Abstract
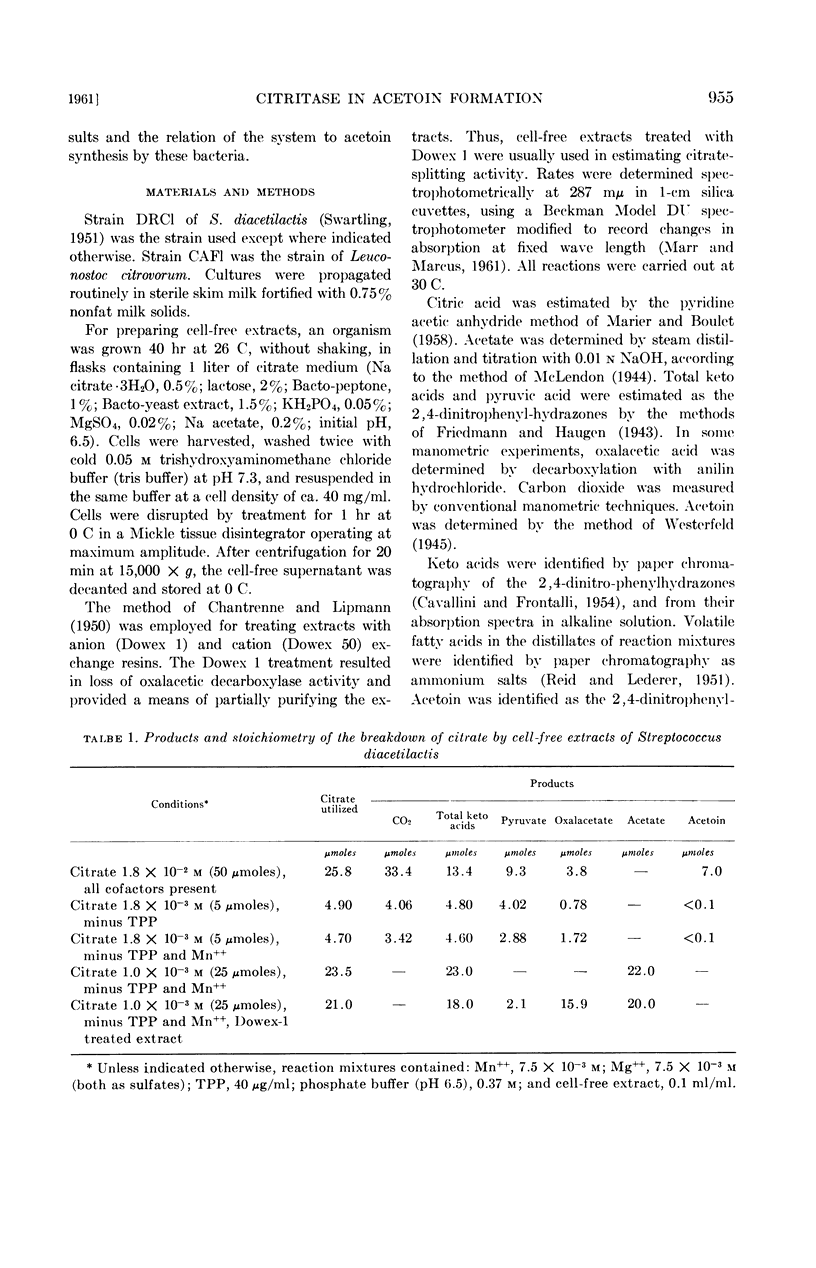
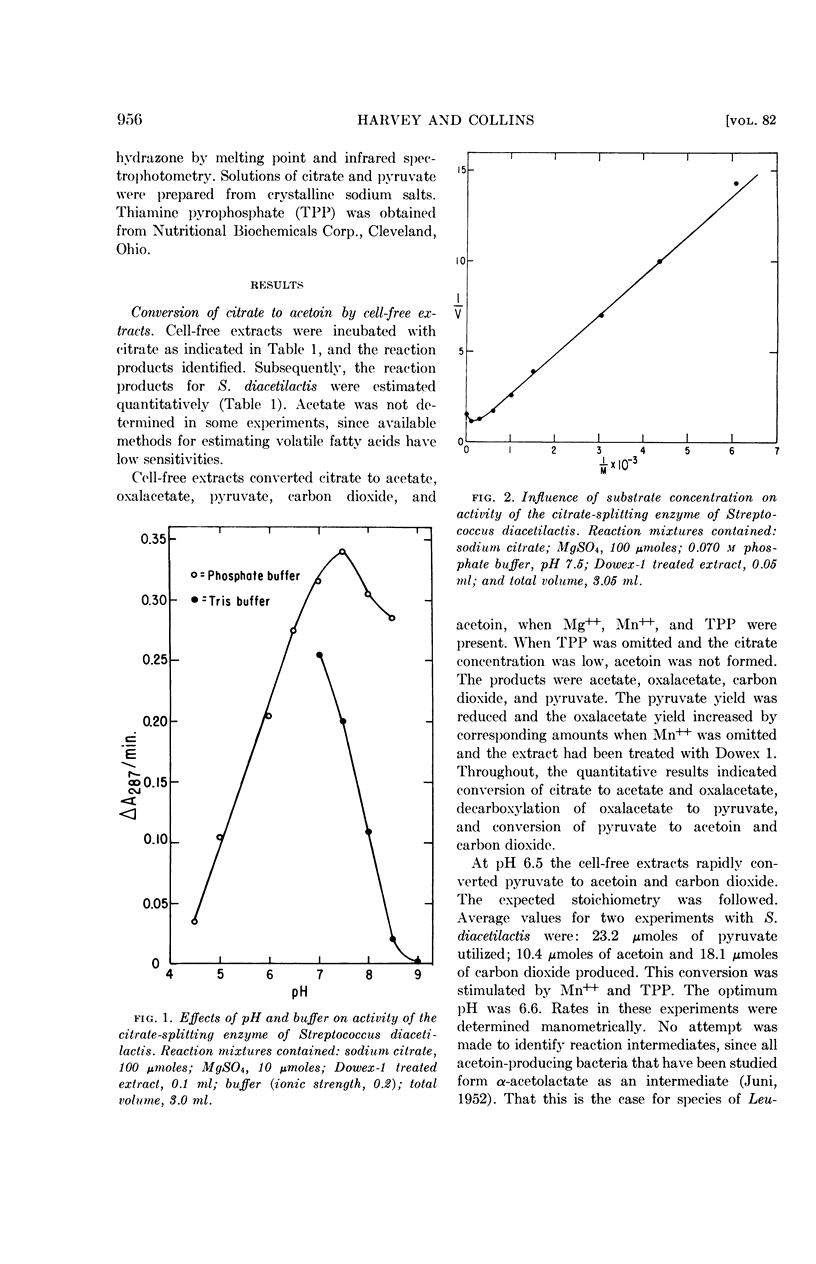
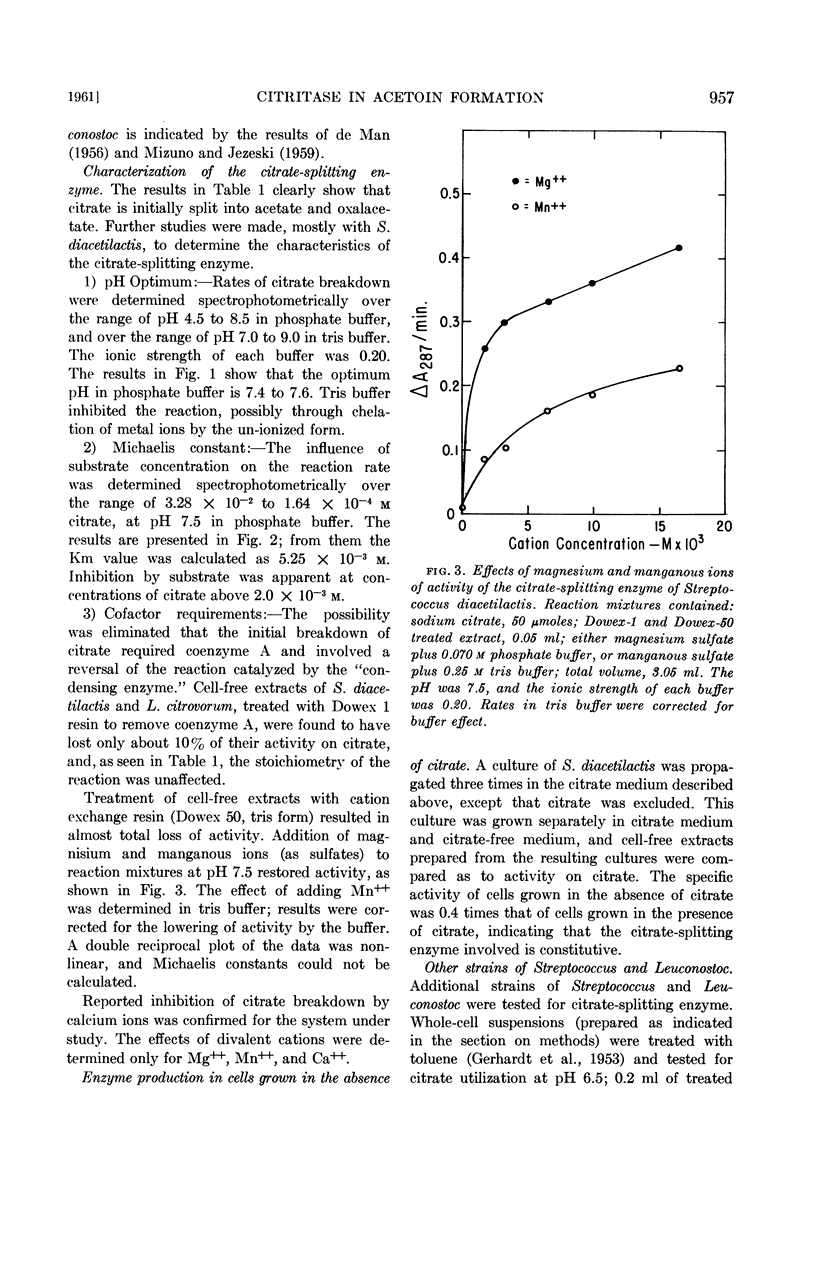
Harvey, R. J. (University of California, Davis) and E. B. Collins. Role of citritase in acetoin formation by Streptococcus diacetilactis and Leuconostoc citrovorum. J. Bacteriol. 82:954–959. 1961.—Cell-free extracts of Streptococcus diacetilactis and Leuconostoc citrovorum converted citrate to acetate, oxalacetate, pyruvate, carbon dioxide, and acetoin. The products, stoichiometry, and cofactor requirements of the citrate-splitting reaction were identical to those reported for citritase. Coenzyme A was not required; the reaction was stimulated by magnesium or manganous ions, and inhibited by calcium ions. In S. diacetilactis the enzyme is constitutive; it has been found inducible in all other organisms that have been studied. Ten strains of S. diacetilactis, three strains of Leuconostoc, and one strain of S. liquefaciens contained the enzyme; 21 strains of S. cremoris and 3 strains of S. lactis did not. Cell-free extracts of S. diacetilactis and L. citrovorum converted pyruvate to acetoin and carbon dioxide in the presence of manganous ions and thiamine pyrophosphate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAVALLINI D., FRONTALI N. Quantitative determination of keto-acids by paper partition chromatography. Biochim Biophys Acta. 1954 Mar;13(3):439–445. doi: 10.1016/0006-3002(54)90351-0. [DOI] [PubMed] [Google Scholar]
- CHANTRENNE H., LIPMANN F. Coenzyme A dependence and acetyl donor function of the pyruvate-formate exchange system. J Biol Chem. 1950 Dec;187(2):757–767. [PubMed] [Google Scholar]
- Campbell J. J., Gunsalus I. C. Citric Acid Fermentation by Streptococci and Lactobacilli. J Bacteriol. 1944 Jul;48(1):71–76. doi: 10.1128/jb.48.1.71-76.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., DAWES E. A. Critic acid metabolism of Aerobacter aerogenes. J Bacteriol. 1953 Sep;66(3):259–265. doi: 10.1128/jb.66.3.259-265.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., MAC GREGOR D. R., MARR A. G., OLSEN C. B., WILSON J. B. The metabolism of brucellae: the role of cellular permeability. J Bacteriol. 1953 May;65(5):581–586. doi: 10.1128/jb.65.5.581-586.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNI E. Mechanisms of formation of acetoin by bacteria. J Biol Chem. 1952 Apr;195(2):715–726. [PubMed] [Google Scholar]
- REID R. L., LEDERER M. Separation and estimation of saturated C2-C7 fatty acids by paper partition chromatography. Biochem J. 1951 Nov;50(1):60–67. doi: 10.1042/bj0500060b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH R. A., STAMER J. R., GUNSALUS I. C. Citritase and isocitritase reactions: equilibria-energetics. Biochim Biophys Acta. 1956 Mar;19(3):567–568. doi: 10.1016/0006-3002(56)90493-0. [DOI] [PubMed] [Google Scholar]
- WHEAT R. W., AJL S. J. Citritase, the citrate-splitting enzyme from Escherichia coli. I. Purification and properties. J Biol Chem. 1955 Dec;217(2):897–907. [PubMed] [Google Scholar]
- WHEAT R. W., AJL S. J. Citritase, the citrate-splitting enzyme from Escherichia coli. II. Reaction mechanisms. J Biol Chem. 1955 Dec;217(2):909–920. [PubMed] [Google Scholar]



