Abstract
Background and Purpose
Presenilin1 (PS1) regulates Notch1 signaling activity, which liberates Notch intracellular domain (NICD). Notch activation promotes neural progenitor cell (NPC) self-renewal in the developing brain. In this study, we tested whether atorvastatin-induced NPC proliferation after stroke is mediated by PS1 and Notch1 activation.
Methods
PS1 and NICD expressions were measured in retired breeder rats subjected to middle cerebral artery occlusion that were left untreated or treated with atorvastatin. To investigate the mechanisms of atorvastatin-induced NPC self-renewal, subventricular zone (SVZ) neurosphere culture and knockdown of Notch1 gene expression by short interfering RNA were used. SVZ neurosphere formation, cell proliferation, real-time polymerase chain reaction, and Western blotting were performed.
Results
Atorvastatin significantly increased the numbers of newly generated neuroblasts and promoted PS1 and NICD expression in the ipsilateral and homologous contralateral SVZ compared with saline-treated control rats. Increased SVZ neurosphere formation and cell proliferation were found in cultured neurospheres derived from normal rat and poststroke rat SVZs treated in vitro with atorvastatin compared with untreated neurospheres (P<0.05). Atorvastatin significantly increased PS1 and hairy and enhancer of split1 (Hes1) gene expression in cultured SVZ neurospheres. Inhibition of PS1 significantly decreased NICD expression. Short interfering RNA knockdown of Notch1 expression, decreased NPC proliferation, and NICD and hairy and enhancer of split1 expression in cultured neurosphere cells.
Conclusions
These data indicate that atorvastatin increases the NPC pool in older rats and that it also upregulates PS1 expression and Notch1 signaling activity, which in turn, facilitate an increase in SVZ NPC proliferation.
Keywords: neural stem/progenitor cells, Notch1, presenilin1, stroke
Neural stem cells (NSCs), which exhibit self-renewal and multipotentiality, are present throughout life.1 In the developing brain, activation of Notch1 signaling is a requirement for survival and proliferation of NSCs.2,3 Notch ligand binding to Notch receptors leads to the cleavage of Notch receptors and the nuclear translocation of Notch intracellular domain (NICD) to induce transcriptional activation of Notch target genes. The hairy/enhancer of split-1 (Hes1) directly affects cell fate decisions as a primary target gene of Notch signaling.4 Hes1 mediates the proliferation of granule neuron precursors.5 Transient administration of Notch ligands to the brains of adult rats increases the numbers of newly generated precursor cells and improves motor skills after ischemic injury.6 An absence of Notch1 and Notch3 decreases Notch activity in the developing central nervous system and leads to increased cell death and decreased neurogenesis.7,8
Processing of Notch1 to produce NICD requires presenilin-1 (PS1), a critical component of γ-secretase.9 Nuclear translocation of NICD is markedly reduced in PS1-deficient cells and is restored by PS1 transfection.10 PS1 also maintains neural progenitor cell (NPC) proliferation via the Notch signaling pathway.11 PS1 is progressively reduced during aging, resulting in a progressive decrease in γ-secretase enzymatic activity, which may cause an age-related decrease in neural cell proliferation.12
Statins, widely used drugs to lower cholesterol, promote NPC proliferation in young adult animals after stroke.13,14 However, the mechanisms underlying statin-induced NPC proliferation have not been fully investigated. In addition, it is unknown whether statins regulate NPC proliferation in the older rat. In this study, we tested the hypothesis that treatment of poststroke rats with atorvastatin promotes NPC proliferation in the retired breeder rat and that atorvastatin increases PS1 expression and activates the Notch1 signaling pathway, which contribute to NPC proliferation.
Materials and Methods
MCAo Model and Atorvastatin Administration
Retired male breeder Wistar rats weighing 550 to 650 g (n=18, age 12 months) were used in all experiments. All experimental procedures were approved by the institutional animal care and use committee of Henry Ford Hospital. Rats were anesthetized with halothane. Permanent middle cerebral artery occlusion (MCAo) was induced by advancing a 3-0 surgical nylon suture to block the origin of the MCA by using a method of intraluminal vascular occlusion modified in our laboratory.15 Sham-operated rats underwent the same surgical procedure without suture insertion. Because 3 mg/kg atorvastatin was found to promote functional outcome after stroke in rats,14 this dose was used in the present study. MCAo and sham-operated rats was gavaged starting 24 hours after MCAo or sham operation, respectively, with saline for control studies or atorvastatin (3 mg/kg) daily for 7 days. To identify newly formed DNA in ischemic brains, rats received injections of bromodeoxyuridine (BrdU, Sigma Chemical; 100 mg/kg IP in 0.007N NaOH physiological saline) starting 1 day after MCAo and daily for the next 14 days. All animals were humanely killed 28 days after MCAo.
Histologic and Immunohistochemical Assessment
Rat brains were fixed by transcardial perfusion with saline followed by perfusion and immersion in 4% paraformaldehyde and embedding in paraffin. The cerebral tissues were cut into 7 equally spaced (2-mm) coronal blocks. A series of adjacent 6-µm-thick sections was cut from each block in the coronal plane. Immunohistochemical staining was used for BrdU (mouse monoclonal antibody, 1:100; Boehringer Mannheim, Indianapolis, Ind), PS1 (1:300 dilution, mouse monoclonal antibody), and NICD (1:3000 dilution, rabbit polyclonal antibody), as previously described.15 Control experiments consisted of staining the brain coronal tissue sections as outlined earlier but without the primary antibodies, as previously described.16
Double Immunohistochemical Staining
To identify the cell type of PS1-reactive cells, double immunofluorescence staining was performed. von Willebrand factor (vWF) is a marker of endothelial cells, glial fibrillary acidic protein (GFAP) is a marker of astrocytes, and β-tubulin III (TuJ1) is a marker of an immature neuronal phenotype. PS1/vWF, PS1/GFAP, PS1/TUJ1, PS1/NICD, NICD/GFAP, NICD/TUJ1, and NICD/vWF double immunohistochemical analyses were performed. A monoclonal antibody against vWF (1:400, Dako, Carpinteria, Calif), a polyclonal antibody against GFAP (1:1000, Dako), and a mouse anti-TUJ1 (1:1000, Novus Biologicals Inc) were used. Fluorescein isothiocyanate (Calbiochem) and cyanine-5.18 (CY5, Jackson Immunoresearch) were used for double-label immunostaining. Each coronal section was first incubated with the primary anti-TUJ1, GFAP, and vWF antibody with Cy5, which was then followed by PS1 or NICD antibody with fluorescein isothiocyanate. Control experiments consisted of staining brain coronal tissue sections as outlined earlier but without the primary antibodies.16
Quantification
BrdU- and NICD-positive cell numbers in the ipsilateral subventricular zone (SVZ) were counted with use of a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system (Imaging Research, St. Catharines, Canada). The total numbers of BrdU- and NICD-positive cells in the ipsilateral SVZ are presented. PS1-positive areas in the ipsilateral SVZ were digitized under a 20X objective (Olympus BX40) with use of a 3-CCD color video camera (Sony DXC-970MD) interfaced with an MCID image analysis system. The digitized images were then contrast enhanced to clearly differentiate positivity from background, and a thresholding procedure was established to determine the proportion of immunoreactive area within the SVZ.14 The data are presented as a percentage of positive immunoreactivity area in the SVZ.
SVZ Neurosphere Culture
SVZ cells were dissociated from normal and poststroke rats 24 hours after MCAo as reported previously.15 Mechanically dissociated ipsilateral SVZ cells were plated at 3×104 cells/mL in Dulbecco’s modified Eagle’s medium/F-12 medium (R&D Systems) containing 20 ng/mL epidermal growth factor (R&D Systems), 20 ng/mL basic fibroblast growth factor (R&D Systems), l-glutamine (2 mmol/L), 0.6% glucose, and B27. The secondary cultured neurosphere cells derived from poststroke and normal rat SVZs were subjected to no treatment for controls or 0.1 µmol/L atorvastatin treatment for 7 days (n=4 per group). The floating neurosphere number (neurosphere diameter >50 µm) was counted at 7 days.
BrdU ELISA Assay for SVZ Neurosphere Cell Proliferation
To evaluate DNA synthesis,17 secondary normal SVZ neurospheres were plated at 5×104 cells/mL in each well of a 96-well plate (Corning), and SVZ neurospheres were left untreated (controls) or treated with 0.1 µmol/L atorvastatin for 2 days (n=4 per group). BrdU (50 µmol/L) was added to the SVZ neurosphere culture medium for 12 hours before fixation, and samples were processed with an ELISA kit for detection of BrdU incorporated into cellular DNA (BrdU labeling and detection kit III, Roche), according to the manufacturer’s instructions.
Real-Time PCR
Cells were harvested and total RNA was isolated from treated cells with TRIzol (Invitrogen), according to a standard protocol.18 Quantitative polymerase chain reaction (PCR) was performed with the SYBR green real-time PCR method. Quantitative reverse transcription–PCR was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, Calif) with 3-stage program parameters provided by the manufacturer. Each sample was tested in triplicate, and samples were obtained from 3 independent experiments that were used for analysis of relative gene expression data with the 2−ΔΔCT method.18 The following primers for reverse transcription–PCR were designed with primer express software (ABI): for glyceraldehyde 3-phosphate dehydrogenase: forward, AGAACATCATCCCTGCATCC and reverse, CACATTGGGGGTAGGAACAC; for Notch1: forward, TCCTCCTGAGAGTTGTCCTAGC and reverse, GTGGTCTAAGTGACCATCAGCA; for PS1: forward, GAGGAAGACGAAGAGCTGACAT and reverse, GAAGCTGACTGACTTGATGGTG; and for Hes1: forward, ACACCGGACAAACCAAAGAC and reverse, ATGCCGGGAGCTATCTTTCT.
Western Blot Assay
To test whether atorvastatin regulates Notch1 signaling activity and whether PS1 mediates atorvastatin-induced Notch activity, for Western blotting15 normal SVZ neurospheres were left untreated (controls) or treated with (1) 0.1 µmol/L atorvastatin, (2) 10 µmol/L γ-secretase inhibitor (γ40-secretsae inhibitor II, Calbiochem), or (3) 0.1 µmol/L atorvastatin with 10 µmol/L γ-secretase inhibitor for 7 days (n=3 per group). Notch1 and NICD expressions were measured. Protein was isolated from cultured cells with Trizol (Invitrogen) according to a standard protocol. Notch1 (1:500, Santa Cruz Biotech) or NICD (1:500) primary antibody was used. For detection, a secondary anti-mouse antibody (1:1000, Bio-Rad) was added. Optical density was quantified with an image processing and analysis program (Scion Image, Ederick, Mass).
Knockdown Notch1 Gene Expression in Neurosphere Cells
Notch1 short interfering (si) RNA (Santa Cruz Biotech) was transfected with Lipofectamine 2000 (Invitrogen) according to a standard protocol. In brief, normal neurosphere cells were plated out in 10-cm plates and allowed to culture until they were 80% confluent. They were then transfected with 8 µg Notch1 siRNA or 8 µg scrambled siRNA (Santa Cruz Biotech) in serum-free medium for 6 hours. Afterward, Dulbecco’s modified Eagle’s medium and 20% fetal bovine serum were added, and the cells were incubated overnight. The following day the medium was changed, and 24 hours later the cells were passaged for subsequent experiments. Notch1 mRNA and protein expression were measured by real-time PCR and Western blotting.
To test whether knockdown (KD) Notch expression may regulate NICD expression and neurosphere cell proliferation, normal SVZ neurosphere cells were cultured with (1) normal neurosphere cells, (2) scrambled neurosphere cells, (3) Notch1-KD neurosphere cells, (4) Notch1-KD neurosphere cells treated with 0.1 µmol/L atorvastatin, or (5) scrambled neurosphere cells treated with 0.1 µmol/L atorvastatin. Notch1, NICD, and Hes1 protein expression was measured by Western blotting 5 days after treatment. In addition, Ki67 (a marker of proliferating cells) immunostaining was performed (1:300 dilution, Novus) 24 hours after treatment. DAPI was used as a nuclear counterstain. All assays were performed in triplicate. Ki67-reactive cells were counted in 5 randomly selected microscopic fields under a 20X objective. The percentage of Ki67-reactive cells within the total number of DAPI-positive cells was calculated.
Statistical Analysis
For in vivo study, the retired breeder rats were allocated to either control or atorvastatin treatment (3 mg/kg). A 2-sample t test was performed to compare BrdU, NICD, and PS1 expression between the 2 groups. In addition, a 2-sample t test was performed to compare PS1, Notch1, and Hes1 gene expression and neurosphere formation between neurosphere cells treated with atorvastatin or left untreated. The data are presented as mean±SE. A value of P<0.05 was taken as significant.
Results
Atorvastatin Increases SVZ Cell Proliferation After Stroke in Retired Breeder Rats
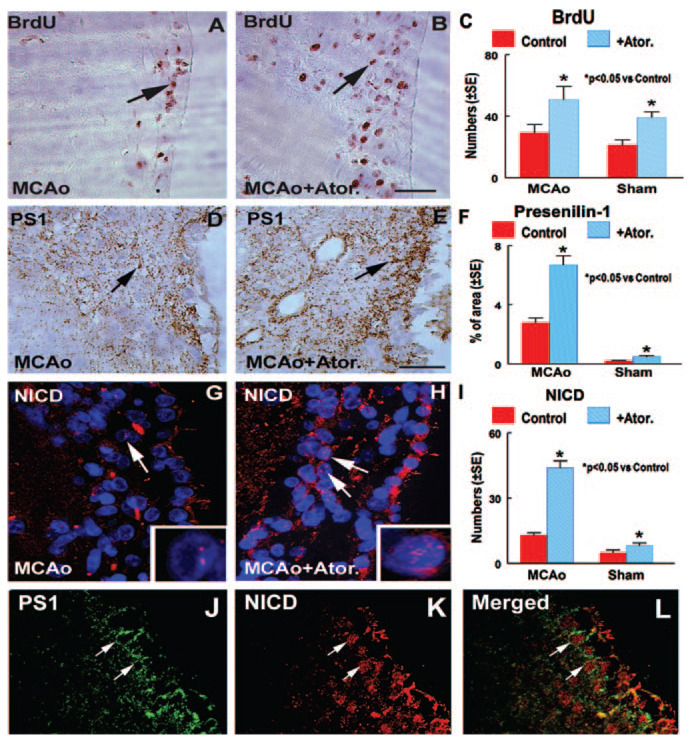
To test whether atorvastatin regulates neural cell proliferation in retired breeder rats after stroke, BrdU immunostaining was performed. Figures 1A through 1C show that atorvastatin (B and C) significantly increased BrdU-positive cell numbers in the ipsilateral SVZ compared with untreated MCAo control or sham control rats (A and C), respectively. This is consistent with our previous findings.14
Figure 1.
Atorvastatin (Ator.) increases NPC proliferation and PS1 and NICD expression after stroke. BrdU, PS1, and NICD expression was measured in the ipsilateral SVZ 28 days after MCAo or sham intervention. A, D, and G show BrdU (A, arrow), PS1 (D, arrow), and NICD (G, arrow) expression, respectively, in the ipsilateral SVZ in MCAo control rats. B, E, and H show BrdU (B, arrow), PS1 (E, arrow), and NICD (H, arrow) expression, respectively, in the ipsilateral SVZ in atorvastatin-treated poststroke rats. G and H inserts are high-magnification views of NICD-positive cells (red); blue color is DAPI nuclear counterstaining. C and I show quantitative data of BrdU- (C) and NICD- (I) positive cell numbers in the ipsilateral SVZ in MCAo or sham-operated control rats treated with or without atorvastatin. F shows quantitative data of the PS1-positive (F) percentage area in the ipsilateral SVZ in MCAo or sham-operated control rats treated with or without atorvastatin. Double immunostaining of PS1 with NICD (J–L) shows colocalization of PS1 (J and L; arrow, cytoplasm) with NICD (K and L; arrow, nucleus). Bar in B and E=50µm
Atorvastatin Promotes PS1 and NICD Expression in the SVZ in Retired Breeder Rats
PS1 is a key molecule regulating the activity of the Notch signaling pathway,9 and Notch activity promotes NPC proliferation.6 To test the mechanism of atorvastatin-induced NPC proliferation, PS1 and NICD expression was measured. Nuclear NICD-positive cell number was measured in the ipsilateral SVZ. PS1-reactive cells are primarily expressed on the cytoplasmic surfaces of membranous organelles,19 and it is difficult to count the total number of PS1-positive cells. Therefore, we measured the PS1-positive area in the ipsilateral SVZ. Figure 1 shows that atorvastatin treatment significantly increased PS1 (D–F) and NICD (G–I) expression in the ipsilateral SVZ compared with MCAo controls or sham controls. Double immunostaining showed that PS1-reactive cells in the cytoplasm (green, J and L) colocalized with NICD nuclear positive cells (red, K and L) in the SVZ. PS1 is expressed in the cytoplasm, and NICD is expressed in the nucleus.
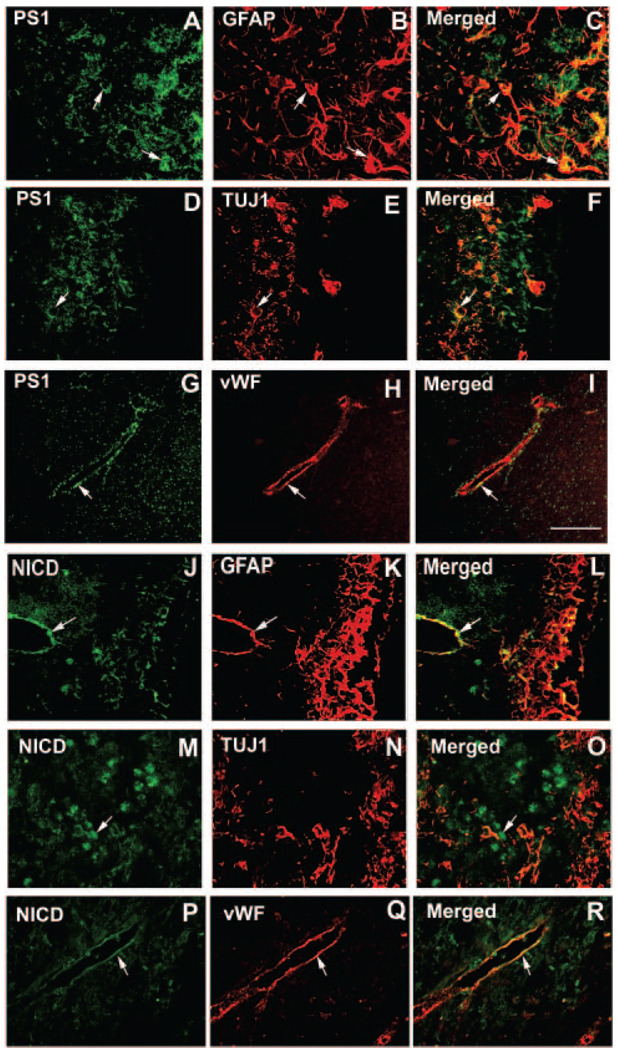
Figure 2 shows that doubly immunostained PS1-reactive cells (green; A, D, and G) primarily colocalized with GFAP-positive cells (red, B and C), and some PS1-reactive cells colocalized with TUJ1- (red, E and F) and vWF- (red, H and I) positive cells. Nuclear NICD-reactive cells (green; J, M, and P) primarily colocalized with GFAP- (red, K and L) and vWF- (Q and R) positive cells but not with TUJ1-positive cells (red, N and O).
Figure 2.
Double immunostaining: The colocalized staining of GFAP and TUJ1 was obtained from SVZs; vWF was obtained from samples adjacent to the SVZ. A–I show double immunostaining for PS1 (A, D, and G; green) and GFAP (B, red), TUJ1 (E, red), and vWF (H, red). A–I show double immunostaining for NICD (J, M, and P; green) and GFAP (K, red), TUJ1 (N, red), and vWF (Q, red). Scale bar in I=50 µm.
Atorvastatin Increases SVZ Neurosphere Formation and NPC Proliferation
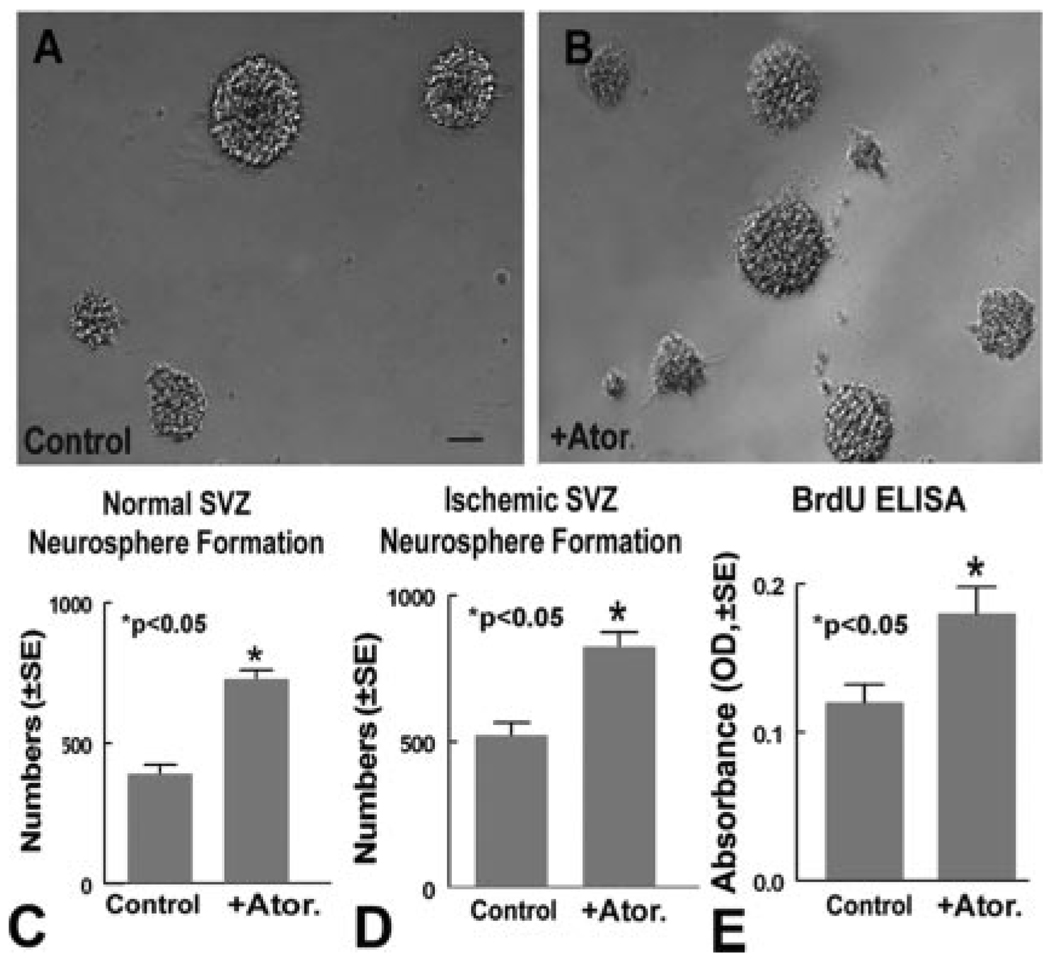
To investigate the mechanisms of atorvastatin-induced NPC proliferation in retired breeder rats, we first tested whether atorvastatin regulates SVZ neurosphere formation in neurosphere cultures derived from poststroke and normal rats. Figure 3 shows that atorvastatin significantly increased neurosphere formation numbers compared with control, untreated neurospheres derived from the normal rat SVZ (A–C, P<0.05) and the poststroke rat SVZ (D). In addition, normal SVZ neurosphere proliferation was measured with a BrdU ELISA assay. Atorvastatin significantly increased SVZ neurosphere cell proliferation compared with untreated control (E, P<0.05). These data suggest that atorvastatin upregulates neurosphere proliferation.
Figure 3.
Atorvastatin (Ator.) increases SVZ neurosphere formation and proliferation. A and B show normal retired breeder rat SVZ neurosphere formation in untreated controls (A) and those treated with 0.1 µmol/L atorvastatin (B). C shows quantitative data of normal SVZ neurosphere formation. D shows quantitative data for ischemic rat SVZ neurosphere formation. E shows quantitative data of the BrdU ELISA assay in normal SVZ neurosphere cells. Scale bar in A=50 µm.
Atorvastatin Increases Notch Signaling and PS1 Gene Expression
Notch signaling activity has been implicated in the maintenance and self-renewal of adult NSCs.3 PS1 cleaves Notch and activates Notch signaling.20 Hes is a basic helix-loop-helix type of transcriptional repressor and is downstream of Notch activation.21 To identify the molecular mechanisms underlying atorvastatin-regulated NPC proliferation, PS1, Notch1, and Hes1 genes were measured in normal SVZ neurospheres. Atorvastatin significantly increased the amount of Hes1 (5.7±0.7-fold) and PS1 (4.7±0.9-fold), but not Notch1 (1.4±0.1-fold), mRNA expression compared with untreated neurosphere controls.
PS1 Regulates Atorvastatin-Induced Notch Signaling Activity
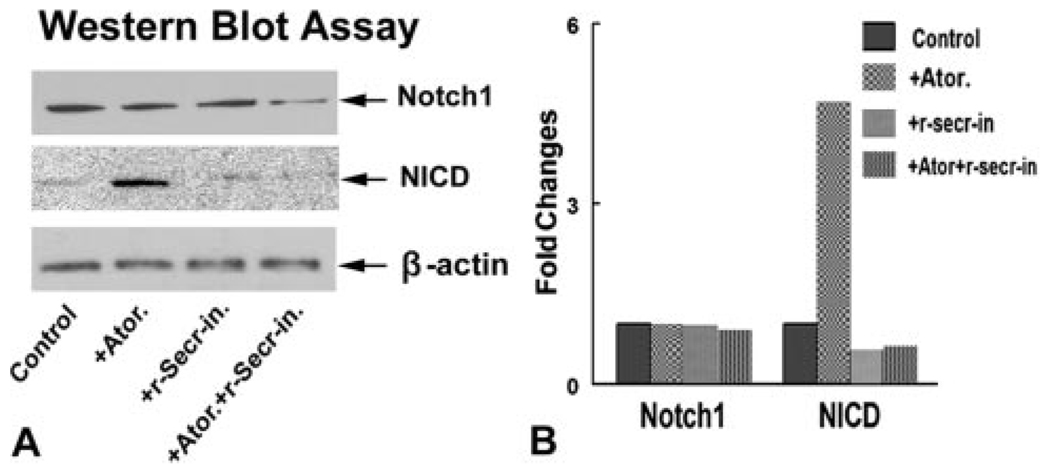
To test whether PS1 is involved in atorvastatin-increased Notch signaling activity, cultured normal SVZ neurosphere cells were treated with or without the γ-secretase inhibitor (γ40-secretase inhibitor II) in vitro, and NICD expression was measured. Figure 4 shows that atorvastatin upregulated SVZ neurosphere NICD protein expression. Inhibition of γ-secretase activity attenuated atorvastatin-induced NICD expression. These data suggest that atorvastatin promotes PS1 expression, which may upregulate Notch1 signaling activity.
Figure 4.
Atorvastatin increases NICD protein expression. Inhibition of PS1 by a γ-secretase inhibitor decreases atorvastatin-induced NICD expression. A shows Western blot assay for Notch1 and NICD protein expression in normal retired breeder rat SVZ neurosphere cells treated with or without atorvastatin (0.1 µmol/L) and/or an γ-secretase inhibitor (10 µmol/L). B shows semiquantitative data for the Western blot.
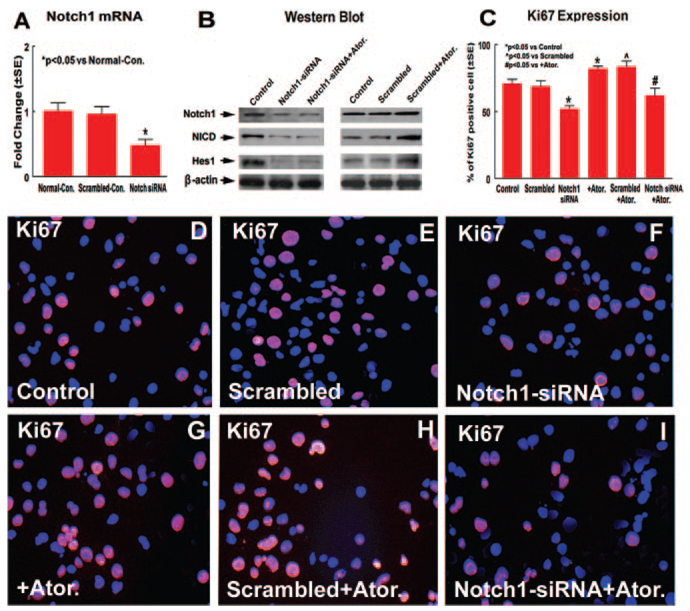
Notch1-KD in SVZ Neurosphere Cells Decreases Neurosphere Cell Proliferation
To test whether Notch signaling regulates SVZ neurosphere cell proliferation, Notch1-KD in SVZ neurosphere cells was used with Notch1 siRNA. Notch1, NICD, and Hes1 expression was measured in Notch1-KD neurosphere cells. Figure 5A shows that Notch1 siRNA significantly (52%) decreased Notch1 mRNA expression in SVZ neurosphere cells compared with normal control neurosphere cells, and Notch1 expression was decreased by 46% compared with scrambled controls. There was no significant difference in scrambled controls compared with normal control neurosphere cells. Figure 5B shows that Notch1-KD in SVZ neurosphere cells decreased Notch1, NICD, and Hes1 expression compared with normal controls and attenuated atorvastatin-induced NICD and Hes1 expression. Scrambled neurospheres did not influence Notch1, NICD, and Hes1 expression compared with control neurospheres. Atorvastatin treatment in scrambled neurospheres increased NICD and Hes1 expression compared with untreated scrambled controls.
Figure 5.
KD of Notch1 gene expression in normal SVZ neurosphere cells decrease neurosphere cell proliferation. A shows real-time PCR assay for Notch1. B shows Western blot assay for Notch1, NICD, and Hes1. C shows quantitative data of Ki67 expression. DI show Ki67 immunostaining in normal, scrambled, or Notch1-KD SVZ neurosphere cells treated with or without atorvastatin (blue color is the DAPI nuclear counterstain; D and G, normal neurosphere cells treated with or without atorvastatin; E and H, scrambled neurosphere cells treated with or without atorvastatin; F and I, Notch1-KD neurosphere cells treated with or without atorvastatin).
Ki67, a nuclear protein expressed in all phases of the cell cycle except the resting phase, is used as a marker of cell proliferation.22 To test whether Notch1-KD influences atorvastatin-induced neurosphere cell proliferation, Ki67 immunostaining and neurosphere formation were measured. Figures 5C through 5I show that atorvastatin treatment of normal or scrambled neurosphere cells significantly increased Ki67 expression compared with untreated normal or scrambled neurosphere cells. Notch1-KD in neurosphere cells significantly decreased Ki67-positive cell numbers compared with normal control neurosphere cells and scrambled controls. Notch1-KD in neurosphere cells and atorvastatin treatment significantly decreased Ki67-positive cell numbers compared with normal neurosphere cells treated with atorvastatin (P<0.05). In addition, owing to incomplete Notch1-KD (52% decrease), Ki67 expression was significantly decreased but not completely inhibited by Notch1-KD. These data suggest that the Notch1 signaling pathway may regulate atorvastatin-induced neurosphere cell proliferation.
Discussion
In this study, we have demonstrated for the first time that atorvastatin treatment of older (12 months) poststroke rats induces NPC proliferation, which coincides with increased PS1 expression and Notch1 signaling activity. Inhibition of PS1 activity attenuated atorvastatin-induced Notch1 activity. KD of Notch1 gene expression attenuated atorvastatin-induced SVZ NPC proliferation. Thus, our data indicate that atorvastatin promotes PS1 expression and Notch signaling activity, which may at least partially facilitate an increase in SVZ NPC proliferation in retired breeder rats.
Atorvastatin Increases NPC Proliferation in Retired Breeder Rats
Neurogenesis, which contributes to the ability of the adult brain to function normally and to adapt to disease, declines with advancing age.23 Atorvastatin treatment increased BrdU incorporation in the ipsilateral SVZ after stroke, which is consistent with in vitro SVZ neurosphere culture data showing that atorvastatin promotes SVZ neurosphere formation and neural cell proliferation. This suggests that atorvastatin increases NPC proliferation and the NPC pool in retired breeder animals.
Atorvastatin Promotes Notch Signaling Activity
NSCs that reside in the adult brain are responsive to environmental demands and appear capable of replacing lost or dysfunctional neurons and glial cells, perhaps even in the aging brain.24 Atorvastatin induces SVZ neural cell proliferation in older brains after stroke and upregulates Notch1 activity (NICD) in the ischemic brain SVZ. Notch1 activation in the murine forebrain promotes radial glial identity. Radial glial cells may function as NPCs in the adult nervous system.25 In addition, Hes, which is expressed by NPCs, is controlled by the transmembrane protein Notch.26 Hes1 is a well-studied downstream target gene in the Notch signaling pathway, which is upregulated by overexpressed NICD.27 Overexpression of Notch1 and Hes1 promotes cell proliferation and self-renewal.28 Hes1- and Hes5-expressing cells are maintained as NSCs, and Hes1 and Hes5 increase NSC proliferation in the embryonic telencephalon but inhibit their differentiation.29 Disruption of Notch/Hes pathway genes results in the reduction of the NPC pool size.3 Our data show that atorvastatin increases activation of Notch1, upregulates Hes1 expression, and promotes neurosphere cell proliferation. Therefore, Notch signaling activity induced by atorvastatin treatment may play a role in enlarging the NPC pool in the retired breeder rat after stroke.
Atorvastatin Increases PS1 Expression in the Ischemic Brain
γ-Secretase activates Notch signaling,30 and PS1 is a critical component of the γ-secretase complex, which cleaves several substrates, including the amyloid precursor protein and Notch1. Our data have shown atorvastatin increases PS1 and NICD expression and induces SVZ cell proliferation in vitro and in vivo. PS1-reactive cells colocalize with NICD-positive cells in the SVZ. Inhibition of γ-secretase activity in cultured SVZ neurospheres attenuates atorvastatin-induced Notch1 signaling activity. Therefore, atorvastatin may upregulate PS1 expression, which promotes Notch1 signaling activity but does not influence Notch1 gene and protein expression. Consistent with our finding, in the ventricular zone of PS1−/− mice, expression of the Notch1 downstream effector gene Hes5 is reduced, whereas expression of Notch1 is unchanged.31 Disruption of Notch1 signaling by a γ-secretase inhibitor or use of mice deficient in PS1 delays neurosphere formation.2,32 Therefore, atorvastatin upregulates PS1 and Notch1 signaling activity, which may partially influence NPC proliferation. In addition to atorvastatin’s upregulation of PS1 and Notch activity, previous studies have shown that statins increase vascular endothelial growth factor and brain-derived neurotrophic factor expression in the ischemic brain, which may also play important roles in regulating NPC proliferation, survival, and neurogenesis.13,15 Although using an in vitro model of SVZ cell culture is an oversimplification of the in vivo condition, it is possible to gain insight into basic biologic mechanisms of atorvastatin-induced endogenous cell proliferation in the SVZ. In addition, PS1-deficient mice exhibit premature differentiation of NPCs31 and increased oligodendroglial cell numbers compared with wild-type mice.33 Therefore, further experiments to test the effects of the atorvastatin-induced PS1/Notch1 pathway on cell fate decisions and neuronal differentiation are warranted.
In summary, we have demonstrated for the first time that atorvastatin increases PS1 expression, activates the Notch1/Hes1 pathway, and promotes NPC proliferation after stroke in retired breeder rats.
Acknowledgments
The authors wish to thank Qinge Lu and Yuping Yang for technical assistance.
Sources of Funding
This work was supported by National Institute of Neurological Disorders and Stroke grants RO1 NS047682 and PO1 NS23393 and American Heart Association grant 0750048Z.
Footnotes
Disclosures
None.
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
References
- 1.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 2.Chojnacki A, Shimazaki T, Gregg C, Weinmaster G, Weiss S. Glycoprotein 130 signaling regulates notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J Neurosci. 2003;23:1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, Conlon RA, Mak TW, Bernstein A, van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iso T, Kedes L, Hamamori Y. Hes and herp families: multiple effectors of the notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 5.Solecki DJ, Liu XL, Tomoda T, Fang Y, Hatten ME. Activated notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 6.Androutsellis-Theotokis A, Leker RR, Soldner F, Hoeppner DJ, Ravin R, Poser SW, Rueger MA, Bae SK, Kittappa R, McKay RD. Notch signaling regulates stem cell numbers in vitro and in vivo. Nature. 2006;442:823–826. doi: 10.1038/nature04940. [DOI] [PubMed] [Google Scholar]
- 7.Mason HA, Rakowiecki SM, Gridley T, Fishell G. Loss of notch activity in the developing central nervous system leads to increased cell death. Dev Neurosci. 2006;28:49–57. doi: 10.1159/000090752. [DOI] [PubMed] [Google Scholar]
- 8.Mason HA, Rakowiecki SM, Raftopoulou M, Nery S, Huang Y, Gridley T, Fishell G. Notch signaling coordinates the patterning of striatal compartments. Development. 2005;132:4247–4258. doi: 10.1242/dev.02008. [DOI] [PubMed] [Google Scholar]
- 9.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent γ-secretase-like protease mediates release of notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 10.Song W, Nadeau P, Yuan M, Yang X, Shen J, Yankner BA. Proteolytic release and nuclear translocation of notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc Natl Acad Sci U S A. 1999;96:6959–6963. doi: 10.1073/pnas.96.12.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wines-Samuelson M, Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- 12.Kern A, Roempp B, Prager K, Walter J, Behl C. Down-regulation of endogenous amyloid precursor protein processing due to cellular aging. J Biol Chem. 2006;281:2405–2413. doi: 10.1074/jbc.M505625200. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Zhang C, Jiang H, Li Y, Zhang L, Robin A, Katakowski M, Lu M, Chopp M. Atorvastatin induction of VEGF and BDNF promotes brain plasticity after stroke in mice. J Cereb Blood Flow Metab. 2005;25:281–290. doi: 10.1038/sj.jcbfm.9600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Zacharek A, Li A, Zhang C, Ding J, Roberts C, Lu M, Kapke A, Chopp M. Vascular endothelial growth factor mediates atorvastatin-induced mammalian achaete-scute homologue-1 gene expression and neuronal differentiation after stroke in retired breeder rats. Neuroscience. 2006;141:737–744. doi: 10.1016/j.neuroscience.2006.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin d1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. discussion 1980-1971. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, Lu M, Kapke A, Chopp M. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci. 2005;25:2366–2375. doi: 10.1523/JNEUROSCI.5071-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Lah JJ, Heilman CJ, Nash NR, Rees HD, Yi H, Counts SE, Levey AI. Light and electron microscopic localization of presenilin-1 in primate brain. J Neurosci. 1997;17:1971–1980. doi: 10.1523/JNEUROSCI.17-06-01971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray WJ, Yao M, Mumm J, Schroeter EH, Saftig P, Wolfe M, Selkoe DJ, Kopan R, Goate AM. Cell surface presenilin-1 participates in the γ-secretase-like proteolysis of notch. J Biol Chem. 1999;274:36801–36807. doi: 10.1074/jbc.274.51.36801. [DOI] [PubMed] [Google Scholar]
- 21.Iso T, Sartorelli V, Chung G, Shichinohe T, Kedes L, Hamamori Y. Herp, a new primary target of notch regulated by ligand binding. Mol Cell Biol. 2001;21:6071–6079. doi: 10.1128/MCB.21.17.6071-6079.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 23.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP, Duan W, Chan SL, Cheng A, Haughey N, Gary DS, Guo Z, Lee J, Furukawa K. Neuroprotective and neurorestorative signal transduction mechanisms in brain aging: modification by genes, diet and behavior. Neurobiol Aging. 2002;23:695–705. doi: 10.1016/s0197-4580(02)00025-8. [DOI] [PubMed] [Google Scholar]
- 25.Fishell G, Kriegstein AR. Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol. 2003;13:34–41. doi: 10.1016/s0959-4388(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama R, Ohtsuka T, Tomita K. The bhlh gene hes1 regulates differentiation of multiple cell types. Mol Cells. 2000;10:1–7. doi: 10.1007/s10059-000-0001-0. [DOI] [PubMed] [Google Scholar]
- 27.Yan B, Raben N, Plotz P. The human acid α-glucosidase gene is a novel target of the notch-1/hes-1 signaling pathway. J Biol Chem. 2002;277:29760–29764. doi: 10.1074/jbc.M204721200. [DOI] [PubMed] [Google Scholar]
- 28.Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- 29.Ohtsuka T, Sakamoto M, Guillemot F, Kageyama R. Roles of the basic helix-loop-helix genes hes1 and hes5 in expansion of neural stem cells of the developing brain. J Biol Chem. 2001;276:30467–30474. doi: 10.1074/jbc.M102420200. [DOI] [PubMed] [Google Scholar]
- 30.Martys-Zage JL, Kim SH, Berechid B, Bingham SJ, Chu S, Sklar J, Nye J, Sisodia SS. Requirement for presenilin 1 in facilitating lagged 2-mediated endoproteolysis and signaling of notch 1. J Mol Neurosci. 2000;15:189–204. doi: 10.1385/jmn:15:3:189. [DOI] [PubMed] [Google Scholar]
- 31.Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- 32.Alexson TO, Hitoshi S, Coles BL, Bernstein A, van der Kooy D. Notch signaling is required to maintain all neural stem cell populations–irrespective of spatial or temporal niche. Dev Neurosci. 2006;28:34–48. doi: 10.1159/000090751. [DOI] [PubMed] [Google Scholar]
- 33.Culvenor JG, Rietze RL, Bartlett PF, Masters CL, Li QX. Oligodendrocytes from neural stem cells express α-synuclein: increased numbers from presenilin 1 deficient mice. Neuroreport. 2002;13:1305–1308. doi: 10.1097/00001756-200207190-00018. [DOI] [PubMed] [Google Scholar]