Abstract
Background
Albuminuria predicts cardiovascular risk, but its function as a marker of endothelial damage and atherosclerosis is uncertain, as is the complex relationship with hypertension and diabetes.
Objective
To determine if hypertension contributes to albuminuria in the absence of marked atherosclerosis, and whether high-normal blood pressure has consistent associations with albuminuria across levels of atherosclerosis and type 2 diabetes.
Methods
Cross-sectional associations of cardiovascular risk factors and albuminuria were examined in 4 groups of 10,113 middle-aged participants from the ARIC study: (1) type 2 diabetics with marked atherosclerosis, (2) type 2 diabetics without marked atherosclerosis, (3) non-diabetics with marked atherosclerosis, and (4) non-diabetics without marked atherosclerosis. Marked atherosclerosis was defined as high levels of carotid atherosclerosis or prevalent coronary heart disease (CHD).
Results
Hyperglycemia and hypertriglyceridemia were associated with albuminuria, but only among type 2 diabetics. In multivariate models, increasing blood pressure levels (but not albuminuria) were significantly associated (p-trend<0.001) with carotid atherosclerosis when stratified by prevalent CHD. Excluding individuals on hypertension medication, higher blood pressure category was associated with albuminuria in all groups (P-trend<0.05). Importantly, the association was strong for even high normal blood pressure among non-diabetics without marked atherosclerosis (OR 2.72, 95% CI: 1.62-4.58) and type 2 diabetics with marked atherosclerosis (OR 11.99, 95% CI: 1.33-108.18).
Conclusion
Blood pressure, even at high-normal levels, is associated with albuminuria. This association is consistent across categories of type 2 diabetes status and atherosclerosis. Though albuminuria may be a marker of generalized vascular damage and endothelial dysfunction, our results suggest that the effects of blood pressure on albuminuria are not solely mediated through generalized vascular damage, as represented by degree of atherosclerosis.
Keywords: blood pressure, nephropathy, diabetes, microalbuminuria, atherosclerosis, hypertension, community-based
Introduction
Microalbuminuria, the excretion of albumin in the urine above normal levels, is a significant predictor of cardiovascular events[14, 16, 21, 30, 43, 47] and renal disease progression [9, 33, 49]. The pathophysiologic mechanism behind the association of albuminuria (micro- and macroalbuminuria) and increased cardiovascular risk is not completely understood, though there is compelling evidence that albuminuria may be a marker of generalized vascular damage and endothelial dysfunction, which may promote atherosclerosis throughout the vascular tree[12, 25, 42, 51]. Diabetes [4] and hypertension [15, 23, 28] are well-established as the two primary risk factors for nephropathy, and also significantly increase the risk of atherosclerosis and endothelial dysfunction [8]. However, the complex relationship between blood pressure, albuminuria, and atherosclerosis is not understood. Studies have shown that the association between albuminuria and atherosclerosis may be partially explained by the effects of blood pressure[5, 36] and other cardiovascular risk factors[31]; however, others have demonstrated the relationship to be independent of these factors [19].
There is evidence that blood pressure, even non-hypertensive levels, is associated with microalbuminuria[24, 35]. Though it is known that increased blood pressure contributes to both albuminuria and atherosclerosis, no study has examined whether the association of blood pressure and albuminuria is different between individuals with and without marked atherosclerosis. Additionally, the pathophysiologic mechanisms behind albuminuria and atherosclerosis among type 2 diabetics and non-diabetics may differ significantly[5]. With these considerations in mind, we conducted a large cross-sectional study of albuminuria nested within a community-based middle-aged cohort of Caucasians and African Americans with and without type 2 diabetes who had measures of clinical and subclinical atherosclerosis. We examined the association of cardiovascular risk factors (with particular emphasis on hypertension) with albuminuria among participants grouped into four risk categories: 1) type 2 diabetics with marked atherosclerosis, 2) type 2 diabetics without marked atherosclerosis, 3) non-diabetics with marked atherosclerosis, and 4) non-diabetics without marked atherosclerosis. Marked atherosclerosis was defined as individuals with high levels of carotid atherosclerosis or prevalent CHD. We hypothesized that the prevalence of albuminuria would be higher among type 2 diabetics and among those with marked atherosclerosis; however, high normal blood pressure would predict albuminuria similarly individuals with and without marked atherosclerosis among both diabetics and non-diabetics.
Methods
Study Population
The study population consisted of 10,113 African Americans and Caucasians with and without type 2 diabetes and carotid atherosclerosis visualized by B-mode ultrasound in the Atheroslcerosis Risk in Communities (ARIC) Study. The ARIC study recruited adults aged 45 to 64 years at baseline in 1987 through 1989. Population samples were selected from 4 US communities: Forsyth County, NC; Jackson, MS; suburbs of Minneapolis, MN, and Washington Co., MD. Participants underwent four standardized examinations in field center clinics, scheduled approximately every three years[1]. At baseline, response rates were 37% of all those eligible in Jackson (all African-American) and 65-66% in the other three communities. However, previous studies have demonstrated that overall, the bias in prevalence estimates related to nonresponse was small (<5%) for most measured characteristics[45]. The current study is a cross-sectional examination of clinical characteristics from visit 4 (1996-1998), except for carotid atherosclerosis which was assessed at either visit 3 (1993-1995) or 4. The institutional review boards of each participating institution approved the study protocol.
Of the 11,625 African American and Caucasian participants who attended visit 4, a single untimed urine sample was collected from 11,482 participants (98.8%). We excluded those missing either urinary albumin or creatinine measurements (n=35) and individuals without a measurement of extracranial carotid atherosclerosis from visit 3 or 4 (n=370). We also excluded individuals without fasting glucose and insulin values (n=511), those missing lipid values (n=149) and serum creatinine (n=117), and those missing information on visit 4 prevalent CHD, diabetes, hypertension medication use, systolic blood pressure (SBP), diastolic blood pressure (DBP), anthropometric data, and cigarette smoking and alcohol use (n=187). Included (n=10113) and excluded individuals (n=1364) with albumin-to-creatinine measurements were similar in visit 4 mean age (62.8 years in each group) and proportion male (43.9% and 46.3%, respectively). However, excluded individuals were more likely to be African American (43.8% vs. 19.4%), type 2 diabetic (46.0% vs. 21.9%), hypertensive (60.7% vs. 45.9%), albuminuric (16.6% vs. 7.3%), and to have prevalent CHD (12.8% vs. 8.0%) compared to included participants.
Assessment of Clinical Characteristics
A standardized interview, clinical examination, and laboratory investigation collected demographic, anthropometric, and cardiovascular risk factor data for participants [37]. Collection of fasting blood samples and processing for serum creatinine and plasma lipids is described in detail elsewhere[37]. Triglyceride levels were loge transformed because of a skewed distribution. Two standardized blood pressure measurements were performed by trained ARIC technicians using a random zero sphygmomanometer and the average of the two was used. Hypertension was defined as a systolic blood pressure (SBP) ≥ 140 mmHg, a diastolic blood pressure (DBP) ≥ 90 mmHg, or the use of antihypertensive medication during the previous 2 weeks. Antihypertensive medications included medications specifically prescribed for blood pressure control or medications with antihypertensive effects based on medication codes. Use of hypertension medication consisted of either utilization of antihypertensive medications based on medication codes or self-reported use of hypertension lowering medication within the past two weeks. Blood pressure was also categorized according to guidelines of the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC6): 1) optimal (SBP<120mmHg and DBP <80mmHg), 2) normal (SBP 120-129mmHg and DBP 80-84mmHg), 3) high normal (SBP 130-139mmHg or DBP 85-89mmHg), 4) stage 1 hypertension (SBP 140-159 mmHg or DBP 90-99mmHg), 5) stage 2 hypertension (SBP 160-179mmHg or DBP 100-109mmHg), and 6) stage 3 hypertension (SBP≥180mmHg or DBP≥110mmHg) [2]. Type 2 diabetes was defined as a fasting glucose of ≥ 126 mg/dL, nonfasting glucose or oral glucose tolerance test 2-hour glucose of ≥ 200 mg/dL, or self-reported history or treatment of type 2 diabetes. Prevalent CHD was defined by history of CHD revascularization procedures, of myocardial infarction, or electrocardiogram evidence of MI at or before visit 4. Baseline glomerular filtration rate (GFR) was estimated from calibrated serum creatinine (SCr) using the simplified equation developed using data from the Modification of Diet in Renal Disease (MDRD) Study[3, 26] as follows:
Bilateral measurement of the combined intimal medial thickness (IMT) of the extracranial carotid arteries was assessed by using B-mode ultrasound and has been described elsewhere[22, 37, 46]. The ARIC study ultrasound methods use a scanning protocol common to field centers, with sonographers and readers receiving centralized training and certification, meeting standards of quality assessment. Intimal medial thickness was measured centrally along the far wall of six different segments: bilaterally in the common carotid artery, the carotid bifurcation, and the internal carotid arteries. An overall mean IMT was utilized, using a maximum likelihood technique when any carotid sites were not visualized[18]. Measurements were adjusted to account for method drift over the visits and systematic differences between readers. Of our study sample, carotid IMT was assessed only during visit 4 in 40.7% and only during visit 3 in 39.9% of participants. Of the 19.4% with carotid IMT measurements from both visits 3 and 4, the more recent measurement was used in our analyses. High levels of carotid atherosclerosis were defined as an increased IMT belonging to the highest quartile[31] and were determined using race, sex, and visit-specific 75th percentile cut points for mean IMT (visit 3: 912μm in white men, 790μm in white women, 865μm in black men, 816μm in black women; visit 4: 962μm in white men, 828μm in white women, 903μm in black men, 834μm in black women). In order to attempt to capture both subclinical and clinical atherosclerosis, individuals were defined as having marked atherosclerosis (n=2902) if they had high levels of carotid atherosclerosis (n=2413) or prevalent CHD (n=810), respectively. Participants with neither high levels of carotid atherosclerosis nor prevalent CHD were classified as not having marked atherosclerosis (n=7211). N=1803 persons without prevalent CHD underwent carotid ultrasound at both visits 3 and 4; within this group, of the 1439 classified as without carotid atherosclerosis in visit 3, N=196 (13.6%) developed de novo carotid atherosclerosis by visit 4. Within the group of 7211 persons without marked atherosclerosis per our definition, 2890 (40.1%) only underwent carotid ultrasound in visit 3; based on the above estimate for developing de novo carotid atherosclerosis between visits 3 and 4, we estimate that approximately 393 (3.9% of the total study population) may be misclassified as not having marked atherosclerosis. We performed sensitivity analysis limiting the sample to those who underwent carotid ultrasound at the most recent visit only (visit 4, n=6083) with similar results (results not shown), demonstrating the robustness of our results despite this potential limitation.
Ascertainment of Albuminuria
An untimed urine sample was collected during the visit 4 clinical examination. Aliquots were frozen within 12 hours and stored at -70°C. Albumin and creatinine levels were measured in the University of Minnesota Physicians Outreach Laboratories, Minneapolis, Minnesota, with albumin by a nephelometric method either on the Dade Behring BN100 (assay sensitivity, 2.0 mg/L) or on the Beckman Immage Nephelometer, and creatinine using the Jaffe method in order to determine the albumin-to-creatinine ratio (ACR; ug/mg) for participants. Blinded samples (n=516) analyzed for quality assurance showed a correlation coefficient (r) of the loge-transformed ACR as r=0.95. The ACR was used to determine levels of albuminuria according to American Diabetes Association [34] and National Kidney Foundation [17] recommendations: normoalbuminuria is ACR<30μg/mg, microalbuminuria is ACR 30-299μg/mg, and macroalbuminuria is ACR≥300μg/mg. Albuminuria cases were defined as participants with either micro- or macroalbuminuria.
Statistical Analysis
Statistical analyses were performed using STATA, version 9 (Stata, College Station, TX). To better differentiate the effects of blood pressure from type 2 diabetes and carotid atherosclerosis, analyses were performed on the study sample stratified into four groups: type 2 diabetics with marked atherosclerosis, type 2 diabetics without marked atherosclerosis, non-diabetics with marked atherosclerosis, and non-diabetics without marked atherosclerosis. In each group, tests of differences in clinical characteristics by albuminuria case status were performed using t-tests, ANOVA, and χ2 tests. P values <0.05 were considered statistically significant.
Multivariate logistic regression was used to calculate the odds ratio (OR) and 95% confidence interval (95% CI) of albuminuria for each JNC6 blood pressure category compared to optimal blood pressure (SBP<120mmHg and DBP<80mmHg), adjusted for kidney disease risk factors. Covariates included age, gender, race, hypertension medication use, prevalent CHD (only among those with atherosclerosis), body mass index (BMI), fasting glucose, fasting insulin, loge triglycerides, HDL cholesterol, current smoking, and GFR categories. GFR was categorized into four categories of renal function[27] (GFR mL/min/1.73m2): high normal (≥120), low normal (90-119), mildly decreased (60-89), and moderately/severely decreased (<60). Additionally, we dichotomized GFR into those with mildly decreased or worse renal function (GFR<90) or at least normal renal function (GFR≥90) when we performed subset analyses of the 4 risk groups among those without hypertension medication. We additionally examined the unadjusted and adjusted associations of various risk factors with carotid atherosclerosis (upper quartile of carotid IMT) as an outcome, stratified by prevalent CHD. In our primary analyses, to examine the association of JNC6 blood pressure categories with albuminuria independent of the effects of hypertension management, we performed multivariate logistic regression in each of the risk groups, excluding individuals using hypertension medication and adjusting for the above covariates, as described. To examine continuous measures of blood pressure and albuminuria among those without hypertension medication use, we also separately analyzed SBP, DBP, pulse pressure (PP; defined as SBP - DBP), and mean arterial pressure (MAP; defined as DBP + (SBP - DBP)/3) as predictors of albuminuria. The prevalence of albuminuria by JNC6 blood pressure category was also determined in each risk group, excluding individuals using hypertension medication and adjusting for covariates mentioned previously. In all individuals without hypertension medication use, we also examined interactions between BP category and 1) type 2 diabetes and 2) marked atherosclerosis by the likelihood ratio test (LRT).
Results
Distribution of ACR by Risk Categories
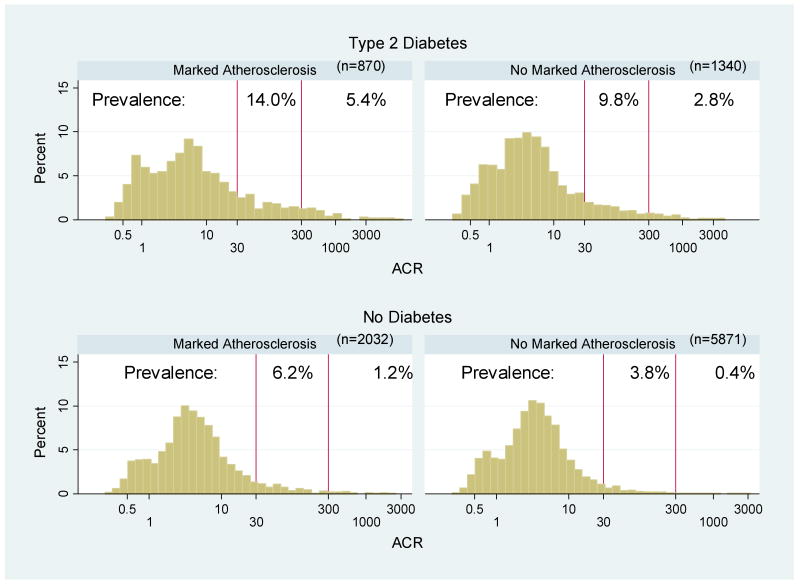
The distribution of measurements of ACR, categorized by the presence or absence of type 2 diabetes and/or marked atherosclerosis, is illustrated in Figure 1. Among the entire study population (n=10,113), the prevalence of albuminuria was 7.3% (microalbuminuria was 6.0% and macroalbuminuria was 1.3%). Among those with type 2 diabetes, the prevalence of albuminuria among those with marked atherosclerosis (n=870) was 19.4% and among those without marked atherosclerosis (n=1340) was 12.6%. Compared to type 2 diabetics, the prevalence among non-diabetics was lower. The prevalence of albuminuria among non-diabetics with (n=2032) and without marked atherosclerosis (n=5871) was 7.4% and 4.2%, respectively. By race, the prevalence of albuminuria among all African-Americans (N=1961) was 11.5% and among all whites (N=8152) was 6.3%. Among non-diabetics (N=7903), albuminuria prevalence was 7.9% and 4.4% for African-Americans and whites, respectively. Similarly, among diabetics (N=2210), there was a greater prevalence of albuminuria among African-Americans (20.5%) than among whites (13.6%).
Figure 1.
Distribution of Albumin-to-Creatinine Ratio (ACR) by Presence of Marked Atherosclerosis and Type 2 Diabetes. Marked therosclerosis was defined as prevalent CHD or the upper quartile of the carotid IMT distribution (race, gender, and visit-specific). The distribution of ACR values was graphed on the natural log scale. Vertical lines correspond to cut-offs for microalbuminuria (30μg/mg) and macroalbuminuria (300μg/mg). Prevalence (%) of microalbuminuria and macroalbuminuria within each risk category is noted.
Clinical Characteristics
Table 1 describes the clinical characteristics associated with albuminuria, categorized by the presence or absence of type 2 diabetes and/or marked atherosclerosis. In each risk group, albuminuria was associated with a worse risk factor profile. Those with albuminuria had a higher prevalence of hypertension medication use, greater SBP and DBP, and worse renal function as estimated by GFR (P<0.05 in all risk categories). Those with albuminuria also had higher fasting glucose and insulin levels, except among marked atherosclerotic non-diabetics, a group in which those with albuminuria had lower BMI compared to individuals with normoalbuminuria. Among those with diabetes, albuminuric participants with marked atherosclerosis were more likely male and to be current smokers while albuminuric participants without marked atherosclerosis were more likely African American. Interestingly, lipid levels were comparable by case status in most risk groups, except albuminuria cases among marked atherosclerotic type 2 diabetics had higher triglyceride levels and lower LDL cholesterol. Among all non-diabetics, cases were older, more likely to be African American, and to be current smokers (P<0.05 among those with and without marked atherosclerosis). Among all those with marked atherosclerosis, cases had a higher prevalence of CHD among both type 2 diabetics and non-diabetics.
Table 1. Clinical characteristics of ARIC participants by Albuminuria and Presence of Type 2 Diabetes and Atherosclerosis.
| Characteristic | Type 2 Diabetes | No Diabetes | ||||||
|---|---|---|---|---|---|---|---|---|
| Marked Atherosclerosis | No Marked Atherosclerosis | Marked Atherosclerosis | No Marked Atherosclerosis | |||||
| No. | No Albuminuria 701 |
Albuminuria 169 |
No Albuminuria 1171 |
Albuminuria 169 |
No Albuminuria 1881 |
Albuminuria 151 |
No Albuminuria 5623 |
Albuminuria 248 |
| Demographic | ||||||||
| Age, mean(SD),y | 65.0 (5.5) | 65.8 (5.0) | 62.8 (5.5) | 62.7 (5.9) | 64.6 (5.5) | 66.2 (5.3)§§§ | 61.8 (5.5) | 63.2 (5.8) ‡‡‡ |
| Male, No. (%) | 330 (47.1) | 99 (58.6) †† | 498 (42.5) | 78 (46.2) | 892 (47.4) | 80 (53.0) | 2361 (42.0) | 97 (39.1) |
| African American, No. (%) | 153 (21.8) | 40 (23.7) | 285 (24.3) | 73 (43.2)*** | 322 (17.1) | 36 (23.8) § | 976 (17.4) | 76 (30.7) ‡‡‡ |
| Prevalent Disease | ||||||||
| Hypertension Medication Use, No. (%) | 468 (66.8) | 139 (82.3) ††† | 616 (52.6) | 124 (73.4)*** | 934 (49.7) | 114 (75.5) §§§ | 1690 (30.1) | 126 (50.8) ‡‡‡ |
| Prevalent CHD, No. (%) | 202 (28.8) | 79 (46.8) ††† | 472 (25.1) | 57 (37.8) §§ | ||||
| Clinical Characteristics | ||||||||
| SBP (mmHg) | 132.1 (19.4) | 139.8 (20.3) ††† | 129.7 (17.5) | 139.5 (21.1) *** | 128.9 (18.9) | 141.1 (25.1) §§§ | 123.6 (17.3) | 137.4 (23.5) ‡‡‡ |
| DBP (mmgHg) | 68.5 (10.6) | 71.3 (12.1) †† | 71.0 (10.0) | 74.2 (11.7) *** | 70.0 (10.5) | 73.0 (13.4) §§ | 70.9 (9.7) | 76.0 (12.5) ‡‡‡ |
| Height (cm) | 166.8 (9.1) | 168.2 (9.0) | 167.5 (9.4) | 168.2 (10.9) | 167.7 (9.4) | 167.4 (9.1) | 167.8 (9.4) | 166.2 (9.2) ‡ |
| Diabetes-Related Characteristics | ||||||||
| Body mass index, kg/m2 | 30.7 (5.7) | 31.4 (6.0) | 30.6 (5.8) | 31.5 (6.3) | 28.2 (5.2) | 26.9 (5.2) §§ | 27.9 (5.2) | 27.7 (5.8) |
| Fasting Glucose (mg/dL) | 139.7 (44.6) | 153.4 (54.5) ††† | 137.0 (49.6) | 156.3 (64.0) *** | 98.8 (8.8) | 99.0 (9.9) | 97.7 (8.7) | 99.1 (9.1)‡ |
| Insulin (μU/mL) | 17.8 (14.2) | 22.0 (17.3) †† | 16.3 (10.7) | 21.9 (24.8) *** | 11.6 (9.4) | 11.5 (6.8) | 10.9 (6.6) | 11.9 (7.5)‡ |
| Lipid Profiles | ||||||||
| Total Cholesterol | 200.1 (39.5) | 196.6 (36.5) | 198.4 (36.1) | 200.1 (42.1) | 202.2 (36.5) | 200.4 (39.9) | 201.0 (35.7) | 200.3 (35.5) |
| Triglycerides mg/dL, median (25, 75%ile) | 145 (107, 201) | 165 (122, 241) †† | 139 (99, 199) | 135 (101, 200) | 123 (91, 169) | 116 (83, 161) | 113 (83, 159) | 114 (87, 161) |
| HDL Cholesterol | 44.0 (13.5) | 42.6 (14.6) | 46.4 (14.5) | 46.5 (15.8) | 49.0 (15.2) | 51.1 (20.3) | 52.7 (17.1) | 52.2 (17.2) |
| LDL Cholesterol | 124.0 (35.1) | 117.9 (32.0) † | 121.2 (32.4) | 121.8 (38.8) | 125.8 (33.6) | 123.6 (37.7) | 122.5 (33.0) | 121.8 (32.0) |
| Renal Function | ||||||||
| GFR (mL/min/1.73m2) | 81.9 (19.5) | 74.8 (26.0) ††† | 85.5 (17.9) | 81.2 (26.0)** | 81.7 (17.8) | 72.2 (23.7) §§§ | 82.4 (16.3) | 79.9 (22.3) ‡ |
| Serum Creatinine (mg/dL) | 0.9 (0.2) | 1.2 (1.1) ††† | 0.9 (0.2) | 1.1 (0.8) *** | 0.9 (0.2) | 1.2 (0.6) §§§ | 0.9 (0.2) | 1.0 (0.5) ‡‡‡ |
| Urinary Albumin (mg/L) | 5.9 (8.1) | 460.2 (1018.3) ††† | 5.0 (7.7) | 272.1 (516.1) *** | 4.5 (6.7) | 172.8 (311.8) §§§ | 3.5 (5.0) | 144.7 (311.3) ‡‡‡ |
| Urinary Creatinine (mg/dL) | 108.0 (65.6) | 109.1 (61.6) | 104.2 (67.2) | 100.5 (57.9) | 97.1 (65.9) | 90.4 (58.1) | 92.1 (67.0) | 92.1 (65.7) |
| Lifestyle Characteristics | ||||||||
| Current Smoking, N(%) | 104 (14.8) | 33 (19.5) | 128 (10.9) | 31 (18.3)** | 328 (17.4) | 50 (33.1) §§§ | 764 (13.6) | 55 (22.2) ‡‡‡ |
Data are means (SD) or number (%).
Comparing Characteristics By Albuminuria Status:
Type 2 Diabetes, Atherosclerosis: †P<0.05; ††P<0.01; †††P<0.001
Type 2 Diabetes, No Atherosclerosis: * P< 0.05; **P<0.01; ***P<0.001
No Diabetes, Atherosclerosis: § P< 0.05; §§P<0.01; §§§P<0.001
No Diabetes, No Atherosclerosis: ‡P< 0.05; ‡‡P<0.01; ‡‡‡P<0.001
Multivariate Analyses
Multivariate logistic regression results for albuminuria are listed in table 2, adjusting for all listed covariates. Demographic characteristics were not consistently associated with albuminuria across risk groups. Age was not independently associated with albuminuria. Among type 2 diabetics with and without marked atherosclerosis, male gender was significantly associated with albuminuria, though it was not associated among non-diabetics. African-American race was associated with albuminuria, but only among those without marked atherosclerosis. In terms of lifestyle characteristics, current smoking was significantly associated with albuminuria across all risk groups.
Table 2. Multivariate Odds Ratios (ORs) with 95% Confidence Intervals (CIs) for Albuminuria by Type 2 Diabetes Status and Presence of Atherosclerosis, Adjusted for All Variables in the Table.
| Type 2 Diabetes | No Diabetes | |||
|---|---|---|---|---|
| Marked Atherosclerosis 870 |
No Marked Atherosclerosis 1340 |
Marked Atherosclerosis 2032 |
No Marked Atherosclerosis 5871 |
|
| Risk Factor | ||||
| Demographic | ||||
| Age (per 5-y increase) | 1.14 (0.94 - 1.38) | 0.95 (0.80 - 1.13) | 1.11 (0.93 - 1.34) | 1.10 (0.97 - 1.25) |
| Male (v female) | 1.89 (1.26 - 2.85)** | 1.61 (1.08 - 2.40)* | 1.30 (0.84 - 1.99) | 0.81 (0.59 - 1.10) |
| African American (v white) | 1.16 (0.72 - 1.89) | 1.98 (1.30 - 3.01)** | 1.19 (0.75 - 1.91) | 1.69 (1.22 - 2.35)** |
| Prevalent Disease | ||||
| Prevalent CHD (yes v no) | 1.91 (1.28 - 2.84)** | 1.72 (1.14 - 2.57)** | ||
| Clinical Characteristics | ||||
| Hypertension Medication Use (yes v no) | 1.54 (0.95 - 2.47) | 1.70 (1.14 - 2.54)** | 2.33 (1.53 - 3.54)*** | 1.60 (1.21 - 2.12)** |
| JNC6 Blood Pressure (mmHg) Categories | ||||
| Optimal (reference) SBP<120 & DBP<80 |
1.00 | 1.00 | 1.00 | 1.00 |
| Normal SBP 120-129 and DBP 80-84 |
0.97 (0.52 - 1.83) | 1.22 (0.71 - 2.11) | 1.57 (0.87 - 2.83) | 1.36 (0.89 - 2.08) |
| High Normal SBP 130-139 or DBP 85-89 |
1.58 (0.87 - 2.89) | 0.80 (0.43 - 1.47) | 1.87 (1.01 - 3.46)* | 2.71 (1.82 - 4.03)*** |
| Stage 1 Hypertension SBP 140-159 or DBP 90-99 |
2.53 (1.47 - 4.35)** | 1.96 (1.17 - 3.29)* | 3.07 (1.77 - 5.31)*** | 2.42 (1.60 - 3.64)*** |
| Stage 2 Hypertension SBP 160-179 or DBP 100-109 |
2.93 (1.46 - 5.90)** | 4.40 (2.30 - 8.40)*** | 4.71 (2.46 - 9.03)*** | 6.79 (4.17 - 11.07)*** |
| Stage 3 Hypertension SBP >=180 or DBP >=110 |
2.34 (0.65 - 8.48) | 5.33 (1.80 - 15.78)** | 7.40 (2.75 - 19.90)*** | 29.31 (12.57 - 68.31)*** |
| Diabetes-Related Characteristics | ||||
| Body mass index, (per 1-unit increase, kg/m2) | 1.02 (0.99 - 1.06) | 1.01 (0.97 - 1.04) | 0.96 (0.92 - 1.00)* | 0.95 (0.93 - 0.98)** |
| Fasting Glucose (per 10 mg/dL increase) | 1.06 (1.02 - 1.10)** | 1.05 (1.02 - 1.09)** | 1.07 (0.86 - 1.31) | 1.12 (0.95 - 1.31) |
| Insulin (per 10 μU/mL increase) | 1.11 (1.00 - 1.24) | 1.22 (1.09 - 1.38)** | 1.03 (0.84 - 1.27) | 1.17 (0.95 - 1.43) |
| Lipid Profiles | ||||
| Loge (Triglycerides, mg/dL) (per unit increase) | 1.72 (1.05 - 2.81)* | 1.46 (0.96 - 2.22) | 0.81 (0.51 - 1.29) | 1.09 (0.78 - 1.52) |
| HDL Cholesterol (per 10 mg/dL increase) | 1.12 (0.96 - 1.31) | 1.13 (0.98 - 1.31) | 1.09 (0.96 - 1.24) | 0.96 (0.87 - 1.06) |
| GFR (mL/min/1.73m2) | ||||
| High Normal (≥120) | 1.22 (0.47 - 3.17) | 1.55 (0.70 - 3.45) | 1.12 (0.40 - 3.14) | 0.75 (0.29 - 1.97) |
| Normal (90 - 119, reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| Mildly Decreased (60 - 89) | 0.73 (0.47 - 1.15) | 1.22 (0.81 - 1.84) | 0.93 (0.59 - 1.44) | 0.80 (0.60 - 1.09) |
| Moderately/Severly Decreased (<60) | 2.29 (1.34 - 3.91)** | 6.19 (3.39 - 11.28)*** | 3.81 (2.27 - 6.41)*** | 2.46 (1.59 - 3.80)*** |
| Lifestyle Characteristics | ||||
| Current Smoking (yes v no) | 2.19 (1.33 - 3.60)** | 2.43 (1.48 - 3.99)*** | 3.22 (2.11 - 4.92)*** | 1.92 (1.37 - 2.68)*** |
P< 0.05;
P<0.01;
P<0.001
When examining prevalent disease, among those with marked atherosclerosis, prevalent CHD was associated with albuminuria to a similar degree among those with type 2 diabetes (OR 1.91, 95% CI: 1.28 - 2.84) and non-diabetics (OR 1.72, 95% CI: 1.14 - 2.57). Use of hypertension medication was independently associated with albuminuria in a statistically significant fashion except among type 2 diabetics with marked atherosclerosis. Blood pressure was strongly associated with albuminuria in all groups. Interestingly, the association appeared stronger among individuals without marked atherosclerosis compared to those with marked atherosclerosis. This was true among both diabetics and non-diabetics. Similarly, across comparable atherosclerosis categories, the association of blood pressure with albuminuria also appeared stronger among non-diabetics compared to diabetics.
The associations of albuminuria with metabolic and lipid characteristics were interesting, particularly when compared between type 2 diabetes strata (table 2). BMI was not associated with albuminuria among type 2 diabetics; however, among non-diabetics a weak inverse association emerged in a model adjusting for all other risk factors. Fasting glucose was significantly associated among all type 2 diabetics, while insulin was only associated among type 2 diabetics without marked atherosclerosis. Neither fasting glucose nor insulin was associated among non-diabetics. The association of triglycerides and albuminuria were also strikingly different by type 2 diabetes status, though not significantly different by atherosclerosis status. Triglycerides predicted albuminuria among type 2 diabetics with marked atherosclerosis (OR 1.72, 95% CI: 1.05 - 2.81). Among type 2 diabetics without marked atherosclerosis, the association of triglycerides and albuminuria was similar in magnitude but not significant (OR 1.46, 95% CI: 0.96 - 2.22). Among non-diabetics, there was no association of triglycerides with albuminuria among those without marked atherosclerosis (OR 1.09, 95% CI: 0.78 - 1.52) and with marked atherosclerosis (OR 0.81, 95% CI: 0.51 - 1.29). Glucose and triglycerides, components of the metabolic syndrome, are associated with albuminuria only among type 2 diabetics.
Decreased kidney function was also independently associated with albuminuria in all four risk groups (table 2). Compared to normal kidney function, moderate-severely decreased renal function (GFR<60 mL/min/1.73m2) was strongly associated with albuminuria. Mildly decreased renal function (GFR 60-89 mL/min/1.73m2) was not associated with albuminuria in any risk group.
We attempted to further explore the relationship between prevalent CHD, carotid atherosclerosis, and albuminuria. In our study there is a strong association between prevalent CHD and carotid atherosclerosis, with an unadjusted odds ratio of 2.26 (95% CI: 1.95 - 2.63) for marked carotid atherosclerosis (upper quartile of carotid IMT) with prevalent CHD as the outcome; the multivariate model was also statistically significant (OR 1.69, 95% CI: 1.43 - 1.99). There was a similar association of albuminuria with CHD, with an unadjusted OR of 2.92 (95% CI: 2.39 - 3.58) and an adjusted OR of 1.77 (95% CI: 1.39 - 2.24). In table 3, to better examine the association between carotid atherosclerosis as an outcome and other risk factors such as albuminuria, blood pressure, and lipid levels, we performed unadjusted and multivariate logistic regression on carotid atherosclerosis, stratified by prevalent CHD. Among those with and without prevalent CHD, univariate and multivariate analyses demonstrated statistically significant associations of carotid atherosclerosis with age (p<0.001). Current smoking and lipid profiles were significantly associated in both unadjusted and adjusted analyses with carotid atherosclerosis only among those without CHD; among those with prevalent CHD, only triglyceride level had an unadjusted association with carotid atherosclerosis. Diabetes-related characteristics and poor renal function only had an association with carotid atherosclerosis among those without CHD in univariate analysis; they were no longer significant after adjustment for other risk factors, though GFR<60 had a slightly increased point estimate (OR 1.16, 95% CI: 0.95 - 1.41). Among those without CHD, type 2 diabetes was significantly associated with carotid atherosclerosis, even after adjustment for other risk factors (OR 1.24, 95% CI: 1.07 - 1.43); interestingly, among those with prevalent CHD, type 2 diabetes had an increased point estimate in univariate analysis (OR 1.27) but had no association after adjustment for other major risk factors (OR 0.96). Among those with prevalent CHD, albuminuria had an increased (though non-significant) unadjusted association (OR 1.20) with carotid atherosclerosis which was no longer present after further adjustment for confounding risk factors. This relationship between albuminuria and carotid atherosclerosis was similarly seen among those without prevalent CHD, with a statistically significant (p<0.001) unadjusted association of OR 1.57 (95% CI: 1.31 - 1.88), which also became a null association after adjustment for confounders (OR 0.96). The increased association between blood pressure and carotid atherosclerosis was more robust and was consistently demonstrated in univariate and multivariate analyses (p-trend<0.001), among those with and without CHD.
Table 3. Univariate and Multivariate Odds Ratios (ORs) with 95% Confidence Intervals (CIs) for Marked Carotid Atherosclerosis (Upper quartile of Carotid IMT) by Prevalent CHD.
| Prevalent CHD (N=810) |
No CHD (N=9303) |
|||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| Risk Factor | ||||
| Albuminuria (yes v no) | 1.20 (0.83 - 1.75) | 0.83 (0.54 - 1.27) | 1.57 (1.31 - 1.88)*** | 0.96 (0.78 - 1.17) |
| Demographic | ||||
| Age (per 5-y increase) | 1.42 (1.24 - 1.62)*** | 1.38 (1.19 - 1.60)*** | 1.60 (1.53 - 1.67)*** | 1.55 (1.48 - 1.63)*** |
| Male (v female) | 0.79 (0.58 - 1.06) | 0.69 (0.48 - 1.01) | 0.94 (0.85 - 1.04) | 0.83 (0.74 - 0.93)** |
| African American (v white) | 0.68 (0.45 - 1.01) | 0.63 (0.39 - 1.00)* | 1.03 (0.91 - 1.17) | 0.97 (0.84 - 1.12) |
| Prevalent Disease | ||||
| Type 2 Diabetes (yes v no) | 1.27 (0.95 - 1.71) | 0.96 (0.65 - 1.44) | 1.72 (1.53 - 1.92)*** | 1.24 (1.07 - 1.43)** |
| Clinical Characteristics | ||||
| Hypertension Medication Use (yes v no) | 1.88 (1.33 - 2.67)*** | 1.59 (1.08 - 2.33)* | 1.79 (1.62 - 1.98)*** | 1.36 (1.22 - 1.52)*** |
| JNC6 Blood Pressure (mmHg) Categories | ||||
| Optimal (reference) SBP<120 & DBP<80 |
1.00 | 1.00 | 1.00 | 1.00 |
| Normal SBP 120-129 and DBP 80-84 |
1.13 (0.77 - 1.66) | 1.13 (0.76 - 1.70) | 1.45 (1.26 - 1.66)*** | 1.27 (1.10 - 1.47)** |
| High Normal SBP 130-139 or DBP 85-89 |
1.23 (0.80 - 1.88) | 1.19 (0.76 - 1.87) | 1.70 (1.47 - 1.97)*** | 1.36 (1.17 - 1.59)*** |
| Stage 1 Hypertension SBP 140-159 or DBP 90-99 |
2.24 (1.49 - 3.37)*** | 2.07 (1.34 - 3.20)** | 2.21 (1.92 - 2.54)*** | 1.63 (1.41 - 1.90)*** |
| Stage 2 Hypertension SBP 160-179 or DBP 100-109 |
2.00 (1.08 - 3.70)* | 1.91 (1.00 - 3.68) | 2.86 (2.31 - 3.55)*** | 1.87 (1.48 - 2.35)*** |
| Stage 3 Hypertension SBP >=180 or DBP >=110 |
5.33 (1.38 - 20.55)* | 4.72 (1.13 - 19.72)* | 3.88 (2.48 - 6.07)*** | 2.40 (1.50 - 3.84)*** |
| Diabetes-Related Characteristics | ||||
| Body mass index, (per 1-unit increase, kg/m2) | 1.01 (0.98 - 1.03) | 1.00 (0.97 - 1.03) | 1.02 (1.01 - 1.03)*** | 1.01 (1.00 - 1.02) |
| Fasting Glucose (per 10 mg/dL increase) | 1.01 (0.98 - 1.05) | 1.01 (0.96 - 1.06) | 1.05 (1.04 - 1.07)*** | 1.01 (0.99 - 1.03) |
| Insulin (per 10 μU/mL increase) | 1.08 (0.99 - 1.19) | 1.07 (0.96 - 1.18) | 1.13 (1.07 - 1.19)*** | 1.01 (0.95 - 1.08) |
| Lipid Profiles | ||||
| Loge (Triglycerides, mg/dL) (per unit increase) | 1.41 (1.04 - 1.91)* | 1.18 (0.80 - 1.74) | 1.42 (1.28 - 1.58)*** | 1.15 (1.01 - 1.31)* |
| HDL Cholesterol (per 10 mg/dL increase) | 0.94 (0.85 - 1.05) | 0.95 (0.82 - 1.10) | 0.92 (0.89 - 0.94)*** | 0.94 (0.91 - 0.98)** |
| GFR (mL/min/1.73m2) | ||||
| High Normal (≥120) | 1.27 (0.56 - 2.89) | 1.46 (0.61 - 3.50) | 1.33 (0.97 - 1.81) | 1.17 (0.84 - 1.62) |
| Normal (90 - 119, reference) | 1.00 | 1.00 | 1.00 | 1.00 |
| Mildly Decreased (60 - 89) | 1.12 (0.80 - 1.56) | 1.00 (0.70 - 1.42) | 0.91 (0.82 - 1.02) | 0.91 (0.81 - 1.01) |
| Moderately/Severly Decreased (<60) | 1.46 (0.95 - 2.23) | 0.93 (0.58 - 1.51) | 1.84 (1.54 - 2.20)*** | 1.16 (0.95 - 1.41) |
| Lifestyle Characteristics | ||||
| Current Smoking (yes v no) | 1.03 (0.70 - 1.53) | 1.38 (0.90 - 2.12) | 1.46 (1.29 - 1.67)*** | 1.83 (1.59 - 2.11)*** |
P< 0.05;
P<0.01;
P<0.001
Blood Pressure and Albuminuria, Excluding Hypertension Medication Use
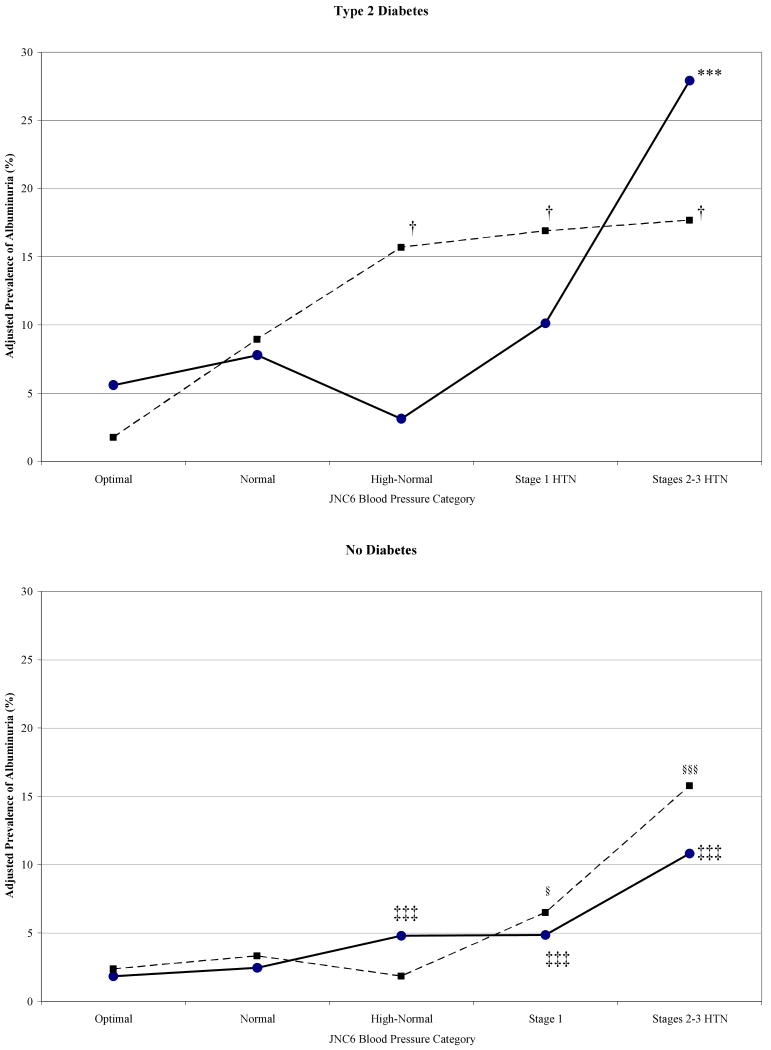
We excluded individuals with self-reported treated hypertension to examine the association of albuminuria with blood pressure, isolated from the effects of hypertension medication, with a subsequent study sample of 5,902 individuals. When examining JNC6 blood pressure categories, individuals with stage 2 and 3 hypertension were combined since the number of untreated hypertensives with stage 3 blood pressure was relatively small. In Figure 2, we estimated the prevalence of albuminuria by blood pressure category in each of the four risk groups, adjusted to the risk factor characteristics of all individuals within each diabetic and atherosclerotic risk group. In all four groups, the prevalence of albuminuria increased with blood pressure, adjusted for all other risk factors. As blood pressure category was increased, there was a greater association with albuminuria in all risk categories (P-trend<0.05). The association of atherosclerosis with albuminuria is most evident among hypertensives. Among non-diabetics without marked atherosclerosis, prevalence of albuminuria shows a consistent dose response, reaching at stage 2 and 3 hypertension a prevalence level comparable to normotensive diabetics with marked atherosclerosis.
Figure 2.
Adjusted prevalence of albuminuria by JNC6 blood pressure categories in those with and without type 2 diabetes by atherosclerosis severity. Prevalence at each blood pressure level was adjusted to the risk factor characteristics of all individuals within that diabetic and atherosclerotic risk group. Adjustment was for age, race, gender, fasting glucose, fasting insulin, BMI, smoking status, GFR <90, loge triglycerides, and HDL among those without atherosclerosis (●), and also for prevalence of CHD among those with atherosclerosis (■).
Compared to optimal blood pressure:
Type 2 Diabetes, Marked Atherosclerosis: †P<0.05; ††P<0.01; †††P<0.001
Type 2 Diabetes, No Marked Atherosclerosis: * P< 0.05; **P<0.01; ***P<0.001
No Diabetes, Marked Atherosclerosis: § P< 0.05; §§P<0.01; §§§P<0.001
No Diabetes, No Marked Atherosclerosis: ‡P< 0.05; ‡‡P<0.01; ‡‡‡P<0.001
We also examined in multivariate logistic regression models the independent associations with albuminuria of blood pressure categories in all risk groups, after excluding treated hypertensives (see table 4). Higher blood pressure category was associated with albuminuria in all groups. Importantly, the association was strong and graded in the group with neither marked atherosclerosis nor diabetes. In this group, high normal blood pressure was independently associated with albuminuria (OR 2.76, 95% CI: 1.61 - 4.73) compared to optimal blood pressure. This relationship was also seen among type 2 diabetics with marked atherosclerosis (n=263), with those with high normal BP having an OR of 11.99 (1.33 - 108.18).
Table 4. Multivariate Odds Ratios (ORs) with 95% Confidence Intervals (CIs) of Albuminuria by Type 2 Diabetes Status and Presence of Atherosclerosis, Excluding Subjects Using Hypertension Medication.
| Type 2 Diabetes | No Diabetes | |||
|---|---|---|---|---|
| Marked Atherosclerosis†‡ 263 |
No Marked Atherosclerosis† 600 |
Marked Atherosclerosis†‡ 984 |
No Marked Atherosclerosis† 4055 |
|
| JNC6 Blood Pressure (mmHg) Categories | P-trend = 0.008 | P-trend = 0.005 | P-trend=0.002 | P-trend<0.001 |
| Optimal (reference) SBP<120 & DBP<80 |
1.00 | 1.00 | 1.00 | 1.00 |
| Normal SBP 120-129 and DBP 80-84 |
5.93 (0.64 - 54.87) | 1.45 (0.59 - 3.59) | 1.42 (0.53 - 3.82) | 1.35 (0.78 - 2.32) |
| High Normal SBP 130-139 or DBP 85-89 |
11.99 (1.33 - 108.18)* | 0.53 (0.15 - 1.84) | 0.77 (0.20 - 2.95) | 2.72 (1.62 - 4.58)*** |
| Stage 1 Hypertension SBP 140-159 or DBP 90-99 |
13.23 (1.44 - 121.88)* | 1.98 (0.78 - 4.98) | 2.94 (1.12 - 7.70)* | 2.76 (1.61 - 4.73)*** |
| Stage 2 and 3 Hypertension SBP≥160 or DBP≥100 |
14.08 (1.36 - 145.83)* | 7.67 (2.57 - 22.90)*** | 8.41 (2.59 - 27.29)*** | 6.68 (3.25 - 13.70)*** |
P< 0.05;
P<0.01;
P<0.001
Adjusted for age, gender, race, BMI, fasting glucose, insulin, triglycerides, HDL, GFR<90, and current smoking
Additionally adjusted for prevalent CHD
To better evaluate the association of blood pressure and albuminuria among individuals not treated for hypertension, multivariate logistic regression results for albuminuria for each 10 mmHg increase in SBP, DBP, PP, and MAP were individually evaluated in each of the four risk groups (table 5). Consistent with previous studies, increases in each metric of BP resulted in a higher prevalence of albuminuria. However, only SBP and PP were consistently significantly associated with albuminuria across all risk categories, though the degree of variability across risk groups may be more a reflection of analyses in subgroups with differing samples sizes.
Table 5. Multivariate Odds Ratios (ORs) with 95% Confidence Intervals (CIs) of Albuminuria by Type 2 Diabetes Status and Presence of Atherosclerosis, Excluding Hypertensive Subjects and Subjects Using Hypertension Medication Per 10 unit increase mmHg.
| Type 2 Diabetes | No Diabetes | |||
|---|---|---|---|---|
| Marked Atherosclerosis†‡ | No Marked Atherosclerosis† | Marked Atherosclerosis†‡ | No Marked Atherosclerosis† | |
| (per 10mmHg increase) | 263 | 600 | 984 | 4055 |
| SBP | 1.33 (1.06 - 1.66)* | 1.36 (1.14 - 1.62)** | 1.36 (1.15 - 1.61)*** | 1.35 (1.23 - 1.48)*** |
| DBP | 1.24 (0.82 - 1.87) | 1.39 (0.98 - 1.97) | 1.25 (0.88 - 1.76) | 1.63 (1.34 - 1.98)*** |
| PP | 1.37 (1.05 - 1.79)* | 1.42 (1.14 - 1.77)** | 1.36 (1.13 - 1.64)** | 1.32 (1.17 - 1.49)*** |
| MAP | 1.43 (0.99 - 2.06) | 1.54 (1.15 - 2.06)** | 1.52 (1.12 - 2.06)** | 1.65 (1.40 - 1.94)*** |
P< 0.05;
P<0.01;
P<0.001
Adjusted for age, gender, race, BMI, fasting glucose, insulin, triglycerides, HDL, GFR<90, and current smoking
Additionally adjusted for prevalent CHD
Formal tests of interaction between JNC6 BP categories and atherosclerosis (LRT (5 d.f.), P=0.57) and BP categories and type 2 diabetes status (LRT (5d.f.), P=0.78) were not significant among individuals who did not use hypertension medication. Among those without hypertension treatment (n=5902), in our multivariate model, marked atherosclerosis was not associated with albuminuria (OR 0.88, 95% CI: 0.63 - 1.24) while both diabetes (OR 1.94, 1.35 - 2.79) and normal/high normal blood pressure were associated with albuminuria, independent of all CVD risk factors [normal BP(SBP 120-129 and DBP 80-84): OR 1.49, 95% CI: 1.00 - 2.22, p=0.052; high normal BP(SBP 130-139 or DBP 85-89): OR 1.97, 95% CI: 1.29 - 3.00, p=0.002 compared to optimal BP]. Sensitivity analyses examining macroalbuminuria (excluding microalbuminurics and those on antihypertensive medication, n=5685) as the outcome in the above multivariate model demonstrated risk estimates of blood pressure categories comparable to the above results, though not significant, except among those with stage 2 and 3 hypertension (OR: 9.38, 95% CI: 1.61 - 54.69). Analyses examining microalbuminuria (excluding macroalbuminurics and those on hypertensive treatment) and blood pressure categories were comparable to results with albuminuria as the outcome (results not shown).
Discussion
The associations of blood pressure and albuminuria were confirmed in our study, independent of type 2 diabetes and level of atherosclerosis. It was evident that even untreated high-normal blood pressure levels were associated with albuminuria, particularly among non-diabetics without marked atherosclerosis (OR 2.72, 95% CI: 1.62-4.58). This relationship of high-normal blood pressure and albuminuria was also evident among diabetics with marked atherosclerosis, though the sample size was smaller (OR 11.99, 95% CI: 1.33 - 108.18). Neither diabetes status nor atherosclerosis interacted with blood pressure to affect the prevalence of albuminuria. Though the effects of blood pressure may contribute in parallel to albuminuria and atherosclerosis, our results suggest the effects on albuminuria may be independent of those on atherosclerosis. Albuminuria is related to hypertension, even in the absence of marked atherosclerosis.
Our results extend previously published studies which did not examine atherosclerosis. Hypertension has been shown to be associated with albuminuria in several studies [11, 20, 29, 32, 38, 39, 41, 50]. However, few studies have examined the association between high normal blood pressure and albuminuria[24, 35]. In the Third National Health and Nutrition Examination Survey (NHANES III), Knight et al. determined that among normotensives without diabetes, compared to optimal blood pressure, those with high normal blood pressure had an increased odds of microalbuminuria (OR 2.13, 95% CI: 1.51 - 3.01)[24]. Similarly, Murtaugh et al. in the Coronary Artery Risk Development in Young Adults (CARDIA) Study demonstrated that prevalent microalbuminuria increased as blood pressure increased, even between strata of optimal blood pressure (OR 1.74, 95% CI 1.09 - 2.77 for SBP 110-119 and DBP 75-79 versus SBP<110 and DBP<75) after adjustment for age, sex, race, education and diabetes. However, when diabetics were excluded, race-specific associations were attenuated and the association between blood pressure and microalbuminuria among those with optimal blood pressure was no longer significant [35].
The mechanism behind increased blood pressure and albuminuria is complex, but increases in blood pressure may increase glomerular filtration, resulting in renal damage and subsequent albuminuria, or it may be that albuminuria is a marker of generalized vascular damage and endothelial dysfunction associated with blood pressure[12, 24, 42]. Albuminuria may also adversely affect traditional cardiovascular risk factors or it may be a proxy for co-clustering of risk factors [12, 14, 21]. Previous studies of blood pressure and albuminuria did not attempt to determine the effects on albuminuria of blood pressure isolated from blood pressure effects on atherosclerosis. Our study suggests that blood pressure does have effects on albuminuria, even prior to the appearance of significant atherosclerosis. However, we can not exclude that albuminuria may still be a marker of endothelial dysfunction associated with blood pressure. Though previous studies have found an association between carotid IMT and microalbuminuria among hypertensives[6, 7], several other studies have demonstrated that the association was no longer significant after adjustment for blood pressure [5, 6, 25, 36, 40], suggesting that the association between carotid IMT and microalbuminuria was due more to a shared risk factor (e.g. blood pressure) or co-clustering of risk factors[31]. When we stratified by prevalent CHD and examined associations with marked carotid atherosclerosis and albuminuria, our results demonstrated that the increased unadjusted association was no longer present after adjustment for major confounders such as blood pressure, diabetes, and lipid levels. However, only increasing blood pressure levels were consistently associated with carotid atherosclerosis among those with and without CHD after adjustment for confounders. These results are suggestive that the relationship of albuminuria with carotid atherosclerosis may be more a reflection of shared and confounding risk factors rather than an independent association, though blood pressure may still have independent effects on albuminuria. However, a recent study found associations with both carotid and femoral artery atherosclerosis of urinary ACR to be independent of major cardiovascular risk factors, including blood pressure [19]. If blood pressure results in both atherosclerosis and microalbuminuria, it is difficult to determine if blood pressure has effects on albuminuria beyond its effects on atherosclerosis. Blood pressure, even high-normal levels, appear to be associated with albuminuria to a similar extent in those with and without marked atherosclerosis and/or type 2 diabetes.
Additionally, the association in the population between decreased kidney function, as measured by GFR, and the presence of kidney damage, as indicated by albuminuria, is not completely understood, though albuminuria can precede the decline in GFR[13, 48]. Our study determined that only a moderate-severe decrease in GFR (<60mL/min/1.73m2) was significantly associated with albuminuria (range of OR across risk categories: 2.27 to 6.37), compared to those with normal renal function (table 4). In our sample, mildly decreased GFR (60-89 mL/min/1.73m2) was not associated with albuminuria after adjustment for all major risk factors. Compared to normal renal function, in the NHANES III sample the unadjusted prevalence was 1.4 times greater among those with mildly decreased GFR and 4.2 times greater among those with moderately decreased GFR[13], similar to our findings in table 2.
Recently, in the NHANES III population, microalbuminuria was also shown to be associated with the metabolic syndrome (defined as the presence of 3 or more of the following risk factors: elevated blood pressure, low high-density lipoprotein cholesterol level, high triglyceride level, elevated glucose level, and abdominal obesity)[10]. However, the reported relationships of microalbuminuria with the individual components of the metabolic syndrome have been variable[44]. In our study, triglycerides, insulin, and fasting glucose were only predictive among those with type 2 diabetes with similar effects by atherosclerosis status, after adjusting for all cardiovascular risk factors including blood pressure (see table 2). In non-diabetics, these factors were not associated with albuminuria regardless of atherosclerosis. Increased BMI had a weakly protective association with albuminuria in non-diabetics, after adjustment for all other measured factors.
Our study has several limitations. The study is cross-sectional and albuminuria was based on a single spot urine albumin-to-creatinine ratio. It has been suggested estimating prevalence of albuminuria using urinary ACR may be artificially elevated by low urinary creatinine among persons with low muscle mass. However, within each sub-group of table 1, there was no statistically significant difference of urinary creatinine concentration by albuminuric status, demonstrating no significant differential effect within each of the risk categories. Additionally, our analyses attempted to account for possible anthropometric differences by adjusting for BMI. Clinical and subclinical atherosclerosis was measured using upper quartile of carotid IMT and prevalent CHD. Additionally, it is not possible to define a group with absolutely no atherosclerosis or endothelial dysfunction. Furthermore, there is a potential of not appropriately classifying up to 3.9% of the study population as having marked atherosclerosis since they only underwent carotid ultrasound at visit 3 (and not visit 4); however, sensitivity analysis limited to individuals from visit 4 was comparable, suggesting the validity of our results. Additionally, the relevant atherosclerosis may be that in the renal artery, and we are using carotid IMT as a proxy for generalized atherosclerosis. However, our study is one of the largest to examine the association of high normal blood pressure and albuminuria[24, 35] and the first to determine the association on albuminuria of blood pressure, in separation from the effects of marked atherosclerosis.
In summary, blood pressure is associated with albuminuria, independent of type 2 diabetes and marked atherosclerosis. Our results suggest that the relationship of blood pressure with albuminuria is relatively continuous and graded, with even high-normal levels of blood pressure associated with albuminuria. This study emphasizes the importance of blood pressure management, even among normotensives, in order to minimize renal damage and cardiovascular risk. Additionally, our study finds that blood pressure is associated with prevalent albuminuria to a comparable degree between those with and without marked atherosclerosis. Furthermore, the correlation of albuminuria (as an indicator of renal structural damage) is only significantly associated with moderate-severely impaired levels of renal function. In addition, the individual components of the metabolic syndrome are only associated with albuminuria in type 2 diabetics (and not non-diabetics) in our sample, after adjusting for blood pressure. Though albuminuria may still be a marker of generalized vascular damage and endothelial dysfunction, our results suggest that the effects of blood pressure on albuminuria are not solely mediated through generalized vascular damage, as represented by atherosclerosis. Consistency of the finding across participants with and without diabetes and atherosclerosis supports the hypothesis that much of nephropathy is multifactorial with common pathways across different diagnostic settings.
Acknowledgments
The ARIC Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. CCH was also supported by grants from the American Diabetes Association, the National Kidney Foundation of Maryland, and NIH Medical Scientist Training Program. The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Potential Conflicts of Interest: None
Reference List
- 1.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 2.The sixth report of the Joint National Committee on prevention, detection evaluation, and treatment of high blood pressure. Arch Intern Med. 1997;157(21):2413–2446. doi: 10.1001/archinte.157.21.2413. [DOI] [PubMed] [Google Scholar]
- 3.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–266. [PubMed] [Google Scholar]
- 4.USRDS 2001 Annual Data Report. Bethesday, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2001. [Google Scholar]
- 5.Agewall S, Bjorn F. Microalbuminuria and intima-media thickness of the carotid artery in clinically healthy men. Atherosclerosis. 2002;164(1):161–166. doi: 10.1016/s0021-9150(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 6.Agewall S, Wikstrand J, Ljungman S, Fagerberg B. Urinary albumin excretion is associated with the intima-media thickness of the carotid artery in hypertensive males with non-insulin-dependent diabetes mellitus. J Hypertens. 1995;13(4):463–469. [PubMed] [Google Scholar]
- 7.Bigazzi R, Bianchi S, Nenci R, Baldari D, Baldari G, Campese VM. Increased thickness of the carotid artery in patients with essential hypertension and microalbuminuria. J Hum Hypertens. 1995;9(10):827–833. [PubMed] [Google Scholar]
- 8.Chambless LE, Folsom AR, Davis V, Sharrett R, Heiss G, Sorlie P, et al. Risk factors for progression of common carotid atherosclerosis: the Atherosclerosis Risk in Communities Study, 1987-1998. Am J Epidemiol. 2002;155(1):38–47. doi: 10.1093/aje/155.1.38. [DOI] [PubMed] [Google Scholar]
- 9.Chavers BM, Bilous RW, Ellis EN, Steffes MW, Mauer SM. Glomerular lesions and urinary albumin excretion in type I diabetes without overt proteinuria. N Engl J Med. 1989;320(15):966–70. doi: 10.1056/NEJM198904133201503. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, et al. The metabolic syndrome and chronic kidney disease in U.S. adults. Ann Intern Med. 2004;140(3):167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 11.Cirillo M, Senigalliesi L, Laurenzi M, Alfieri R, Stamler J, Stamler R, et al. Microalbuminuria in nondiabetic adults: relation of blood pressure, body mass index, plasma cholesterol levels, and smoking: The Gubbio Population Study. Arch Intern Med. 1998;158(17):1933–1939. doi: 10.1001/archinte.158.17.1933. [DOI] [PubMed] [Google Scholar]
- 12.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004;109(25 Suppl 1):IV31–IV46. doi: 10.1161/01.CIR.0000133442.99186.39. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 14.Damsgaard EM, Froland A, Jorgensen OD, Mogensen CE. Microalbuminuria as predictor of increased mortality in elderly people. BMJ. 1990;300(6720):297–300. doi: 10.1136/bmj.300.6720.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond JR, Karnovsky MJ. Focal and segmental glomerulosclerosis: analogies to atherosclerosis. Kidney Int. 1988;33(5):917–924. doi: 10.1038/ki.1988.87. [DOI] [PubMed] [Google Scholar]
- 16.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin- dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157(13):1413–8. [PubMed] [Google Scholar]
- 17.Eknoyan G, Hostetter T, Bakris GL, Hebert L, Levey AS, Parving HH, et al. Proteinuria and other markers of chronic kidney disease: a position statement of the national kidney foundation (NKF) and the national institute of diabetes and digestive and kidney diseases (NIDDK) Am J Kidney Dis. 2003;42(4):617–622. doi: 10.1016/s0272-6386(03)00826-6. [DOI] [PubMed] [Google Scholar]
- 18.Espeland MA, Byington RP, Hire D, Davis VG, Hartwell T, Probstfield J. Analysis strategies for serial multivariate ultrasonographic data that are incomplete. Stat Med. 1992;11(8):1041–1056. doi: 10.1002/sim.4780110806. [DOI] [PubMed] [Google Scholar]
- 19.Furtner M, Kiechl S, Mair A, Seppi K, Weger S, Oberhollenzer F, et al. Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Eur Heart J. 2005;26(3):279–287. doi: 10.1093/eurheartj/ehi014. [DOI] [PubMed] [Google Scholar]
- 20.Goetz FC, Jacobs DR, Jr, Chavers B, Roel J, Yelle M, Sprafka JM. Risk factors for kidney damage in the adult population of Wadena, Minnesota. A prospective study. Am J Epidemiol. 1997;145(2):91–102. doi: 10.1093/oxfordjournals.aje.a009091. [DOI] [PubMed] [Google Scholar]
- 21.Hallan S, Astor B, Romundstad S, Aasarod K, Kvenild K, Coresh J. Association of kidney function and albuminuria with cardiovascular mortality in older vs younger individuals: The HUNT II Study. Arch Intern Med. 2007;167(22):2490–2496. doi: 10.1001/archinte.167.22.2490. [DOI] [PubMed] [Google Scholar]
- 22.Heiss G, Sharrett AR, Barnes R, Chambless LE, Szklo M, Alzola C. Carotid atherosclerosis measured by B-mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134(3):250–256. doi: 10.1093/oxfordjournals.aje.a116078. [DOI] [PubMed] [Google Scholar]
- 23.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–8. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 24.Knight EL, Kramer HM, Curhan GC. High-normal blood pressure and microalbuminuria. Am J Kidney Dis. 2003;41(3):588–595. doi: 10.1053/ajkd.2003.50120. [DOI] [PubMed] [Google Scholar]
- 25.Kramer H, Jacobs DR, Jr, Bild D, Post W, Saad MF, Detrano R, et al. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46(1):38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Greene T, Kusek JW, Beck GJ, MDRD Study Group A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. abstrA0828. [Google Scholar]
- 27.Levey ASCJeal. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. American Journal of Kidney Diseases. 2002;39(2 Suppl 1) [PubMed] [Google Scholar]
- 28.Ma KW, Greene EL, Raij L. Cardiovascular risk factors in chronic renal failure and hemodialysis populations. Am J Kidney Dis. 1992;19(6):505–13. doi: 10.1016/s0272-6386(12)80827-4. [DOI] [PubMed] [Google Scholar]
- 29.Martinez MA, Moreno A, Aguirre dC, Cabrera R, Rocha R, Torre A, et al. Frequency and determinants of microalbuminuria in mild hypertension: a primary-care-based study. J Hypertens. 2001;19(2):319–326. doi: 10.1097/00004872-200102000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Mattock MB, Morrish NJ, Viberti G, Keen H, Fitzgerald AP, Jackson G. Prospective study of microalbuminuria as predictor of mortality in NIDDM. Diabetes. 1992;41(6):736–41. doi: 10.2337/diab.41.6.736. [DOI] [PubMed] [Google Scholar]
- 31.McDonald SP, Maguire GP, Duarte N, Wang XL, Hoy WE. Carotid intima-media thickness, cardiovascular risk factors and albuminuria in a remote Australian Aboriginal community. Atherosclerosis. 2004;177(2):423–431. doi: 10.1016/j.atherosclerosis.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Metcalf P, Baker J, Scott A, Wild C, Scragg R, Dryson E. Albuminuria in people at least 40 years old: effect of obesity, hypertension, and hyperlipidemia. Clin Chem. 1992;38(9):1802–1808. [PubMed] [Google Scholar]
- 33.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311(2):89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 34.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. Nephropathy in diabetes. Diabetes Care. 2004;27 1:S79–S83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 35.Murtaugh MA, Jacobs DR, Jr, Yu X, Gross MD, Steffes M. Correlates of urinary albumin excretion in young adult blacks and whites: the Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2003;158(7):676–686. doi: 10.1093/aje/kwg208. [DOI] [PubMed] [Google Scholar]
- 36.Mykkanen L, Zaccaro DJ, O'Leary DH, Howard G, Robbins DC, Haffner SM. Microalbuminuria and carotid artery intima-media thickness in nondiabetic and NIDDM subjects. The Insulin Resistance Atherosclerosis Study (IRAS) Stroke. 1997;28(9):1710–1716. doi: 10.1161/01.str.28.9.1710. [DOI] [PubMed] [Google Scholar]
- 37.National Heart LaBI. ARIC Manual of Operation: no 1, general description and study management; no 2, cohort component procedures; no 3, surveillance ocmponent procedures; no 4, pulmonary function assessment; no 5, electrocardiography; no 6, ultrasound assessment; no 7, blood collection and processing; no 8, lipid and lipprotein determinations; no 9. Heomstasis determinations; no 10, clinical chemistry determinations; no 11, sitting blood pressure and postural changes in blood pressure and heart rate; no 12, quality assurance. ARIC Coordinating Center, School of Public Health, University of North Carolina; Suite 203, NCNB Plaza, 137 E. Franklin St., Chapel Hilll, NC 27514: 1987. [Google Scholar]
- 38.Palatini P, Graniero GR, Mormino P, Mattarei M, Sanzuol F, Cignacco GB, et al. Prevalence and clinical correlates of microalbuminuria in stage I hypertension. Results from the Hypertension and Ambulatory Recording Venetia Study (HARVEST Study) Am J Hypertens. 1996;9(4 Pt 1):334–341. doi: 10.1016/0895-7061(95)00391-6. [DOI] [PubMed] [Google Scholar]
- 39.Parving HH, Mogensen CE, Jensen HA, Evrin PE. Increased urinary albumin-excretion rate in benign essential hypertension. Lancet. 1974;1(7868):1190–1192. doi: 10.1016/s0140-6736(74)91002-2. [DOI] [PubMed] [Google Scholar]
- 40.Pedrinelli R, Dell'Omo G, Penno G, Bandinelli S, Giannini D, Balbarini A, et al. Dissociation between microalbuminuria and common carotid thickness in essential hypertensive men. J Hum Hypertens. 2000;14(12):831–835. doi: 10.1038/sj.jhh.1001123. [DOI] [PubMed] [Google Scholar]
- 41.Pontremoli R, Sofia A, Ravera M, Nicolella C, Viazzi F, Tirotta A, et al. Prevalence and clinical correlates of microalbuminuria in essential hypertension: the MAGIC Study. Microalbuminuria: A Genoa Investigation on Complications. Hypertension. 1997;30(5):1135–1143. doi: 10.1161/01.hyp.30.5.1135. [DOI] [PubMed] [Google Scholar]
- 42.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med. 1998;339(20):1448–1456. doi: 10.1056/NEJM199811123392007. [DOI] [PubMed] [Google Scholar]
- 43.Rossing P, Hougaard P, Borch-Johnsen K, Parving HH. Predictors of mortality in insulin dependent diabetes: 10 year observational follow up study. Bmj. 1996;313(7060):779–84. doi: 10.1136/bmj.313.7060.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rowley K, O'Dea K, Best JD. Association of albuminuria and the metabolic syndrome. Curr Diab Rep. 2003;3(1):80–86. doi: 10.1007/s11892-003-0058-1. [DOI] [PubMed] [Google Scholar]
- 45.Shahar E, Folsom AR, Jackson R. The effect of nonresponse on prevalence estimates for a referent population: insights from a population-based cohort study. Atherosclerosis Risk in Communities (ARIC) Study Investigators. Ann Epidemiol. 1996;6(6):498–506. doi: 10.1016/s1047-2797(96)00104-4. [DOI] [PubMed] [Google Scholar]
- 46.Sharrett AR, Coady SA, Folsom AR, Couper DJ, Heiss G. Smoking and diabetes differ in their associations with subclinical atherosclerosis and coronary heart disease-the ARIC Study. Atherosclerosis. 2004;172(1):143–149. doi: 10.1016/j.atherosclerosis.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 47.Valmadrid CT, Klein R, Moss SE, Klein BE. The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med. 2000;160(8):1093–100. doi: 10.1001/archinte.160.8.1093. [DOI] [PubMed] [Google Scholar]
- 48.Verhave JC, Gansevoort RT, Hillege HL, Bakker SJ, De ZD, de Jong PE. An elevated urinary albumin excretion predicts de novo development of renal function impairment in the general population. Kidney Int Suppl. 2004;(92):S18–S21. doi: 10.1111/j.1523-1755.2004.09205.x. [DOI] [PubMed] [Google Scholar]
- 49.Viberti GC, Hill RD, Jarrett RJ, Argyropoulos A, Mahmud U, Keen H. Microalbuminuria as a predictor of clinical nephropathy in insulin- dependent diabetes mellitus. Lancet. 1982;1(8287):1430–2. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 50.Winocour PH, Harland JO, Millar JP, Laker MF, Alberti KG. Microalbuminuria and associated cardiovascular risk factors in the community. Atherosclerosis. 1992;93(12):71–81. doi: 10.1016/0021-9150(92)90201-q. [DOI] [PubMed] [Google Scholar]
- 51.Yokoyama H, Aoki T, Imahori M, Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004;66(1):448–454. doi: 10.1111/j.1523-1755.2004.00752.x. [DOI] [PubMed] [Google Scholar]