A 25 year old Caucasian female presented with a painless enlarging mass in the right breast. She first noticed it 18 to 24 months ago when her right breast became firmer and larger. She was seen by her primary care provider who attributed it to hormonal changes, and continued to only observe it. Three months ago she noticed another but smaller breast mass superficial to the first mass. In addition, she has noted a 10 to 15 pound weight loss over the year. She denied any breast pain, skin thickening, nipple discharge or other nipple changes. Family history was negative for breast and ovarian cancer. On physical examination, the right breast was approximately twice as large as the left breast with distended veins seen at the medial aspect of the right breast. A large soft mass associated with 2 smaller superficial masses occupied most of the right breast. No lymph nodes were palpated. X-ray mammography, ultrasound (US) and magnetic resonance imaging (MRI) examinations were performed.
Findings
X-ray mammography showed a large radio-dense mass measuring approximately 14 cm. Multiple scattered amorphous calcifications were seen throughout the mass.
On US, the dominant mass was circumscribed, hypo-echoic and contained many cystic spaces with dependent echogenic foci suggestive of milk of calcium. Two circumscribed oval iso- to hypo-echoic lesions were detected with US measuring about 3 cm each in diameter, with benign orientation (wider than tall).
On MRI, the dominant mass had a well defined smooth border and showed low signal on axial T1 weighted images (Fig. 1A). On the axial T2 weighted images, the mass was hyper-intense with dark internal septations (Fig. 1B). Axial T1 weighted post-contrast images and subtraction images showed heterogeneous enhancement with internal non-enhancing septations (Fig. 1C and D). On dynamic contrast enhanced (DCE) MRI, the time enhancement curve showed a persistently enhancing pattern (Fig. 1G). Pharmaco-kinetic parameters were derived from post processing of DCE-MRI data and revealed permeability (mean 0.2 min-1) and Kep (0.6 min-1) (Fig. 1E). Diffusion weighted imaging (DWI) was done and apparent diffusion coefficient (ADC) maps were generated. The average ADC value of the mass was 2.9 × 10−3 mm2/sec (Fig. 1F).
Figure 1.
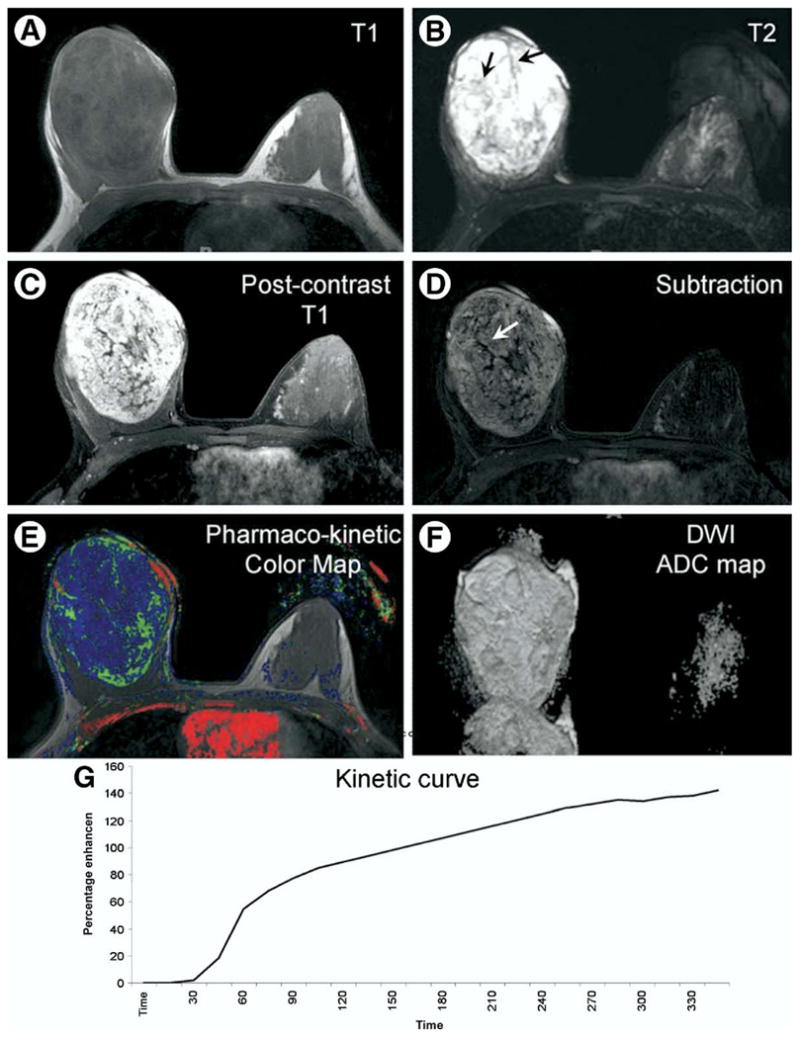
A 25 year old Caucasian female presented with a painless enlarging right breast mass. (A) Axial T1 weighted image shows a markedly enlarged right breast with a large oval well defined low signal mass. (B) Axial T2 weighted image with fat suppression shows the mass exhibiting bright T2 signal and dark internal septations (black arrows). (C, D) Axial post-contrast T1 weighted image with fat suppression and subtraction image show a heterogeneous enhancement pattern of the mass with non-enhancing septations (white arrow). (E) Axial pharmaco-kinetic post-processing color map shows the lesion with blue and green colors denoting low permeability and Kep values. (F) Axial ADC map shows th mass has high signal denoting high ADC value. (G) The corresponding kinetic curve of the lesion shows an enhancement pattern of the persistently enhancing (type 1a) type.
Differential diagnosis included giant fibroadenoma and phyllodes tumor. Biopsy was performed with 14 gauge needle using ultrasound guidance. Complete surgical excision was performed 3 weeks later.
Diagnosis
Giant fibroadenoma.
Discussion
Fibroadenomas are the most common benign tumors of the breast and are more common under the age of 30. In rare occasions, fibroadenomas can show rapid and massive growth resulting in what is called giant fibroadenomas. Giant fibroadenomas are rare representing less than 4% of all fibroadenomas. They present as a rapidly growing unilateral mass which is well circumscribed. Histologically the tumor is composed of ducts and fibrous connective tissue and can be treated with simple enucleation.1
Phyllodes tumors of the breast are an uncommon fibroepithelial tumor with an epithelial and a more cellular stromal component, and comprise only 1% of all breast tumors. They are sharply demarcated and typically are freely mobile. They occur in all age groups, but are uncommon in adolescents, and are more likely to occur in women over 35 years.2 Phyllodes tumor can be benign, borderline or malignant depending on histological features including stroma, cellularity, mitotic activity, and infiltration along the tumor’s border. About 90% of the tumors are low grade or benign, and although they rarely metastasize,3 they do tend to grow aggressively and recur locally. Recurrence is lower in the older patient. Benign tumors with no residual disease have a good prognosis. Borderline and malignant tumors show better results with total mastectomy than breast conserving surgery.3
There are no clinical or imaging features that clearly distinguish between the phyllodes tumor and the giant fibroadenoma.
On the non-contrast MRI scan, the tumor was ovoid and well defined with dark T1 and bright T2 signals and dark T2 internal septations, all suggestive of a benign entity. After contrast injection, the tumor exhibited a heterogeneous enhancement pattern with non-enhancing septations, an appearance typical of fibroadenomas.4 However, this appearance has been reported in the phyllodes tumor as well.2 On DCE MRI, the tumor showed a persistently enhancing pattern (type 1a) suggestive of benign dynamics.5 Pharmaco-kinetic measurements showed the mass to have low permeability and low Kep denoting a low likelihood of malignancy.6 On DWI, the mass showed a high ADC value (2.9 × 10−3 mm2/sec), which is similar to the suggested cutoff points published in the literature (1.37 and 1.68,9) and suggests a benign diagnosis with the values approaching that of a cyst (3×10−3 mm2/sec).
In summary, even after using both standard and advanced MRI protocols, we were still not able to determine whether the mass was a giant fibroadenoma or a phyllodes tumor, but we were able to conclude that the mass contained benign features. A benign diagnosis was confirmed on histopathology.
References
- 1.Dolmans GH, Hoogbergen MM, van Rappard JH. Giant fibroadenoma of one breast: Immediate bilateral reconstruction. J Plast Reconstr Aesthet Surg. 2007;60:1156–1157. doi: 10.1016/j.bjps.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Wurdinger S, Herzog AB, Fischer DR, et al. Differentiation of phyllodes breast tumors from fibroadenomas on MRI. AJR Am J Roentgenol. 2005;185:1317–1321. doi: 10.2214/AJR.04.1620. [DOI] [PubMed] [Google Scholar]
- 3.Belkacemi Y, Bousquet G, Marsiglia H, et al. Phyllodes tumor of the breast. Int J Radiat Oncol Biol Phys. 2008;70:492–500. doi: 10.1016/j.ijrobp.2007.06.059. [DOI] [PubMed] [Google Scholar]
- 4.Nunes LW, Schnall MD, Orel SG. Update of breast MR imaging architectural interpretation model. Radiology. 2001;219:484–494. doi: 10.1148/radiology.219.2.r01ma44484. [DOI] [PubMed] [Google Scholar]
- 5.Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–110. doi: 10.1148/radiology.211.1.r99ap38101. [DOI] [PubMed] [Google Scholar]
- 6.Pediconi F, Catalano C, Venditti F, et al. Color-coded automated signal intensity curves for detection and characterization of breast lesions: preliminary evaluation of a new software package for integrated magnetic resonance based breast imaging. Invest Radiol. 2005;40:448–457. doi: 10.1097/01.rli.0000167427.33581.f3. [DOI] [PubMed] [Google Scholar]
- 7.Rubesova E, Grell AS, De Maertelaer V, et al. Quantitative diffusion imaging in breast cancer: a clinical prospective study. J Magn Reson Imaging. 2006;24:319–324. doi: 10.1002/jmri.20643. [DOI] [PubMed] [Google Scholar]
- 8.Woodhams R, Matsunaga K, Iwabuchi K, et al. Diffusion-weighted imaging of malignant breast tumors: the usefulness of apparent diffusion coefficient (ADC) value and ADC map for the detection of malignant breast tumors and evaluation of cancer extension. J Comput Assist Tomogr. 2005;29:644–649. doi: 10.1097/01.rct.0000171913.74086.1b. [DOI] [PubMed] [Google Scholar]
- 9.Woodhams R, Matsunaga K, Kan S, et al. ADC mapping of benign and malignant breast tumors. Magn Reson Med Sci. 2005;4:35–42. doi: 10.2463/mrms.4.35. [DOI] [PubMed] [Google Scholar]


