Abstract
Human T lymphotropic virus type 1 (HTLV-1) infects 10–20 million people worldwide. The majority of infected individuals are asymptomatic; however, approximately 3% develop the debilitating neurological disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). There is also currently no cure, vaccine or effective therapy for HTLV-1 infection, and the mechanisms for progression to HAM/TSP remain unclear. NK T cells are an immunoregulatory T cell subset whose frequencies and effector functions are associated critically with immunity against infectious diseases. We hypothesized that NK T cells are associated with HAM/TSP progression. We measured NK T cell frequencies and absolute numbers in individuals with HAM/TSP infection from two cohorts on two continents: São Paulo, Brazil and San Francisco, CA, USA, and found significantly lower levels when compared with healthy subjects and/or asymptomatic carriers. Also, the circulating NK T cell compartment in HAM/TSP subjects is comprised of significantly more CD4+ and fewer CD8+ cells than healthy controls. These findings suggest that lower numbers of circulating NK T cells and enrichment of the CD4+ NK T subset are associated with HTLV-1 disease progression.
Keywords: CD8, HTLV-1 infection, innate immunity, NK T cells, CD4
Introduction
Most human T lymphotropic virus type 1 (HTLV-1)-infected subjects are asymptomatic, but the chronic neurodegenerative disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) develops in about 3% of carriers [1–3]. The factors mediating progression to HAM/TSP remain largely unresolved. Hypothesized pathogenic mechanisms include direct toxic effects of higher proviral loads and viral tax proteins, an abnormally high CD8+ T cell response to HTLV-1 with concomitant damage to neural tissue, and molecular mimicry with HTLV-1 antigens resembling myelin basic protein stimulating an autoimmune response [4–8].
Natural killer T (NK T) cells are an immunoregulatory subset of T cells whose frequencies and effector functions are often associated strongly with autoimmunity, tumour immunity and immunity against infectious diseases [9]. NK T cells have been implicated in the control of many infections, including Mycobacterium tuberculosis[10–12] and human immunodeficiency virus (HIV) [13–16]. In HIV-infected individuals NK T cell numbers are lower in the circulation than healthy controls [14,15], and the remaining cells are severely functionally impaired [17,18]. We hypothesized that NK T cells are involved in the progression to HAM/TSP among HTLV-1-infected individuals. We measured the frequencies, absolute counts and CD4, CD8 and CD4-/CD8- double negative cell (DN) subsets of NK T cells within the peripheral blood mononuclear cells (PBMC) of individuals with HAM/TSP, HTLV-1-infected asymptomatic and healthy control subjects from cohorts in two different geographical locations, São Paulo, Brazil and San Francisco, USA.
Materials and methods
Human subjects
We obtained biological specimens from subjects with HAM/TSP, HTLV-1 infection without neurological disease and age- and sex-matched subjects with no evidence of HTLV-1 infection (healthy controls). Subjects were identified from two separate HTLV-1 cohorts derived from the University of São Paulo in Brazil (Brazilian cohort) and from former blood donors in the multi-centre US HTLV Outcomes Study (HOST) of the University of California San Francisco (American cohort) (Table 1). HTLV-1 infection status was determined by serology [enzyme immunoassay (EIA) and Western blot]; polymerase chain reaction (PCR) or type-specific serology was used to differentiate HTLV-1 from HTLV-II. HIV status of all subjects were tested and known to be seronegative in both cohorts. PBMC were isolated and cyropreserved in liquid nitrogen until use. Absolute lymphocyte counts were determined on fresh blood samples using automated clinical haematology analysers at both locations, and used to calculate the numbers of NK T cells per µl blood. This study was approved by the Institutional Review Boards and Ethical Committees of the University of California, San Francisco (UCSF Committee on Human Research) and the University of São Paulo.
Table 1.
Patient cohort demographics.
| Patient characteristics |
|||
|---|---|---|---|
| Groups | Number (n) | Gender (male, female) | Age: median (IQR)* |
| American cohort | 50 | ||
| Uninfected controls | 20 | 6, 14 | 47 (43, 52) |
| HTLV-1 carriers | 20 | 5, 15 | 54 (51, 63) |
| HAM/TSP | 10 | 2, 8 | 52 (47, 61) |
| Brazilian cohort | 68 | ||
| Uninfected controls | 18 | 9, 9 | 27 (24, 49·5) |
| HTLV-1 carriers | 31 | 10, 21 | 46 (36, 53) |
| HAM/TSP I | 19 | 6, 13 | 57 (46, 62) |
Interquartile range.
HTLV-1, human T lymphotropic virus type 1; HAM/TSP, HTLV-1-associated myelopathy/tropical spastic paraparesis.
Flow cytometry
PBMCs were thawed rapidly in RPMI-1640 with 10% fetal bovine serum and washed in fluorescence activated cell sorter (FACS) buffer [phosphate-buffered saline (PBS), with 0·5% bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA)]. The NK T cells were defined as follows: for the American cohort, gating included cells positive for CD1d PBS-57-loaded tetramers (NIH Tetramer Facility, Atlanta, GA, USA), anti-CD3 (BD Biosciences, San Jose, CA, USA) and anti-Vα24 antibodies (Beckman Coulter, Fullerton, CA, USA); for the Brazilian cohort, gating included anti-CD3 (BD Biosciences) anti-Vα24 (Beckman Coulter) and anti-Vβ11 (Beckman Coulter) positive cells. NK T cell subsets were defined using allophycocyanin (APC) anti-CD4 and APC-Cy7 anti-CD8 antibodies (both from BD Biosciences). The Amine Aqua dye (Invitrogen, Carlsbad, CA, USA) was used to exclude dead cells in all samples from the American (HOST) cohort. Flow cytometry for the American and Brazilian cohorts were performed on a LSR-II (BD Biosciences) and FACSCanto (BD Biosciences), respectively. All data were analysed using FlowJo software (version 6·4·7; Tree Star Inc., Ashland, OR, USA).
Statistical analysis
We employed non-parametric test statistics in all cases. The Mann–Whitney U-test was used to compare NK T percentages between the different groups. Correlations were assessed between continuous variables by use of the Spearman's rank correlation test. Statistical tests were performed on GraphPad Prism statistical software (GraphPad Software, San Diego, CA, USA) and used to display results from this study.
Results
Lower proportion of NK T cells in HTLV-1 associated HAM/TSP infection
We compared the NK T cell levels of the three subject groups from both our American and Brazilian cohorts using a panel of NK T cell markers (Fig. 1a,b). The NK T cell frequencies were significantly lower in HAM/TSP subjects compared to HTLV-1 asymptomatic carriers in the American cohort (Fig. 2a). Similar results were found from the Brazilian cohort, with significantly lower NK T cell frequencies in HAM/TSP subjects compared to either the healthy or HTLV-1 asymptomatic groups (Fig. 2b). The absolute numbers of NK T cells were calculated from a subset of subjects from both cohorts. Significantly lower numbers were found in HAM/TSP compared to HTLV-asymptomatic subjects in the American cohort (Fig. 2c). In the Brazilian cohort, significantly fewer NK T cells were present in both HTLV-1-infected groups compared to healthy controls, regardless of disease status (Fig. 2d). However, the lowest numbers of circulating NK T cells were found in the HAM/TSP subjects in both cohorts (Fig. 2c,d).
Fig. 1.

Gating strategy for the natural killer T (NK T) cells. Gating strategy for NK T cells from the (a) San Francisco HTLV Outcomes Study (HOST) cohort and (b) Sao Paulo cohort. One representative donor from each cohort is shown.
Fig. 2.
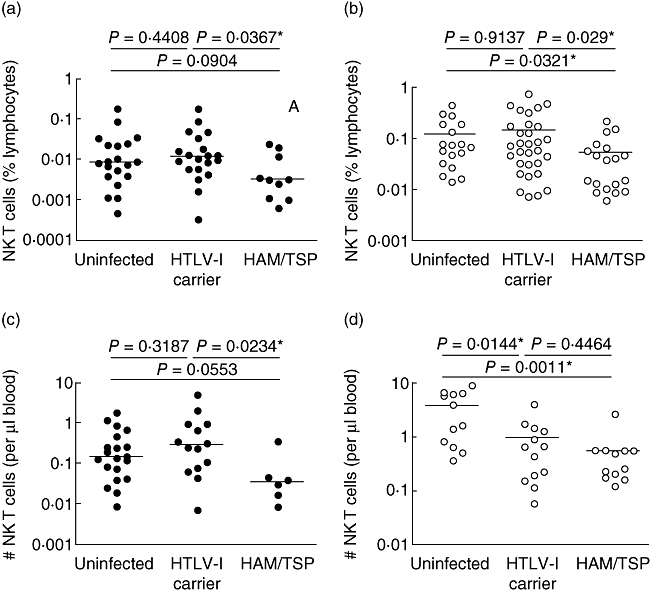
Proportion of natural killer T (NK T) cells in patients and controls. The NK T cell frequencies of three subject groups is shown from the American cohort (a) (black dots) and Brazilian cohort (b) (white dots). Absolute numbers of NK T cells for the American cohort (c) (black dots) and Brazilian cohort (d) (white dots).
Shift in the CD4+ and CD8+ NK T cell subsets in HAM/TSP patients
NK T cell subsets, defined using CD4 and CD8 antigens, can produce distinct patterns of T helper type 1 (Th1) and Th2 cytokines, with CD4+ cells reported to secrete more Th2 cytokines than their CD4- counterparts [19,20]. Furthermore, CD4 CD8 DN NK T cells represent the second dominant NK T cell subset (50–20% of NK T cells) [21,22]. We therefore evaluated whether the CD4 single positive (SP), CD8 SP and CD4 and CD8 DN subset distribution is altered in the NK T cell compartment of HAM/TSP subjects from the Brazilian cohort. The higher numbers of NK T cell frequencies in this cohort allowed an accurate assessment of subset distribution. Among the Brazilian HAM/TSP patients, we observed a significantly higher proportion of CD4+ (SP) NK T cells, and a significantly lower percentage of CD8+ (SP) cells (Fig. 3a). No difference in the frequency of DN NK T cells was observed among all subject groups (Fig. 3a). Whereas the distribution of total (all CD3+) CD4+ and CD8+ T cells was similar between all subject groups, we observed a reduction in the frequency of total DN cells between the healthy subjects and HAM/TSP population (Fig. 3b). Taken together, these results indicate that the CD4/CD8 ratio shift in the NK T cell compartment is specific to NK T cells.
Fig. 3.
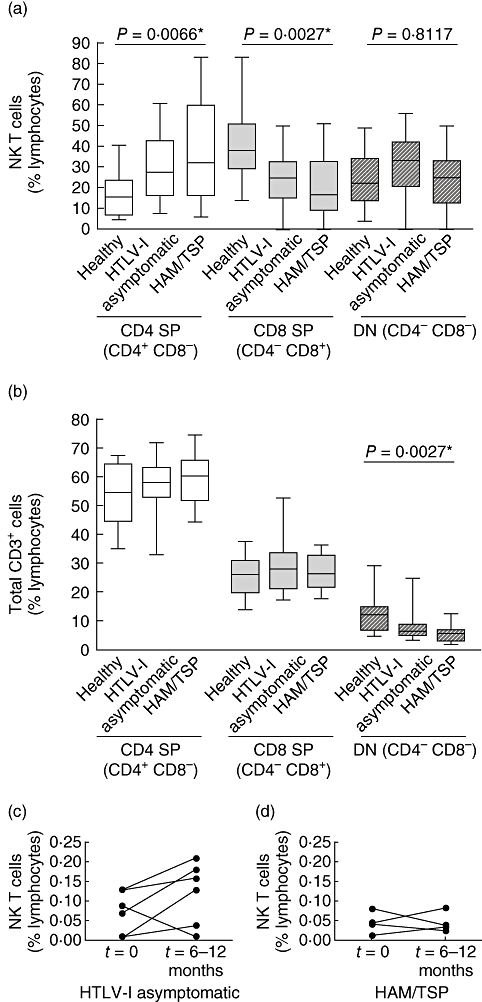
Proportion of natural killer T (NK T) cell subsets in patients. The frequencies of total (a) NK T cell lymphocyte subsets and (b) CD3+ cells expressing single positive (SP) CD4 and CD8 cells together with CD4-/CD8- double negative (DN) cells from the Brazilian cohort. (c,d) The frequency of NK T cells from two time-points in 10 human T lymphotropic virus type 1 (HTLV-1) infected individuals; HTLV-1 carriers (c) and those with HAM/TSP (d) from the Brazilian cohort. *P < 0·05 statistically significant.
Assessment of NK T cells in HTLV-1-infected patients over time
We next compared the NK T cell frequencies longitudinally from a subset of individuals from the Brazilian cohort. Overall, we did not detect any significant differences in NK T cell frequencies among either the HTLV-1 asymptomatic or the HAM/TSP individuals over a 6-months–1 year interval (Fig. 3c,d). Our longitudinal measurements were of a relatively short interval for this chronic disease, and thus analysis of the dynamics of the NK T cell population in long-term longitudinal studies is an important next step to assess the prognostic value of monitoring the NK T cells during the course of HTLV-1 infection.
Discussion
This is, to our knowledge, the first study to assess NK T cells in the peripheral blood of HTLV-1-infected individuals. The high variability of NK T cell frequencies, between both individuals and groups from different geographical locations, renders our similar results from two different continents striking.
NK T cells can produce abundant proinflammatory cytokines, but are also implicated strongly in the suppression/containment of activated T cells. Therefore, a selective loss of immunosuppressive NK T cells could lead to an unabated proinflammatory HTLV-1-specific T cell response and potentially fuel immunopathogenesis and disease progression. It is unknown whether the low NK T cell numbers in the circulation of HAM/TSP subjects is a cause or a consequence of disease progression. In HIV-infected patients, NK T cells express receptors necessary for viral entry such as CD4, CXCR4 or CCR5. Also, compared with classical CD4+ T cells, NK T cells are depleted preferentially in vitro by HIV [15]. Whether NK T cells or specific NK T cell subsets are more prone to HTLV-1 infection remains unclear. Thus, analysis of NK T cell populations in long-term longitudinal studies is an important next step to assess the prognostic value of monitoring the NK T cells during the course of HTLV-1 infection. Also, it will also be important to determine if the residual circulating NK T cells have retained both proinflammatory and/or regulatory activity in future studies.
NK T cell populations were measured using different flow cytometric panels in the two cohorts. Comparisons of the reliability of detection of NK T cells with either a combination of Vα24- and Vβ11-expressing cells and CD1d-tetramer+ cells differ as to which is a better surrogate marker for this population [23,24]. We have not seen significant differences in direct comparative studies [10], and therefore believe that we are observing similar populations in both cohorts. That said, we concede that to confirm unequivocally if the geographical or genetic differences are responsible for the differences in total NK T frequencies between the cohorts, a larger sampling with the same reagents will be needed.
We observed an apparent shift in the NK T cell subsets based on CD4+ and CD8+ expression in HTLV-1 infection. It is possible that the CD4- NK T cells have selectively left the circulation to the central nervous system (CNS) tissue or cerebrospinal fluid (CSF) or, alternatively, this subset-skewing reflects preferential expansion of the CD4+ immunosuppressive NK T subset in response to inflammation from HAM/TSP. Also, the frequency of the CD4+ NK T cells among healthy controls in the Brazilian cohort is lower compared to previous studies (40–60% mean range) [21,22], and this difference may be due to unknown differences in NK T subset distributions in different geographical locations. An apparent enrichment of the CD4+ subset among NK T cells (and relative decrease of other subsets) is interesting, and has been observed in other chronic diseases such as chronic hepatitis C virus (HCV) [25], mycobacterial infections [21] and also in the liver of patients with primary biliary cirrhosis [26] or patients with intrahepatic malignant tumours [27].
Currently there is no cure, vaccine or effective therapy for HTLV-1 infection. Our findings suggest that therapies modulating NK T cells could hold promise in preventing progression from asymptomatic HTLV-1 infection to neurodegenerative disease.
Acknowledgments
The project described was supported by grants R37AI052731 from the National Institute of Allergy and Infectious Diseases, 2R01-HL-62235 from the National Heart Lung and Blood Institute and by funds the AIDS Research Institute of University of California San Francisco, the Brazilian Program for STD and AIDS, Ministry of Health (914/BRA/3014 – UNESCO/Kallas), the São Paulo City Health Department (2004-0·168.922-7/Kallas), Fundação de Amparo a Pesquisa do Estado de São Paulo (04/15856-9/Kallas) and the John E. Fogarty International Center (D43 TW00003). The authors would like to thank Dr Johan Sandberg for a critical review of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Disclosure
All authors declare no conflicts of interest.
References
- 1.Orland JR, Engstrom J, Fridey J, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes study. Neurology. 2003;61:1588–94. doi: 10.1212/01.wnl.0000096011.92542.da. [DOI] [PubMed] [Google Scholar]
- 2.Osame M, Usuku K, Izumo S, et al. HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031–2. doi: 10.1016/s0140-6736(86)91298-5. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Barin F, Vernant JC, et al. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet. 1985;2:407–10. doi: 10.1016/s0140-6736(85)92734-5. [DOI] [PubMed] [Google Scholar]
- 4.Itoyama Y, Minato S, Kira J, et al. Spontaneous proliferation of peripheral blood lymphocytes increased in patients with HTLV-I-associated myelopathy. Neurology. 1988;38:1302–7. doi: 10.1212/wnl.38.8.1302. [DOI] [PubMed] [Google Scholar]
- 5.Bangham CR. Human T-cell leukaemia virus type I and neurological disease. Curr Opin Neurobiol. 1993;3:773–8. doi: 10.1016/0959-4388(93)90152-o. [DOI] [PubMed] [Google Scholar]
- 6.Sakai JA, Nagai M, Brennan MB, Mora CA, Jacobson S. In vitro spontaneous lymphoproliferation in patients with human T-cell lymphotropic virus type I-associated neurologic disease: predominant expansion of CD8+ T cells. Blood. 2001;98:1506–11. doi: 10.1182/blood.v98.5.1506. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson S. Immunopathogenesis of human T cell lymphotropic virus type I-associated neurologic disease. J. Infect Dis. 2002;186(Suppl. 2):S187–92. doi: 10.1086/344269. [DOI] [PubMed] [Google Scholar]
- 8.Levin MC, Lee SM, Kalume F, et al. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8:509–13. doi: 10.1038/nm0502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 10.Snyder-Cappione JE, Nixon DF, Loo CP, et al. Individuals with pulmonary tuberculosis have lower levels of circulating CD1d-restricted NKT cells. J Infect Dis. 2007;195:1361–4. doi: 10.1086/513567. [DOI] [PubMed] [Google Scholar]
- 11.Sugawara I, Yamada H, Mizuno S, Li CY, Nakayama T, Taniguchi M. Mycobacterial infection in natural killer T cell knockout mice. Tuberculosis. 2002;82:97–104. doi: 10.1054/tube.2002.0331. [DOI] [PubMed] [Google Scholar]
- 12.Sada-Ovalle I, Chiba A, Gonzales A, Brenner MB, Behar SM. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathol. 2008;4:e1000239. doi: 10.1371/journal.ppat.1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li D, Xu XN. NKT cells in HIV-1 infection. Cell Res. 2008;18:817–22. doi: 10.1038/cr.2008.85. [DOI] [PubMed] [Google Scholar]
- 14.van der Vliet HJ, von Blomberg BM, Hazenberg MD, et al. Selective decrease in circulating V alpha 24+V beta 11+ NKT cells during HIV type 1 infection. J Immunol. 2002;168:1490–5. doi: 10.4049/jimmunol.168.3.1490. [DOI] [PubMed] [Google Scholar]
- 15.Sandberg JK, Fast NM, Palacios EH, et al. Selective loss of innate CD4(+) V alpha 24 natural killer T cells in human immunodeficiency virus infection. J Virol. 2002;76:7528–34. doi: 10.1128/JVI.76.15.7528-7534.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Unutmaz D. NKT cells and HIV infection. Microb Infect. 2003;5:1041–7. doi: 10.1016/s1286-4579(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 17.Moll M, Kuylenstierna C, Gonzalez VD, et al. Severe functional impairment and elevated PD-1 expression in CD1d-restricted NKT cells retained during chronic HIV-1 infection. Eur J Immunol. 2009;39:902–11. doi: 10.1002/eji.200838780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snyder-Cappione JE, Loo CP, Carvalho KI, et al. Lower cytokine secretion ex vivo by natural killer T cells in HIV-infected individuals is associated with higher CD161 expression. AIDS. 2009;15:1965–70. doi: 10.1097/QAD.0b013e32832b5134. [DOI] [PubMed] [Google Scholar]
- 19.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med. 2002;195:637–41. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Im JS, Kang TJ, Lee SB, et al. Alteration of the relative levels of iNKT cell subsets is associated with chronic mycobacterial infections. Clin Immunol. 127:214–24. doi: 10.1016/j.clim.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Valpha24 NKT cell compartment. Eur J Immunol. 2003;33:588–96. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]
- 23.Brigl M, van den Elzen P, Chen X, et al. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol. 2006;176:3625–34. doi: 10.4049/jimmunol.176.6.3625. [DOI] [PubMed] [Google Scholar]
- 24.Exley MA, Hou R, Shaulov A, et al. Selective activation, expansion, and monitoring of human iNKT cells with a monoclonal antibody specific for the TCR alpha-chain CDR3 loop. Eur J Immunol. 2008;38:1756–66. doi: 10.1002/eji.200737389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas M, Gadola S, Meier U, et al. Frequency and phenotype of circulating Valpha24/Vbeta11 double-positive natural killer T cells during hepatitis C virus infection. J Virol. 2003;77:2251–7. doi: 10.1128/JVI.77.3.2251-2257.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kita H, Naidenko OV, Kronenberg M, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123:1031–43. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 27.Bricard G, Cesson V, Devevre E, et al. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009;182:5140–51. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]


