth, 3) the condition is either rarely seen in association with live births or, when seen in live births, results in a significant increase in neonatal death, 4) a dose–response relationship exists so that the greater the “dose” of the condition, the greater the likelihood of fetal death, 5) the condition is associated with evidence of fetal compromise, and 6) the stillbirth likely would not have occurred if that condition had not been present, ie, lethality.
Using these criteria, we examined the conditions listed in Box 1 as potential probable causes of death. Our ultimate goal was to develop agreement on the conditions that should be considered potential causes of stillbirth and, when possible, the dose (severity) of that condition necessary to consider that condition a cause of stillbirth.
BOX 1. CONDITIONS ASSOCIATED WITH STILLBIRTH.
Infection
Severe maternal illness
Placental infection leading to hypoxemia
Fetal infection leading to congenital deformity
Fetal infection leading damage of a vital organ
Precipitating preterm labor with the fetus dying in labor
Maternal medical conditions
Hypertensive disorders
Diabetes mellitus
Thyroid disease
Renal disease
Liver disease
Connective tissue disease (systemic lupus erythematosus)
Cholestasis
Antiphospholipid syndrome
Heritable thrombophilias
Red cell alloimmunization
Platelet alloimmunization
Congenital anomaly and malformations
Chromosomal abnormalities including confined placental mosaicism
Fetomaternal hemorrhage
Fetal growth restriction
Placental abnormalities including vasa previa and placental abruption
Umbilical cord pathology including velamentous insertion, prolapse, occlusion and entanglement
Multifetal gestation including twin–twin transfusion syndrome and twin reverse arterial perfusion
Amniotic band sequence
Central nervous system lesions
INFECTION
Stillbirths have been associated with bacterial, viral, and protozoal infections (Table 1, available online at http://links.lww.com/AOG/A128).3 Infection is believed to be associated with approximately 10% to 20% of stillbirths in developed countries and with a much greater percentage in developing countries. Infection accounts for a greater percentage of preterm stillbirths than term stillbirths. Nevertheless, the role of infection as the cause of death is difficult to prove for several reasons. First, organisms such as urea-plasma and certain viruses that are causal may not be identified easily. Second, finding organisms in the placenta or fetus does not prove causality. Third, because infection may initiate a chain of events leading to stillbirth, its importance might not be appreciated (eg, parvovirus causing hydrops). Lastly, positive serologic tests do not prove causality.3
Placental and fetal infections likely originate from two predominant pathways. The most common is an ascending infection from the vagina in the space between the maternal decidua and the fetal membranes. Further spread may result in organisms reaching the amniotic fluid or the fetus. An immune response brings inflammatory cells into the chorioamnion (chorioamnionitis), into the amniotic fluid (amnionitis), to the umbilical cord (funisitis), or to the fetus (usually pneumonitis). Infections also may arise systemically in the woman, spread hematogenously, and reach the fetus through the placental villi (villitis). These types of infection typically involve the fetal liver because that is the first major fetal organ reached, but other organs often are involved.3
Infection may cause stillbirth by several mechanisms: 1) causing severe maternal illness, 2) infecting the placenta and preventing oxygen and nutrients from crossing to the fetus, 3) infecting the fetus and causing a congenital deformity that is incompatible with life, 4) infecting the fetus and damaging a vital organ such as the brain or heart, and 5) precipitating preterm labor, with the fetus dying in labor.
Severe maternal infection is defined as an illness requiring hospital treatment, usually marked by high fever and the need for treatments such as intravenous antibiotics, surgery, or ventilator support. Reports of stillbirth associated with severe maternal infections including influenza, polio, varicella pneumonia, and other bacterial and viral diseases in the absence of fetal or placental involvement are well-documented.4,5 Other examples in this category include stillbirths that occur in the presence of pyelonephritis and appendicitis.
Infection of the placenta with an organism likely to cause a decrease in placental function may lead to stillbirth. Histologic evidence of placental infection of sufficient severity to cause stillbirth is seen with malaria and syphilis.6,7 A placental infection may be invoked as a cause of death when there is evidence of maternal infection with an organism known to cause a decrease in placental function and evidence of placental histologic changes compatible with that infection. Infection by Listeria monocytogenes with multiple microabscesses involving the placental parenchyma is an example of acute placentitis, which can be lethal to the fetus.8
Fetal infection with an organism that may cause a fetal deformity or other abnormality may lead to stillbirth. A classic example of this category is rubella.9 This mechanism for cause of death may be deduced by the presence of a congenital deformity or other condition known to be caused by a certain organism. Proof of maternal infection with that organism should be documented.
Infection of the fetus with damage to a vital organ such as the brain or heart may result in stillbirth. Examples include fetal infection with group B Streptococcus, Escherichia coli, and Enterococcus.10 Bacterial infection can be regarded as the cause of death if signs of infection are present in the fetus (eg, pneumonia) or if there is a positive culture of fetal heart blood or of maternal blood combined with signs of infection in the placenta, ie, chorioamnionitis or funisitis. Viral infection can be regarded as the cause of death if there is a positive viral polymerase chain reaction in the placenta or fetal tissue plus signs of viral infection in the fetus or histopathologic examination of the placenta showing signs of viral infection (eg, viral inclusions or villitis). To invoke this mechanism as a cause, there should be evidence on fetal autopsy of extensive organ involvement as well as culture evidence of fetal organ involvement with an organism known to or potentially able to cause stillbirth. Chorioamnionitis by itself should not be considered a cause of stillbirth except as noted below.
Organisms may precipitate preterm labor, with the fetus dying in labor. In developed countries, this occurs most often at previable gestational ages when a decision is made to not intervene (eg, with cesarean delivery) for fetal indications. To invoke this mechanism of fetal death, histologic chorioamnionitis should be present.
MATERNAL MEDICAL CONDITIONS
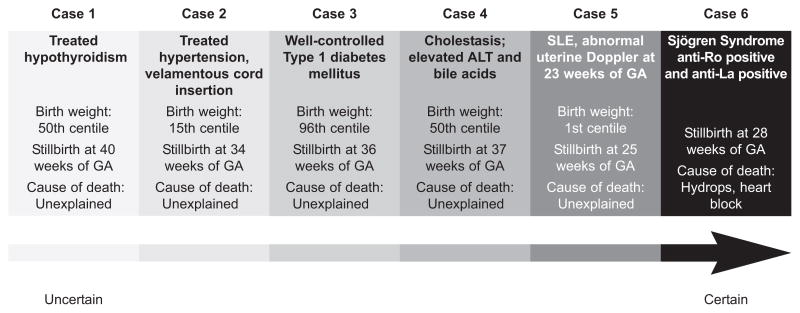
The associations between preexisting and acquired medical conditions in the woman and the risk of stillbirth are a good illustration of the problems of classifying stillbirth in general (Table 2, available online at http://links.lww.com/AOG/A128). Stillbirths in the presence of maternal medical conditions represent a continuum of risk (Fig. 1), from those where there is little evidence to support a causal association through to those where the maternal condition can provide a likely explanation for the death of the fetus.
Fig. 1.
Continuum of certainty in pathophysiology of maternal medical conditions as a cause of stillbirth. On the extreme left, a stillbirth in a woman with treated hypothyroidism and a normal birth weight should be regarded as unexplained. As one progresses to the right on the continuum, there are increasing levels of certainty as to the role of the pathophysiology of a particular condition in causing the stillbirth. As the extreme right, Sjogren syndrome with anti-Ro and anti-La antibodies leading to hydrops may be regarded as a certain cause of death. The cause of death is considered unexplained in five cases despite clear disposing factors because the exact cause of death is unknown. GA, gestational age; ALT, alanine amino-transferase. Figure courtesy of Prof Gordon Smith.
Reddy. Stillbirth Classification of Cause of Death. Obstet Gynecol 2009.
HYPERTENSIVE DISORDERS
Hypertensive disorders are divided into preexisting conditions (chronic hypertension) and disorders acquired during pregnancy (preeclampsia). Overall, the stillbirth rate associated with mild essential hypertension is low and is similar to the rate in the general population.11 The risk of stillbirth is increased in chronic hypertension when there is associated placental insufficiency. Consequent stillbirths are related to abruption, placental insufficiency associated with fetal growth restriction, and superimposed preeclampsia/eclampsia (the major cause of morbidity in this group). In the 1950s, preeclampsia/eclampsia was associated with about one fifth of stillbirths.12 More recently, the Scottish Stillbirth & Infant Death Enquiry (1985–2004) related 6–7% of stillbirths to pre-eclampsia, which is consistent with other international studies (4–9%).13 The risk of stillbirth increases with multisystem disease: 21 per 1,000 with severe pre-eclampsia without hemolysis, elevated liver enzymes, low platelets syndrome, 22 per 1,000 with eclampsia, and 50 per 1,000 with hemolysis, elevated liver enzymes, low platelets syndrome.14 Preeclampsia may be considered as a cause of death if it progresses to eclampsia or if it is associated with placental abruption or fetal growth restriction. Whether preeclampsia of lesser degrees of severity should be considered the cause of death is unclear. Some authorities consider preeclampsia as a placental cause of death and do not generally consider preeclampsia itself as a cause of stillbirth.
DIABETES MELLITUS
Diabetes mellitus leads to an increased risk of stillbirth through multiple pathways: congenital abnormality, placental insufficiency/fetal growth restriction, macrosomia, and intrapartum stillbirth due to obstructed labor. The link between excessive fetal growth and stillbirth likely involves maternal hyperglycemia leading to fetal hyperglycemia, which in turn triggers excess fetal insulin production to maintain fetal plasma glucose levels in the physiologic range. In the fetus, insulin stimulates fetal growth, which may result in metabolic acidosis if excessive and if the placental oxygen supply is insufficient. The endpoint of this process may be stillbirth. The risk of stillbirth is increased in women with poor glycemic control. However, the exact relationship between quantitative measures of glycemic control and the risk of stillbirth is not well-characterized.
Stillbirth rates in women with diabetes are relatively low in case series from tertiary care referral centers. However, population-based studies in both the United Kingdom and the United States demonstrate increased risks of stillbirth among women with diabetes. A study of U.S. birth-certificate data describes a 2.5-fold increased risk of stillbirth,15 and a national audit of pregnant women with diabetes in the United Kingdom reports a fourfold to fivefold increased risk (ie, 25–30/10,000).16 Stillbirth rates were similar for type 1 and non–insulin-dependent diabetes mellitus.16
Diabetes mellitus may be considered the cause of death when there is previously known (or clinically diagnosed at birth) insulin-dependent diabetes mellitus and signs of either intrauterine or intrapartum asphyxia, large for gestational age fetus, small for gestational age (SGA) fetus, or severe malformation. Diabetes also can be considered as the cause of death in non–insulin-dependent diabetes mellitus or gestational diabetes when there are signs of intrauterine or intrapartum asphyxia or if the placenta demonstrates characteristic histopathologic findings such as immature villi with mild stromal edema, enlarged villous diameters, and an increase in the prominence of cytotrophoblast. However, stillbirth should not be ascribed to diabetes when non–insulin-dependent diabetes mellitus or gestational diabetes is the sole positive finding.
THYROID DISEASE
Treated thyroid disease carries a low risk of stillbirth and should not be considered a cause of death. The exception is Graves’ disease, with thyroid-stimulating hormone receptor antibody (thyroid stimulation immunoglobulin) leading to fetal thyrotoxicosis. This may occur even if the woman is euthyroid. Even then, Graves’ disease is a rare cause of stillbirth; only 1% of neonates exhibit hyperthyroidism with the presence of maternal antibody.17 There is an increased risk of stillbirth among women with untreated hyperthyroidism (100/1,000).18 Untreated hypothyroidism also is associated with a higher risk of stillbirth (40/1,000) when it is symptomatic and associated with abruption and pregnancy-induced hypertension.18
RENAL DISEASE
The association of renal disease with stillbirth depends on the severity of renal impairment and the presence of hypertension. The risk of stillbirth increases with increasing severity of renal impairment. There is a positive linear relationship between maternal creatinine levels and stillbirth rates: creatinine <1.3 mg/dL (115 micromol/L), stillbirth rate 15 per 1,000; creatinine=1.3–1.9 mg/dL (115–168 micro-mol/L), stillbirth rate 30–100 per 1,000; creatinine >1.9 mg/dL (168 micromol/L), stillbirth rate 200–800 per 1,000.19
LIVER DISEASE
Cholestasis of pregnancy, the most common form of noninfectious liver disease presenting in pregnancy, is associated with stillbirth. Raised bile acid levels (40–70 micromol/L) plus a history of pruritus historically are associated with high rates of stillbirth (60–70/1,000).20 Acute fatty liver of pregnancy is a rare condition that is an accepted cause of fetal death.21
Connective tissue disease such as systemic lupus erythematosus is associated with increased rates of stillbirth of about 40–70 per 1,000.18 Stillbirth rates are increased further if there is active disease at the start of pregnancy, associated hypertension, lupus nephritis (up to 300/1,000), and antiphospholipid syndrome.22 Women with systemic lupus erythematosus and those with Sjögren’s syndrome may have circulating auto-antibodies, anti-Ro and anti-La. These antibodies can cross the placenta and react with antigens in the fetal cardiac-conduction system, leading to congenital heart block. This can lead to hydrops and fetal death.22
ANTIPHOSPHOLIPID SYNDROME
Antiphospholipid syndrome is an autoimmune disorder characterized by the presence of antiphospholipid antibodies and one or more clinical features, including pregnancy morbidity such as recurrent early pregnancy loss, fetal death, and preterm birth due to placental insufficiency or thrombosis.23 The three best-characterized antiphospholipid antibodies are lupus anticoagulant, anticardiolipin antibodies, and anti-beta2-glycoprotein-I antibodies. Lupus anticoagulant is considered either positive or negative. Moderate or high positive levels of immunoglobulin G or immunoglobulin M anticardiolipin or anti-β-2-glyco-protein-I antibodies (two occasions 12 weeks apart at 99% or higher) are considered criteria for the syndrome.23,24 The mechanism of pregnancy loss remains uncertain but includes inflammation, thrombosis, and infarction in the placenta. Treatment with thromboprophylactic doses of heparin and low-dose aspirin appears to improve obstetric outcome in women with antiphospholipid syndrome.25
Antiphospholipid syndrome may be considered a probable cause of death when antiphospholipid syndrome is diagnosed in the woman and there is either 1) clear histopathologic evidence of placental insufficiency (eg, examination of the placenta shows more than 30% infarction or thrombosis in fetal placental vessels) or 2) clear clinical evidence of placental insufficiency (eg, birth weight less than the 3rd percentile for gestational age, severe preeclampsia, or abnormal fetal testing).
HERITABLE THROMBOPHILIAS
The role of heritable coagulopathies or thrombophilias involving deficiencies or abnormalities in anticoagulant proteins or an increase in procoagulant proteins in stillbirth is unclear. Case series and retrospective studies have reported an increased stillbirth risk associated with the factor V Leiden mutation (associated with abnormal factor V resistance to the anticoagulant effects of protein C), the G20210A mutation in the promoter of the prothrombin gene, and deficiencies of the anticoagulant proteins anti-thrombin III, protein C, and protein S.26–28 Similar to antiphospholipid syndrome, the histologic findings of placental infarction, necrosis, and vascular thrombosis observed in some cases of stillbirth have led to the hypothesis that thrombophilia also may be associated with thrombosis in the uteroplacental circulation. Excessive thrombosis of placental or fetal stem vessels or placental infarction may lead to uteroplacental insufficiency and hypoxia.
Two large prospective cohort studies, however, found no association between the factor V Leiden mutation and either pregnancy loss or obstetric complications characterized by placental insufficiency.29,30 The lack of an association between thrombophilias and pregnancy loss in these prospective cohorts raises questions about the validity of an association between thrombophilia and stillbirth. Also, thrombophilias are extremely common in healthy women with normal pregnancy outcomes. Thrombophilia in association with the following conditions may be associated with the occurrence of stillbirth: 1) clinical evidence of placental insufficiency such as fetal growth restriction or placental infarction and 2) recurrent fetal loss. However, thrombophilia alone should not be considered a cause of stillbirth; in this situation, it may be considered a risk factor.
RED CELL ALLOIMMUNIZATION
More than 50 different red-cell antigens have been reported to be associated with hemolytic disease of the fetus and newborn. However, three antibodies—anti-Rhesus D, anti-Rhesus C, and anti-Kell—account for the majority of fetuses with severe disease, ie, those requiring intrauterine transfusion or resulting in hydropic stillbirth. The widespread use of Rhesus immune globulin has resulted in a large reduction in the incidence of Rhesus alloimmunization in pregnancy. However, prophylactic immune globulins to prevent maternal antibody formation to other red-cell antigens are not available currently. For this reason, maternal alloimmunization to non-Rhesus antigens continues to contribute to the incidence of stillbirth.31
For red cell alloimmunization to be considered a cause of stillbirth, the following criteria should be present: 1) maternal antibodies against the pertinent antigen (positive indirect Coombs’ test), 2) antibody titers 1:16 or more of the pertinent antibody (for antibodies against Kell, 1:8 or more is considered significant), 3) clear evidence of fetal anemia with hydrops, and 4) evidence of fetal extramedullary hematopiesis.
PLATELET ALLOIMMUNIZATION
Platelet alloimmunization may be a cause of stillbirth. Fetal/neonatal alloimmune thrombocytopenia results from maternal alloimmunization against fetal platelet antigens inherited from the father (absent on maternal platelets). When severe, fetal alloimmune thrombocytopenia results in intracranial hemorrhage and stillbirth. In Caucasians, HPA-1a is the most frequently implicated platelet antigen, followed by HPA-5a; for Asians, HPA-4 antigen is most commonly linked to fetal/neonatal alloimmune thrombocytopenia.32 For platelet alloimmunization to be considered a cause of stillbirth, the following criteria should be present: 1) maternal antibodies against pertinent paternal and fetal platelet antigen, 2) parental platelet antigen incompatibility for the pertinent platelet antigen, 3) clear evidence of fetal thrombocytopenia (<50×109/L), and 4) evidence of massive intracranial hemorrhage.
CONGENITAL MALFORMATIONS
Although some have recommended that extensive post-natal evaluation of the fetus be performed only in selected cases, the frequent occurrence of unexpected and unanticipated findings is argument for routine, comprehensive assessment of all stillbirths.33 Using such an approach, around 20% of stillborns will have detectable congenital anomalies as fetal causes of death.34 There is heterogeneity of diagnoses, with more than 90 associated disorders and no single diagnosis accounting for more than 1.5% of all occurrences (Table 3, available online at http://links.lww.com/AOG/A128).
A malformation can be considered the cause of death based on specific criteria; fulfilling any one of the following is sufficient to postulate that a particular process is causal:
There are epidemiologic data demonstrating an excess of intrauterine mortality (eg, Turner syndrome, Down syndrome, trisomy 18, and Smith Lemli Opitz syndrome).
The process is seen rarely in liveborn neonates (eg, lower mesodermal defects, triploidy, and early amnion disruption).
When the process is seen in liveborn neonates, it frequently results in neonatal death (anencephaly, congenital cardiomyopathy, hydranencephaly, and limb-body wall disruption sequence).
There is biologic plausibility that it can result in death (aprosencephaly syndrome, Meckel syndrome, and jugulolymphatic obstruction sequence).
If multiple criteria are met, the likelihood that the process caused death is increased.
CHROMOSOMAL ABNORMALITIES
Chromosomal abnormalities of either the fetus or the placenta meet the criteria for causality. Although it is well-accepted that additional or missing genetic material can lead to fetal death, the exact molecular mechanisms by which this occurs for any specific imbalance is unknown. Factors that appear to have an effect on lethality include the specific chromosome error involved, the associated fetal structural anomalies, and the distribution of the abnormal cell line within the fetus, the placenta, or both.
Overall, fetal cytogenetic abnormalities account for 6–13% of all stillbirths,35,36 but the proportion is higher with macerated or malformed fetuses. In a large study of 750 stillbirths, 38% of stillborns with morphologic abnormalities had chromosomal abnormalities compared with 4.6% in those without morphologic abnormalities.35 The distribution of chromosomal abnormalities associated with stillbirth is similar to that seen in live births (monosomy X: 23%, trisomy 21: 23%, trisomy 18: 21%, and trisomy 13: 8%).36 Of fetuses older than 20 weeks of gestation with trisomy 21 or trisomy 18, 10% and 32%, respectively, result in stillbirth with no gestational-age clustering of the time of fetal death.37
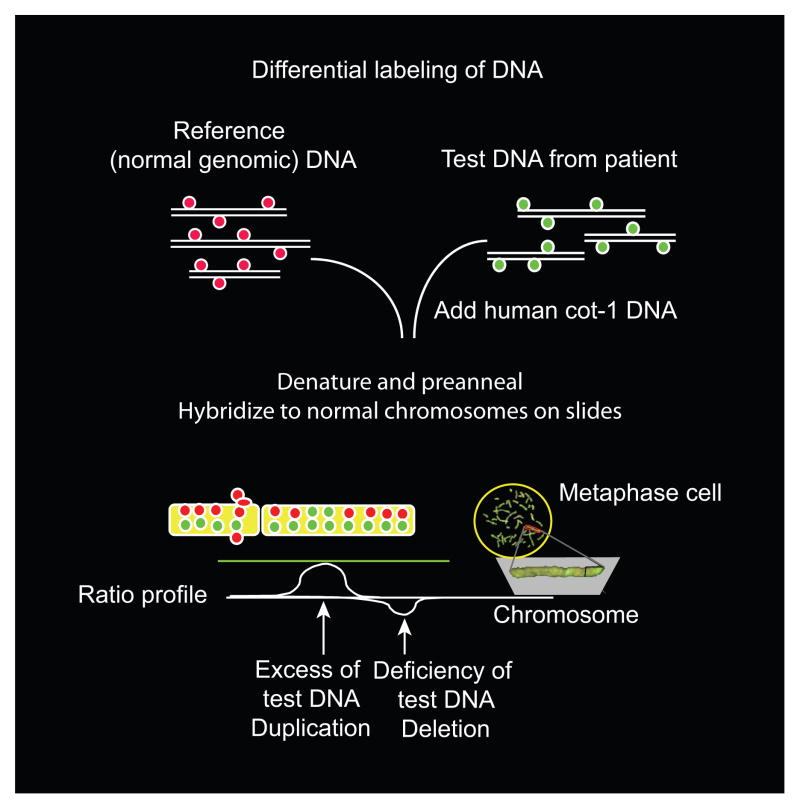
The frequency of cytogenetic abnormalities in stillbirths may be higher than suggested originally because 40–50% of attempted karyotypes fail as a result of inadequate cell growth. Use of newer cytogenetic techniques in which tissue culture is not required should result in a more precise evaluation of the effect of cytogenetic imbalances on fetal death. In addition, older series used lower resolution banding techniques, which will miss smaller but phenotypically significant defects such as microdeletions or duplications. Recently, molecular karyotyping by array-based analysis has provided exceedingly high-resolution genomic analysis independent of the ability of the cells to grow or generate good metaphase spreads (Fig. 2). Although small genomic changes have been demonstrated to be important in a number of genetic disorders,38 their significance as a cause of stillbirth has not been evaluated.
Fig. 2.
The potential use of comparative genomic hybridization to diagnose structural genomic changes in stillbirth. This figure illustrates the general principle underlying comparative genomic hybridization, which is used to identify DNA deletions or duplications. In this technique, fluorescently labeled DNA from a normal control sample is mixed with a test sample that is labeled with a different-colored dye. The mixture then is hybridized to normal metaphase chromosomes. Regional differences in the fluorescence ratio represent gains and losses in the sample DNA compared with the control DNA. Arrays containing hundreds of thousands of small, well-defined DNA probes have replaced normal metaphase chromosomes, allowing more precise and detailed analysis of small, underrepresented or overrepresented areas. Figure courtesy of Dr. Ronald Wapner.
Reddy. Stillbirth Classification of Cause of Death. Obstet Gynecol 2009.
Confined placental mosaicism, in which the karyotype of the fetus is euploid despite an abnormal cell line in the placenta, has been appreciated only recently as a cause of stillbirth (Fig. 3, available online at http://links.lww.com/AOG/A128). Confined placental mosaicism occurs in approximately 1–2% of first-trimester chorionic villus samples. In most of these cases, the abnormal cell line has no phenotypic consequence, but it is associated with an altered perinatal outcome such as spontaneous abortion, stillbirth, or fetal growth restriction in 15–20% of affected pregnancies. Factors that predict pregnancy outcome in confined placental mosaicism include the specific chromosome involved (eg, chromosomes 2, 3, 9, 14, 15, 16, and 18 are known to alter outcome), the persistence of the abnormal cell line throughout pregnancy, the percentage of aneuploid cells in the placenta, the cell lineage containing the aneuploid cells, and possibly the presence of uniparental disomy.39 For example, confined placental mosaicism of trisomy 16 is associated with a particularly high probability of fetal death, preterm delivery, fetal growth restriction, and fetal anomalies, with less than one third of affected pregnancies resulting in a full-term, normally grown neonate.40
Fetal single gene and mendelian disorders also may result in stillbirth. Autosomal recessive disorders such as hemoglobinopathies (eg, alpha thalassemia), metabolic diseases such as Smith Lemli Opitz syndrome, glycogen storage diseases, peroxisomal disorders, and amino acid disorders all have been associated with stillbirth, albeit by different mechanisms. Unfortunately, it may be difficult to identify autosomal recessive disorders as the cause of stillbirth because the phenotype in lethal cases may differ from that seen in live births and will not be familiar to clinical dysmorphologists accustomed to examining liveborns. This has resulted in a relative paucity of information on lethal fetal phenotypes. This is compounded by the fact that there is not a detailed registry of recognizable stillbirth syndromes.
Autosomal dominant disorders also may result in stillbirth and usually result from spontaneous mutations (eg, skeletal dysplasias) but on rare occasions may occur secondary to a parental mutation with in utero consequences (eg, prolonged QT interval). In addition, X-linked dominant mutations may be lethal in male fetuses. Because of skewed X inactivation, affected women will not be affected as severely and, unless examined by an experienced geneticist, their altered phenotype may be missed.
FETOMATERNAL HEMORRHAGE
Fetomaternal hemorrhage is defined as the transplacental passage of fetal cells to the maternal circulation and has been attributed as the cause of 4% of all stillbirths.41–43 It is important to distinguish between the physiologic fetomaternal hemorrhage that commonly occurs during normal pregnancy and pathologic fetomaternal hemorrhage. There is often some transplacental passage of fetal cells into the maternal circulation during normal pregnancy, occurring as early as 8 weeks of gestation.44 Although fetal cells are detectable in about 50% of women after delivery,45 they are less than 1% of the total fetal-placental blood volume in the vast majority of cases.
The most consistent risk factors associated with fetomaternal hemorrhage appear to be abruption and abdominal trauma. Other risk factors are cesarean delivery, operative vaginal delivery, retained placenta, multiple gestations, and abnormal fetal testing.
The threshold for fetomaternal hemorrhage severe enough to cause stillbirth is unknown. The clinical effect of fetal bleeding is influenced by whether the hemorrhage is acute or chronic. Acute fetomaternal hemorrhage leads to severe fetal anemia, ultimately resulting in cardiovascular decompensation, stroke, disseminated intravascular coagulation, and stillbirth. In contrast, chronic bleeding is associated with chronic hypoxia leading to neurologic impairment or stillbirth. Chronic bleeding may be established through the identification of erythroblasts and reticulocytes in fetal blood using flow cytometry.44
Gestational age (and consequently the fetal-placental blood volume) at the time of the fetomaternal hemorrhage also influences the risk of stillbirth. Estimation of the fetomaternal hemorrhage volume can be performed (Box 2). Consider that a 30-mL loss from a 600-g fetus represents 42% of the total fetal blood volume, whereas the same bleed in a 3,600-g fetus is only 7%.46
BOX 2. ESTIMATION OF THE FETOMATERNAL HEMORRHAGE VOLUME (FETAL RED CELLS IN THE MATERNAL CIRCULATION).
FMH red cell volume=fetal red cell volume=maternal blood volume×maternal Hct×fetal red cell percent in maternal blood
FMH whole blood volume=fetal whole blood volume=(maternal blood volume× maternal Hct×fetal red cell percent in maternal blood)/fetal Hct
Assuming:
Maternal whole blood volume ≈5,000 mL
Maternal Hct ≈0.35
Fetal Hct ≈0.50
For example, the assay for fetal cells (eg, Kleihauer Betke) determines that fetal cells are 2% of cells in maternal blood.
Fetal red cell volume in maternal circulation: 5,000×0.35×0.02=35 mL red cells
Fetal whole blood volume in maternal circulation: 5,000×0.35×0.02/0.5=70 mL whole blood
Seventy milliliters of whole blood would be approximately 60% of the fetoplacental blood volume at 30 weeks of gestation—objective evidence that fetomaternal hemorrhage was the likely cause of the stillbirth. Nomograms are available for fetoplacental blood volume over different gestational ages.
FMH, fetomaternal hemorrhage; Hct, hematocrit.
A large fetomaternal hemorrhage will cause severe fetal anemia and, in some cases, fetal death due to exsanguination. A 50% mortality rate in fetuses with acute bleeds of 20% or more of their total blood volumes has been noted.46 Using a threshold of 20 mL/kg of fetal bleeding, the rate of severe fetomaternal hemorrhage was 1.1 per 1,000 in a cohort of 45,180 deliveries. This threshold was associated with an increased risk of stillbirth, induced preterm delivery, neonatal intensive care unit admission, and neonatal anemia requiring transfusion.47
There is no gold standard for attributing stillbirth to fetomaternal hemorrhage. A threshold of more than 20 mL/kg appears to be a reasonable criterion to attribute fetomaternal hemorrhage as the cause of death. In addition to the Kleihauer-Betke or flow cytometry results, there should be autopsy confirmation of fetal anemia and hypoxia to attribute stillbirth to fetomaternal hemorrhage.
FETAL GROWTH RESTRICTION
Fetal growth restriction is not an actual cause of stillbirth but a highly relevant condition that is found in a significant proportion of stillbirths and in the majority of the cases that currently are classified as unexplained.48 Pathologic associations with fetal growth restriction include fetal abnormalities (eg, chromosomal and structural anomalies); multifetal pregnancy; maternal conditions such as infection (eg, cytomegalovirus), hypertensive disease, malnutrition, and smoking; placental disease; and cord abnormalities such as velamentous cord insertion.
Fetal growth restriction can be defined as the failure of the fetus to reach its growth potential. The definition of fetal growth restriction used in clinical practice differs widely: estimated fetal weight or birth weight lower than the 10th or lower than the 5th percentile of the population or less than 2 standard deviations below the mean (3rd percentile). However, the use of population-based percentiles does not account for an individual fetus’s inherent growth potential.
Customized growth curves adjust for physiologic variation such as maternal height, weight, parity, and ethnic origin and thus are better able to distinguish between normal small and pathologically small fetuses (Fig. 4A and B, available online at http://links.lww.com/AOG/A128). In a comparative study of a large Swedish database,49 there was a sixfold increase in stillbirth when a fetus was SGA below the 10th percentile by customized growth charts, and such customization resulted in a larger proportion of fetuses being identified as at risk. In contrast, there was no increase in the risk of stillbirth when a fetus was SGA by population-based curves alone, ie, not SGA by customized standards. There was also a dose–response effect, with the more profound the SGA, the greater the risk for fetal death. Although the majority of stillbirths are preterm, fetuses that die at earlier gestational ages are also more severely growth restricted.50
Diagnosis of fetal growth restriction with reference to stillbirth may be: 1) antenatal—usually by prospective assessment with ultrasonography, 2) post-natal—by birth weight compared with growth potential, and 3) pathology—postmortem, placenta. The association between antenatally detected fetal growth restriction and stillbirth probably is underestimated because the resulting delivery, when severe fetal growth restriction is detected, will prevent the occurrence of stillbirth. In addition, the antenatal diagnosis of fetal growth restriction is unreliable, with studies suggesting that, in unselected populations, less than a third of liveborn SGA neonates (lower than the 10th percentile) are detected to be small antenatally.51
On postmortem examination (when available), histopathologic evidence can support the diagnosis of fetal growth restriction. Markers of redistribution of blood flow to maintain brain growth, a key finding in fetal growth restriction, include: 1) head-circumference percentile higher than weight percentile, 2) brain weight appropriate for gestation but other organs appropriate for body weight, and 3) altered brain weight:liver weight ratio.
PLACENTAL CAUSES
Placental examination may reveal underlying probable causes of stillbirth, including those related to circulatory abnormalities such as abruption, infection/inflammation, genetic/chromosomal abnormalities such as confined placental mosaicism, hydrops, and vascular lesions. Probable placental causes of stillbirth include developmental abnormalities of the placenta such as placenta previa, vasa previa, and neoplasms.
Of migrational disorders, vasa previa occurs when submembranous fetal vessels cross the endocervical os. It may cause stillbirth as a result of rupture of fetal vessels during labor or rupture of membranes leading to fetal exsanguination or both. Fetal blood may pass through the vagina rather than entering the maternal circulation. Histologic evaluation of the placenta and cord confirms the diagnosis.
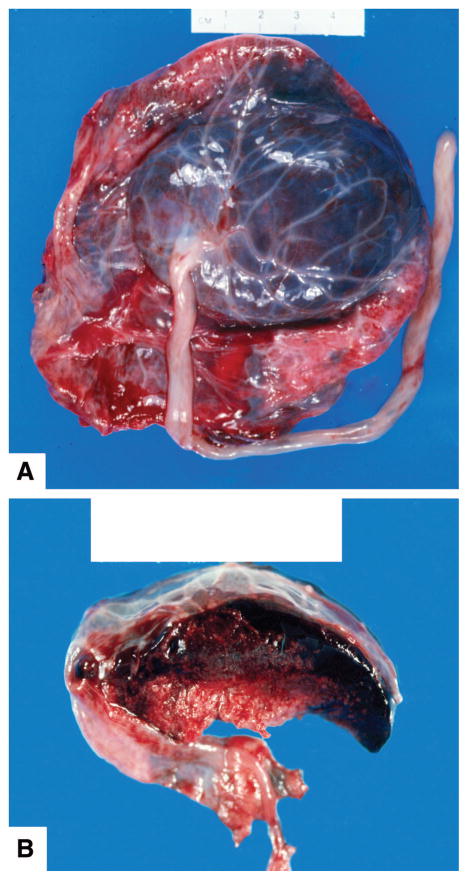
Circulatory disorders of the placenta related to the occurrence of stillbirth may be on the maternal or fetal side (Fig. 5). A major maternal circulatory disorder is abruptio placenta, which may be considered a cause of death when there are clinical signs of a large abruption or histopathologic examination of the placenta showing extensive signs of abruption (Fig. 6, available online at http://links.lww.com/AOG/A128). The adjusted relative risk was 8.9 (95% confidence interval [CI], 6.0–13.0) for stillbirth in a cohort of women with abruption. The subset of women with more than 75% placental separation had an adjusted relative risk for stillbirth of 31.5 (95% CI 17.0–58.4).52
Fig. 5.
Massive subchorial hematoma, also known as Breus’ mole, located under the chorion. A. Fetal surface. B. Cut section illustrating the subchorionic location. Figure courtesy of Dr. Halit Pinar, Brown University.
Reddy. Stillbirth Classification of Cause of Death. Obstet Gynecol 2009.
The diagnosis of abruption often is made based on clinical parameters. The most common is vaginal bleeding, which occurs in a majority of women with abruption. However, some patients will have a concealed abruption in which blood from premature placental separation remains trapped behind the placenta, never passing through the vagina where it becomes obvious to the clinician. Other clinical signs of abruption include abdominal pain, abnormal fetal heart rate tracings indicative of hypoxia, and abnormal uterine contraction patterns with either titanic contractions and hyperstimulation or tachysystole. Clinicians often estimate the degree of placental detachment based on gross evaluation of the placenta. However, it may be difficult to determine whether the detachment occurred before or after death in cases of antepartum stillbirth. These clinical parameters are central to the diagnosis of abruption, but they are inherently subjective. Placental abruption may be considered the cause of death when there are clinical signs of a large abruption or histopathologic examination of the placenta showing extensive signs of abruption (more than 30%).
Histologic evaluation of the placenta can be helpful in documenting abruption. In cases of chronic abruption, there may be hemosiderin deposits in the placenta. After time, both perivillous and marginal fibrin deposition and decidual necrosis may be present.53 In other cases, there may be evidence of abnormal placental vasculature, thrombosis, and reduced placental perfusion (eg, infarction).
Placental pathology resulting from underlying maternal or fetal disease also may result in stillbirth. An SGA placenta, defined as being less than 5% of the expected weight for corresponding gestational age, usually is due to reduced uteroplacental blood flow and associated impaired villous growth and development. This finding often is associated with maternal vascular diseases such as preeclampsia/eclampsia, hypertension, and diabetes mellitus with renal disease. In chronic infections and aneuploidies, placental growth also is impaired. In maternal hypertensive conditions, the placenta often shows multiple infarcts and decidual vasculopathy with acute atherosis.
Large for gestational age placenta is defined as being more than 95% of the expected weight for corresponding gestational age. Relatively common causes of large placentas associated with stillbirth include hydrops fetalis as a result of immune or nonimmune causes, maternal diabetes mellitus, and specific infections such as syphilis.54,55
UMBILICAL CORD PATHOLOGY
Umbilical cord abnormalities account for 3.4–15% of stillbirths.56,57 Velamentous insertion of the umbilical cord occurs when vessels insert on the membranes rather than the placenta (Fig. 7A and B, available online at http://links.lww.com/AOG/A128). It may cause stillbirth if it leads to a vasa previa. With furcate insertion of the umbilical cord, the umbilical cord blood vessels lose the protective cover of Wharton’s substance before entering the chorionic plate. Owing to splaying of the vessels and their wide distribution, the vessels are exposed to external trauma. During labor and delivery, they may rupture, twist, and consequently compromise the placental circulation, resulting in stillbirth.
Umbilical cord prolapse is an obstetric emergency that causes stillbirths and is defined as presentation of the cord in advance of the presenting fetal part. Cord prolapse is associated with abnormal presentation, prematurity, multiparity, obstetric manipulation, and abnormally long umbilical cords.58,59
Umbilical cord occlusion results in cessation of blood flow to the fetus; there are several potential mechanisms whereby cord accidents could lead to stillbirth. These include intermittent disruption of blood flow such as cord prolapse, fetal blood loss through cord hemorrhage, intrinsic cord abnormalities, and entanglement of the cords in the case of monochorionic twins.57 Umbilical cord torsion has been reported as a cause of fetal death and is seen most frequently at the fetal end of the cord. If the torsion occurred antemortem, the cord should remain twisted after separation of the fetus from the placenta. The involved cord is congested and edematous, often with evidence of thrombosis of the cord vessels.60 Other uncommon causes of death include rupture, strictures, and hematomas of the umbilical cord.
Cord entanglement in the form of nuchal cords occurs in up to 30% of uncomplicated pregnancies. In a cohort–control study of almost 14,000 deliveries, single nuchal cords were present at birth in 23.6% and multiple nuchal cords in 3.7% of deliveries.61 Nuchal cords were not associated with an increased risk of stillbirth in this cohort (odds ratio for stillbirth of 1.03, 95% CI 0.64–1.60).61 Similarly, true knots also are common in live births. Examination of a tight knot may show grooving of the cord and constriction of the umbilical vessels in longstanding cases and edema, congestion, or thrombosis in more acute ones. It is difficult to attribute any adverse outcome to the presence of a knot in the absence of such changes. Thus, the isolated finding of a nuchal cord or true knot at the time of birth is insufficient evidence that cord accident is the cause of the stillbirth.
Ideally, corroborating evidence should be present to conclude that a cord accident is the likely cause of death. First, other recognized causes of stillbirth should be excluded through a careful and systematic evaluation. Second, there should be evidence of cord occlusion and hypoxia on perinatal postmortem examination and histologic examination of the placenta and umbilical cord. Recently, Parast et al suggested histologic criteria for the diagnosis of cord accident.62 They propose vascular ectasia and thrombosis within the umbilical cord, chorionic plate, or stem villi as minimal histologic criteria suggestive of cord accident. For a probable diagnosis, they require the previous findings as well as regional distribution of avascular villi or villi showing stromal karyorrhexis.62
COMPLICATIONS OF MULTIFETAL GESTATION
Specific placental abnormalities are associated with multiple gestations, mostly monochorionic placentation. The most common of these complications is twin–twin transfusion, which occurs in 9% of monochorionic diamniotic gestations as a result of arteriovenous anastomoses within the placenta63 (Fig. 8, available online at http://links.lww.com/AOG/A128). Ultrasonographic findings vary but typically involve impaired growth, oligohydramnios, absent bladder filling, and increased resistance to flow in the umbilical cord of the donor. The recipient is usually normally grown with hydramnios. If severe, either twin may exhibit cardiac dysfunction or hydrops (often in the recipient) or may die. The mortality rate can be 90% in untreated cases.
Monochorionic monoamniotic twins occur in 5% of monochorionic twins. There is a high stillbirth rate, in large part due to cord entanglement because both fetuses and both cords are in the same amniotic sac. Other potential contributors to fetal death include preterm birth, growth impairment, malformations, genetic abnormalities, and vascular anastomoses.
Twin reverse arterial perfusion sequence is a rare complication of monochorionic twins and occurs in up to 1% of monochorionic pregnancies, resulting from artery–artery anastomoses with reverse perfusion in one of the twins. Reverse flow of deoxygenated blood leads to the abnormal development of one twin so that the heart develops abnormally and cannot function. This “acardiac” twin cannot survive. However, the pump twin also is at risk of stillbirth because of the additional cardiac demands of perfusing the acardiac twin. Mortality in untreated cases has been reported to be as high as 50–75%.64
AMNIOTIC BAND SEQUENCE
Amniotic band sequence is a sporadic condition of uncertain cause that refers to the entrapment of fetal parts by disrupted amnion and often results in stillbirth. Findings are variable and include amputations, constrictions, clefts, and deformations as well as amniotic bands in contact with deformities.
UTERINE COMPLICATIONS
Uterine rupture may be regarded as the cause of stillbirth when clinical signs of obstructed circulation occur. There is also an increased risk for uterine abnormalities in women with recurrent pregnancy loss including stillbirth.65,66 The mechanism leading to fetal death is uncertain but is thought to be a result of poorly vascularized uterine tissue or space constraints. In addition to directly causing stillbirth, uterine malformations may lead to stillbirth indirectly by increasing the risk for preterm premature rupture of membranes, cervical insufficiency, and preterm labor. Septate uterus is the anomaly most commonly associated with stillbirth. The septum has a relatively high proportion of avascular connective tissue relative to muscle (compared with the vascular uterine fundus). Thus, implantation on the septum may lead to decreased blood flow and pregnancy loss. Implantation on a uterine septum also increases the risk for placental abruption, another cause of stillbirth.
INTRAPARTUM STILLBIRTH
Intrapartum stillbirth rates (the death of a fetus during labor and delivery) are about 1 per 1,000 births in developed countries compared with 7.3 per 1,000 births in developing countries and range as high as 20–25 per 1,000 births for some countries in southern Africa and Asia. About one tenth of stillbirths in developed countries are intrapartum; in some developing countries, with much higher overall stillbirth rates, 50% or more of stillbirths are intrapartum.67 Causes of intrapartum stillbirth include shoulder dystocia, malpresentation, cord prolapse, severe birth trauma, and fetal hypoxia as occurs with abruption or uterine rupture. In addition, there is an increased risk of intrapartum stillbirth for the second twins regardless of chorionicity.68
CENTRAL NERVOUS SYSTEM
When considering the relationship of central nervous system (CNS) findings in stillbirth to the cause of death, it is often difficult to determine whether they are 1) a direct cause of death, 2) associated with a systemic process or disease with the direct cause of death in another organ system, 3) indicators of a pathophysiologic process leading to death (such as hypoxia), or 4) incidental.
Central nervous system infection, hypoxic-ischemic injury, or intracranial hemorrhage can lead to stillbirth, but the underlying cause resides outside the CNS. Infections that produce significant necrosis and inflammation, such as cytomegalovirus, varicella, toxoplasmosis, and Listeria, may lead to death by destructive lesions in the CNS. However, these infections often result in injury to other organs as well.3 Ischemic brain lesions may be associated with abnormalities of placental–fetal blood flow or intrauterine infection. Hypoxia-ischemic injury in the brain of a stillborn can be expressed as germinal matrix hemorrhage, white-matter injury, or neuronal injury, and the vulnerability of different brain regions is strongly related to gestational age.69 Maternal trauma or coagulopathy may lead to large subdural hemorrhages that impair survival.70,71 Fetal CNS neoplasms are rare but can be a cause of stillbirth.72
In summary, we attempted to define the medical and obstetric conditions that cause stillbirths and, when feasible, the characteristics of those conditions—when present—that one could use to attribute that cause to a specific stillbirth. However, there was universal agreement among the conference attendees that, in virtually all cases, there will be a degree of uncertainty regarding whether a specific condition was indeed the cause of death. To deal with this uncertainty, some existing classification systems note which potentially relevant conditions were present in cases of stillbirth, whereas other classification systems code each condition as a certain, probable, or possible cause. Although there is no standard method to deal with the inherent uncertainty, each classification system should recognize this uncertainty as an important issue and address it in some manner. In this article, we developed definitions by which a condition should be considered a cause of death, regardless of the system used.
There is still varying and considerable uncertainty in establishing the cause of death for many cases of stillbirth because of our incomplete understanding of the underlying pathophysiology. Given the involvement of the woman, the fetus, and the placenta, a shared organ, it is imperative that studies to elucidate cause involve thorough investigation of how a disease process affects all three components. This knowledge is needed to design interventions that will reduce the incidence of stillbirth and lead to the birth of a healthy newborn.
Supplementary Material
Footnotes
For a listing of workshop participants, see the Appendix online at http://links.lww.com/AOG/A127.
The article as it appears in print is abridged. Selected figures and tables appear online at http://links.lww.com/AOG/A128.
This workshop was cosponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, First Candle and the Office of Rare Diseases, National Institutes of Health, Bethesda, Maryland.
Financial Disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet. 2006;367:1487–94. doi: 10.1016/S0140-6736(06)68586-3. [DOI] [PubMed] [Google Scholar]
- 2.MacDorman MF, Kirmeyer S. National vital statistics reports. 8. Vol. 57. Hyattsville (MD): National Center for Health Statistics; 2009. Fetal and perinatal mortality, United States, 2005. [PubMed] [Google Scholar]
- 3.Goldenberg RL, Thompson C. The infectious origins of stillbirth. Am J Obstet Gynecol. 2003;189:861–73. doi: 10.1067/s0002-9378(03)00470-8. [DOI] [PubMed] [Google Scholar]
- 4.Hardy JM, Azarowicz EN, Mannini A, Medearis DN, Jr, Cooke RE. The effect of Asian influenza on the outcome of pregnancy: Baltimore 1957–1958. Am J Public Health Nations Health. 1961;51:1182–8. doi: 10.2105/ajph.51.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horn P. Poliomyelitis in pregnancy: a twenty-year report from Los Angeles County, California. Obstet Gynecol. 1955;6:121–37. [PubMed] [Google Scholar]
- 6.Steketee RW, Wirima JJ, Slutsker L, Heymann DL, Breman JG. The problem of malaria and malaria control in pregnancy in sub-Saharan Africa. Am J Trop Med Hyg. 1996;55(suppl):2–7. doi: 10.4269/ajtmh.1996.55.2. [DOI] [PubMed] [Google Scholar]
- 7.Sheffield JS, Sanchez PJ, Wendel GD, Jr, Fong DW, Margraf LR, Zeray F, et al. Placental histopathology of congenital syphilis. Obstet Gynecol. 2002;100:126–33. doi: 10.1016/s0029-7844(02)02010-0. [DOI] [PubMed] [Google Scholar]
- 8.Plaza MC, Gilbert-Barness E. Fetal death in utero secondary to Listeria monocytogenes placental infection. Pediatr Pathol Mol Med. 2001;20:433–7. [PubMed] [Google Scholar]
- 9.De Santis M, Cavaliere AF, Straface G, Caruso A. Rubella infection in pregnancy. Reprod Toxicol. 2006;21:390–8. doi: 10.1016/j.reprotox.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Petersson K, Bremme K, Bottinga R, Hofsjö A, Hulthén-Varli I, Kublickas M, et al. Diagnostic evaluation of intrauterine fetal deaths in Stockholm 1998–99 [published erratum appears in Acta Obstet Gynecol Scand 2003;82:102] Acta Obstet Gynecol Scand. 2002;81:284–92. doi: 10.1034/j.1600-0412.2002.810402.x. [DOI] [PubMed] [Google Scholar]
- 11.Ananth CV, Savitz DA, Bowes WA., Jr Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet Gynecol Scand. 1995;74:788–93. doi: 10.3109/00016349509021198. [DOI] [PubMed] [Google Scholar]
- 12.Tricomi V, Kohl SG. Fetal death in utero. Am J Obstet Gynecol. 1957;74:1092–7. doi: 10.1016/0002-9378(57)90162-x. [DOI] [PubMed] [Google Scholar]
- 13.Information and Statistics Division NHS Scotland. Scottish perinatal and infant mortality report 2000. Edinburgh (Scotland): ISD Scotland Publications; 2001. [Google Scholar]
- 14.Martin JN, Jr, Rinehart BK, May WL, Magann EF, Terrone DA, Blake PG. The spectrum of severe preeclampsia: comparative analysis by HELLP (hemolysis, elevated liver enzyme levels, and low platelet count) syndrome classification. Am J Obstet Gynecol. 1999;180:1373–84. doi: 10.1016/s0002-9378(99)70022-0. [DOI] [PubMed] [Google Scholar]
- 15.Smulian JC, Ananth CV, Vintzileos AM, Scorza WE, Knuppel RA. Fetal deaths in the United States. Influence of high-risk conditions and implications for management. Obstet Gynecol. 2002;100:1183–9. doi: 10.1016/s0029-7844(02)02389-x. [DOI] [PubMed] [Google Scholar]
- 16.Macintosh MC, Fleming KM, Bailey JA, Doyle P, Modder J, Acolet D, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ. 2006;333:177. doi: 10.1136/bmj.38856.692986.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neale D, Burrow G. Thyroid disease in pregnancy. Obstet Gynecol Clin North Am. 2004;31:893–905. doi: 10.1016/j.ogc.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Simpson LL. Maternal medical disease: risk of antepartum fetal death. Semin Perinatol. 2002;26:42–50. doi: 10.1053/sper.2002.29838. [DOI] [PubMed] [Google Scholar]
- 19.Fischer MJ. Chronic kidney disease and pregnancy: maternal and fetal outcomes. Adv Chronic Kidney Dis. 2007;14:132–45. doi: 10.1053/j.ackd.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Fisk NM, Bye WB, Storey GN. Maternal features of obstetric cholestasis: 20 years experience at King George V Hospital. Aust N Z J Obstet Gynaecol. 1988;28:172–6. doi: 10.1111/j.1479-828x.1988.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 21.Ko H, Yoshida EM. Acute fatty liver of pregnancy. Can J Gastroenterol. 2006;20:25–30. doi: 10.1155/2006/638131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molad Y. Systemic lupus erythematosus and pregnancy. Curr Opin Obstet Gynecol. 2006;18:613–7. doi: 10.1097/GCO.0b013e32800ff5c5. [DOI] [PubMed] [Google Scholar]
- 23.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 24.Antiphospholipid syndrome. ACOG Practice Bulletin No. 68. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106:1113–21. doi: 10.1097/00006250-200511000-00056. [DOI] [PubMed] [Google Scholar]
- 25.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. The Cochrane Database of Systemic Reviews. 2005;(2) doi: 10.1002/14651858.CD002859.pub2. Art. No.: CD002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rey E, Kahn SR, David M, Shrier I. Thrombophilic disorders and fetal loss: a meta-analysis. Lancet. 2003;361:901–8. doi: 10.1016/S0140-6736(03)12771-7. [DOI] [PubMed] [Google Scholar]
- 27.Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. Eur J Obstet Gynecol Reprod Biol. 2002;101:6–14. doi: 10.1016/s0301-2115(01)00496-1. [DOI] [PubMed] [Google Scholar]
- 28.Lockwood C, Silver R. Thrombophilias in pregnancy. In: Creasy R, Resnick R, Iams J, editors. Maternal-fetal medicine: principles and practice. 5. Philadelphia (PA): WB Saunders Company; 2003. pp. 1005–22. [Google Scholar]
- 29.Lindqvist PG, Svensson PJ, Marsaál K, Grennert L, Luterkort M, Dahlbäck B. Activated protein C resistance (FV:Q506) and pregnancy. Thromb Haemost. 1999;81:532–7. [PubMed] [Google Scholar]
- 30.Dizon-Townson D, Miller C, Sibai B, Spong CY, Thom E, Wendel G, Jr, et al. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517–24. doi: 10.1097/01.AOG.0000173986.32528.ca. [DOI] [PubMed] [Google Scholar]
- 31.Moise KJ. Fetal anemia due to non-Rhesus-D red-cell alloimmunization. Semin Fetal Neonatal Med. 2008;13:207–14. doi: 10.1016/j.siny.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan C. Alloimmune thrombocytopenia of the fetus and the newborn. Blood Rev. 2002;16:69–72. doi: 10.1054/blre.2001.0187. [DOI] [PubMed] [Google Scholar]
- 33.Pauli RM, Reiser CA, Lebovitz RM, Kirkpatrick SJ. Wisconsin Stillbirth Service Program: I. Establishment and assessment of a community-based program for etiologic investigation of intrauterine deaths. Am J Med Genet. 1994;50:116–34. doi: 10.1002/ajmg.1320500204. [DOI] [PubMed] [Google Scholar]
- 34.Pauli RM, Reiser CA. Wisconsin Stillbirth Service Program: II. Analysis of diagnoses and diagnostic categories in the first 1,000 referrals. Am J Med Genet. 1994;50:135–53. doi: 10.1002/ajmg.1320500205. [DOI] [PubMed] [Google Scholar]
- 35.Korteweg FJ, Bouman K, Erwich JJ, Timmer A, Veeger NJ, Ravise JM, et al. Cytogenetic analysis after evaluation of 750 fetal deaths: proposal for diagnostic workup. Obstet Gynecol. 2008;111:865–74. doi: 10.1097/AOG.0b013e31816a4ee3. [DOI] [PubMed] [Google Scholar]
- 36.Wapner RJ, Lewis D. Genetics and metabolic causes of stillbirth. Semin Perinatol. 2002;26:70–4. doi: 10.1053/sper.2002.29853. [DOI] [PubMed] [Google Scholar]
- 37.Won RH, Currier RJ, Lorey F, Towner DR. The timing of demise in fetuses with trisomy 21 and trisomy 18. Prenat Diagn. 2005;25:608–11. doi: 10.1002/pd.1243. [DOI] [PubMed] [Google Scholar]
- 38.Shaffer LG, Coppinger J, Alliman S, Torchia BA, Theisen A, Ballif BC, et al. Comparison of microarray-based detection rates for cytogenetic abnormalities in prenatal and neonatal specimens. Prenat Diagn. 2008;28:789–95. doi: 10.1002/pd.2053. [DOI] [PubMed] [Google Scholar]
- 39.Kalousek DK, Barrett IJ, McGillivray BC. Placental mosaicism and intrauterine survival of trisomies 13 and 18. Am J Hum Genet. 1989;44:338–43. [PMC free article] [PubMed] [Google Scholar]
- 40.Benn P. Trisomy 16 and trisomy 16 Mosaicism: a review. Am J Med Genet. 1998;79:121–33. [PubMed] [Google Scholar]
- 41.Laube DW, Schauberger CW. Fetomaternal bleeding as a cause for “unexplained” fetal death. Obstet Gynecol. 1982;60:649–51. [PubMed] [Google Scholar]
- 42.Marions L, Thomassen P. Six cases of massive feto-maternal bleeding causing intra-uterine fetal death. Acta Obstet Gynecol Scand. 1991;70:85–8. doi: 10.3109/00016349109006184. [DOI] [PubMed] [Google Scholar]
- 43.Samadi R, Greenspoon JS, Gviazda I, Settlage RH, Goodwin TM. Massive fetomaternal hemorrhage and fetal death: are they predictable? J Perinatol. 1999;19:227–9. doi: 10.1038/sj.jp.7200144. [DOI] [PubMed] [Google Scholar]
- 44.Sebring ES, Polesky HF. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion. 1990;30:344–57. doi: 10.1046/j.1537-2995.1990.30490273444.x. [DOI] [PubMed] [Google Scholar]
- 45.Cohen F, Zuelzer WW, Gustafson DC, Evans MM. Mechanisms of isoimmunization. I. The transplacental passage of fetal erythrocytes in homospecific pregnancies. Blood. 1964;23:621–46. [PubMed] [Google Scholar]
- 46.Dziegiel MH, Nielsen LK, Berkowicz A. Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol. 2006;13:490–5. doi: 10.1097/01.moh.0000245687.09215.c4. [DOI] [PubMed] [Google Scholar]
- 47.Rubod C, Deruelle P, Le Goueff F, Tunez V, Fournier M, Subtil D. Long-term prognosis for infants after massive feto-maternal hemorrhage. Obstet Gynecol. 2007;110:256–60. doi: 10.1097/01.AOG.0000271212.66040.70. [DOI] [PubMed] [Google Scholar]
- 48.Gardosi J, Kady SM, McGeown P, Francis A, Tonks A. Classification of stillbirth by relevant condition at death (ReCoDe): population based cohort study. BMJ. 2005;331:1113–7. doi: 10.1136/bmj.38629.587639.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clausson B, Gardosi J, Francis A, Cnattingius S. Perinatal outcome in SGA births defined by customised versus population-based birthweight standards. BJOG. 2001;108:830–4. doi: 10.1111/j.1471-0528.2001.00205.x. [DOI] [PubMed] [Google Scholar]
- 50.Gardosi J, Mul T, Mongelli M, Fagan D. Analysis of birth weight and gestational age in antepartum stillbirths. Br J Obstet Gynaecol. 1998;105:524–30. doi: 10.1111/j.1471-0528.1998.tb10153.x. [DOI] [PubMed] [Google Scholar]
- 51.Hepburn M, Rosenberg K. An audit of the detection and management of small-for-gestational age babies. Br J Obstet Gynaecol. 1986;93:212–6. doi: 10.1111/j.1471-0528.1986.tb07895.x. [DOI] [PubMed] [Google Scholar]
- 52.Ananth CV, Berkowitz GS, Savitz DA, Lapinski RH. Placental abruption and adverse perinatal outcomes. JAMA. 1999;282:1646–51. doi: 10.1001/jama.282.17.1646. [DOI] [PubMed] [Google Scholar]
- 53.Elliott JP, Gilpin B, Strong TH, Jr, Finberg HJ. Chronic abruption-oligohydramnios sequence. J Reprod Med. 1998;43:418–22. [PubMed] [Google Scholar]
- 54.Naeye RL. Functionally important disorders of the placenta, umbilical cord and fetal membranes. Hum Pathol. 1987;18:680–91. doi: 10.1016/s0046-8177(87)80239-3. [DOI] [PubMed] [Google Scholar]
- 55.Naeye RL. Do placental weights have clinical significance? Hum Pathol. 1987;18:387–91. doi: 10.1016/s0046-8177(87)80170-3. [DOI] [PubMed] [Google Scholar]
- 56.Korteweg FJ, Gordijn SJ, Timmer A, Erwich JJ, Bergman KA, Bouman K, et al. The TULIP classification of perinatal mortality: introduction and multidisciplinary inter-rater agreement. BJOG. 2006;113:393–401. doi: 10.1111/j.1471-0528.2006.00881.x. [DOI] [PubMed] [Google Scholar]
- 57.Collins JH. Umbilical cord accidents: human studies. Semin Perinatol. 2002;26:79–82. doi: 10.1053/sper.2002.29860. [DOI] [PubMed] [Google Scholar]
- 58.Naeye RL. Umbilical cord length: clinical significance. J Pediatr. 1985;107:278–81. doi: 10.1016/s0022-3476(85)80149-9. [DOI] [PubMed] [Google Scholar]
- 59.Rayburn WF, Beynen A, Brinkman DL. Umbilical cord length and intrapartum complications. Obstet Gynecol. 1981;57:450–2. [PubMed] [Google Scholar]
- 60.Bakotic BW, Boyd T, Poppiti R, Pflueger S. Recurrent umbilical cord torsion leading to fetal death in 3 subsequent pregnancies: a case report and review of the literature. Arch Pathol Lab Med. 2000;124:1352–5. doi: 10.5858/2000-124-1352-RUCTLT. [DOI] [PubMed] [Google Scholar]
- 61.Carey JC, Rayburn WF. Nuchal cord encirclements and risk of stillbirth. Int J Gynaecol Obstet. 2000;69:173–4. doi: 10.1016/s0020-7292(99)00219-2. [DOI] [PubMed] [Google Scholar]
- 62.Parast MM, Crum CP, Boyd TK. Placental histologic criteria for umbilical blood flow restriction in unexplained stillbirth. Hum Pathol. 2008;39:948–53. doi: 10.1016/j.humpath.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 63.Lewi L, Jani J, Blickstein I, Huber A, Gucciardo L, Van Mieghem T, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol. 2008;199:514.e1–8. doi: 10.1016/j.ajog.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 64.Sullivan AE, Varner MW, Ball RH, Jackson M, Silver RM. The management of acardiac twins: a conservative approach. Am J Obstet Gynecol. 2003;189:1310–3. doi: 10.1067/s0002-9378(03)00597-0. [DOI] [PubMed] [Google Scholar]
- 65.Stray-Pedersen B, Stray-Pedersen S. Etiologic factors and subsequent reproductive performance in 195 couples with a prior history of habitual abortion. Am J Obstet Gynecol. 1984;148:140–6. doi: 10.1016/s0002-9378(84)80164-7. [DOI] [PubMed] [Google Scholar]
- 66.Acién P. Reproductive performance of women with uterine malformations. Hum Reprod. 1993;8:122–6. doi: 10.1093/oxfordjournals.humrep.a137860. [DOI] [PubMed] [Google Scholar]
- 67.Goldenberg RL, McClure EM, Bann CM. The relationship of intrapartum and antepartum stillbirth rates to measures of obstetric care in developed and developing countries. Acta Obstet Gynecol Scand. 2007;86:1303–9. doi: 10.1080/00016340701644876. [DOI] [PubMed] [Google Scholar]
- 68.Smith GC, Shah I, White IR, Pell JP, Dobbie R. Mode of delivery and the risk of delivery-related perinatal death among twins at term: a retrospective cohort study of 8073 births. BJOG. 2005;112:1139–44. doi: 10.1111/j.1471-0528.2005.00631.x. [DOI] [PubMed] [Google Scholar]
- 69.Grafe MR, Kinney HC. Neuropathology associated with stillbirth. Semin Perinatol. 2002;26:83–8. doi: 10.1053/sper.2002.29862. [DOI] [PubMed] [Google Scholar]
- 70.Akman CI, Cracco J. Intrauterine subdural hemorrhage. Dev Med Child Neurol. 2000;42:843–6. doi: 10.1017/s0012162200001559. [DOI] [PubMed] [Google Scholar]
- 71.Oswal K, Agarwal A. Warfarin-induced fetal intracranial sub-dural hematoma. J Clin Ultrasound. 2008;36:451–3. doi: 10.1002/jcu.20464. [DOI] [PubMed] [Google Scholar]
- 72.Janisch W, Haas JF, Schreiber D, Gerlach H. Primary central nervous system tumors in stillborns and infants. Epidemiological considerations. J Neurooncol. 1984;2:113–6. doi: 10.1007/BF00177895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.