Abstract
Patients with allergic rhinitis have traditionally been placed into ‘seasonal’ and ‘perennial’ categories, which do not account for the subclinical inflammatory state that exists in many patients. In subjects with seasonal and perennial allergic rhinitis, even subthreshold doses of allergen have been found to cause inflammatory cell infiltration in the nasal mucosa, including increases in expression of cellular adhesion molecules, nasal and conjunctival eosinophilia, and other markers of inflammation, which do not result in overt allergy symptoms. This state – which has been termed ‘minimal persistent inflammation’– may contribute to hyperreactivity and increased susceptibility to development of clinical symptoms as well as common co-morbidities of allergic rhinitis, such as asthma. Treating overt allergy symptoms as well as this underlying inflammatory state requires agents that have well-established clinical efficacy, convenient administration, potent anti-inflammatory effects and proven long-term safety, so that long-term continuous administration is feasible. Of the three major classes of commonly used allergic rhinitis medications – intranasal corticosteroids, anti-histamines, and anti-leukotrienes – intranasal corticosteroids appear to represent the most reasonable therapeutic option in patients who would benefit from continuous inhibition of persistent inflammation.
Keywords: allergic rhinitis, intranasal corticosteroids, minimal persistent inflammation, nasal allergy inflammation
Introduction
Allergic rhinitis (AR) is an inflammatory condition of the nasal mucosa elicited by an interaction between environmental allergens and immunoglobulin (Ig)E in sensitized individuals. It is characterized by nasal symptoms including congestion, sneezing, itching and rhinorrhoea, as well as ocular effects such as eye itching, tearing and redness. The rate of self-reported AR across Europe is as high as 18·7% [1]. In the United States, AR affects approximately 10–30% of adults [2] and up to 40% of children, or an estimated 20–40 million patients [2], making it the sixth most common chronic illness [3]. Moreover, up to one-third of patients with allergies never visit a physician, suggesting that AR's true prevalence may be underestimated [4]. AR prevalence rates have increased in recent decades [5], most notably in low-prevalence countries [6] and among children [5,6]. Although long-term changes in pollen levels will probably vary by region, current climate models predict an earlier onset [7,8] and extended duration [9,10] of seasonal allergens. Warmer temperatures also increase pollen quantity [8,11–15]; thus, the prevalence and severity of allergic diseases is likely to increase over time.
Pollen allergens are seasonal while dust mites and animal dander are present year-round, and AR has been classified traditionally as seasonal (SAR) or perennial (PAR) [16]. However, individuals sensitized to seasonal allergens may experience symptoms throughout the year, and those sensitized to perennial allergens may experience intermittent symptoms. Because of these findings, the Allergic Rhinitis and its Impact on Asthma (ARIA) group has proposed the new classifications of intermittent AR, in which symptoms occur < 4 days per week or < 4 consecutive weeks per year, and persistent AR, in which symptoms are present > 4 days per week and > 4 consecutive weeks per year [6]. Many practitioners utilize the ARIA classification because it focuses on relevant characteristics of patients' symptoms [17].
Allergen exposure varies depending on the time of year and the success of allergen-avoidance measures. Patients may appear asymptomatic during periods of low allergen exposure; however, chronic up-regulation of inflammatory cells and mediators has been observed in nasal passages of AR patients during symptom-free periods [6]. This ‘minimal persistent inflammation’[6,18] primes the nasal mucosa, leading to increased sensitivity to allergens and non-specific irritants, and increased inflammatory response to a given level of allergen exposure [19–24].
While AR treatment is guided typically by the need to reduce symptoms, e.g. at the start of allergy season, symptom-based therapy does not address inflammation that is present during symptom-free periods [e.g. minimal persistent inflammation (MPI)]. A number of authors have suggested that treatment strategies that reduce inflammation during asymptomatic periods may have positive effects on the onset, progression and severity of AR [25–28]. This paper reviews the pathophysiological processes underlying MPI and discusses the potential impact of treatment strategies to address these processes.
Pathophysiology
AR is a prototypical immediate hypersensitivity reaction, wherein the binding of allergen to mast cell-bound IgE results in rapid mast cell degranulation, increased levels of inflammatory mediators, local infiltration of inflammatory cells and, in many cases, a recurrence of symptoms several hours after initial allergen exposure [29]. This response can be described as an initial allergen sensitization during which individuals with genetic and environmental risk factors develop hypersensitivity to specific allergen(s), followed by triggering of the acute response in which subsequent allergen exposure results in the rapid release of inflammatory mediators [18,30].
Sensitization
Atopy begins with the establishment of allergen sensitization. Initial sensitizing exposure may occur in utero[31], and sensitivity is often established very early in childhood [32]. Intensity and persistence of exposure during the first years of life appears to influence whether the initial sensitization will progress to frank allergic disease or regress to a non-atopic phenotype [33]. After sensitization has been established [30], interleukin (IL)-4 interacts with the antigen-major histocompatibility complex (MHC) on activated antigen-presenting cells (APC) to stimulate the differentiation of naive T cells [T helper type 0 (Th0)] into Th2 cells. Atopy-promoting Th2 cells release a number of proinflammatory cytokines (IL-2, IL-3, IL-4, IL-5, IL-9, IL-10, IL-13) and granulocyte–macrophage colony-stimulating factor (GM-CSF) [30,34], whose effects include differentiation and localization of immune cells to the site of exposure; IgE-type class switching of B cells; and increased synthesis of IgE, which binds to specific receptors on mast and other immune cells [30] (Fig. 1).
Fig. 1.
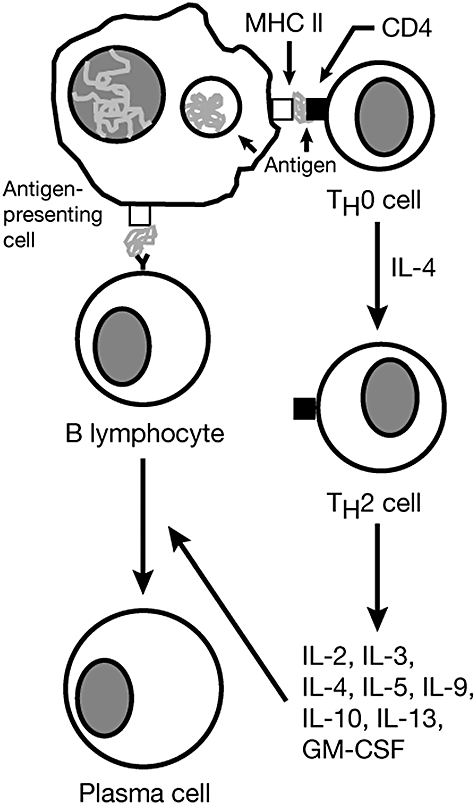
Simplified schema of the differentiation of T helper type 2 (Th2) cells and activation of B lymphocytes in the establishment of sensitization to airway allergens. Reprinted from Journal of Allergy and Clinical Immunology, volume 104, number 4, part 1, DS Pearlman, Pathophysiology of the inflammatory response, pages S132–S137, copyright © 1999, with permission from Elsevier [30]. GM-CSF: granulocyte macrophage colony-stimulating factor; MHC II: major histocompatibility complex II.
Acute- and late-phase response
Asymptomatic up-regulation of inflammation occurring during the sensitization phase makes possible the symptomatic acute-phase response. Mast cell-derived mediators (histamine, leukotrienes, platelet-activating factor, bradykinin, etc.) cause the classic early-phase symptoms of AR (congestion, itching, sneezing and rhinorrhoea) [35]. While acute symptoms often disappear within 1 h [36], these early-phase mediators also initiate a complex network of inflammatory phenomena in the nasal mucosa – involving adhesion molecules, Th2 cells, cytokines and other inflammatory mediators [35]– that evolves over several hours following allergen provocation [30]. Components of this inflammatory cascade, including cytokines, chemokines and leukotrienes, stimulate proliferation and inhibit apoptosis of immune cells [37]. They also act as chemoattractants, promoting migration and infiltration of immune cells at the challenge site [37]. In addition, early-phase mediators increase expression of cell-surface adhesion molecules [intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1)] on endothelial and epithelial cells in the nasal mucosa [38,39], which promote migration of inflammatory cells (eosinophils, basophils and neutrophils) from the circulation and cell adhesion to the inflammation site [27,30,35] (Fig. 2).
Fig. 2.
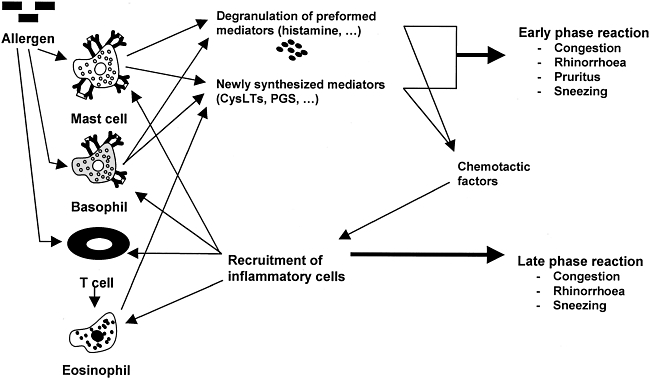
Allergen-induced mast cell degranulation initiates the inflammatory cascade. Histamine and other mast cell-derived mediators (CysLTs, etc.) cause early phase symptoms within seconds. Local infiltration of inflammatory cells (eosinophils, basophils) occurs in response to up-regulation of chemokines [regulated upon activation normal T cell expressed and secreted (RANTES), eotaxin, etc.], adhesion molecules [intercellular adhesion molecule-1 (ICAM-1), etc.], and growth factors [interleukin (IL)-4, granulocyte–macrophage colony-stimulating factor (GM-CSF), etc.]. Infiltration leads to further release of inflammatory mediators and hypersensitivity of the nasal mucosa, which contribute to late phase symptoms and priming. Adapted with permission from Storms WW. Minimal persistent inflammation, an emerging concept in the nature and treatment of allergic rhinitis: the possible role of leukotrienes. Ann Allergy Asthma Immunol 2003; 91:131–40. Copyright 2003 by American College of Allergy, Asthma and Immunology [27]. CysLT: cysteinyl leukotriene; PG: prostaglandin; VCAM: vascular cell adhesion molecule.
Inflammatory cell infiltration and accumulation of activated eosinophil products is credited with inducing the late-phase response [30], characterized by a recurrence of symptoms 3–11 h following initial challenge, in up to 80% of patients with AR [40,41]. Subjects who develop late-phase symptoms have been found to have significantly higher numbers of eosinophils and neutrophils in nasal lavage samples [42–44]. Activated eosinophils secrete eosinophil cationic protein (ECP) and other mediators that stimulate eosinophil proliferation, migration and adhesion [30]; amplify production of Th2 cytokines [37,27]; and damage endothelial cells. ECP levels in nasal lavage samples have also been shown to correlate with symptoms 24 h later [45].
Repeated exposure to nasal allergens leads to long-term changes in local and systemic inflammation, including up-regulation of circulating eosinophils and allergen-specific IgE [46], increased levels of adhesion molecules in airway mucosa [18] and enhanced systemic response to allergen challenge [46]. Furthermore, in children with asthma, early sensitization and chronic exposure to perennial allergens may be significantly detrimental to long-term lung function [33].
Minimal persistent inflammation
In studies in the late 1960s, Connell identified and characterized ‘priming’, a local, reversible and non-specific up-regulation of sensitivity and responsiveness to allergen that follows repeated allergen exposure [47]. These experiments assessed changes in post-challenge nasal symptoms and allergen threshold dose, defined as a 33–50% reduction in nasal patency, in pollen-sensitive subjects who underwent repeated allergen challenge. Subjects with out-of-season AR were challenged on 4 consecutive days; with each successive daily challenge, post-challenge symptoms occurred earlier and were more severe, even as the allergen threshold dose decreased. Subjects were then challenged weekly throughout the pollen season. An inverse relationship between environmental allergen levels and allergen threshold dose was noted; allergen threshold dose decreased from preseason to midseason and increased from midseason to end of season.
When allergen exposure ceased, subjects reverted to a non-hypersensitive, non-primed state, with recovery rates varying by intensity of the priming process. In controlled challenge experiments, allergen threshold dose decreased and recovery interval increased with successive weekly priming episodes. Following priming by environmental allergens during that allergy season, increased sensitivity could be demonstrated for up to 2 months after the end of pollen season. These experiments also showed that priming with one allergen results in hypersensitivity to other allergens and that priming is a local phenomenon; subjects who underwent unilateral nasal challenge demonstrated hypersensitivity only in the challenged nostril.
In the intervening 4 decades, our understanding of the biochemical and cellular mechanisms involved in priming has increased substantially. More recently, the term ‘MPI’ has been used to describe a phenomenon whereby repeated exposure to low levels of allergen produces no allergy symptoms but does elicit a state of heightened sensitivity to subsequent allergen exposure [18,48] (Fig. 3). In the nasal mucosa, MPI is characterized by the presence of inflammatory cells (eosinophils, neutrophils) and increased ICAM-1 expression on epithelial cells [49], which have been documented in patients with SAR and PAR during symptom-free periods [18,42,50]. Building on Connell's work [19,47], MPI offers a theoretical construct to explain the priming effect, and suggests that patients with MPI may be at increased risk for developing allergy symptoms and therefore may benefit from anti-inflammatory treatment during symptom-free periods.
Fig. 3.
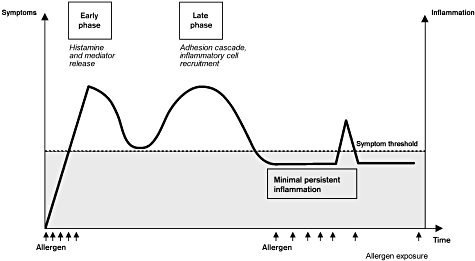
The paradigm of minimal persistent inflammation. When subliminal exposure to an allergen occurs, the patient is symptom free but subclinical inflammation is present. Reprinted with permission from Passalacqua G, Ciprandi G, Canonica GW. The nose-lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol 2001; 1:7–13, copyright Lippincott Williams & Wilkins [48].
Patients with PAR, typically sensitized to allergens that are present in the environment year-round (animal dander, household dust mites, etc.) are subject to persistent natural allergen exposure even when AR symptoms are not clinically evident [18]. Indeed, studies have repeatedly found evidence of significant nasal inflammation, including increased numbers of eosinophils and neutrophils, increased markers of inflammatory cell activation (tryptase, eosinophil protein X and myeloperoxidase) and increased ICAM-1 expression in samples obtained from nasal airways of asymptomatic subjects with PAR [18,42,44,51]. Data suggest that, although clinically silent, MPI reduces the dose of allergen required to provoke allergy symptoms. Allergen threshold dose was lower in mite-sensitive subjects compared with out-of-season pollen-sensitive subjects [42]. In symptom-free subjects with PAR, reduction in threshold dose was inversely correlated with prechallenge eosinophil levels [44], and occurrence of late-phase symptoms was also associated with higher numbers of prechallenge inflammatory cells in these subjects [42,44]. However, no correlation between prechallenge nasal ICAM-1 levels and occurrence of a late-phase response was noted [42].
Unlike mite- and dander-sensitive patients, individuals with SAR are typically sensitized to pollen and other allergens that are essentially absent outside of the allergy season. In studies performed during the winter months, no significant differences between pollen-sensitive subjects and non-allergic controls were noted regarding numbers of inflammatory cells (eosinophils, mast cells), markers of eosinophil activation or expression of ICAM-1 [42,44,52] in nasal lavage or brush samples. However, number of prechallenge mast cells correlated positively with severity of post-challenge sneezing and congestion, as well as with the number of late-phase eosinophils [52]. Subjects with out-of-season SAR also demonstrated increased levels of histamine and ECP in nasal lavage samples when challenged repeatedly with 1/100th of the allergen dose required to elicit symptoms [53].
A different picture emerges when subjects with SAR are examined proximal to the onset of seasonal allergen exposure. Significant nasal inflammation has been demonstrated in asymptomatic subjects with SAR who were assessed during the first week of the season and after the end of seasonal allergy symptoms [50,54]. Ricca et al. demonstrated that inflammation is present prior to the initial onset of allergy symptoms, during symptom-free periods and for at least 4 weeks after pollen counts and symptoms had returned to baseline levels (Figs 4 and 5) [50]. Increases in eosinophils, neutrophils and ICAM-1 expression preceded the onset of allergy symptoms, which were clinically evident only after pollen counts increased dramatically from day 10 onward. Similarly, Bachert et al. found that IL-1, leukotrienes, ECP and histamine levels in nasal secretions remained elevated significantly 6 weeks after pollen levels and symptom scores had returned to preseason levels [44,54].
Fig. 4.
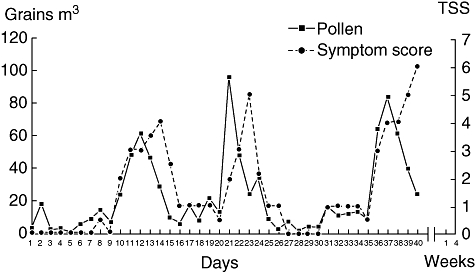
Mode of daily total symptom scores and pollen counts in subjects with seasonal allergic rhinitis. Reprinted from Ricca V, Landi M, Ferrero P et al. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol 2000; 105:54–7, with permission from Elsevier [50]. TSS: total symptom score.
Fig. 5.
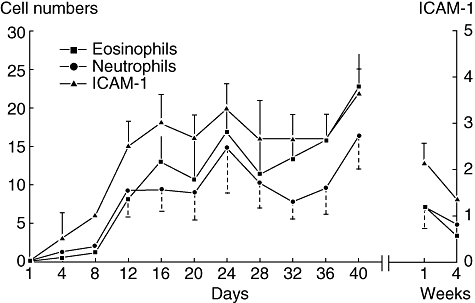
Eosinophil and neutrophil counts in nasal scrapings and intercellular adhesion molecule-1 (ICAM-1) positivity on nasal epithelial cells (± standard deviation) in subjects with seasonal allergic rhinitis. Reprinted from Ricca V, Landi M, Ferrero P et al. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol 2000; 105:54–7, with permission from Elsevier [50].
ICAM-1 and asthma in MPI
By stimulating migration and adherence of inflammatory cells, ICAM-1 mediates local infiltration at the site of allergen challenge; increased expression following allergen challenge is essential to the enduring up-regulation of inflammatory cells in AR [35,55]. Patients with AR show an increased risk of developing asthma and vice versa [56–59], and it has been suggested that up-regulation of ICAM-1 may be an important mechanistic linkage between these two diseases [60,61]. Functionally, subjects with AR, with or without co-morbid asthma, demonstrate increased lower airway constriction, increased sensitivity to bronchoconstricting agents and increased inflammatory cells in sputum samples following nasal allergen challenge [62,63]. Severity of allergen-induced nasal inflammation appears to correlate with the resulting pulmonary response, in that there is an inverse correlation between nasal lavage ICAM-1 and IL-6 levels and pulmonary function [62]. In addition, subjects with active AR symptoms demonstrated increased lower airway eosinophils [60,61], whereas subjects with out-of-season SAR did not [63]. Sputum samples from the latter group did show increased early markers of inflammation, including ICAM-1 and ECP [63], suggesting that MPI may be present throughout the upper and lower airways during symptom-free periods.
In addition to being a receptor for T cell-specific ligands [64], ICAM-1 is also the major receptor for human rhinoviruses [65], a frequent cause of upper respiratory infections in children. Up-regulation of ICAM-1 is associated with increased susceptibility to infections [35], which are an important trigger of asthma exacerbations. Some authors have suggested that chronic up-regulation of ICAM-1 may increase susceptibility to asthma exacerbation [66–68].
Patients with PAR have a more pronounced and sustained inflammation of nasal mucosa, which may explain the higher risk of asthma in patients with AR sensitive to perennial compared with seasonal allergens [69]. Studies have also shown the clinical relevance of upper airway inflammation in lower airway disease. Patients with co-morbid AR and asthma who received treatment for their AR symptoms had lower utilization of healthcare resources compared with those who did not receive AR-specific therapy [70].
Clinical implications
These data suggest that MPI may have a priming effect, resulting in a hyperreactive state in which the threshold dose of allergen is reduced and the severity and duration of allergic response is increased [27,47]. When exposure to allergen is too low to provoke symptoms, a weak inflammatory infiltration occurs in the nasal mucosa [71]. Some authors have suggested that clinically apparent symptoms represent only the ‘tip of the iceberg’ of the allergic reaction, although the consequences of occult inflammation and hyperreactivity may be substantial [50]. From a clinical perspective, while allergen avoidance is an essential part of disease management, avoidance measures alone are generally ineffective at improving symptoms [6]. This suggests that therapeutic strategies for AR should be revised and aimed at reducing inflammatory phenomena as well as symptoms, i.e. continuous treatment throughout the entire period of allergen exposure rather than on a symptomatic, as-needed basis [50]. Currently, three major options that may have effects on MPI – the anti-histamines, the anti-leukotrienes and intranasal corticosteroids (INS) – are available for treating AR.
Anti-histamines
Anti-histamines improve early-phase H1-receptor-mediated symptoms such as sneezing, itching, rhinorrhoea and, to a lesser degree, nasal congestion [72]. In in vitro studies, loratadine and desloratadine inhibited significantly histamine-induced expression of ICAM-1, P-selectin, IL-6 and IL-8 [73,74], with desloratadine demonstrating more potent cytokine inhibition than loratadine [74]. Desloratadine has also been shown to inhibit phorbol 12-myristate 13-acetate-induced expression of IL-4 [75] and decrease eosinophil viability [76]. Jiinquan et al. assessed ex vivo leucocyte migration and ECP production in subjects who received high-dose cetirizine (10–20 mg/day) [77]. Although cetirizine inhibited migration significantly, there was no effect on ECP levels. In clinical trials of patients with symptomatic AR, significantly decreased levels of nasal mucosal inflammatory cells and mediators have been observed after treatment with two different second-generation anti-histamines [1,78,79].
Although anti-histamines are used commonly on demand, some authors have suggested that continuous use of anti-histamines may reduce MPI by reducing the infiltration of inflammatory cells [49,80]. The effects of continuous anti-histamine therapy on allergen sensitivity or responsiveness in symptom-free patients with AR have not been evaluated, but studies have compared inflammatory markers in patients receiving continuous versus on-demand therapy. In pollen-allergic subjects, cetirizine [81] and azelastine [82] administered continuously for 4 and 12 weeks, respectively, were significantly more effective than on-demand treatment in reducing nasal eosinophils and neutrophils; azelastine also inhibited ICAM-1 expression [82]. Greater reductions in adhesion molecules and eosinophils were also observed with daily versus on-demand treatment with loratadine or cetirizine in patients sensitized to perennial allergens [83,84]. No difference in nasal lavage ECP levels was observed after 1 month of continuous compared with on-demand desloratadine in pollen-sensitive children [85]. Similarly, no significant difference in nasal eosinophils or ICAM-1 levels was observed after 6 months of continuous or on-demand levocetirizine in patients with persistent AR [86]. Twelve months of continuous terfenadine was compared with placebo in mite-allergic children with AR and/or asthma (on-demand therapy was not assessed) [87]. Nasal eosinophils, neutrophils and ICAM-1 were reduced with terfenadine versus placebo at some but not all monthly assessments.
Second-generation anti-histamines effectively reduce early-phase symptoms, but their effects on inflammation are less consistent [27]. In addition, greater improvement in nasal symptoms with continuous versus on-demand anti-histamine treatment has not been demonstrated consistently [81,82,85,86]. Decreased bronchial hyperreactivity was also observed in two studies [81,85]. These data suggest that continuous therapy results in better clinical outcomes compared with on-demand therapy. However, because allergy symptoms were present when treatment was initiated in the studies cited [81,82,85,86], these data reflect the impact of continuous treatment on clinically apparent inflammation rather than on changes in subclinical MPI levels.
Anti-leukotrienes
Cysteinyl leukotrienes are important mediators of nasal allergy symptoms, particularly nasal congestion [88] and leukotriene receptor antagonists are recommended for treatment of moderate-to-severe AR and asthma/AR co-morbidity [89]. These agents have been shown to reduce levels of IL-4 and IL-13 and to increase interferon-γ, a Th1 cytokine, thereby shifting an atopic Th2 cytokine pattern towards a non-atopic Th1 pattern [90]. Leukotriene receptor antagonists also reduce peripheral eosinophilia in patients with AR [91]; because local infiltration is dependent upon circulating eosinophils, it has been suggested that this confers a beneficial effect on MPI [27]. However, studies assessing the effects of leukotriene receptor antagonists on nasal infiltration are, as yet, unpublished.
Intranasal corticosteroids
Perhaps the strongest evidence exists for treating MPI with INS. These agents are the most potent medications available for management of AR, particularly in patients with moderate-to-severe disease [2,6,16,89]. Intranasal corticosteroids are highly effective in reducing early- and late-phase nasal congestion, rhinorrhoea, sneezing and nasal itching in SAR and PAR, without the side effects associated with systemic glucocorticosteroids [92]. Two large meta-analyses found superior efficacy for INS compared with oral or topical anti-histamines in reducing nasal symptoms and at least equal efficacy at relieving ocular symptoms [93,94].
The mechanisms by which glucocorticoids inhibit allergic inflammation are complex and not understood completely; however, efficacy is thought to owe to their effects on regulating expression of proteins associated with inflammation [95]. In the cytoplasm, glucocorticoids bind to and activate glucocorticoid receptors. This glucocorticoid receptor complex regulates DNA transcription by binding to positive and negative glucocorticoid response elements in promoter-activator regions of target genes [95]. Inhibition of gene expression also occurs via interactions between the glucocorticoid receptor complex and cytoplasmic transcription factors such as nuclear factor (NF)-κB and activator protein-1 (AP-1) [95–97].
Although the exact target genes are unknown [97], the downstream effect of INS appears to be down-regulation of the expression of a number of cytokines (IL-1, IL-3, IL-4, IL-5, IL-6, Il-10, IL-13, TNF-α, GM-CSF) and chemokines [IL-8, regulated upon activation normal T cell expressed and secreted (RANTES), eotaxin] that promote the proliferation, infiltration and activation of inflammatory cells [95,98–101]. Pretreatment with fluticasone propionate (FP) has also been shown to inhibit post-challenge cytokine mRNA levels in nasal biopsy samples from subjects with AR [102]. Differences in cytokine inhibition have been demonstrated among INS agents, with greater potency in vitro against atopy-promoting Th2 cytokines (IL-4, IL-5) observed with mometasone furoate (MF) and FP compared with older agents such as beclomethasone dipropionate (BDP), budesonide (BUD) and triamcinolone [98]. In addition, pretreatment with INS may shift post-challenge cytokine expression from a Th2 pattern towards a non-atopic Th1 pattern [103,104].
The impact of INS on inflammatory mediators and cells that are the basis for nasal priming and hyperresponsiveness has been well described. In clinical trials of subjects with AR, INS inhibited allergen-induced expression of ICAM-1 on nasal epithelial cells [100,105]. Nasal airway infiltration, activation and survival of inflammatory cells such as eosinophils, basophils and mast cells were also reduced with INS treatment [104,106–110]. Although INS such as MF, FP and fluticasone furoate have exceedingly low systemic absorption [111,112], INS also inhibit systemic up-regulation of the inflammatory markers, including post-challenge circulating allergen-specific IgE antibodies [113] and eosinophil-progenitor cells [114,115].
INS also decrease specific and non-specific sensitivity in atopic nasal tissue, suggesting inhibition of the underlying inflammation. Although not classed as mast cell stabilizers, INS inhibit the allergen-induced release of histamine [99] and other mast cell-derived mediators in patients with AR [37,99,116–120]. In addition, INS increase the threshold dose of allergen [116] and histamine [117,121] required to elicit allergy symptoms. INS dramatically reduce or eliminate antigen-presenting Langerhans cells in the nasal epithelia [106,122–124] which may, in part, explain the effect of INS to eliminate the increased sensitivity seen in untreated, allergen-primed subjects with AR [116]. Furthermore, treatment with INS has been shown to reduce the number of emergency room visits in patients with both AR and asthma [70,125].
Because subjects with MPI display increased sensitivity to allergen challenge [20], delay in onset of seasonal symptoms may be a clinical end-point for MPI inhibition. Prophylactic administration of INS has been shown to delay onset and reduce symptom severity in adults with SAR [25,26,28,126,127]. Randomized, placebo-controlled trials have compared once-daily MF with once-daily BUD or twice-daily BDP, initiated 4 weeks prior to the start of allergy season and continuing for 4 weeks into the season [25,26]. In both studies, prophylaxis with MF, BUD or BDP delayed significantly the onset of non-minimal symptoms (MF 27 days, BDP 27 days [25]; MF 26 days, BUD 34 days [26]) and resulted in a significantly higher proportion of days with no or minimal symptoms after the start of allergy season (MF 83%, BDP 77% [25]; MF 81%, BUD 82% [26]) versus placebo (64% [25]; 63% [26]). While no significant differences between active groups were observed for the above outcomes in either study, one study found that preseason nasal symptoms were significantly lower with MF versus BDP, as well as a trend towards longer delay to moderate-to-severe symptoms (P = 0·08) [25].
In a dose-ranging trial of BUD in SAR prophylaxis, 364 subjects were randomized to begin BUD therapy (200 µg or 400 µg) or placebo 4 weeks prior to the pollen season, and then continued on one of the above doses of BUD after the start of the pollen season for 6 more weeks [127]. Subjects who received BUD prophylaxis had significantly lower symptom scores during only the first week of the pollen season compared with those who received preseason placebo. However, compared with subjects who received low-dose pre- and in-season treatment, subjects who received high-dose (400 µg) followed by low-dose (200 µg) treatment had numerically lower total and individual nasal symptoms for an additional 2–5 weeks. These results suggest that more potent suppression of inflammation in MPI can have lasting effects on symptoms during the pollen season [127].
Studies have also compared the efficacy of INS versus other AR treatment options for prophylaxis of SAR. Pullertis et al. compared FP, montelukast, montelukast with loratadine and placebo, each administered once daily, in 62 subjects with SAR. Treatment was initiated 2–3 weeks prior to the anticipated start of allergy season and continued throughout the season [128]. Daytime and night-time symptom scores were consistently lower with FP compared with the two other active treatment arms throughout the study [128], and these differences were increasingly evident as the pollen season progressed. In addition, treatment with FP abolished completely the pollen-induced increases in nasal eosinophils that were observed in the active- and placebo-treated arms [128]. Two studies have compared INS and mast cell stabilizers, which are indicated outside the United States for prophylaxis of SAR [129]. In a study by Bousquet et al., subjects received disodium cromoglycate four times daily or FP once daily for 6 weeks, starting at least 1 week prior to the allergy season [126]. Subjects receiving FP reported significantly higher percentages of days without nasal symptoms, while reduction in ocular symptoms favoured disodium cromoglycate. However, approximately one-quarter of subjects were excluded from analysis, primarily for non-adherence, due probably to the required four-times-daily administration in both groups [126]. In addition, treatment was initiated after the start of pollen season in 18·3% of subjects included in the analysis; therefore, these results are not from a rigorously defined prophylactic regimen. In a recent study by Pitsios et al., treatment was initiated 2–4 weeks before allergy season with once-daily MF or thrice-daily nedocromil and continued for up to 4 months [28]. Subjects receiving MF reported significantly more minimal-symptom days (77·6% versus 57·3%, P < 0·01) and lower mean nasal symptom scores (1·46 versus 3·02). In contrast to the study by Bousquet et al., all subjects completed the study; furthermore, all subjects were asymptomatic when treatment was initiated. MF was approved for prophylactic use in adults with allergen-identified SAR [130] and it is the only INS with this indication [131].
Conclusion
Allergen provocation in AR results in an acute- and late-phase inflammatory response characterized by up-regulation of inflammatory mediators and inflammatory cell infiltration of the nasal mucosa. A subclinical inflammatory state has also been described in symptom-free patients allergic to house dust mites or pollens, in which subthreshold doses of allergen stimulate small but significant increases in expression of cellular adhesion molecules, nasal and conjunctival eosinophilia and other inflammatory markers. This MPI appears to be present year-round in PAR and periseasonally in SAR, and may have a priming effect by increasing allergen sensitivity as well as an inflammatory response to allergen provocation. Therefore, treatment options that target underlying inflammation along with symptom relief should be considered. Further research is needed regarding the clinical relevance of MPI, and the timing and duration of treatment of subclinical inflammation. In light of the clear relationship between the upper and the lower airways [6], the relevance of nasal MPI to the lower airway inflammation has to be considered. Concerns have been raised regarding patient compliance in the absence of symptoms, but patients who are prone to more frequent exacerbations would be more likely to adhere to continuous therapy [27].
Acknowledgments
Editorial support was provided by Karl Torbey, MD. This assistance was funded by Schering-Plough.
Disclosure
Dr G.W. Canonica reports having received honoraria for educational presentations, and/or funding for research, and/or travel expenses, and/or for service in advisory boards from: Menarini, Alk Abello, Almirall, Altana, Astra Zeneca, Boeringher Ingelheim, Chiesi Farmaceutici, Gentili, GSK, Lofarma, MSD, Novartis, Pfizer, Schering Plough, Stallergenes, UCB Pharma, Uriach Valeas. Enrico Campalati reports no conflicts of interests.
References
- 1.Canonica GW, Tarantini F, Compalati E, Penagos M. Efficacy of desloratadine in the treatment of allergic rhinitis: a meta-analysis of randomized, double-blind, controlled trials. Allergy. 2007;62:359–66. doi: 10.1111/j.1398-9995.2006.01277.x. [DOI] [PubMed] [Google Scholar]
- 2.Dykewicz MS, Fineman S. Executive summary of joint task force practice parameters on diagnosis and management of rhinitis. Ann Allergy Asthma Immunol. 1998;81:463–8. doi: 10.1016/S1081-1206(10)63152-3. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Management of allergic and nonallergic rhinitis: summary. Rockville, MD: Agency for Healthcare Research and Quality; 2002. Evidence report/technical assessment number 54. [Google Scholar]
- 4.McMenamin P. Costs of hay fever in the United States in 1990. Ann Allergy. 1994;73:35–9. [PubMed] [Google Scholar]
- 5.Nimmagadda SR, Evans R., III Allergy: etiology and epidemiology. Pediatr Rev. 1999;20:111–15. doi: 10.1542/pir.20-4-110. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA2LEN and AllerGen) Allergy. 2008;63(Suppl. 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Mozo H, Galan C, Jato V, et al. Quercus pollen season dynamics in the Iberian peninsula: response to meteorological parameters and possible consequences of climate change. Ann Agric Environ Med. 2006;13:209–24. [PubMed] [Google Scholar]
- 8.Frei T, Gassner E. Climate change and its impact on birch pollen quantities and the start of the pollen season an example from Switzerland for the period 1969–2006. Int J Biometeorol. 2008;52:667–74. doi: 10.1007/s00484-008-0159-2. [DOI] [PubMed] [Google Scholar]
- 9.Emberlin J, Detandt M, Gehrig R, Jaeger S, Nolard N, Rantio-Lehtimäki A. Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int J Biometeorol. 2002;46:159–70. doi: 10.1007/s00484-002-0139-x. [DOI] [PubMed] [Google Scholar]
- 10.Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science. 2002;296:1689–91. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 11.Wayne P, Foster S, Connolly J, Bazzaz F, Epstein P. Production of allergenic pollen by ragweed (Ambrosia artemisiifolia L.) is increased in CO2-enriched atmospheres. Ann Allergy Asthma Immunol. 2002;88:279–82. doi: 10.1016/S1081-1206(10)62009-1. [DOI] [PubMed] [Google Scholar]
- 12.Ziska LH, Gebhard DE, Frenz DA, Faulkner S, Singer BD, Straka JG. Cities as harbingers of climate change: common ragweed, urbanization, and public health. J Allergy Clin Immunol. 2003;111:290–5. doi: 10.1067/mai.2003.53. [DOI] [PubMed] [Google Scholar]
- 13.Stach A, Emberlin J, Smith M, Adams-Groom B. Factors that determine the severity of Betula spp. pollen seasons in Poland (Poznań and Krakow) and the United Kingdom (Worcester and London) Int J Biometeorol. 2008;52:311–21. doi: 10.1007/s00484-007-0127-2. [DOI] [PubMed] [Google Scholar]
- 14.Emberlin J, Smith M, Close R, Adams-Groom B. Changes in the pollen seasons of the early flowering trees Alnus spp. and Corylus spp. in Worcester, United Kingdom, 1996–2005. Int J Biometeorol. 2007;51:181–91. doi: 10.1007/s00484-006-0059-2. [DOI] [PubMed] [Google Scholar]
- 15.D'Amato G. Environmental urban factors (air pollution and allergens) and the rising trends in allergic respiratory diseases. Allergy. 2002;57(Suppl. 72):30–3. doi: 10.1034/j.1398-9995.57.s72.5.x. [DOI] [PubMed] [Google Scholar]
- 16.van Cauwenberge P, Bachert C, Passalacqua G, et al. Consensus statement on the treatment of allergic rhinitis: European Academy of Allergology and Clinical Immunology. Allergy. 2000;55:116–34. doi: 10.1034/j.1398-9995.2000.00526.x. [DOI] [PubMed] [Google Scholar]
- 17.Demoly P, Allaert FA, Lecasble M, Bousquet J, PRAGMA Validation of the classification of ARIA (Allergic Rhinitis and its Impact on Asthma) Allergy. 2003;58:672–5. doi: 10.1034/j.1398-9995.2003.t01-1-00202.x. [DOI] [PubMed] [Google Scholar]
- 18.Ciprandi G, Buscaglia S, Pesce G, et al. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol. 1995;96:971–9. doi: 10.1016/s0091-6749(95)70235-0. [DOI] [PubMed] [Google Scholar]
- 19.Connell JT. Quantitative intranasal pollen challenges. 3. The priming effect in allergic rhinitis. J Allergy. 1969;43:33–44. doi: 10.1016/0021-8707(69)90018-5. [DOI] [PubMed] [Google Scholar]
- 20.Juliusson S, Bende M. Priming effect of a birch pollen season studied with laser Doppler flowmetry in patients with allergic rhinitis. Clin Allergy. 1988;18:615–8. doi: 10.1111/j.1365-2222.1988.tb02913.x. [DOI] [PubMed] [Google Scholar]
- 21.Wachs M, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Observations on the pathogenesis of nasal priming. J Allergy Clin Immunol. 1989;84:492–501. doi: 10.1016/0091-6749(89)90362-x. [DOI] [PubMed] [Google Scholar]
- 22.Naito K, Ishihara M, Senoh Y, Takeda N, Yokoyama N, Iwata S. Seasonal variations of nasal resistance in allergic rhinitis and environmental pollen counts. II. Efficacy of preseasonal therapy. Auris Nasus Larynx. 1993;20:31–8. doi: 10.1016/s0385-8146(12)80208-2. [DOI] [PubMed] [Google Scholar]
- 23.Koh YY, Lim HS, Min KU, Min YG. Airways of allergic rhinitics are ‘primed’ to repeated allergen inhalation challenge. Clin Exp Allergy. 1994;24:337–46. doi: 10.1111/j.1365-2222.1994.tb00244.x. [DOI] [PubMed] [Google Scholar]
- 24.Assing K, Bodtger U, Poulsen LK, Malling HJ. Grass pollen symptoms interfere with the recollection of birch pollen symptoms – a prospective study of suspected, asymptomatic skin sensitization. Allergy. 2007;62:373–7. doi: 10.1111/j.1398-9995.2006.01280.x. [DOI] [PubMed] [Google Scholar]
- 25.Graft D, Aaronson D, Chervinsky P, et al. A placebo- and active-controlled randomized trial of prophylactic treatment of seasonal allergic rhinitis with mometasone furoate aqueous nasal spray. J Allergy Clin Immunol. 1996;98:724–31. doi: 10.1016/s0091-6749(96)70119-7. [DOI] [PubMed] [Google Scholar]
- 26.Marazzi P, Nolop K, Lutsky BN, et al. Prophylactic use of once-daily mometasone furoate (Nasonex) aqueous nasal spray in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 1997;99:S440. Pt 2. [Google Scholar]
- 27.Storms WW. Minimal persistent inflammation, an emerging concept in the nature and treatment of allergic rhinitis: the possible role of leukotrienes. Ann Allergy Asthma Immunol. 2003;91:131–40. doi: 10.1016/S1081-1206(10)62167-9. [DOI] [PubMed] [Google Scholar]
- 28.Pitsios C, Papadopoulos D, Kompoti E, et al. Efficacy and safety of mometasone furoate vs nedocromil sodium as prophylactic treatment for moderate/severe seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96:673–8. doi: 10.1016/S1081-1206(10)61064-2. [DOI] [PubMed] [Google Scholar]
- 29.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin Exp Allergy. 2006;36:689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pearlman DS. Pathophysiology of the inflammatory response. J Allergy Clin Immunol. 1999;104:S132–7. doi: 10.1016/s0091-6749(99)70308-8. [DOI] [PubMed] [Google Scholar]
- 31.Kay AB. Allergy and allergic diseases. First of two parts. N Engl J Med. 2001;344:30–7. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 32.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 33.Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, the Multicentre Allergy Study (MAS) group Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368:763–70. doi: 10.1016/S0140-6736(06)69286-6. [DOI] [PubMed] [Google Scholar]
- 34.Baraniuk JN. Pathogenesis of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S763–72. doi: 10.1016/s0091-6749(97)70125-8. [DOI] [PubMed] [Google Scholar]
- 35.Canonica GW. Introduction to nasal and pulmonary allergy cascade. Allergy. 2002;57(Suppl. 75):8–12. doi: 10.1034/j.1398-9995.57.s75.2.x. [DOI] [PubMed] [Google Scholar]
- 36.Dahl R. Early- and late-phase reaction in the bronchi and the nose. In: Mygind N, Pipkorn J, Dahl R, editors. Rhinitis and asthma. Copenhagen: Munksgaard; 1990. pp. 203–12. [Google Scholar]
- 37.Bousquet J, Van Cauwenberge P, Khaltaev N, the ARIA Workshop Group and the World Health Organization Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(Suppl. 5):S147–334. doi: 10.1067/mai.2001.118891. [DOI] [PubMed] [Google Scholar]
- 38.Bachert C, Hauser U, Prem B, Rudack C, Ganzer U. Proinflammatory cytokines in allergic rhinitis. Eur Arch Otorhinolaryngol. 1995;252(Suppl. 1):S44–9. doi: 10.1007/BF02484434. [DOI] [PubMed] [Google Scholar]
- 39.Montefort S, Feather IH, Wilson SJ, et al. The expression of leukocyte-endothelial adhesion molecules is increased in perennial allergic rhinitis. Am J Respir Cell Mol Biol. 1992;7:393–8. doi: 10.1165/ajrcmb/7.4.393. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Clement P, Smitz J, De Waele M, Derde MP. Correlations between complaints, inflammatory cells and mediator concentrations in nasal secretions after nasal allergen challenge and during natural allergen exposure. Int Arch Allergy Immunol. 1995;106:278–85. doi: 10.1159/000236855. [DOI] [PubMed] [Google Scholar]
- 41.Miyahara S, Miyahara N, Matsubara S, Takeda K, Koya T, Gelfand EW. IL-13 is essential to the late-phase response in allergic rhinitis. J Allergy Clin Immunol. 2006;118:1110–16. doi: 10.1016/j.jaci.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Ciprandi G, Pronzato C, Ricca V, Passalacqua G, Bagnasco M, Canonica GW. Allergen-specific challenge induces intercellular adhesion molecule 1 (ICAM-1 or CD54) on nasal epithelial cells in allergic subjects: relationships with early and late inflammatory phenomena. Am J Respir Crit Care Med. 1994;150:1653–9. doi: 10.1164/ajrccm.150.6.7524984. [DOI] [PubMed] [Google Scholar]
- 43.Pastorello EA, Riario-Sforza GG, Incorvaia C, Segala M, Fumagalli M, Gandini R. Comparison of rhinomanometry, symptom score, and inflammatory cell counts in assessing the nasal late-phase reaction to allergen challenge. J Allergy Clin Immunol. 1994;93:85–92. doi: 10.1016/0091-6749(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 44.Milanese M, Ricca V, Canonica GW, Ciprandi G. Eosinophils, specific hyperreactivity and occurrence of late phase reaction in allergic rhinitis. Eur Ann Allergy Clin Immunol. 2005;37:7–10. [PubMed] [Google Scholar]
- 45.Linder A, Venge P, Deuschl H. Eosinophil cationic protein and myeloperoxidase in nasal secretion as markers of inflammation in allergic rhinitis. Allergy. 1987;42:583–90. doi: 10.1111/j.1398-9995.1987.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 46.Niederberger V, Ring J, Rakoski J, et al. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int Arch Allergy Immunol. 2007;142:133–44. doi: 10.1159/000096439. [DOI] [PubMed] [Google Scholar]
- 47.Connell JT. Quantitative intranasal pollen challenge. II. Effect of daily pollen challenge, environmental pollen exposure, and placebo challenge on the nasal membrane. J Allergy. 1968;41:123–39. doi: 10.1016/0021-8707(68)90053-1. [DOI] [PubMed] [Google Scholar]
- 48.Passalacqua G, Ciprandi G, Canonica GW. The nose–lung interaction in allergic rhinitis and asthma: united airways disease. Curr Opin Allergy Clin Immunol. 2001;1:7–13. doi: 10.1097/01.all.0000010978.62527.4e. [DOI] [PubMed] [Google Scholar]
- 49.Montoro J, Sastre J, Jáuregui I, et al. Allergic rhinitis: continuous or on demand antihistamine therapy? J Investig Allergol Clin Immunol. 2007;17(Suppl. 2):21–7. [PubMed] [Google Scholar]
- 50.Ricca V, Landi M, Ferrero P, et al. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 2000;105:54–7. doi: 10.1016/s0091-6749(00)90177-5. [DOI] [PubMed] [Google Scholar]
- 51.Knani J, Campbell A, Enander I, Peterson CG, Michel FB, Bousquet J. Indirect evidence of nasal inflammation assessed by titration of inflammatory mediators and enumeration of cells in nasal secretions of patients with chronic rhinitis. J Allergy Clin Immunol. 1992;90:880–9. doi: 10.1016/0091-6749(92)90460-j. [DOI] [PubMed] [Google Scholar]
- 52.Juliusson S, Pipkorn U, Karlsson G, Enerbäck L. Mast cells and eosinophils in the allergic mucosal response to allergen challenge: changes in distribution and signs of activation in relation to symptoms. J Allergy Clin Immunol. 1992;90:898–909. doi: 10.1016/0091-6749(92)90462-b. [DOI] [PubMed] [Google Scholar]
- 53.Roquat A, Ihre E, van Hage-Hamsten M, Halldén G, Zetterström O. Allergen-induced inflammation in the nose: a comparison of acute and repeated low-dose allergen exposure. Allergy. 1996;51:42–8. doi: 10.1111/j.1398-9995.1996.tb04548.x. [DOI] [PubMed] [Google Scholar]
- 54.Bachert C, van Kempen M, Van Cauwenberge P. Regulation of proinflammatory cytokines in seasonal allergic rhinitis. Int Arch Allergy Immunol. 1999;118:375–9. doi: 10.1159/000024141. [DOI] [PubMed] [Google Scholar]
- 55.Canonica GW. Introduction: expanding the anti-allergy therapeutic horizon. Allergy. 2002;57(Suppl. 75):5–7. doi: 10.1034/j.1398-9995.57.s75.1.x. [DOI] [PubMed] [Google Scholar]
- 56.Huovinen E, Kaprio J, Laitinen LA, Koskenvuo M. Incidence and prevalence of asthma among adult Finnish men and women of the Finnish Twin Cohort from 1975 to 1990, and their relation to hay fever and chronic bronchitis. Chest. 1999;115:928–36. doi: 10.1378/chest.115.4.928. [DOI] [PubMed] [Google Scholar]
- 57.Guerra S, Sherrill DL, Martinez FD, Barbee RA. Rhinitis as an independent risk factor for adult-onset asthma. J Allergy Clin Immunol. 2002;109:419–25. doi: 10.1067/mai.2002.121701. [DOI] [PubMed] [Google Scholar]
- 58.Greisner WA, Settipane RJ, Settipane GA. The course of asthma parallels that of allergic rhinitis: a 23-year follow-up study of college students. Allergy and Asthma Proc. 2000;21:371–6. doi: 10.2500/108854100778249123. [DOI] [PubMed] [Google Scholar]
- 59.Rzehak P, Schoefer Y, Wichmann HE, Heinrich J. A prospective study on the association between hay fever among children and incidence of asthma in East Germany. Eur J Epidemiol. 2008;23:17–22. doi: 10.1007/s10654-007-9205-3. [DOI] [PubMed] [Google Scholar]
- 60.Braunstahl GJ, Overbeek SE, Kleinjan A, Prins JB, Hoogsteden HC, Fokkens WJ. Nasal allergen provocation induces adhesion molecule expression and tissue eosinophilia in upper and lower airways. J Allergy Clin Immunol. 2001;107:469–76. doi: 10.1067/mai.2001.113046. [DOI] [PubMed] [Google Scholar]
- 61.Braunstahl GJ, Overbeek SE, Fokkens WJ, et al. Segmental bronchoprovocation in allergic rhinitis patients affects mast cell and basophil numbers in nasal and bronchial mucosa. Am J Respir Crit Care Med. 2001;164:858–65. doi: 10.1164/ajrccm.164.5.2006082. [DOI] [PubMed] [Google Scholar]
- 62.Zhang QG, Zheng DS, Yao YT, Zhang XH, Yu HL, Liang DP. Relationship between levels of intercellular adhesion molecule-1, interleukin-6 and airway hyperresponsiveness in patients with allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2004;39:617–20. [PubMed] [Google Scholar]
- 63.Beeh KM, Beier J, Kornmann O, Meier C, Taeumer T, Buhl R. A single nasal allergen challenge increases induced sputum inflammatory markers in non-asthmatic subjects with seasonal allergic rhinitis: correlation with plasma interleukin-5. Clin Exp Allergy. 2003;33:475–82. doi: 10.1046/j.1365-2222.2003.01632.x. [DOI] [PubMed] [Google Scholar]
- 64.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–34. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 65.Greve JM, Davis G, Meyer AM, et al. The major human rhinovirus receptor is ICAM-1. Cell. 1989;56:839–47. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- 66.Johnston SL, Pattemore PK, Sanderson G, et al. The relationship between upper respiratory infections and hospital admissions for asthma: a time–trend analysis. Am J Respir Crit Care Med. 1996;154:654–60. doi: 10.1164/ajrccm.154.3.8810601. 3 pt 1. [DOI] [PubMed] [Google Scholar]
- 67.Passalacqua G, Ciprandi G, Pasquali M, Guerra L, Canonica GW. An update on the asthma–rhinitis link. Curr Opin Allergy Clin Immunol. 2004;4:177–83. doi: 10.1097/00130832-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 68.Gorska-Ciebiada M, Ciebiada M, Gorska MM, Gorski P, Grzelewska-Rzymowska I. Intercellular adhesion molecule 1 and tumor necrosis factor alpha in asthma and persistent allergic rhinitis: relationship with disease severity. Ann Allergy Asthma Immunol. 2006;97:66–72. doi: 10.1016/S1081-1206(10)61372-5. [DOI] [PubMed] [Google Scholar]
- 69.Linneberg A, Henrik Nielsen N, Frølund L, Madsen F, Dirksen A, Jørgensen T, the Copenhagen Allergy Study The link between allergic rhinitis and allergic asthma: a prospective population-based study. The Copenhagen Allergy Study. Allergy. 2002;57:1048–52. doi: 10.1034/j.1398-9995.2002.23664.x. [DOI] [PubMed] [Google Scholar]
- 70.Crystal-Peters J, Neslusan C, Crown WH, Torres A. Treating allergic rhinitis in patients with comorbid asthma: the risk of asthma-related hospitalizations and emergency department visits. J Allergy Clin Immunol. 2002;109:57–62. doi: 10.1067/mai.2002.120554. [DOI] [PubMed] [Google Scholar]
- 71.Passalacqua G, Canonica GW. Impact of rhinitis on airway inflammation: biological and therapeutic implications. Respir Res. 2001;2:320–3. doi: 10.1186/rr80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salmun LM. Antihistamines in late-phase clinical development for allergic disease. Exp Opin Investig Drugs. 2002;11:259–73. doi: 10.1517/13543784.11.2.259. [DOI] [PubMed] [Google Scholar]
- 73.Vignola AM, Crampette L, Mondain M, et al. Inhibitory activity of loratadine and descarboethoxyloratadine on expression of ICAM-1 and HLA-DR by nasal epithelial cells. Allergy. 1995;50:200–3. doi: 10.1111/j.1398-9995.1995.tb01133.x. [DOI] [PubMed] [Google Scholar]
- 74.Molet S, Gosset P, Lassalle P, Czarlewski W, Tonnel AB. Inhibitory activity of loratadine and descarboxyethoxyloratadine on histamine-induced activation of endothelial cells. Clin Exp Allergy. 1997;27:1167–74. [PubMed] [Google Scholar]
- 75.Zhao Y, Leung PC, Woo KS, et al. Inhibitory effects of budesonide, desloratadine and dexamethasone on cytokine release from human mast cell line (HMC-1) Inflamm Res. 2004;53:664–49. doi: 10.1007/s00011-004-1309-6. [DOI] [PubMed] [Google Scholar]
- 76.Mullol J, Roca-Ferrer J, Alobid I, et al. Effect of desloratadine on epithelial cell granulocyte-macrophage colony-stimulating factor secretion and eosinophil survival. Clin Exp Allergy. 2006;36:52–8. doi: 10.1111/j.1365-2222.2005.02403.x. [DOI] [PubMed] [Google Scholar]
- 77.Jinquan T, Reimert CM, Deleuran B, Zachariae C, Simonsen C, Thestrup-Pedersen K. Cetirizine inhibits the in vitro and ex vivo chemotactic response of T lymphocytes and monocytes. J Allergy Clin Immunol. 1995;95:979–86. doi: 10.1016/s0091-6749(95)70098-6. 5 pt 1. [DOI] [PubMed] [Google Scholar]
- 78.Pasquali M, Baiardini I, Rogkakou A, et al. Levocetirizine in persistent allergic rhinitis and asthma: effects on symptoms, quality of life and inflammatory parameters. Clin Exp Allergy. 2006;36:1161–7. doi: 10.1111/j.1365-2222.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 79.Ciprandi G, Tosca MA, Milanese M, Ricca V. Cetirizine reduces cytokines and inflammatory cells in children with perennial allergic rhinitis. Eur Ann Allergy Clin Immunol. 2004;36:237–40. [PubMed] [Google Scholar]
- 80.Fujikura T. New therapeutic approaches to the treatment of nasal allergy: antiinflammatory effects of H1 receptor antagonists. Drugs Today (Barc. 2001;37:455–61. doi: 10.1358/dot.2001.37.7.844188. [DOI] [PubMed] [Google Scholar]
- 81.Ciprandi G, Passalacqua G, Mincarini M, Ricca V, Canonica GW. Continuous versus on demand treatment with cetirizine for allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:507–11. doi: 10.1016/S1081-1206(10)63057-8. [DOI] [PubMed] [Google Scholar]
- 82.Ciprandi G, Ricca V, Passalacqua G, et al. Seasonal rhinitis and azelastine: long- or short-term treatment? J Allergy Clin Immunol. 1997;99:301–7. doi: 10.1016/s0091-6749(97)70046-0. [DOI] [PubMed] [Google Scholar]
- 83.Ferreira MB, Santos MC, Pregal AL, et al. Effect of specific immunotherapy versus loratadine on serum adhesion molecules. Allerg Immunol (Paris) 2001;33:319–22. [PubMed] [Google Scholar]
- 84.Lauriello M, Muzi P, Di Rienzo L, Di Stanislao C, Tirelli GC, Bologna M. A two-year course of specific immunotherapy or of continuous antihistamine treatment reverse eosinophilic inflammation in severe persistent allergic rhinitis. Acta Otorhinolaryngol Ital. 2005;25:284–91. [PMC free article] [PubMed] [Google Scholar]
- 85.Dizdar EA, Sekerel BE, Keskin O, et al. The effect of regular versus on-demand desloratadine treatment in children with allergic rhinitis. Int J Pediatr Otorhinolaryngol. 2007;71:843–9. doi: 10.1016/j.ijporl.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 86.Canonica GW, Fumagalli F, Guerra L, et al. Global Allergy and Asthma European Network. Levocetirizine in persistent allergic rhinitis: continuous or on-demand use? A pilot study. Curr Med Res Opin. 2008;24:2829–39. doi: 10.1185/03007990802395927. [DOI] [PubMed] [Google Scholar]
- 87.Ciprandi G, Ricca V, Tosca M, Landi M, Passalacqua G, Canonica GW. Continuous antihistamine treatment controls allergic inflammation and reduces respiratory morbidity in children with mite allergy. Allergy. 1999;54:358–65. doi: 10.1034/j.1398-9995.1999.00920.x. [DOI] [PubMed] [Google Scholar]
- 88.Okubo K, Baba K. Therapeutic effect of montelukast a cysteinyl leukotriene receptor 1 antagonist, on Japanese patients with seasonal allergic rhinitis. Allergol Int. 2008;57:247–55. doi: 10.2332/allergolint.O-07-515. [DOI] [PubMed] [Google Scholar]
- 89.Price D, Bond C, Bouchard J, et al. International Primary Care Respiratory Group (IPCRG) Guidelines: management of allergic rhinitis. Prim Care Respir J. 2006;15:58–70. doi: 10.1016/j.pcrj.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ciprandi G, Frati F, Marcucci F, et al. Nasal cytokine modulation by montelukast in allergic children: a pilot study. Eur Ann Allergy Clin Immunol. 2003;35:295–9. [PubMed] [Google Scholar]
- 91.Nayak AS, Philip G, Lu S, Malice MP, Reiss TF, the Montelukast Fall Rhinitis Investigator Group Efficacy and tolerability of montelukast alone or in combination with loratadine in seasonal allergic rhinitis: a multicenter, randomized, double-blind, placebo-controlled trial performed in the fall. Ann Allergy Asthma Immunol. 2002;88:592–600. doi: 10.1016/S1081-1206(10)61891-1. [DOI] [PubMed] [Google Scholar]
- 92.Mygind N, Nielsen LP, Hoffmann HJ, et al. Mode of action of intranasal corticosteroids. J Allergy Clin Immunol. 2001;108(Suppl. 1):S16–25. doi: 10.1067/mai.2001.115561. [DOI] [PubMed] [Google Scholar]
- 93.Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317:1624–9. doi: 10.1136/bmj.317.7173.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yáñez A, Rodrigo GJ. Intranasal corticosteroids versus topical H1 receptor antagonists for the treatment of allergic rhinitis: a systematic review with meta-analysis. Ann Allergy Asthma Immunol. 2002;89:479–84. doi: 10.1016/S1081-1206(10)62085-6. [DOI] [PubMed] [Google Scholar]
- 95.Umland SP, Schleimer RP, Johnston SL. Review of the molecular and cellular mechanisms of action of glucocorticoids for use in asthma. Pulm Pharmacol Ther. 2002;15:35–50. doi: 10.1006/pupt.2001.0312. [DOI] [PubMed] [Google Scholar]
- 96.Newton R. Molecular mechanisms of glucocorticoid action: what is important? Thorax. 2000;55:603–13. doi: 10.1136/thorax.55.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Leung DY, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111:3–22. doi: 10.1067/mai.2003.97. [DOI] [PubMed] [Google Scholar]
- 98.Umland SP, Nahrebne DK, Razac S, et al. The inhibitory effects of topically active glucocorticoids on IL-4, IL-5, and interferon-gamma production by cultured primary CD4+ T cells. J Allergy Clin Immunol. 1997;100:511–19. doi: 10.1016/s0091-6749(97)70144-1. [DOI] [PubMed] [Google Scholar]
- 99.Frieri M, Therattil J, Chavarria V, et al. Effect of mometasone furoate on early and late phase inflammation in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1998;81:431–7. doi: 10.1016/S1081-1206(10)63141-9. 5 pt 1. [DOI] [PubMed] [Google Scholar]
- 100.Ciprandi G, Tosca MA, Passalacqua G, Canonica GW. Intranasal mometasone furoate reduces late-phase inflammation after allergen challenge. Ann Allergy Asthma Immunol. 2001;86:433–8. doi: 10.1016/S1081-1206(10)62491-X. [DOI] [PubMed] [Google Scholar]
- 101.Erin EM, Leaker BR, Zacharasiewicz AS, et al. Single dose topical corticosteroid inhibits IL-5 and IL-13 in nasal lavage following grass pollen challenge. Allergy. 2005;60:1524–9. doi: 10.1111/j.1398-9995.2005.00928.x. [DOI] [PubMed] [Google Scholar]
- 102.Kleinjan A, Holm AF, Dijkstra MD, et al. Preventive treatment of intranasal fluticasone propionate reduces cytokine mRNA expressing cells before and during a single nasal allergen provocation. Clin Exp Allergy. 2000;30:1476–85. doi: 10.1046/j.1365-2222.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- 103.Wright ED, Christodoulopoulos P, Small P, Frenkiel S, Hamid Q. Th-2 type cytokine receptors in allergic rhinitis and in response to topical steroids. Laryngoscope. 1999;109:551–6. doi: 10.1097/00005537-199904000-00006. [DOI] [PubMed] [Google Scholar]
- 104.Erin EM, Zacharasiewicz AS, Nicholson GC, et al. Topical corticosteroid inhibits interleukin-4, -5 and -13 in nasal secretions following allergen challenge. Clin Exp Allergy. 2005;35:1608–14. doi: 10.1111/j.1365-2222.2005.02381.x. [DOI] [PubMed] [Google Scholar]
- 105.Ciprandi G, Ricca V, Passalacqua G, Fasolo A, Canonica GW. Intranasal fluticasone propionate reduces ICAM-1 on nasal epithelial cells both during early and late phase after allergen challenge. Clin Exp Allergy. 1998;28:293–9. doi: 10.1046/j.1365-2222.1998.00239.x. [DOI] [PubMed] [Google Scholar]
- 106.Holm A, Dijkstra M, Kleinjan A, et al. Fluticasone propionate aqueous nasal spray reduces inflammatory cells in unchallenged allergic nasal mucosa: effects of single allergen challenge. J Allergy Clin Immunol. 2001;107:627–33. doi: 10.1067/mai.2001.113520. [DOI] [PubMed] [Google Scholar]
- 107.Baroody FM, Shenaq D, DeTineo M, Wang J, Naclerio RM. Fluticasone furoate nasal spray reduces the nasal–ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptoms. J Allergy Clin Immunol. 2009;123:1342–8. doi: 10.1016/j.jaci.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 108.Lange B, Lukat KF, Rettig K, Holtappels G, Bachert C. Efficacy, cost-effectiveness, and tolerability of mometasone furoate, levocabastine, and disodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 2005;95:272–82. doi: 10.1016/S1081-1206(10)61225-2. [DOI] [PubMed] [Google Scholar]
- 109.Bascom R, Wachs M, Naclerio RM, Pipkorn U, Galli SJ, Lichtenstein LM. Basophil influx occurs after nasal antigen challenge: effects of topical corticosteroid pretreatment. J Allergy Clin Immunol. 1988;81:580–9. [PubMed] [Google Scholar]
- 110.Mullol J, Xaubet A, López E, Roca-Ferrer J, Picado C. Comparative study of the effects of different glucocorticosteroids on eosinophil survival primed by cultured epithelial cell supernatants obtained from nasal mucosa and nasal polyps. Thorax. 1995;50:270–4. doi: 10.1136/thx.50.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Crim C, Pierre LN, Daley-Yates PT. A review of the pharmacology and pharmacokinetics of inhaled fluticasone propionate and mometasone furoate. Clin Ther. 2001;23:1339–54. doi: 10.1016/s0149-2918(01)80113-2. [DOI] [PubMed] [Google Scholar]
- 112.Martin BG, Ratner PH, Hampel FC, et al. Optimal dose selection of fluticasone furoate nasal spray for the treatment of seasonal allergic rhinitis in adults and adolescents. Allergy Asthma Proc. 2007;28:216–25. doi: 10.2500/aap.2007.28.2983. [DOI] [PubMed] [Google Scholar]
- 113.Pullerits T, Praks L, Sjöstrand M, Rak S, Skoogh BE, Lötvall J. An intranasal glucocorticoid inhibits the increase of specific IgE initiated during birch pollen season. J Allergy Clin Immunol. 1997;100:601–5. doi: 10.1016/s0091-6749(97)70162-3. [DOI] [PubMed] [Google Scholar]
- 114.Mastrandrea F, Coradduzza G, De Vita L, et al. CD34+ cells in peripheral blood of healthy human beings and allergic subjects: clue to acute and minimal persistent inflammation. Allergol Immunopathol (Madr) 2002;30:209–17. doi: 10.1016/s0301-0546(02)79123-4. [DOI] [PubMed] [Google Scholar]
- 115.Sergejeva S, Malmhäll C, Lötvall J, Pullerits T. Increased number of CD34+ cells in nasal mucosa of allergic rhinitis patients: inhibition by a local corticosteroid. Clin Exp Allergy. 2005;35:34–8. doi: 10.1111/j.1365-2222.2004.02038.x. [DOI] [PubMed] [Google Scholar]
- 116.Pipkorn U, Proud D, Lichtenstein LM, Kagey-Sobotka A, Norman PS, Naclerio RM. Inhibition of mediator release in allergic rhinitis by pretreatment with topical glucocorticosteroids. N Engl J Med. 1987;316:1506–10. doi: 10.1056/NEJM198706113162403. [DOI] [PubMed] [Google Scholar]
- 117.Baroody FM, Cruz AA, Lichtenstein LM, Kagey-Sobotka A, Proud D, Naclerio RM. Intranasal beclomethasone inhibits antigen-induced nasal hyperresponsiveness to histamine. J Allergy Clin Immunol. 1992;90:373–6. doi: 10.1016/s0091-6749(05)80017-x. [DOI] [PubMed] [Google Scholar]
- 118.Wang D, Smitz J, De Waele M, Clement P. Effect of topical applications of budesonide and azelastine on nasal symptoms, eosinophil count and mediator release in atopic patients after nasal allergen challenge during the pollen season. Int Arch Allergy Immunol. 1997;114:185–92. doi: 10.1159/000237665. [DOI] [PubMed] [Google Scholar]
- 119.Karaman O, Günbay A, Uzuner N, et al. The comparison of the efficacy of fluticasone propionate with cetirizine in perennial allergic rhinitis. Allergol Immunopathol (Madr) 2001;29:55–9. doi: 10.1016/s0301-0546(01)79018-0. [DOI] [PubMed] [Google Scholar]
- 120.Ahlström Emanuelsson C, Andersson M, Persson CG, Thorsson L, Greiff L. Effects of topical formoterol alone and in combination with budesonide in a pollen season model of allergic rhinitis. Respir Med. 2007;101:1106–12. doi: 10.1016/j.rmed.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 121.Konno A, Yamakoshi T, Terada N, Fujita Y. Mode of action of a topical steroid on immediate phase reaction after antigen challenge and nonspecific nasal hyperreactivity in nasal allergy. Int Arch Allergy Immunol. 1994;103:79–87. doi: 10.1159/000236609. [DOI] [PubMed] [Google Scholar]
- 122.Holm AF, Godthelp T, Fokkens WJ, et al. Long-term effects of corticosteroid nasal spray on nasal inflammatory cells in patients with perennial allergic rhinitis. Clin Exp Allergy. 1999;29:1356–66. doi: 10.1046/j.1365-2222.1999.00665.x. [DOI] [PubMed] [Google Scholar]
- 123.Fokkens WJ, Godthelp T, Holm AF, Klein-Jan A. Local corticosteroid treatment: the effect on cells and cytokines in nasal allergic inflammation. Am J Rhinol. 1998;12:21–6. doi: 10.2500/105065898782102990. [DOI] [PubMed] [Google Scholar]
- 124.Till SJ, Jacobson MR, O'Brien F, et al. Recruitment of CD1a+ Langerhans cells to the nasal mucosa in seasonal allergic rhinitis and effects of topical corticosteroid therapy. Allergy. 2001;56:126–31. doi: 10.1034/j.1398-9995.2001.056002126.x. [DOI] [PubMed] [Google Scholar]
- 125.Corren J, Manning BE, Thompson SF. Rhinitis therapy and the prevention of hospital care for asthma: a case-control study. J Allergy Clin Immunol. 2004;113:415–19. doi: 10.1016/j.jaci.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 126.Bousquet J, Chanal I, Alquié MC, et al. Prevention of pollen rhinitis symptoms: comparison of fluticasone propionate aqueous nasal spray and disodium cromoglycate aqueous nasal spray: a multicenter, double-blind, double-dummy, parallel-group study. Allergy. 1993;48:327–33. doi: 10.1111/j.1398-9995.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 127.Morelli MC, Bordonaro S, Hedbys L, Romagnani S, the Italian Study Group Effect of pre-seasonal and seasonal treatment with budesonide topical nasal powder in patients with seasonal allergic rhinitis. Allergol Int. 1996;45:151–7. [Google Scholar]
- 128.Pullerits T, Praks L, Ristioja V, Lötvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 2002;109:949–55. doi: 10.1067/mai.2002.124467. [DOI] [PubMed] [Google Scholar]
- 129.New Zealand Medicines and Medical Devices Safety Authority. Tilarin Nasal Spray Data Sheet. Available at http://www.medsafe.govt.nz/Profs/Datasheet/t/Tilarinnasalspray.htm (accessed 10 October 2008.
- 130.Schering Corporation. Nasonex [package insert. Kenilworth, NJ: Schering Corporation; 2005. [Google Scholar]
- 131.Schering Corporation. Nasonex.com/Benefits and Side Effects. Available at: http://www.nasonex.com/nasx/application?namespace=main&event=content_display&event_input=benefitsofusing (accessed 28 August 2008.


