Abstract
BACKGROUND
High-quality external validation studies have recently been high-lighted to be of paramount importance for proper translation of prognostic markers into the clinical setting. To that end, the authors examined associations of the insulin-like growth factor-II mRNA binding protein, IMP3, with clinical and pathologic features of clear cell renal cell carcinoma (ccRCC) in an independent cohort of patients to validate recent work showing IMP3 as a prognostic marker for RCC progression and death.
METHODS
The authors studied 716 consecutive tumor specimens from patients treated with surgery at the study institution for unilateral, sporadic, noncystic ccRCC between 1990 and 1999. Tissues were stained and scored for IMP3 expression and these expression levels were correlated with clinical and pathologic features as well as clinical outcomes including progression to distant metastases and death from RCC.
RESULTS
It was observed that 213 ccRCC specimens (29.8%) expressed tumor cell IMP3. IMP3 expression was associated with advanced stage and grade of primary tumors as well as other adverse features including coagulative tumor necrosis and sarcomatoid differentiation. After multivariate adjustment for ccRCC prognostic features, positive IMP3 expression was still found to be associated with a 42% increase in the risk of death from RCC (hazards ratio [HR], 1.42; P = .024). Among the subset of patients with clinically localized disease, positive IMP3 expression was associated with a nearly 5-fold increased risk of distant metastases (HR, 4.71; P <.001).
CONCLUSIONS
Using a large and independent cohort of ccRCC patients, the authors confirmed that tumor cell IMP3 expression represents an independent predictor of aggressive ccRCC tumor behavior.
Keywords: IMP3, mRNA binding protein, insulin-like growth factor, kidney cancer, biomarker, cancer progression
Great emphasis has recently been placed on the elucidation of molecular expression profiles that define so-called ‘signatures’ associated with high-risk forms of cancer, either localized cancers that are predisposed to disease progression after attempted extirpation or advanced malignancies that may respond poorly to systemic therapy. This is the case for renal cell carcinoma (RCC), especially RCC of the clear cell subtype (ccRCC). The importance of identifying tumor-associated molecules that collectively define at-risk populations is that such molecules could potentially be utilized to identify patients most apt to fail therapy and therefore might benefit from early adjunctive intervention to preempt impending cancer progression. In addition, such tumor-associated molecules might reveal important mechanisms driving malignant inception and progression. Finally, some prognostic molecules may doubly serve as useful targets for antitumoral therapy.
To our knowledge to date, several molecules have been reported as independent prognostic markers for RCC, including, but not limited to, nuclear antigen Ki-67, survivin, carbonic anhydrase IX, and IMP3.1–4 In addition, we have recently reported that the T-cell coregulatory ligands, B7-H1 and B7-H4, independently portend aggressive ccRCC tumor behavior and adverse patient outcome.5,6 However, to our knowledge, most of these prognostic markers, with the exception of Ki-67,7 have not undergone large-scale, external validation by outside investigators. Recent reviews of this process have suggested that single studies of a given biomarker within 1 cohort of patients are often inadequate to firmly establish its prognostic value. For example, Altmann and Royston8 have stated that prognostic models are not generalizable until validated in additional and, ideally, independent cohorts of patients. Bleeker et al.9 have also echoed this sentiment, demonstrating that prognostic models perform best when applied to datasets from which the prognostic model was originally constructed, but perform much less well when used to assess outcomes for external cohorts of patients not incorporated into the prognostic model during its derivation. This deterioration of prognostic model performance with broader application over time could be, in part, because of the finding that different studies often collect and control for different sets of variables not taken into account during the original derivation of the prognostic model.10 In addition, there can be difficult-to-quantify biases associated with study populations arising from a single institution. Such biases may stem from, but are certainly not limited to, genetic clustering of study populations, as well as aspects of treatment and follow-up that are highly unique to regional or institution-specific standards of care. As such, Justice et al.10 concluded that diverse assessment of any prognostic tool is imperative to confirm its ability to make predictions in an otherwise untested setting. Prompted by this, we initiated the current study to provide external validation of IMP3 expression as an independent prognostic marker of poor outcome among patients treated surgically for ccRCC.
IMP3 is a member of the insulin-like growth factor-II (IGF-II) mRNA binding protein family. Although IMP3 is expressed within developing epithelia, myocytes, and placenta during human and mouse embryogenesis, its expression is low or undetectable in postnatal tissues and virtually absent in adult tissues. IMP3 is believed to participate in the protection and intracellular distribution of IGF-II mRNA and thus has been implicated in regulating the production of IGF-II. It is interesting to note that some reports suggest that RCC tumors, especially aggressive tumors exhibiting sarcomatoid differentiation, express increased IGF-II expression. Most recently, Jiang et al.1 systematically studied the expression of IMP3 in a cohort of 371 patients with localized tumors of the clear cell, papillary, chromophobe, or unclassified RCC subtypes. In that study, Jiang et al.1 reported that tumor cell IMP3 expression was significantly associated with progression to distant metastases and death, even after multivariate adjustment for patient age, sex, tumor size, stage, grade, and histologic subtype.
In keeping with the aforementioned imperative for external validation of prognostic markers pertaining to human malignancy, we report an independent and large-scale analysis of IMP3 expression using 716 consecutive ccRCC specimens. We demonstrate that IMP3 expression correlates with advanced tumor stage and grade, as well as the presence of several other adverse pathologic features including coagulative tumor necrosis and sarcomatoid differentiation. We further show that even after multivariate adjustment for well-known ccRCC prognostic features, IMP3 expression is independently associated with death from RCC. For patients who present with TNM stage I disease, ccRCC IMP3 expression is associated with a 6-fold increased risk of progression to distant metastases. This risk of metastases is similarly increased for patients presenting with stage II and III ccRCC tumors that express IMP3. Hence, we confirm the findings of Jiang et al,1 thereby validating tumor cell IMP3 expression as an independent predictor of aggressive ccRCC tumor behavior.
MATERIALS AND METHODS
Patient Selection
Upon approval from the Institutional Review Board, we identified 716 patients treated with open radical nephrectomy or nephron-sparing surgery for unilateral, sporadic, noncystic ccRCC between 1990 and 1999 from the Mayo Clinic Nephrectomy Registry.
Clinical and Pathologic Features
Clinical features studied included age, sex, symptoms at presentation, Eastern Cooperative Oncology Group performance status, and tumor thrombus level. Patients with a palpable flank or abdominal mass; discomfort; macroscopic hematuria; acute onset varicocele; or constitutional symptoms including rash, sweats, weight loss, fatigue, early satiety, and anorexia were considered symptomatic. Pathologic features studied included histologic subtype classified according to the International Union Against Cancer, the American Joint Committee on Cancer (AJCC), and Heidelberg guidelines; tumor size; the 2002 AJCC primary tumor classification; regional lymph node involvement; distant metastases; the 2002 AJCC TNM stage groupings; nuclear grade; coagulative tumor necrosis; and sarcomatoid differentiation.11,12
Patient Outcome
Disease status for patients in the Mayo Clinic Nephrectomy Registry is updated each year. If a patient has not been seen at our institution within the previous year, the patient is sent a disease status questionnaire. If there is evidence of disease progression in this questionnaire, the date, location, and treatment are verified in writing with the patient’s local physician. Patient vital status is similarly updated on a yearly basis. If a patient has died within the previous year, a death certificate is ordered to determine the cause of death. A visit to our institution within 6 months of the date of death for metastatic RCC is good documentation that RCC was the cause of death. If the death certificate does not support this, the medical history is reviewed by a urologist to determine the cause of death. If a death certificate cannot be obtained, the cause of death must be verified with the patient’s family or local physician.
IMP3 Immunohistochemical Staining
Immunohistochemical studies were performed by the Department of Pathology at the University of Massachusetts on 5-µm sections of formalin-fixed, paraffin-embedded tissue as previously described.1 Antigen retrieval was performed with 0.01 mol/L of citrate buffer (pH 6.0) in an 800-watt microwave oven for 15 minutes before immunostaining. The slides were stained on the DAKO Autostainer (Dako Corporation, Carpinteria, Calif) using the EnVision (Dako) staining reagents. The sections were first blocked for endogenous protein binding and peroxidase activity with an application of Dual Endogenous Block (Dako) for 10 minutes, followed by a buffer wash. The sections were then incubated with a mouse monoclonal antibody specific for IMP3 (L523S, Corixa, Seattle, Wash) at a concentration of 2.0 µg/mL for 30 minutes, followed again by a buffer wash. Sections were then incubated with the EnVision + Dual Link reagent (a polymer conjugated with goat-antimouse immunoglobulin [Ig] and horse-radish peroxidase) for 30 minutes. The sections were then washed and treated with diaminobenzidine (DAB) and hydrogen peroxide, which reacted to observe the endproduct. A toning solution (DAB Enhancer, Dako) was used to enrich the final color. The sections were counterstained with hematoxylin, dehydrated, and coverslipped with permanent media. Sections of urothelial carcinoma with known positivity of IMP3 were used as positive controls for the L523S mouse monoclonal antibody (MoAb) specific for IMP3/KOC (Corixa) staining. Negative control sections were stained by replacing the primary antibody with nonimmune mouse IgG (Vector Laboratories, Burlingame, Calif) at 2.0 µg/mL.
IMP3 Quantitation
IMP3 tumor expression was evaluated visually by a Mayo Clinic pathologist (Y.S.) without knowledge of patient outcome. The proportions of tumor cells that demonstrated unequivocal cytoplasmic immunoreactivity for IMP3 were recorded as <30%, 30% to 60%, or >60%. A negative score was assigned to sections that had no evidence of specific staining. This is similar to the criteria used previously.1
Statistical Methods
Associations of IMP3 expression with clinical and pathologic features were evaluated using chi-square and Fisher exact tests. Kaplan-Meier curves were used to observe the association of IMP3 expression and patient outcome. To estimate the magnitude of the association between IMP3 expression and death from RCC, we employed Cox proportional hazards regression modeling in both a univariate setting and after multivariate adjustment for the Mayo Clinic SSIGN score, a composite scoring system developed specifically for patients with ccRCC that accounts for primary tumor classification, regional lymph node involvement, distant metastases at presentation, tumor size, nuclear grade, and coagulative tumor necrosis.13 Among the subset of patients with localized disease at presentation (ie, pNX/pN0; pM0; stage Groups I, II, or III), we evaluated the association between IMP3 expression and progression to distant metastases univariately and after adjusting for the Mayo Clinic metastases-free survival score.14 Associations with outcome were summarized with hazard ratios (HRs) and 95% confidence intervals (95% CIs). Statistical analyses were performed using the SAS software package (SAS Institute, Cary, NC). All tests were 2-sided and P values <.05 were considered statistically significant.
RESULTS
All Patients
At last follow-up 381 patients had died, including 225 patients who died from RCC at an average of 3.5 years after nephrectomy (median, 2.2 years; range, 0.2–14.1 years). Among the 335 patients who were still alive at last follow-up, the average duration of follow-up was 9.5 years (median, 9.3 years; range, 0.1–16.7 years); only 15 patients (4.5%) had <5 years of follow-up. Cancer-specific survival rates (standard error [SE], number still at risk) at 5 years and 10 years after nephrectomy were 75.4% (1.7%, 463) and 66.8% (2.0%, 185), respectively.
There were 503 ccRCC tumors (70.3%) with negative IMP3 expression, 84 (11.7%) with <30% IMP3-positive tumor cells, 58 (8.1%) with 30% to 60% positive tumor cells, and 71 (9.9%) with >60% IMP3-positive tumor cells (Fig. 1). For patients with IMP3-positive tumors, there was no statistically significant difference noted with regard to cancer-specific survival among those with <30%, 30% to 60%, and >60% IMP3-positive tumor cells (P = .719, log-rank test). Thus, for further analyses, the tumors were grouped into those that were IMP3-positive and IMP3-negative. Comparisons of clinical and pathologic features by IMP3 expression are summarized in Table 1. Positive IMP3 tumor expression was found to be significantly associated with symptoms at presentation, tumor thrombus, larger tumor size, advanced tumor stage and grade, coagulative tumor necrosis, and sarcomatoid differentiation.
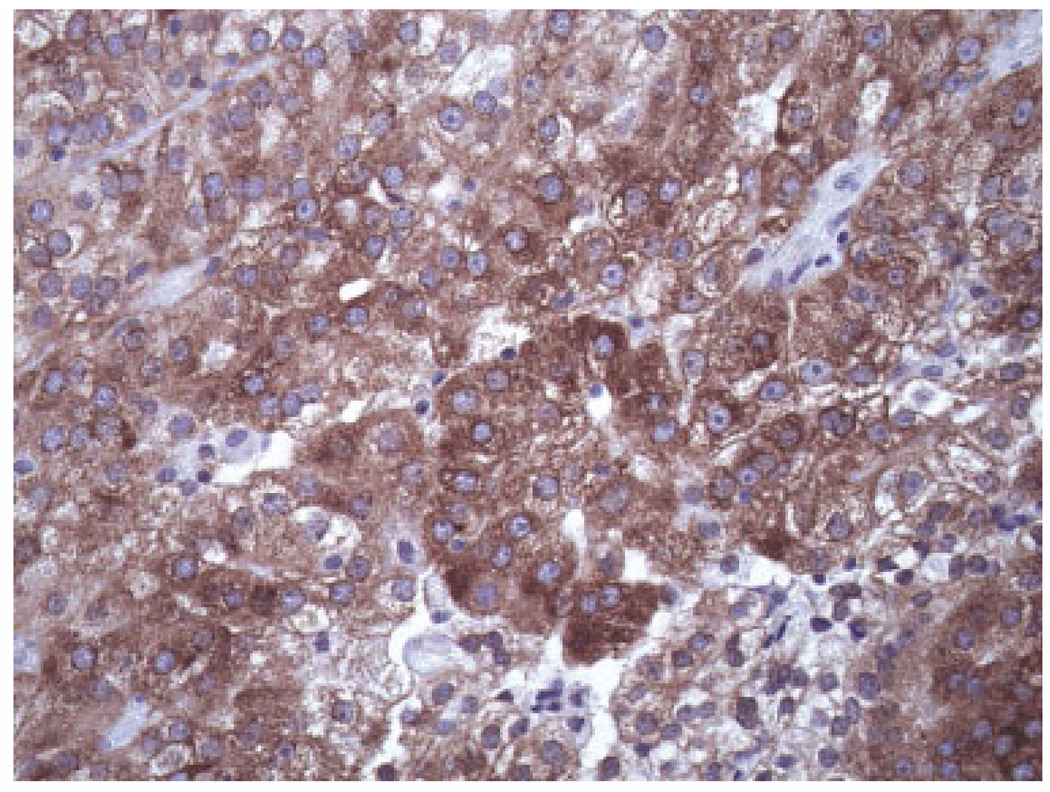
FIGURE 1.
Photomicrograph demonstrating high (>60%) IMP3 staining by clear cell renal cell carcinoma (×400 magnification).
TABLE 1.
Comparison of Clinical and Pathologic Features by IMP3 Tumor Expression for 716 Patients With ccRCC
| Tumor IMP3 expression |
|||
|---|---|---|---|
| Negative N = 503 Positive N = 213 |
|||
| Feature | No. (%) | P | |
| Age at surgery, y | |||
| <65 | 257 (51.1) | 112 (52.6) | .716 |
| ≥65 | 246 (48.9) | 101 (47.4) | |
| Sex | |||
| Female | 198 (39.4) | 62 (29.1) | .009 |
| Male | 305 (60.6) | 151 (70.9) | |
| Symptoms at presentation | |||
| No | 215 (42.7) | 48 (22.5) | <.001 |
| Yes | 288 (57.3) | 165 (77.5) | |
| Constitutional symptoms at presentation | |||
| No | 408 (81.1) | 132 (62.0) | <.001 |
| Yes | 95 (18.9) | 81 (38.0) | |
| ECOG performance status | |||
| 0 | 454 (90.3) | 196 (92.0) | .457 |
| ≥1 | 49 (9.7) | 17 (8.0) | |
| Tumor thrombus | |||
| None | 452 (89.9) | 131 (61.5) | <.001 |
| Level 0 | 33 (6.6) | 40 (18.8) | |
| Level I–IV | 18 (3.6) | 42 (19.7) | |
| Primary tumor size, cm | |||
| <5 | 215 (42.7) | 23 (10.8) | <.001 |
| 5 to <7 | 122 (24.3) | 36 (16.9) | |
| 7 to <10 | 90 (17.9) | 64 (30.1) | |
| ≥10 | 76 (15.1) | 90 (42.3) | |
| 2002 primary tumor classification | |||
| pT1a | 170 (33.8) | 11 (5.2) | <.001 |
| pT1b | 158 (31.4) | 40 (18.8) | |
| pT2 | 89 (17.7) | 55 (25.8) | |
| pT3a | 34 (6.8) | 24 (11.3) | |
| pT3b | 47 (9.3) | 71 (33.3) | |
| pT3c | 4 (0.8) | 7 (3.3) | |
| pT4 | 1 (0.2) | 5 (2.4) | |
| Regional lymph node involvement | |||
| pNX and pN0 | 494 (98.2) | 199 (93.4) | <.001 |
| pN1 and pN2 | 9 (1.8) | 14 (6.6) | |
| Distant metastases at presentation | |||
| pM0 | 473 (94.0) | 172 (80.8) | <.001 |
| pM1 | 30 (6.0) | 41 (19.2) | |
| 2002 TNM stage groupings | |||
| I | 317 (63.0) | 47 (22.1) | <.001 |
| II | 80 (15.9) | 39 (18.3) | |
| III | 71 (14.1) | 80 (37.6) | |
| IV | 35 (7.0) | 47 (22.1) | |
| Nuclear grade | |||
| 1 | 52 (10.3) | 0 | <.001 |
| 2 | 305 (60.6) | 28 (13.1) | |
| 3 | 138 (27.4) | 142 (66.7) | |
| 4 | 8 (1.6) | 43 (20.2) | |
| Coagulative tumor necrosis | |||
| No | 442 (87.9) | 78 (36.6) | <.001 |
| Yes | 61 (12.1) | 135 (63.4) | |
| Sarcomatoid differentiation | |||
| No | 498 (99.0) | 190 (89.2) | <.001 |
| Yes | 5 (1.0) | 23 (10.8) | |
ccRCC indicates clear cell renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group.
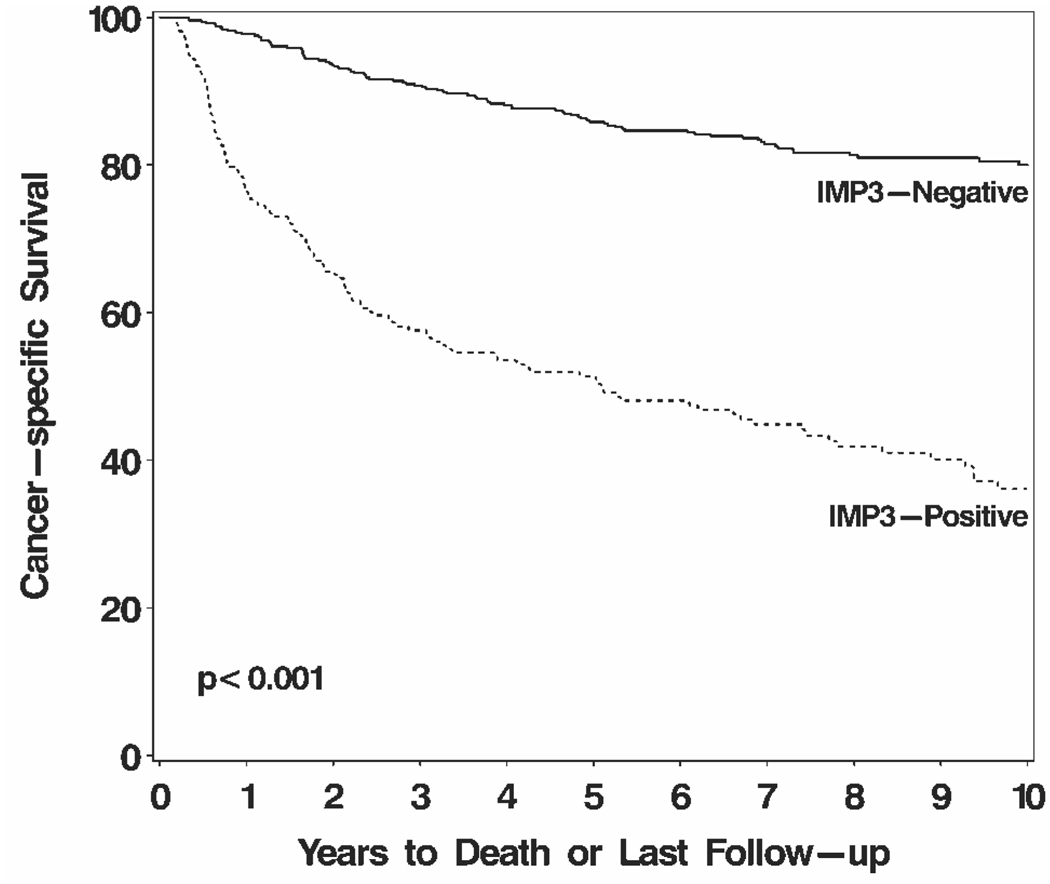
On univariate analysis, patients with IMP3-positive tumors were nearly 5 times more likely to die from RCC compared with patients with IMP3-negative tumors (HR, 4.60; 95% CI, 3.53–6.00 [P < .001]). Cancer-specific survival rates (SE, number still at risk) at 5 years and 10 years after nephrectomy were 51.4% (3.5%, 93) and 36.1% (3.8%, 33), respectively, for patients with IMP3-positive tumors compared with 85.8% (1.6%, 370) and 80.0% (2.0%, 152), respectively, for patients with IMP3-negative tumors (Fig. 2). After adjusting for the SSIGN score13 (median, 3; range, 0–15), this significant association between IMP3 expression and death from RCC persisted (HR, 1.42; 95% CI, 1.05–1.91 [P = .024]). The SSIGN score encompasses most of the clinical and pathologic features associated with poor prognosis from ccRCC. Two features, symptoms at presentation and sarcomatoid differentiation, have been previously shown to negatively impact prognosis and were also associated with IMP3 expression.13 Even after controlling for those 2 features along with the SSIGN score, IMP3 expression was still associated with a 41% increased risk of death from RCC (HR, 1.41; 95% CI, 1.04–1.91 [P = .026]).
FIGURE 2.
Association between IMP3 tumor expression and death from renal cell carcinoma (RCC) for 716 patients with clear cell RCC. Cancer-specific survival rates (standard error, number still at risk) at 5 years and 10 years after nephrectomy were 51.4% (3.5%, 93) and 36.1% (3.8%, 33), respectively, for patients with IMP3-positive tumors compared with 85.8% (1.6%, 370) and 80.0% (2.0%, 152), respectively, for patients with IMP3-negative tumors.
Patients With Localized Disease
Among the 629 patients with localized disease at the time of presentation (ie, pNX/pN0; pM0; TNM stage Groups I, II, or III), 161 progressed to distant metastases at an average of 3.2 years after nephrectomy (median, 1.9 years; range, 0.1–14.6 years). Distant metastases-free survival rates (SE, number still at risk) at 5 years and 10 years after nephrectomy were 79.2% (1.7%, 414) and 71.5% (2.1%, 165), respectively. There were 466 localized ccRCC tumors (74.1%) with negative IMP3 expression, 64 (10.2%) with <30% IMP3-positive tumor cells, 47 (7.5%) with 30% to 60% positive tumor cells, and 52 (8.3%) with >60% IMP3-positive tumor cells. Comparisons of clinical and pathologic features by IMP3 expression for this subset are summarized in Table 2.
TABLE 2.
Comparison of Clinical and Pathologic Features by IMP3 Tumor Expression for 629 Patients With Localized ccRCC
| Tumor IMP3 expression |
|||
|---|---|---|---|
| Negative N = 466 Positive N = 163 |
|||
| Feature | No. (%) | P | |
| Age at surgery, y | |||
| <65 | 234 (50.2) | 73 (44.8) | .233 |
| ≥65 | 232 (49.8) | 90 (55.2) | |
| Sex | |||
| Female | 185 (39.7) | 45 (27.6) | .006 |
| Male | 281 (60.3) | 118 (72.4) | |
| Symptoms at presentation | |||
| No | 202 (43.4) | 35 (21.5) | <.001 |
| Yes | 264 (56.6) | 128 (78.5) | |
| Constitutional symptoms at presentation | |||
| No | 384 (82.4) | 105 (64.4) | <.001 |
| Yes | 82 (17.6) | 58 (35.6) | |
| ECOG performance status | |||
| 0 | 419 (89.9) | 153 (93.9) | .130 |
| ≥1 | 47 (10.1) | 10 (6.1) | |
| Tumor thrombus | |||
| None | 424 (91.0) | 104 (63.8) | <.001 |
| Level 0 | 27 (5.8) | 28 (17.2) | |
| Level I–IV | 15 (3.2) | 31 (19.0) | |
| Primary tumor size, cm | |||
| <5 | 210 (45.1) | 20 (12.3) | <.001 |
| 5 to <7 | 118 (25.3) | 32 (19.6) | |
| 7 to <10 | 77 (16.5) | 51 (31.3) | |
| ≥10 | 61 (13.1) | 60 (36.8) | |
| 2002 primary tumor classification | |||
| pT1a | 169 (36.3) | 10 (6.1) | <.001 |
| pT1b | 148 (31.8) | 37 (22.7) | |
| pT2 | 80 (17.2) | 39 (23.9) | |
| pT3a | 27 (5.8) | 18 (11.0) | |
| pT3b | 39 (8.4) | 53 (32.5) | |
| pT3c | 3 (0.6) | 6 (3.7) | |
| 2002 TNM stage groupings | |||
| I | 317 (68.0) | 47 (28.8) | <.001 |
| II | 80 (17.2) | 39 (23.9) | |
| III | 69 (14.8) | 77 (47.2) | |
| Nuclear grade | |||
| 1 | 48 (10.3) | 0 | <.001 |
| 2 | 295 (63.3) | 27 (16.6) | |
| 3 | 118 (25.3) | 106 (65.0) | |
| 4 | 5 (1.1) | 30 (18.4) | |
| Coagulative tumor necrosis | |||
| No | 417 (89.5) | 66 (40.5) | <.001 |
| Yes | 49 (10.5) | 97 (59.5) | |
| Sarcomatoid differentiation | |||
| No | 463 (99.4) | 150 (92.0) | <.001 |
| Yes | 3 (0.6) | 13 (8.0) | |
ccRCC indicates clear cell renal cell carcinoma; ECOG, Eastern Cooperative Oncology Group.
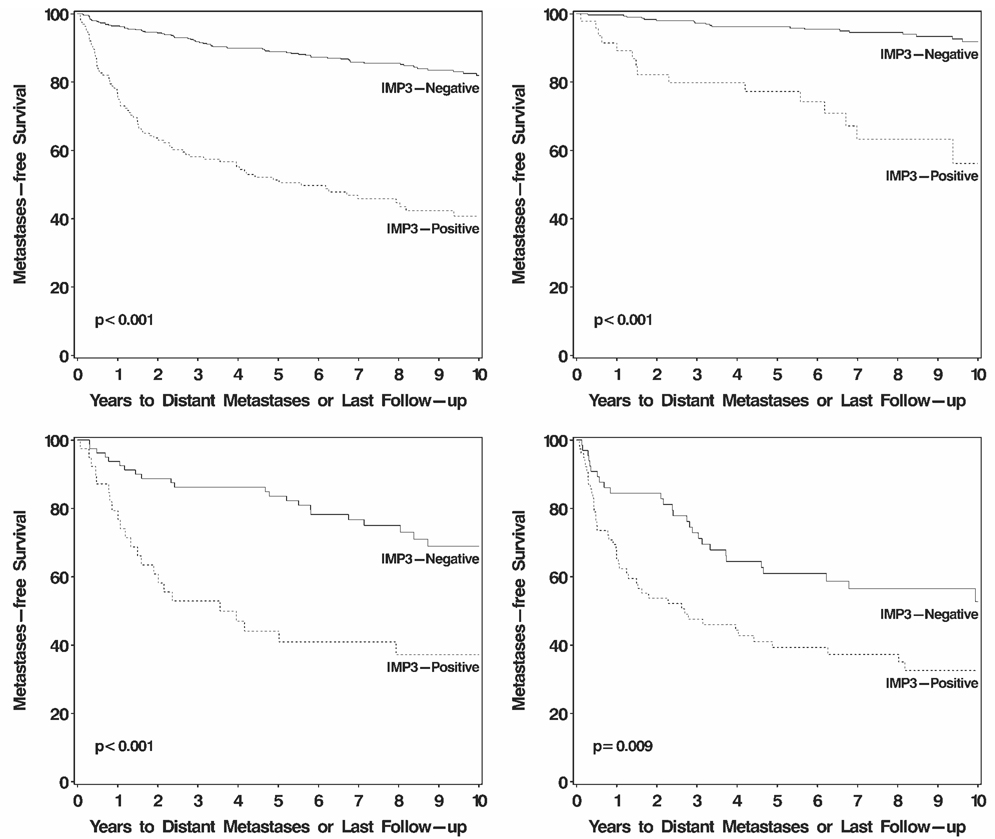
For patients with IMP3-positive tumors, there was no statistically significant difference in distant metastases-free survival among those with <30%, 30% to 60%, and >60% IMP3-positive tumor cells (P = .993, log-rank test). On univariate analysis, patients with IMP3-positive tumors were nearly 5 times more likely to progress to distant metastases compared with patients with IMP3-negative tumors (HR, 4.71; 95% CI, 3.44–6.43 [P < .001]). Distant metastases-free survival rates (SE, number still at risk) at 5 years and 10 years after nephrectomy were 51.4% (4.1%, 64) and 40.8% (4.5%, 23), respectively, for patients with IMP3-positive tumors compared with 88.9% (1.5%, 350) and 81.9% (2.1%, 142), respectively, for patients with IMP3-negative tumors (Fig. 3, top left). After adjusting for the metastases-free survival score14 (median, 3; range, 0–9), patients with IMP3-positive tumors were still 52% more likely to develop disease progression compared with patients with IMP3-negative tumors (HR, 1.52; 95% CI, 1.05–2.20 [P = .025]). This significant association persisted after adjusting for the metastases-free survival score, symptoms, and sarcomatoid differentiation (HR, 1.53; 95% CI, 1.06–2.21 [P = .025]).
FIGURE 3.
Association between IMP3 tumor expression and progression to distant metastases for 629 patients with localized clear cell renal cell carcinoma (top left). Distant metastases-free survival rates (standard error, number still at risk) at 10 years after nephrectomy were 40.8% (4.5%, 23) and 81.9% (2.1%, 142), respectively, for patients with IMP3-positive and IMP3-negative tumors. Comparing patients with IMP3-positive tumors with those with IMP3-negative tumors, the 10-year distant metastases-free survival rates were 56.2% (9.9%, 7) and 91.8% (1.9%, 105), respectively, for patients with stage I disease (top right); 37.2% (8.2%, 8) and 68.9% (5.7%, 23), respectively, for patients with stage II disease (bottom left); and 32.5% (6.0%, 23) and 52.7% (7.1%, 14), respectively, for patients with stage III disease (bottom right).
Among the subset of 364 patients with stage I ccRCC, positive IMP3 tumor expression was associated with an over 6-fold increased risk of progression to distant metastases (HR, 6.46; 95% CI, 3.33–12.53 [P < .001]) (Fig. 3, top right). This association remained statistically significant among the 119 stage II patients (HR, 3.04; 95% CI, 1.72–5.39 [P < .001]) (Fig. 3, bottom left) as well as among the 146 stage III patients (HR, 1.85; 95% CI, 1.16–2.96 [P =.009]) (Fig. 3, bottom right). We found similarly significant associations between positive IMP3 tumor expression and death from RCC among patients with stage I (HR, 2.17; 95% CI, 1.44–3.27 [P <.001]), stage II (HR, 2.02; 95% CI, 1.20–3.40 [P = .009]), and stage III (HR, 1.50; 95% CI, 1.00–2.23 [P = .048]) localized ccRCC.
DISCUSSION
In parallel with the ever-increasing number of adjunctive therapies available for treating ccRCC, there has been a rising demand for approaches to identify patients at increased risk for cancer progression after primary ccRCC tumor resection. Tumor cell IMP3 protein expression has previously been shown to represent a robust indicator for predicting metastases in patients treated for clinically localized RCC. Specifically, in a study of 371 patients with RCC, 54 of whom (14.6%) had IMP3-positive tumors, Jiang et al.1 reported that among patients with TNM stage I RCC, the 5-year metastases-free survival rate was 44% for patients with IMP3-positive tumors compared with 98% for patients with IMP3-negative tumors. Similar associations were demonstrated for patients presenting with stage II tumors (5-year metastases-free survival rates of 41% and 94% for IMP3-positive and IMP3-negative tumors, respectively) and stage III tumors (5-year metastases-free survival rates of 16% and 62% for IMP3-positive and IMP3-negative tumors, respectively).1
In the current study, 213 (30%) of 716 RCC tumors of the clear cell subtype were observed to express the IMP3 protein. Consistent with what was reported by Jiang et al.,1 the results of the current study demonstrated that IMP3 expression was significantly associated with advanced ccRCC stage and grade, increased regional lymph node involvement, and distant metastases, as well as an increased likelihood for coexistent coagulative tumor necrosis and sarcomatoid differentiation. In the current study, TNM stage I patients with IMP3-positive ccRCC tumors exhibited a 10-year metastases-free survival rate of 56.2% compared with 91.8% for patients with IMP3-negative tumors. Likewise, IMP3 expression was an indicator of decreased 10-year metastases-free survival for stage II patients (37.2% and 68.9% for IMP3-positive and IMP3-negative tumors, respectively) and stage III patients (32.5% and 52.7% for IMP3-positive and IMP3-negative tumors, respectively). In addition, among all 716 patients studied, we demonstrated that even after multivariate adjustment for ccRCC prognostic features comprising the SSIGN score,13 positive IMP3 expression was independently associated with an increased risk of death from RCC. Hence, our study confirms the findings of Jiang et al.1 and thereby provides external validation of tumor cell IMP3 expression as an independent predictor of aggressive ccRCC tumor behavior.
External validation has garnered increasing attention as more potential prognostic markers are identified, and as more of these markers have proven to fail in secondary studies and in the setting of clinical trial testing.15,16 Extrapolating from the prognostic model literature, it is reasonable to conclude that the more often a given marker or predictive algorithm is independently demonstrated to be prognostic, the more apt it is to secure widespread and meaningful utility in the clinical setting.10 IMP3 has now proven to be a strong predictor of metastases and cancer-specific death in 2 distinct studies, conducted using 2 independent cohorts of RCC patients that encompass a diverse range of disease and clinical management environments. Related to this, several rigorous guidelines (REMARK recommendations) pertaining to the study of prognostic instruments have recently been published in multiple journals to prevent relatively nonmeritorious biomarkers and prognostic algorithms from being introduced and then propagated within the scientific and medical literature.16 As such, it is interesting to note that our current study of IMP3 as a prognostic biomarker closely adheres to these guidelines.16
There are some limitations to the current study that warrant discussion. First, it would have been preferable to employ an independent laboratory to conduct the immunohistochemical staining of ccRCC specimens for the current study. At the time these studies were conducted, however, anti-IMP3 was not commercially available and was only accessible through our collaborator (Z.J.). Second, the cohort of patients that we report has been extensively studied for the purposes of elucidating other prognostic markers and, currently, the importance of IMP3 relative to these other prognostic markers remains unclear and is an area of future study. Nevertheless, we view external validation of IMP3 as a prognostic marker as a necessary first step before embarking on further investigations testing whether IMP3 continues to perform as an independent predictor of ccRCC outcome when adjusting for other markers we have previously reported (namely, B7-H1, B7-H4, and Ki-67). Finally, the current study does not readily reveal an obvious mechanism whereby IMP3 increases the aggressiveness of ccRCC, although some mechanisms might be inferred.
As noted earlier, the significance of IMP3 expression by ccRCC remains, for the most part, underexplored. IMP3 is a regulatory binding protein believed to be involved in the stabilization and intracellular trafficking of IGF-II mRNA to facilitate IGF-II production. It should be noted, however, that this is not the only purported function of IMP3 because IMP3 has also been implicated inhibiting degradation and modulating translation of other intracellular nucleotide sequences.17 In addition, to our knowledge, the role of IMP3 in IGF-II production is not completely established at the current time. Nevertheless, it does appear to be mechanistically plausible that abnormalities of the IGF cell signaling pathway may be somehow be associated with the emergence of various features of aggressive ccRCC. Further evidence supporting that other portions of the IGF cell signaling pathway, in general, may increase ccRCC aggressiveness has recently been provided by Parker et al.18 Specifically, this group examined IGF-IR as a potential prognostic and therapeutic moiety for ccRCC. In the studies by Parker et al.,18 tumor cell IGF-IR expression was surveyed in whole tissue specimens derived from 280 ccRCC patients. Expression of IGF-IR on the cell surface of ccRCC tumor cells was observed in 54% of the specimens studied and was reported to correlate with male sex, advanced tumor stage and grade, and a greater likelihood of distant metastases and coagulative tumor necrosis. Furthermore, Parker et al.18 reported that IGF-IR expression was associated with an increased risk of death from ccRCC. Hence, there appears to be some degree of overlap in the prognostic capabilities of IGF-IR and IMP3 expression as predictors of pathologic features and clinical outcomes associated with ccRCC. In addition, some case reports have shown that aggressive RCC tumors, especially those exhibiting sarcomatoid differentiation, express increased levels of IGF-II.19,20 Given that several key components of the IGF pathway are apparently expressed by aggressive forms of ccRCC, it is tempting to speculate that this pathway may contribute to the adverse clinicopathologic behavior of this malignancy.
However, not all studies support a role for IGF-II in mediating the pathobiology of ccRCC tumors. For instance, in 1 tissue microarray study involving 137 ccRCC specimens, Schips et al.21 observed that only 30% of ccRCC tumors expressed IGF-IR, and further reported that IGF-IR expression failed to correlate with tumor stage, grade, or mortality. In addition, none of the ccRCC tumors examined by this group were reported to exhibit immunohistochemical evidence of IGF-II protein expression. However, Schips et al.21 did report that 82% of ccRCC tumors studied expressed IGF-I at the protein level. Thus, a role for IGF-I in modulating ccRCC tumor behavior via the IGF pathway remains possible but still leaves in question the role, if any, of IGF-II. Without question, further systematic investigations of the IGF pathway will be required to more clearly elucidate how, and to what extent, this pathway regulates ccRCC pathobiology.
Conclusions
IMP3 expression is a predictor of metastatic progression and death from RCC, as is clearly supported by the current external validation study. Assessment of IMP3 expression may prove useful to identify ‘at risk’ patients who might benefit from aggressive adjunctive therapy after primary tumor resection. Ultimately, IMP3 and the IGF pathway may provide useful targets to improve ccRCC therapy; however, further studies will be warranted to more firmly establish this supposition.
Acknowledgments
Supported in part by generous gifts from The Richard M. Schulze Family Foundation and The Commonwealth Foundation for Cancer Research and very kind donations provided by The Helen and Martin Kimmel Foundation.
We thank Karen Dresser for assistance with immunohistochemical staining and Catherine Lehman for assistance with patient follow-up.
REFERENCES
- 1.Jiang Z, Chu PG, Woda BA, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 2006;7:556–564. doi: 10.1016/S1470-2045(06)70732-X. [DOI] [PubMed] [Google Scholar]
- 2.Bui MH, Visapaa H, Seligson D, et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171:2461–2466. doi: 10.1097/01.ju.0000116444.08690.e2. [DOI] [PubMed] [Google Scholar]
- 3.Krambeck AE, Dong H, Thompson RH, et al. Survivin and b7-h1 are collaborative predictors of survival and represent potential therapeutic targets for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1749–1756. doi: 10.1158/1078-0432.CCR-06-2129. [DOI] [PubMed] [Google Scholar]
- 4.Byun SS, Yeo WG, Lee SE, et al. Expression of survivin in renal cell carcinomas: association with pathologic features and clinical outcome. Urology. 2007;69:34–37. doi: 10.1016/j.urology.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Krambeck AE, Thompson RH, Dong H, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci U S A. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tollefson MK, Thompson RH, Sheinin Y, et al. KI-67 and coagulative tumor necrosis are independent predictors of poor outcome for patients with clear cell renal cell carcinoma and not surrogates for each other. Cancer. 2007;110:783–790. doi: 10.1002/cncr.22840. [DOI] [PubMed] [Google Scholar]
- 8.Altman DG, Royston P. What do we mean by validating a prognostic model? Stat Med. 2000;19:453–473. doi: 10.1002/(sici)1097-0258(20000229)19:4<453::aid-sim350>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 9.Bleeker SE, Moll HA, Steyerberg EW, et al. External validation is necessary in prediction research: a clinical example. J Clin Epidemiol. 2003;56:826–832. doi: 10.1016/s0895-4356(03)00207-5. [DOI] [PubMed] [Google Scholar]
- 10.Justice AC, Covinsky KE, Berlin JA. Assessing the generalizability of prognostic information. Ann Intern Med. 1999;130:515–524. doi: 10.7326/0003-4819-130-6-199903160-00016. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual. 6th ed. New York: Springer Press; 2002. [Google Scholar]
- 12.Kovacs G, Akhtar M, Beckwith BJ, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–133. doi: 10.1002/(SICI)1096-9896(199710)183:2<131::AID-PATH931>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 13.Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168:2395–2400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 14.Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–1671. doi: 10.1002/cncr.11234. [DOI] [PubMed] [Google Scholar]
- 15.Kyzas PA, Denaxa-Kyza D, Ioannidis JP. Quality of reporting of cancer prognostic marker studies: association with reported prognostic effect. J Natl Cancer Inst. 2007;99:236–243. doi: 10.1093/jnci/djk032. [DOI] [PubMed] [Google Scholar]
- 16.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–1184. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen FC, Nielsen J, Christiansen J. A family of IGF-II mRNA binding proteins (IMP) involved in RNA trafficking. Scand J Clin Lab Invest Suppl. 2001;234:93–99. [PubMed] [Google Scholar]
- 18.Parker A, Cheville JC, Lohse C, et al. Expression of insulin-like growth factor I receptor and survival in patients with clear cell renal cell carcinoma. J Urol. 2003;170:420–424. doi: 10.1097/01.ju.0000071474.70103.92. [DOI] [PubMed] [Google Scholar]
- 19.Holt RI, Teale JD, Jones JS, et al. Gene expression and serum levels of insulin-like growth factors (IGFs) and IGF-binding proteins in a case of non-islet cell tumour hypoglycaemia. Growth Horm IGF Res. 1998;8:447–454. doi: 10.1016/s1096-6374(98)80297-9. [DOI] [PubMed] [Google Scholar]
- 20.Fernando HS, Hawkyard SJ, Poon P, et al. Renal cell carcinoma with non-islet cell tumor hypoglycemia. Int J Urol. 2006;13:985–986. doi: 10.1111/j.1442-2042.2006.01452.x. [DOI] [PubMed] [Google Scholar]
- 21.Schips L, Zigeuner R, Ratschek M, et al. Analysis of insulin-like growth factors and insulin-like growth factor I receptor expression in renal cell carcinoma. Am J Clin Pathol. 2004;122:931–937. doi: 10.1309/G7PY-0RE7-T86H-HQYV. [DOI] [PubMed] [Google Scholar]