Abstract
Placental malaria infection affects the T helper type 1 (Th1)/Th2 balance in neonatal children. We investigated a potential role of regulatory T cells in this balance by comparing T cell responses of cord blood mononuclear cells (CBMC) from parasitized and non-parasitized placenta of Gambian women. CBMC were depleted of CD4+CD25+ forkhead box P3 (FoxP3)+ regulatory T cells and analysed in vitro for their ability to produce interferon (IFN)-γ, sCD30 and interleukin (IL)-10 in response to phytohaemagglutinin (PHA), live Plasmodium falciparum,schizont extracts and the recombinant P. falciparum blood stage antigen merozoite surface protein 1 (MSP119). As expected, lower IFN-γ and higher sCD30 responses were observed for the cells from the parasitized group. In addition, higher IL-10 levels were produced by CBMC from the parasitized group. Depletion of regulatory T cells decreased IL-10 production, which resulted in a restoration of IFN-γ expression in response to all stimuli. The Th2 marker sCD30 remained significantly higher in the parasitized group in response to malaria protein antigens while similar levels were recovered between both groups in response to live P. falciparum. Similar effects were observed by adding an antibody that blocks IL-10 function. These results suggest that the impact of P. falciparum infection on Th1 differentiation of neonatal T cells can be ascribed to regulatory T cells through production of IL-10.
Keywords: CBMC, placental malaria, Th1/Th2, Tregs
Introduction
Malaria parasites cause approximately 300–600 million clinical attacks and 2 million deaths per year [1]. Pregnant women are particularly susceptible. The prevalence of placental malaria, in which parasitized red blood cells are sequestered in the placenta, ranges from 30 to 70% of gravid women in many malaria endemic areas, including The Gambia. This condition is associated with increased risk of abortion, stillbirths, low birth weight and infant mortality [2,3]. Using a birth cohort in 2001 that enrolled pregnant Gambian women, the immunological responses to mitogens and Plasmodium falciparum schizont extracts were compared for cord blood mononuclear cells (CBMC) from parasitized and non-parasitized placentas [4]. We found that placental malaria infection also impacts on the newborn infant's immunological response [4]. Placental infection with P. falciparum inhibited production of the T helper type 1 (Th1) cytokines interferon (IFN)-γ and interleukin (IL)-12 and stimulated expression of the Th2 marker sCD30 when the lymphocytes of neonatal children were examined. These results suggested that the parasite has acquired an ability to manipulate T cell immunity by affecting the Th1/Th2 balance and that an active mechanism of T cell suppression may operate during the course of malaria infection. Regulatory T cells (Tregs) have been shown recently to contribute to the maintenance of P. falciparum infection [5,6]. Moreover, parasite antigen-specific CD4+ regulatory cells are generated in utero as a consequence of P. falciparum infection of the placenta [7]. The functional role of Tregs in the balance of Th1/Th2 immune responses during P. falciparum infection in newborns is still poorly understood. We therefore examined the possible role of Tregs in immune dysregulation during active placental P. falciparum infection, using frozen cord blood samples collected from the 2001 Sukuta cohort (pilot study). We compared both the magnitude and the Th1/Th2 balance of cytokines induced in vitro by mitogen, malaria antigens and live parasites, both before and after depletion of Tregs or the addition of anti-IL-10 blocking antibodies to CBMC cultures.
Materials and methods
Population
Details of the study population have already been published [4]. Briefly, in collaboration with the Gambian Ministry of Health, a study was undertaken in the peri-urban village of Sukuta 11 km from the Medical Research Council (MRC) Laboratories in Fajara during the period of September 2001 to January 2002. Primiparous and multiparous pregnant women were recruited into the study. After obtaining written informed consent, up to 50 ml of cord blood was collected upon delivery. Active malaria infection was assessed by microscopic examination of Giemsa-stained placental imprints and cord bloods. All slides were read twice by experienced microscopists and discrepancies resolved by a third reader (limit of detection, approximately 2 parasites/µl) [8]. The study was approved by the Joint Gambia Government/MRC Ethics Committee.
Parasite cultivation and schizont collection
P. falciparum parasites (Pf164 line) were cultured in vitro, as described previously [9]. Parasites at a parasitaemia of approximately 5% ring stages were synchronized using repeated treatments with 5% sorbitol for 20 min at 37°C [10] and then mature parasite-infected erythrocytes were harvested by flotation on a cushion of Plasmagel (a final concentration of 2% gelatine in incomplete culture medium) [11]. The harvested parasites were mixed with fresh uninfected red blood cells and cultured to a final parasitaemia of 10%. The stages of parasite development were assessed by microscopy, as described previously [12].
Parasite cultures were tested at regular intervals for contamination with Mycoplasma spp. by using a polymerase chain reaction (PCR)-based mycoplasma-detection kit (American Type Culture Collection, Manassas, VA, USA). A crude preparation of P. falciparum antigens was obtained by freezing and thawing of parasite-infected erythrocytes. The extracts were titrated on cells from semi-immune adults to identify conditions for optimal induction of cell activation, which was set as a concentration of 10 µg/ml. Extracts from uninfected erythrocytes were used as negative controls.
Culture of cord blood mononuclear cells
Selected frozen samples of CBMC of the Sukuta cohort were thawed, washed with RPMI-1640 medium (Sigma, St Louis, MO, USA) and resuspended in RPMI-1640 medium supplemented with 20 µg/ml gentamycin (Sigma), 2 mM L-glutamine (Sigma) and 10% human antibody serum (Sigma). CBMC (viability reached 85%) were cultured (2 × 105 cells in 200 µl complete medium) for either 3 days in the presence of phytohaemagglutinin (PHA-L, 2·5 µg/ml; Sigma) or 6 days in the presence of erythrocytes infected with P. falciparum (Pf164) at 10% parasitaemia or schizont extracts at 10 µg/ml. Uninfected erythrocytes or extracts from them were used as negative controls, respectively. Merozoite surface protein 1 (MSP119) [13] at a final concentration of 5 µg/ml was used to examine the T cell response to a blood stage antigen. All assays were performed in triplicate. Cytokines (IFN-γ, IL-10 and soluble sCD30) in the culture supernatants were measured using enzyme-linked immunosorbent assay (ELISA).
CD4+CD25+ T cell depletion and analysis
CBMC were depleted of CD4+CD25+ cells using Miltenyi beads (Miltenyi Biotec, Bergisch Gladbach, Germany), following the manufacturer's instructions. Briefly, the depletion was performed in two steps. First, a cocktail of biotinylated antibodies [with specificity for CD16, CD14, CD8, CD19, CD56 and γδ T cell receptor (TCR)] together with anti-biotin microbeads (microbeads conjugated to monoclonal anti-biotin antibody) was used to separate the non-CD4+ T cells from the CD4+ T cells. Then anti-CD25 antibody and anti-biotin microbeads were added to the CD4+ fraction to deplete the CD4+CD25+ cells. The resultant CD4+CD25- T cells were then added back to the non-CD4+ fraction to reconstitute the cell population for in vitro stimulation. The positively selected CD4+CD25+ T cells were also eluted from the columns and cultured (106/ml) overnight in vitro with PHA to assess their IL-10 production in comparison with CD4+CD25- fraction.
Anti-CD25-PE and anti-CD4-fluorescein isothiocyanate (FITC) antibodies (Becton-Dickinson, UK) were used for quality control of the separations and also to compare frequency of CD4+CD25+ population in groups from parasitized and non-parasitized placenta. Briefly, in this later case, selected CBMC samples were thawed and stained for 20 min at 4°C, washed with phosphate-buffered saline–bovine serum albumin (PBS–BSA) 2% and analysed using a fluorescence activated cell sorter (FACS)Calibur (Becton-Dickinson).
RNA extraction and reverse transcription
Total RNA was extracted from CD4+CD25+ T cell fraction and CD4+CD25- cell fraction using a total RNA isolation system (Promega Corporation, Madison, WI, USA). The reaction was incubated at 37°C for 1·5 h, and stopped by incubation at 95°C for 5 min. Semi-quantitative reverse transcription–polymerase chain reaction (RT–PCR) was used to analyse forkhead box P3 (FoxP3) expression using specific primers. Glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) served as a positive control for each cDNA sample. Amplification reactions were performed in a volume of 25 µl containing 1 µl cDNA as template, 0·2 µmol of each primer, 0·5 U of Taq polymerase (Boehringer Mannheim, Mannheim, Germany), 0·1 µmol of each dNTP and 1×Taq polymerase buffer. GAPDH primer sequences were 5′-GCAAATTCCATGGCACCGT (forward) and 5′-TCGCCCCACTTGATTTTGG (reverse). FoxP3 primer sequences were 5′-AGCTGGAGTTCCGCAAGAAAC (forward) and 5′-TGTTCGTCCATCCTCCTTTCC (reverse).
Human cytokine determinations
IFN-γ, IL-10 and sCD30 levels in culture supernatants were determined using ELISA kits from Biosource (IFN-γ, IL-10; BioSource Europe, Fleurus, Belgium), MedSystems (sCD30; MedSystems Diagnostics GmbH, Vienna, Austria), following the manufacturer's instructions.
Statistical analysis
Paired and non-paired t-tests were performed on log-transformed data (which normalized the distribution) to compare the significance of results between the groups with and without detectable parasitaemia.
Results
CD4+CD25+ Fox-P3+ T cell frequency in the CBMC
Detailed epidemiological parameters on the Sukuta pilot study have been reported previously [4]. Briefly, one-third of the women (25 of 73, 34·3%) enrolled into the study had active malaria infection in the placenta at delivery. Fifty per cent of selected cord blood samples from parasitized placenta were also positive for the P. falciparum; however, the parasitaemia was very low (mean = 2500 parasites/µl) and all infants were aparasitaemic at 1 week of age.
No association was found with cord blood parasitaemia and Th differentiation [4]. The parasitized and non-parasitized groups were similar for maternal age, parity, placental weight, birth weight and reported chloroquine use. Cord blood samples from both groups were collected and used for evaluation of the Th1/Th2 T cell ratio [4]. A fraction of each sample had also been kept frozen. We used some of these frozen samples to assess the role of Tregs in the changed T cell ratio.
We excluded specific selection of Tregs due to the freezing process, as frozen samples and fresh CBMC displayed similar responses to PHA, protein antigens or allo-mixed lymphocyte reaction (allo-MLR) (not shown).
We first compared frequencies of CD4+CD25+ FoxP3+ T cells in both groups, then depleted these cells from the population and cultured the residual CBMC in the presence of mitogen, live parasite or malaria protein antigen. CD4+CD25+FoxP3+ and CD4+CD25+FoxP3low fractions were stimulated overnight with PHA and the higher IL-10 production by CD4+CD25+ T cells compared to CD4- CD25- T cells suggested strongly that all or part of CD4+CD25+ FoxP3+ cells are Tregsand not effector cells (Fig. 1).
Fig. 1.
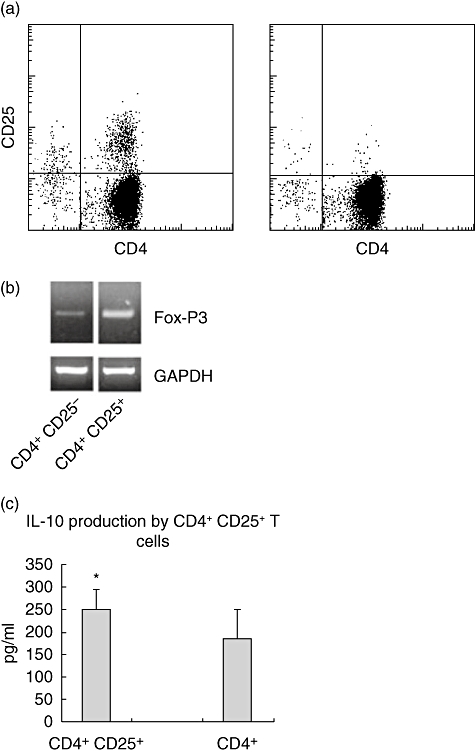
(a) Quality control of CD4+CD25+ T cell depletion and (b) their forkhead box P3 (FoxP3) expression and (c) interleukin (IL)-10 production after stimulation with phytohaemagglutinin (PHA) (18 h). Significantly higher FoxP3 mRNA and IL-10 amounts were produced by CD4+CD25+ (n = 5) compared to CD4+CD25- T cells (n = 6) (*P = 0·0427).
Frequencies of CD4+CD25+ FoxP3+ T cells were low and similar in samples from both groups (1·2% ± 0·2 compared to 1·4% ± 0·3, respectively; P = 0·11). Approximately 10% of CD4+ T cells expressed the α-chain of IL-2 receptor CD25, whereas only 1–2% of the CD4+ T cells were CD25+ in both groups of donors (Fig. 2).
Fig. 2.
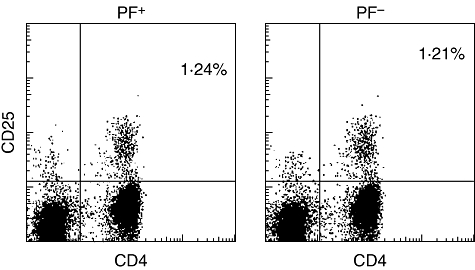
Selected cord blood mononuclear cells (CBMC) samples from parasitized (n = 8) and non-parasitized groups (n = 8) were stained with anti-CD25-phycoerythrin and anti-CD4-fluorescein isothiocyanate and analysed using a fluorescence activated cell sorter (FACS)Calibur. One of eight samples is shown.
Effect of Tregs on Th1/Th2 cytokines produced in response to PHA
As expected from the previous study [4], placental infection decreased IFN-γ production significantly (p2 = 0·03) from cord blood cells in vitro in response to PHA without affecting expression of sCD30 (Fig. 3). Interestingly, we found higher levels of IL-10 in cultures of CBMC from parasitized placenta (p5 = 0·025). Depletion of Tregs resulted in a significant decrease in IL-10 production of both groups (p3 = 0·028; p4 = 0·035) and a significant increase in some of the other cytokines measured. In particular, depletion of Tregs resulted in recovery of IFN-γ production in cultures of cells from the parasitized group (p1 = 0·02). A lower but non-significant decrease in IL-13 production by cells from the parasitized group (not shown) and slightly higher levels of sCD30 in both groups were observed subsequent to depletion of Tregs (Fig. 3).
Fig. 3.
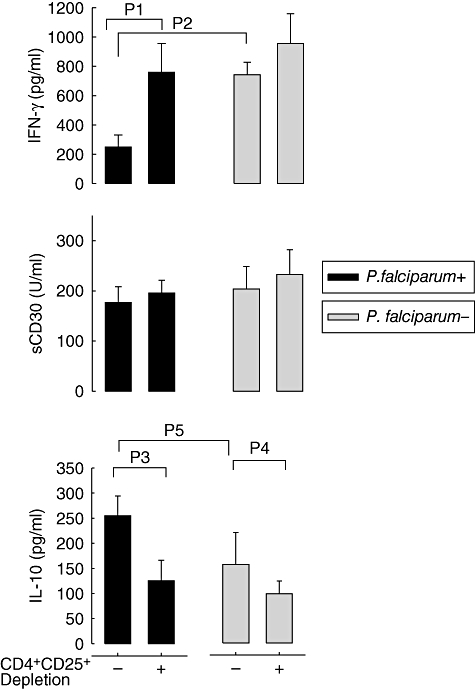
Cytokine production by cord blood mononuclear cells (CBMC) in response to phytohaemagglutinin (PHA). CBMC from newborns whose mothers had malaria infection of the placenta at delivery (black bars, n = 21) or were free of infection (grey bars, n = 34) were cultured for 3 days with PHA and assessed for their cytokine production and sCD30 release. T helper type 1 (Th1) [interferon (IFN)-γ] and Th2 (sCD30) markers, as well as the anti-inflammatory cytokine interleukin (IL)-10 were measured using enzyme-linked immunosorbent assay (ELISA). Measurements were made with (+) or without (−) CD4+CD25+ regulatory T cell depletion. As already reported [4], significantly less IFN-γ was produced by CBMC obtain from the group with parasitized placenta (P2 = 0·003), while the levels of the Th2 markers were similar between groups. Higher IL-10 levels were produced by cells from the parasitized placenta group (P5 = 0·025). Depletion of regulatory T cells decreased IL-10 production in cells from both groups (p3 = 0·028, p4 = 0·035) and restored IFN-γ production (p1 = 0·02). Results are shown as mean ± standard error of the mean.
Effects of Tregs on the Th1/Th2 CBMC cytokines measured in response to live parasites and malaria antigens
CBMC were also tested for their responses to live P. falciparum-infected red blood cells, Pf164 schizont extracts, and purified MSP119 protein after 6 days of culture. CBMC cultured in the presence of uninfected red blood cells, in the presence of extracts of red blood cells and in medium alone were used as negative controls, respectively (Fig. 4).
Fig. 4.
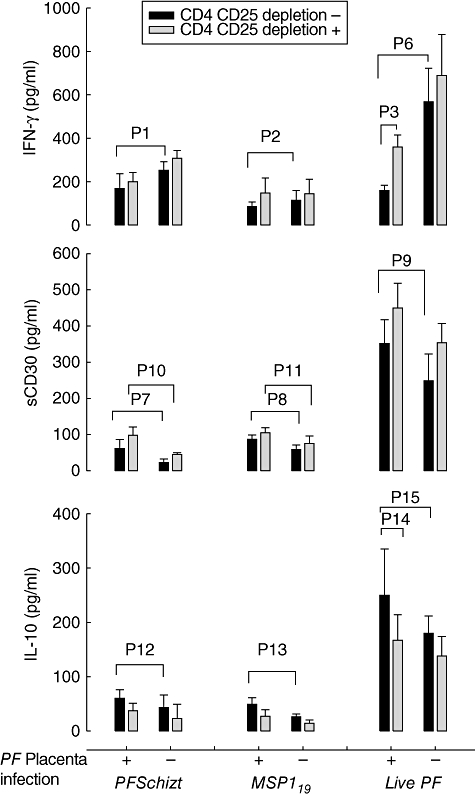
Cytokine production by cord blood mononuclear cells (CBMC) in response to live Plasmodium falciparumand P. falciparumantigens. CBMC from non parasitized (−) (n = 21) and parasitized (+) (n = 25) placenta were assessed for cytokine production and sCD30 release in response to treatment with the merozoite surface protein 1 (MSP119) protein antigen and an extract from P. falciparumPf164 schizonts, or upon stimulation with live parasitized red blood cells. Unstimulated CBMC or CBMC stimulated with either uninfected red blood cell extracts or uninfected intact erythrocytes were used as negative controls, respectively. Measurements were made with (grey bars) or without (black bars) CD4+CD25+ regulatory T cell depletion. Lower interferon (IFN)-γ production (Pf164schizonts p1 = 0·0485; MSP119 p2 = 0·0497, live Pfp6 = 0·015), higher sCD30 (Pf164schizonts p7 = 0·0245; MSP119 p8 = 0·021; livepf p9 = 0·028) and higher interleukin (IL)-10 production (Pf164schizonts p12 = 0·048; MSP119 p13 = 0·0478; live Pfp15 = 0·038) in response to malaria protein antigens Pf164schizonts and MSP119 and live parasite. CD4+CD25+ T cell depletion decreased IL-10 in both groups and significantly in response to live Pf(p14 = 0·0315), maintained the higher sCD30 production in response to malaria protein antigens in the parasitized group (Pf164schizonts (p10 = 0·032; MSP119 p11 = 0·041) and restored IFN-γ production in response to live parasite (p3 = 0·0209). Results are shown as mean ± standard error of the mean (minus backgrounds).
Lower IFN-γ production (Pf164schizonts p1 = 0·0485; MSP119 p2 = 0·00497) and higher IL-10 levels (Pf164schizonts p12 = 0·048; MSP119 p13 = 0·0478) were observed in response to malaria antigens Pf164 schizonts and MSP119 in the parasitized group compared to the non-parasitized group. Moreover, higher levels of sCD30 were observed in response to malaria antigens (Pf164schizonts p7 = 0·0245; MSP119 p8 = 0·021) in the parasitized group. CD4+ CD25+ T cell depletion restored levels of IFN-γ production, decreased IL-10 and increased sCD30 production in both groups. In this later case the difference between both groups remained significant (Pf164schizonts p10 = 0·032; MSP119 p11 = 0·041).
Live parasites induced higher concentrations of all tested cytokines compared to protein antigen. It induced significantly lower levels of IFN-γ (p6 = 0·015), higher levels of IL-10 (p15 = 0·038) and higher amounts of sCD30 (p9 = 0·028) in the parasitized group. CD4+ CD25+ T cell depletion decreased IL-10 production (p14 = 0·049), restored IFN-γ production (p3 = 0·0209) and increased sCD30 in both groups leading to a similar production between both groups.
Effect of IL-10 production on the Th1/Th2 CBMC cytokines
As shown in Figs 3 and 4, higher levels of IL-10 were detected in CBMC cultures derived from the parasitized group and depletion of CD4 T cells resulted in lower IL-10 production and restoration of IFN-γ expression in CBMC from the parasitized group. To examine the direct role of IL-10, we compared cultures of CBMC from both groups in the presence of live parasites, either after depletion of Tregs or in the presence of neutralizing anti-IL-10 antibodies. As shown in Fig. 5, both depletion of Tregs and anti-IL-10 treatment restored IFN-γ production in cells from the parasitized group. In contrast, anti-IL-10 did not have a significant impact on sCD30 (data not shown).
Fig. 5.
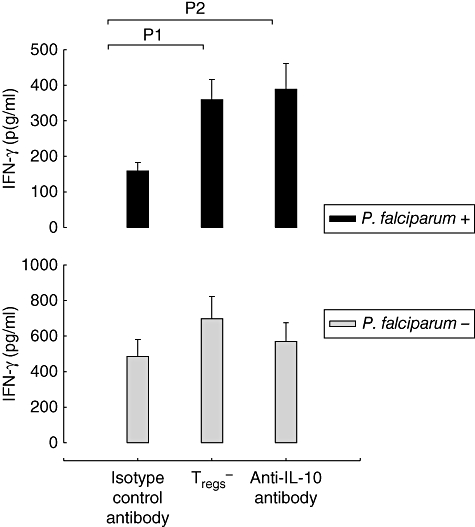
Treatment of cell cultures with anti-interleukin (IL)-10 antibody restored interferon (IFN)-γ production in the cultures of cells from the group with parasitized placenta. Cord blood mononuclear cells (CBMC) from parasitized (upper figure, black bars; n = 10) and non-parasitized (lower figure, grey bars; n = 12) placenta were either depleted of regulatory T cells (Tregs-) or left untreated and cultured in the presence of anti-IL-10 antibody or an isotype control antibody. Following stimulation with live Plasmodium falciparum Pf164-infected red blood cells, IFN-γ release was measured. Significant IFN-γ recovery was observed after regulatory T cell depletion (P1 = 0·014) and anti-IL-10 blocking antibody (P2 = 0·021). Results are shown as the mean ± standard error of the mean (minus backgrounds).
Discussion
P. falciparum placental infection impacts on newborn's immune responses, as both Th1 and Th2 differentiation of newborn's T cells have been reported [4,14]. Mice models versus human malaria, maternal immunity and placental damage may explain these discrepancies. In our hands, previous results from a Sukuta cohort of Gambian primi- and multiparous pregnant women indicated that active placental infection impacts on IFN-γ, IL-12 and sCD30 production of neonatal T cells (lower IFN-γ and IL-12 and higher sCD30) [4]. This study, together with the reported data on impaired IL-12 function in neonates [15], and the inhibitory effect of P. falciparum on dendritic cell (DC) maturation [16], suggested that the reduced production of IFN-γ could be attributed to an inhibition of antigen-presenting cells (APC) in the cultures from the parasitized group. Recent reports indicated that Tregs might be also incriminated in the maintenance of malaria infection through production of high levels of IL-10 and transforming growth factor (TGF)-β. These cytokines, common to APC and Tregs, are known immunomodulatory molecules and may be involved in the inhibition of Th1 cytokine production by the CBMCs from the parasitized placenta group.
We therefore focused on the possible role of this T cell population in the observed Th1/Th2 imbalance. Using the same cohort, we compared CD4+CD25+ FoxP3+ T cell frequencies and cultured selected samples of CBMC before and after depletion of CD4+CD25+ FoxP3low cells in the presence of PHA, Pf164 schizont extracts, MSP119 and live parasites and tested for their ability to elicit IFN-γ, sCD30 and IL-10 secretion. The results confirm our previous observation that active infection of the placenta by P. falciparum exacerbates the Th2 bias of neonatal lymphocytes, as evidenced by the lower IFN-γ in response to all antigens, and higher sCD30 production in response to protein antigens and live Pf by cells from the parasitized group. We have found a higher IL-10 production in response to all antigens as well as higher IL-10 production by the CD4+CD25+ population in response to PHA, which supports their regulatory phenotype. As stated above, IL-10 production may be attributed to APC but CD4+ CD25+ FoxP3+ Tregs may also be important [5,6]. To discriminate between the different cell populations we depleted CD4+CD25+ T cells from the CBMC and reassessed the Th1/Th2 cytokines in vitro. The depletion resulted in lower IL-10 production and restoration of IFN-γ production in response to all tested stimuli. No significant post-depletion effect was observed on IL-13 (not shown). Interestingly, an antigen-dependent response was observed for sCD30, e.g. depletion did not affect the response to protein antigens as the higher sCD30 level observed in the parasitized group remained significant. In contrast, it re-established similar levels between both groups in response to live Pf.
The higher levels of cytokines induced in response to live Pf compared to protein antigens as well as the antigen-associated Th2 differentiation suggest a different and probably a complex mechanism of interaction of APC with these different stimuli.
Despite the similar frequencies of CD4+CD25+ T cells between both groups, which is in contrast to previous reported data [7], depletion of this population impacted on Th1/Th2 cytokines in vitro. The frequency differences may be ascribed to different placental parasitaemia levels, active and/or chronic infection between both cohorts [7,9], and the post-depletion effects observed in our cohort may be attributed to a different CD4+CD25+ activation state, a synergistic effect with APC or the interaction with other T cell populations. Furthermore [17], using a mouse model, Mold et al. have shown that Tregs of maternal origin cross the placenta. However, in our hands depletion of Tregs (CD25+ T cells) did not affect allo-MLR against maternal samples, as reported by Mold's group, suggesting that P. falciparum antigens and placental inflammatory cytokines primed mainly Tregs from CBMC.
To assess the role of IL-10 on Th1 and Th2 cytokines or markers, live Pf stimulation was privileged to protein antigens, as it induced higher concentrations of all tested cytokines and showed a significant difference before and after CD4+CD25+ FoxP3+ T cell depletion. Blockade of IL-10 function with a monoclonal antibody produced a similar profile of cytokine production to depletion of the Tregs, e.g. restoration of IFN-γ production. However, anti-IL-10 antibody had no significant effect on sCD30, e.g. higher levels were still observed in the parasitized group after this treatment (data not shown). This result suggests that the CD4+CD25+ FoxP3+ T cells in the parasitized group, at least through high levels of IL-10, may have reduced the IL-12 [18] production of APC which, in turn, decreased IFN-γ and increased sCD30 production by T cells. IL-10 antibodies were able to attribute the impact of CD4+CD25+ FoxP3+ population on Th1 cytokines to IL-10. These findings on Treg effects on Th1 and Th2 differentiation highlight a complex strategy used by P. falciparum and require further investigation.
In summary, Th1/Th2 neonatal T cell imbalance to active P. falciparum infection of the placenta, a possible strategy used by P. falciparum to escape immune responses, implicates Tregs through IL-10 production, as both depletion of Tregs and blockade of IL-10 function restored IFN-γ production. Previous studies [4], as well as the current results, incriminate both APC through lower IL12 production and CD4+CD25+ FoxP3+ Tregs through IL-10 in IFN-γ decrease, and therefore exacerbation of the Th2 bias.
Acknowledgments
This study was funded by the Medical Research Council, UK and by the European Commission through the EUROMALVAC Consortium, contract QLK2-CT-2002-01197. We are again grateful to the mothers and their babies enrolled into the 2001 study, to the staff of Sukuta Health Centre and to the Department of State for Health. We thank Simon Correa and Saikou Keita for the technical support.
Disclosure
None.
References
- 1.Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–17. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okoko BJ, Ota MO, Yamuah LK, et al. Influence of placental malaria infection on foetal outcome in The Gambia: twenty years after Ian McGregor. J Health Popul Nutr. 2002;20:4–11. [PubMed] [Google Scholar]
- 3.McGregor IA, Wilson ME, Billewicz WZ. Malaria infection of the placenta in The Gambia, West Africa; its incidence and relationship to stillbirth, birth weight and placental weight. Trans R Soc Trop Med Hyg. 1983;77:232–44. doi: 10.1016/0035-9203(83)90081-0. [DOI] [PubMed] [Google Scholar]
- 4.Ismaili J, Van der Sande M, Holland MJ, et al. Plasmodium falciparum infection of the placenta affects newborn immune responses. Clin Exp Immunol. 2003;133:414–21. doi: 10.1046/j.1365-2249.2003.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walther M, Tongren JE, Andrews L, et al. Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria. Infect Immun. 2005;23:287–96. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Hisaeda H, Maekawa Y, Iwakawa D, et al. Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 7.Brustoski K, Moller U, Kramer M, et al. Reduced cord blood immune effector-cell responsiveness mediated by CD4+ cells induced in utero as a consequence of placental Plasmodium falciparum infection. J Infect Dis. 2006;193:146–54. doi: 10.1086/498578. [DOI] [PubMed] [Google Scholar]
- 8.Greenwood BM, Amstrong JRM. Comparison of two simple methods for determining malaria parasite density. Trans R Soc Trop Med Hyg. 1991;85:186–8. doi: 10.1016/0035-9203(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 9.Gardner JP, Pinches RA, Roberts DJ, et al. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum. Proc Natl Acad Sci USA. 1996;93:3503–8. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–20. [PubMed] [Google Scholar]
- 11.Pasvol G, Wilson RJ, Smalley ME, et al. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978;72:87–8. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- 12.Freeman RR, Holder AA. Surface antigens of malaria merozoites A high molecular weight precursor is processed to an 83,000 mol wt form expressed on the surface of Plasmodium falciparum merozoites. J Exp Med. 1983;158:1647–53. doi: 10.1084/jem.158.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan WDB, Birdsall TA, Frenkiel MG, et al. Solution structure of an EGF module pair from the Plasmodium falciparum merozoite surface protein 1. J Mol Biol. 1999;289:113–22. doi: 10.1006/jmbi.1999.2753. [DOI] [PubMed] [Google Scholar]
- 14.Malhotra AN, Wamachi PL, Mungai EM, et al. Fine specificity of neonatal lymphocytes to an abundant malaria blood-stage antigen: epitope mapping of Plasmodium falciparum MSP133. J Immunol. 2008;180:3383–90. doi: 10.4049/jimmunol.180.5.3383. [DOI] [PubMed] [Google Scholar]
- 15.Goriely S, Vincart B, Stordeur P, et al. Deficient IL-12 (p35) gene expression by dendritic cells derived from neonatal monocytes. J Immunol. 2001;166:2141–6. doi: 10.4049/jimmunol.166.3.2141. [DOI] [PubMed] [Google Scholar]
- 16.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–7. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 17.Jeff EM, Jakob M, Trevor D, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng JC, Abu Bakar S, Richardson MM, et al. IL-10 and IL-12B polymorphisms each influence IL-12p70 secretion by dendritic cells in response to LPS. Immunol Cell Biol. 2006;84:227–32. doi: 10.1111/j.1440-1711.2006.01419.x. [DOI] [PubMed] [Google Scholar]


