Abstract
16α-Bromoepiandrosterone (HE2000) is a synthetic steroid that limits non-productive inflammation, enhances protective immunity and improves survival in clinical studies of patients with human immunodeficiency virus (HIV), malaria and tuberculosis infections. We now show that HE2000 decreased nitric oxide production by lipopolysaccharide (LPS)-stimulated RAW264·7 cells. Treatment with HE2000 also reduced non-productive inflammation associated with carrageenan-induced pleurisy and LPS-induced lung injury in mice. In the hapten-carrier reporter antigen popliteal lymph node assay, HE2000 increased absolute numbers of lymphocytes, antigen-presenting cells, hapten-specific immunoglobulin (Ig)M antibody-forming cells and shifted the interferon (IFN)-γ/interleukin (IL)-4 balance towards IFN-γ production. In the cystic fibrosis transmembrane conductance regulator (CFTR−/−) mouse model of acute Pseudomonas aeruginosa infection, treatment with HE2000 consistently reduced bacterial burden in lungs. All HE2000 effects were dose-dependent. In H1N1 infection in mice, HE2000 was safe but not effective as a monotherapy, as treatment did not effect survival. HE2000 reduced mortality related to excessive inflammation and opportunistic lung infections in animals and patients, and this might extend to those with H1N1 influenza infection.
Keywords: immune function, influenza, lung inflammation, steroid
Introduction
16α-bromoepiandrosterone (HE2000) is a synthetic immune stimulating steroid with demonstrated ability to benefit patients with malaria [1], tuberculosis [2] and human immunodeficiency virus (HIV) infection [3]. In a randomized, double-blind, placebo-controlled study of the safety, tolerance, immunological effect and anti-HIV activity of subcutaneously administered HE2000 as a monotherapy, in HIV-1-infected, treatment-naive patients, treatment (50 mg) was safe and well tolerated. Treatment was associated with improved immunity, as evidenced by significant increases (comparison to placebo) in numbers of circulating dendritic cells, early activated (CD69+CD25-) CD8 T cells, and T natural killer (T NK) cells as well as anti-HIV-1 T cell responses and half-log decreases in viral load. Treatment also attenuated non-productive (i.e. not associated with effective immunity) inflammation, as evidenced by significant decreases in transcripts for interleukin (IL)-1β, tumour necrosis factor (TNF)-α, IL-6 and cyclooxygenase-2 (COX-2) [3]. In a subsequent study in patients with acquired immune deficiency syndrome (AIDS), HE2000 treatment reduced both the incidence of tuberculosis co-infection by 42·2% and the cumulative incidence of opportunistic infections [2]. These clinical findings were presaged by studies in animals, including feline immunodeficiency virus (FIV) [4] and a murine model of tuberculosis [5]. In those studies, treatment with HE2000 was associated with reduced inflammation, lower pathogen loads and improved immunity.
In the present studies, we tested the ability of HE2000 to inhibit nitric oxide (NO) production in vitro, to limit non-productive lung inflammation in vivo and to enhance cellular and molecular mediators of innate immunity, including IFN-γ signalling. Finally we tested the ability of HE2000 to reduce bacterial burden in lungs in the cystic fibrosis transmembrane conductance regulator (CFTR−/−) mouse model of opportunistic bacterial infection of the lung, and established safety of HE2000 treatment in mice challenged with lethal H1N1 infection. Our observations suggest that HE2000 treatments attenuate non-productive inflammation, enhance effective immunity and may be useful as adjunct therapy to reduce influenza-related mortality.
Materials and methods
Steroids
16α-Bromoepiandrosterone (HE2000) was produced by HollisEden Pharmaceuticals (San Diego, CA, USA). Vehicle (HERF405) contains 0·1% carboxymethylcellulose, 2% polysorbate 80 and 0·1% metabisulphite in phosphate-buffered saline pH 7·4. Control animals were treated with an equal volume of vehicle.
Nitric oxide production by RAW cells
Cell culture
RAW264·7 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) (Invitrogen Corporation, Carlsbad, CA, USA). HE2000 was dissolved in dimethylsulphoxide (DMSO) and added at a maximum of 0·1% v/v. Subconfluent layers of RAW264·7 cells grown in 24-well plates in phenol-red free DMEM + 10% FCS were stimulated with 100 ng/ml lipopolysaccharide (LPS) (Salmonella Minnesota Re595; Calbiochem, San Diego, CA, USA) in the presence or absence of serial dilutions of HE2000.
Measurement of NO
After 6–48 h, nitric oxide in the supernatants was determined using the Griess reagent (Solon, OH, USA), as described by Chao [5]. Cell viability was also assessed by exclusion of propidium iodide. For this purpose cells were incubated with 2 µg/ml propidium iodide (Invitrogen) for 15 min at room temperature and the fluorescence intensity was detected on the FL2 channel of a fluorescence activated cell sorter (FACScan; Becton Dickinson, San Jose, CA, USA). Cells permeabilized with 0·1% saponin were used as a positive control.
Carrageenan-induced pleurisy
Animals
Six to 8-week-old CD1 male mice (Charles River, Calco, Italy) were used for the carrageenan (CAR)-induced pleurisy studies. The animals were maintained in a pathogen-free vivarium at the University of Catania, School of Medicine (Catania, Italy) receiving ad libitum sterilized food and water, and were adapted to the ambient environment for at least 7 days before use. Animal care and use was in compliance with all applicable regulations on protection of animals used for experimental and other scientific purposes.
Experimental groups
Mice were allocated into one of the following groups: (i) administration of CAR only (CAR group, n = 8); (ii) mice treated with either 0·3 or 3 mg HE2000 (s.c.) 24 h and 1 h before CAR (n = 8 per dose); (iii) mice treated with vehicle [subcutaneously (s.c.)] 24 h and 1 h before CAR (n = 8); and (iv) mice treated with a rabbit anti-mouse polyclonal anti-TNF-α antibody (200 µg) given as an intraperitoneal (i.p.) bolus 24 h and 1 h before CAR (n = 8). All treatments were given in a volume of 100 µl. In studies where cytokines were measured in exudates, two to four animals per group were considered.
Pleurisy
Mice were anaesthetized with isoflurane and the skin was incised at the level of the left sixth intercostal space. The underlying muscle was dissected and saline (0·1 ml) or saline containing 2% λ-CAR (0·1 ml; Sigma Chimica, Milan, Italy) was injected into the pleural cavity. The skin incision was closed with a suture and the animals were allowed to recover. At 4 h after the injection of CAR (peak of the neutrophil and exudate response), the animals were euthanized by inhalation of CO2. The chest was opened carefully and the pleural cavity rinsed with 1 ml of saline solution containing heparin (5 U/ml) and indomethacin (10 µg/ml). The exudate and washing solution were removed by aspiration and the total volume measured. Any exudate that was contaminated with blood was discarded. The amount of exudate was calculated by subtracting the volume injected (1 ml) from the total volume recovered. The leucocytes in the exudate were suspended in phosphate-buffered saline (PBS) and counted with an optical microscope in a Burker's chamber after vital Trypan blue staining. IL-6 levels in exudates were determined by enzyme-linked immunosorbent assay (ELISA) (Biosource International, Camarillo, CA, USA).
Statistical analysis
All values in the figures are expressed as mean ± standard deviation. Cell and exudate results were analysed by one-way analysis of variance (anova) followed by a Bonferroni post-hoc test for multiple comparisons. IL-6 results were analysed by two-sided Student's t-test. A P-value of less than 0·05 was considered significant.
LPS-induced lung injury
Animals
Six to 8-week old C57 black/six male mice (approximately 25–30 g; Harlan, San Diego, CA, USA) were used in these studies (at least eight animals per group). These mice were acclimated for at least 3 days prior to the start of the experiment. The animals were housed in an American Association for Laboratory Animal Care accredited facility. Animals were purchased and housed in accordance with institutional guidelines and requirements of the relevant regulatory agencies.
Chemicals and reagents
LPS (Escherichia coli 0111:B4) and dexamethasone (Dex) were obtained from Sigma-Aldrich (St Louis, MO, USA). TNF-α and IL-6 ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA).
Lung injury model
Animals were treated with HE2000 via a single gavage administration (100 µl) 24 h and 1 h before LPS challenge. After light anaesthesia with isofluorane inhalation, LPS (100 µg) was instilled into the trachea under direct visualization. Forty-eight hours after LPS instillation, the mice were euthanized. To obtain the bronchoalveolar lavage fluid (BAL), the euthanized mice were immobilized and a mid-line incision was performed in the neck to isolate the trachea. Three aliquots of PBS (500 µl) were instilled sequentially and aspirated via a blunt-ended 20-gauge needle. The aspirated BAL was assessed for cell counts.
Statistical analysis
Data were analysed using two-sided Student's t-tests.
Popliteal lymph node assay
Chemicals and reagents
Chemicals were obtained from Sigma-Aldrich (Zwijndrect, the Netherlands) unless stated otherwise. Saline (0·9%; B. Braun Melsungen, Melsungen, Germany) and citrate buffer (0·1 M citric acid and 0·1 M disodium hydrogen phospate, pH 6) were used to dilute test chemicals. Trinitrophenol–ovalbumin (TNP–OVA) was prepared as described previously [6].
Mice
Non-specific pathogen-free female BALB/c mice (6–12 weeks old) were obtained from the Utrecht University breeding facility (Gemeenschappelyk Dier Laboratorium, Utrecht, the Netherlands) and assigned randomly to specific treatment groups. Animals were purchased and housed at Utrecht University Animal Facilities in accordance with respective institutional guidelines and requirements of the relevant regulatory agencies.
Popliteal lymph node (PLN) assay
Naive mice (three to five mice per group) were injected s.c. into the right hind footpad with 50 µl of a freshly prepared mixture of the drug together with a subsensitizing dose (10 µg) of TNP–OVA conjugate. Seven days after drug injection, the PLN was excised and separated from adherent fatty tissue. PLNs were isolated in ice-cold PBS/1% bovine serum albumin (BSA) and single-cell suspensions were prepared, washed (350 g at 4°C), resuspended in 1 ml PBS/1% BSA, counted using a Coulter counter (Coulter Electronics, Luton, UK) and adjusted to 1 × 106 cells/ml.
Enzyme-linked immunospot (ELISPOT) assay
Immobilon-P membranes (Immobilon PVDF Transfer; Millipore, Etten-Leur, the Netherlands) were coated overnight with PBS/0·05%Tween/TNP/BSA (10 µg/ml) and blocked for 1 h with PBS/Tween/1% BSA. These membranes were clamped in spot blocks (made in-house) and 5 × 105 cells were centrifuged onto the membranes and incubated for 4 h at 37°C. Membranes were removed from the spot block, washed with PBS and PBS/Tween, and incubated overnight at 4°C with alkaline phosphatase-conjugated goat anti-mouse IgG1 and IgM antibodies (Southern Biotechnologies Associates, Birmingham, AL, USA) in PBS/Tween. For spot development, membranes were washed and incubated in fresh stock solutions of para-nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate toluidine salt in dimethylformamide (BDH Laboratory Supplies, Poole, UK), prepared and diluted in Tris buffer (100 mM Trizma base, 100 mM NaCl and 5 mM MgCl2*6H2O, pH 9·5). The spots were counted in blinded fashion by two independent observers using a stereomicroscope.
Cell culture and cytokine measurements
Cell suspensions (1 × 105 cells in 100 µl complete RPMI-1640 from Life Technologies supplemented with 10% FCS, 50 µM Eagle's basal medium and 200 mM L-glutamine) were incubated with 50 µl concanavalin A (Con A) (15 µg/ml) or medium in 96-well plates (Highbond 3590; Costar, Cambridge, MA, USA) overnight in 5% CO2. After centrifugation for 10 min at 350 g, supernatant was collected and stored at −70°C until analysis. IFN-γ and IL-4 levels were determined by sandwich ELISA. Plates were coated overnight at 4°C with 1 µg/ml of rat anti-mouse IFN-γ or rat anti-mouse IL-4 in 0·05 M carbonate buffer (pH 9·6), washed with PBS/Tween and blocked with PBS/Tween/casein for 4 h at room temperature. Samples and IL-4 and IFN-γ standards (100 µl) were added in several dilutions and incubated overnight at 4°C. After washing, plates were incubated with 0·25 µg/ml biotinylated rat anti-mouse IFN-γ or IL-4 conjugate diluted to optimal concentration in PBS/Tween/casein for 1 h at room temperature. Plates were washed and incubated with streptavidin–horseradish peroxidase (HRP) (0·3 µg/ml) diluted in PBS/Tween/casein for 45 min at room temperature. After final washes, tetramethylbenzidine substrate (0·1 mg/ml) was added and the colour reaction was stopped after 10 min with 2 M H2SO4. Absorbance was measured at 450 nm using an ELISA plate reader ELX800 (Bio-Tek Instruments, Winooski, VT, USA). IL-4 and IFN-γ capture and detecting antibodies were obtained from BD PharMingen (Hamburg, Germany). Streptavidin coupled to HRP was obtained from CLB (Amsterdam, the Netherlands) and casein was purchased from BDH Laboratory Supplies.
Flow cytometry
For flow cytometric analysis, 1 × 105 cells in PBS/BSA were centrifuged, resuspended and incubated with predetermined dilutions of fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, cyclophosphamide (CY)- conjugated monoclonal antibodies in 96-well plates (30 min in darkness at 4°C). Cells were washed, resuspended, stored in formalin (0·1%) and analysed within 18 h. Samples were analysed on a FACScan with standard FACSflow using CellQuest software (BD Biosciences, Franklin Lakes, NJ, USA). The following antibodies used for surface marker staining in tri-colour flow cytometry were purchased from BD PharMingen: CD3 CY (145-2C11), CD4 FITC (RM4-5), CD8 PE (53-6·7), CD19 PE (1D3), CD80 FITC (16-10A1), CD86 FITC (GL1) and CD11c FITC (HL3).
Statistical analysis
Data were analysed using two-tailed Student's t-tests. All P-values < 0·05 were considered statistically significant. The data presented are representative for two series of studies, each with similar results.
Studies in CFTR knockout mice
Animals
Stock Cftrtm1Unc-TgN(FABPCFTR)#Jaw mice were used as in our previous studies [7]. Male mice (nine per group) 6–8 weeks of age, with body weight of at least 16 g, were used in these experiments and bred and housed under standard laboratory conditions. Animals were bred and/or housed in accordance with respective institutional guidelines and requirements of the relevant regulatory agencies at Case Western University, Cleveland, OH.
Infection model
Pseudomonas aeruginosa-laden agarose beads were made and used as described previously [7,8], with minor differences. Mice were inoculated with a 1:35 dilution of the beads. This was established to be an LD50 dose. The slow-growing mucoid clinical strain M57-15 was used in these studies.
Drug treatments
HE2000 or vehicle (0·1 ml) was delivered by various routes (s.c. injection, oral gavage or buccal administration) 24 h before and 1 h after bacterial challenge.
Measurements of bacterial burden in lung
These measurements were performed as in our previous studies [9].
Statistical analysis
Data were analysed by one-way anova.
H1N1 influenza infection model
Animals
Female BALB/c mice (18–21 g) were obtained from Harlan Sprague–Dawley Laboratories (Indianapolis, IN, USA). The animals were quarantined for 24 h prior to use and fed standard mouse chow and tap water ad libitum.
Virus
Influenza A/NWS/33 (H1N1) was obtained from Dr Kennath Cochran of the University of Michigan (Ann Arbor, MI, USA). It was passaged nine times through MDCK cells and titrated in mice prior to use in this study.
Experimental
Mice were infected with a lethal dose 95% (LD)95 of virus. Groups of 19 infected mice were treated with HE2000 (1, 10 or 100 mg/kg given s.c.), Ribavirin (75 mg/kg given i.p.) or with 100 µl of HERF405 (HE2000 placebo given s.c.). Treatments with HE2000 or placebo were given on days −1, +1, +3 and +5. Treatments with Ribavirin were given twice daily for 5 days beginning 4 h before viral infection. Thirty-five infected mice in each drug-treated group and 20 placebo-treated controls were observed for death for 21 days and SaO2 levels determined on days 3–11. Of the remaining animals in each group, three drug-treated and five placebo-treated controls were killed on days 3, 6 and 9; their lungs were weighed, assigned a consolidation score ranging from 0 (normal) to 4 (maximal plum coloration) and assayed for virus titre. Three toxicity control mice were used for each drug dose, treated concomitantly with the infected mice, weighed immediately prior to first treatment, again 18 h after the final treatment, and observed for signs of toxicity for 21 days. Three normal mice were weighed in parallel and held with these animals. Nine additional normal controls were killed on days 3, 6 and 9 to provide background lung data.
Statistical analysis
Survivor numbers were compared using χ2 analysis with Yates' correction. Differences in mean day to death and daily mean SaO2, mean lung weights and mean virus titres were analysed using two-sided Student's t-test. Lung scores were evaluated using Wilcoxon's ranked sum analysis.
Results
HE2000 inhibits nitric oxide production by LPS-stimulated RAW 264·7 cells. HE2000 (0·03–3 µM) had no significant effect on either cell proliferation or NO production by resting (unstimulated) RAW264·7 cells. HE2000 alone did not effect cell proliferation in LPS-stimulated cultures (data not shown). When HE2000 was cultured with LPS-stimulated RAW264·7 cells for 6, 28 h or 48 h, a dose-dependent inhibition of NO production was observed. Optimal inhibition was observed at the 24-h time point (Fig. 1). HE2000 appears to limit NO production with an inhibitory concentration 50% (IC50) of approximately 1 µM. There was a significant (α = 0·05) correlation between HE2000 concentration and reduced NO production (R2 = 0·84). Data are from one of three similar experiments yielding similar results.
Fig. 1.
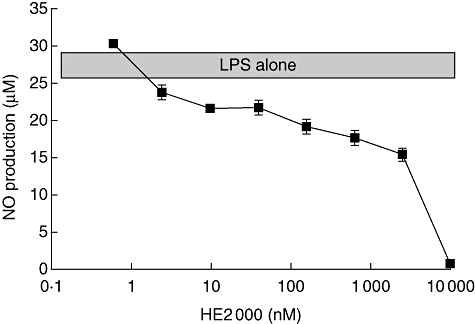
Effect of HE2000 on nitric oxide production by RAW 264·7 cells. Cultures were stimulated with 100 ng/ml lipopolysaccharide in the presence or absence of serial dilutions of HE2000 (n ≥ 3 per culture condition). After 24 h, nitric oxide in the supernatants was determined using Griess reagent. Data are from one of three independent studies with similar results and are expressed as nitric oxide concentration ± standard deviation.
HE2000 inhibits carrageenan-induced pleurisy
All mice that received CAR and were treated with vehicle alone, or were left untreated, developed an acute pleurisy. These mice produced turbid exudates containing increased numbers of polymorphonuclear cells (PMNs) (Fig. 2). The peak of the neutrophil and exudate response was between 4 and 5 h after administration of carrageenan (data not shown). Relative to the two groups of negative control mice (CAR and CAR + vehicle), treatment with either 120 or 12, but not 1·2 mg/kg HE2000 reduce significantly both the degree of lung inflammatory cell infiltration (Fig. 2 top) and the volume of pleural exudate (Fig. 2 bottom). The effects achieved by high-dose HE2000 were comparable to those observed with the polyclonal anti-mouse TNF-α antibody (positive control). Similar results were obtained when HE2000 was given by gavage (data not shown). Treatment with HE2000 was also associated with decreased IL-6 in the pleural exudate (Fig. 3). Similar decreases were also observed in IL-1β and macrophage inflammatory protein-2 (MIP-2), but not in TNF-α or prostaglandin E2 (PGE2) (data not shown). Similar kinetic studies were performed twice yielding similar results.
Fig. 2.
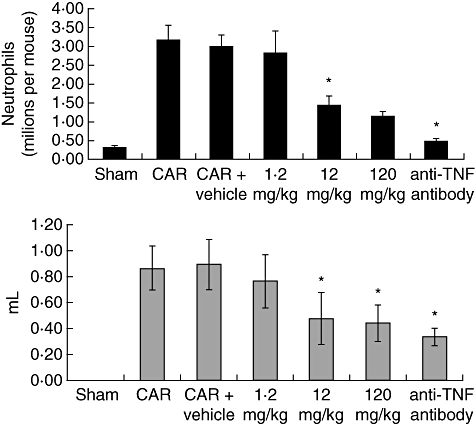
Effect of HE2000 on numbers of polymorphonuclear cells and exudate volume in murine model of carrageenan (CAR)-induced pleurisy. Male CD-1 mice (n = 8 per group) were anaesthetized and either saline alone (0·1 ml) or saline containing 2% λ-CAR (0·1 ml) was injected into the pleural cavity. Mice were treated subcutaneously with HE2000, vehicle or intraperitoneally with anti-tumour necrosis factor-α antibody 24 h and 1 h before CAR challenge. At 4 h after the injection of CAR, the pleural cavity rinsed with 1 ml of saline solution. The exudate and washing solution were removed by aspiration. The leucocytes in the exudate were counted with an optical microscope in a Burker's chamber after vital Trypan blue staining. All values in the figures are expressed as mean ± standard deviation (s.d.). Similar studies were performed twice and yielded similar results. *Significant (P < 0·0001) difference compared to control (vehicle) values.
Fig. 3.
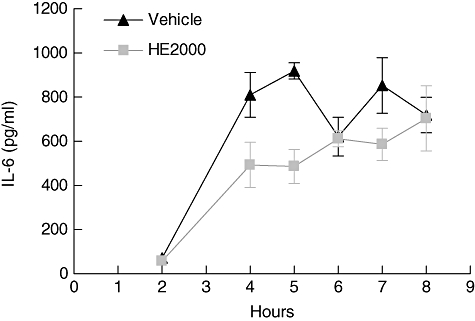
Effect of HE2000 on exudate levels of interleukin (IL)-6 in the murine model of carrageenan (CAR)-induced pleurisy. Male CD-1 mice (n = 2–4 per group) were anaesthetized and either saline (0·1 ml) or saline containing 2% λ-CAR (0·1 ml) was injected into the pleural cavity. Mice were treated subcutaneously with HE2000 (40 mg/kg) or with vehicle 24 h and 1 h before CAR challenge. At 2–8 h after the injection of CAR, the pleural cavity was rinsed with 1 ml of saline solution. The exudate and washing solution were removed by aspiration. Levels of IL-6 were measured by enzyme-linked immunosorbent assay. Data are expressed as pg/ml ± standard deviation. Differences trended (P = 0·06) in favour of HE2000 treatment in terms of reduced IL-6. Similar studies were performed twice and yielded similar results.
HE2000 reduces inflammation in LPS-induced lung injury
When mice (three to five per group) treated orally with HE2000 (40 mg/kg given) were challenged with LPS, the numbers of infiltrating cells in BAL at 48 h were reduced significantly (P < 0·05) (Fig. 4). The vast majority (90%) of these cells were neutrophils, as judged visually and by reduced levels of myloperoxidase. No significant changes in levels of IL-6 or TNF-α in BAL at this 48 h time-point were detected (data not shown).
Fig. 4.
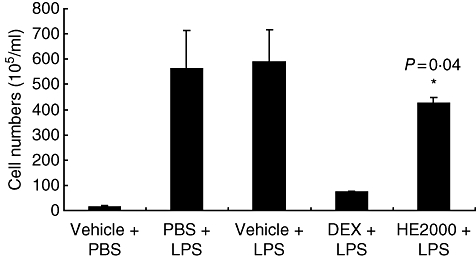
Effect of HE2000 on cellular infiltrates in the mouse model of lipopolysaccharide (LPS)-induced lung injury. Male C57 black/six mice (n = 3–6 per group) were pretreated (24 h before LPS challenge) with HE2000 (oral gavage; n = 6), dexamethasone (Dex) (5 mg/kg; n = 3) or vehicle and with a second dose of HE2000, Dex or vehicle 60 min after intratracheal administration of 100 µg of LPS. Two days later, bronchoalveolar lavage fluid (BAL) was obtained. Cellular infiltrates were enumerated under light microscopy using a cell counter. Data are expressed as numbers of cells per ml of BAL ± standard deviation.
HE2000 enhances elements of innate immunity in the RA-PLN assay
Effects on T and B cells
HE2000 (120 mg/kg) increased significantly numbers of lymphocytes in the PLN 7 days after the footpad was injected with the compound and a non-sensitizing dose of TNP–OVA (Fig. 5a). Both CD4+ and CD8+ T cell numbers were increased (data not shown), although CD4+/CD8+ cell ratios were decreased compared to control levels (Fig. 5b). The CD8+/CD19+ cell ratio was increased significantly in the HE2000-treated group (Fig. 5c). These results suggest that HE2000 is primarily favourable for T cell proliferation and/or chemotaxis, especially for CD8+ cells.
Fig. 5.
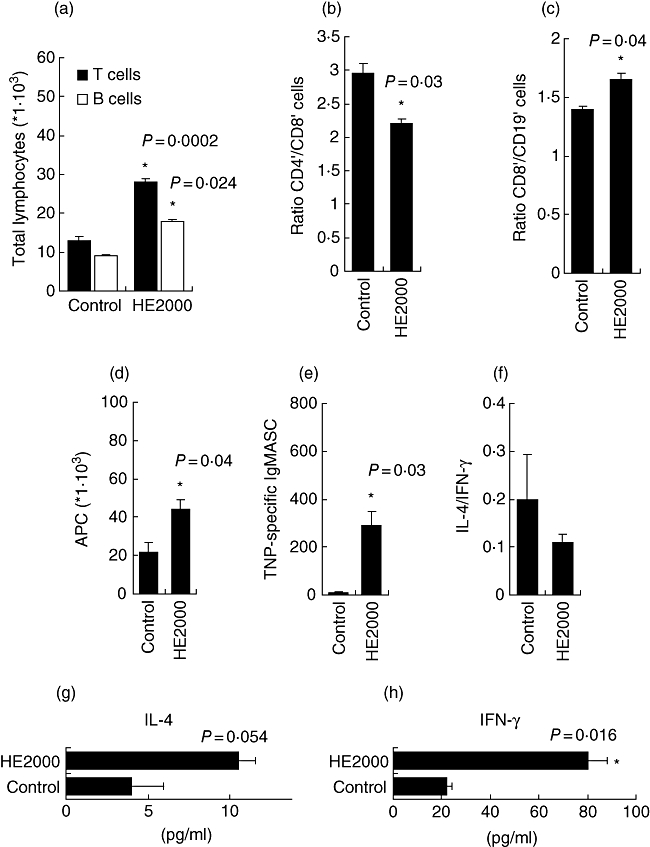
Effect of HE2000 in RA-popliteal lymph node (PLN) assay. HE2000 (3 mg) was injected into the right footpad along with a subsensitizing dose of trinitrophenol–ovalbumin (TNP–OVA) conjugate (10 µg in a total volume of 50 µl). Seven days later, PLN were harvested. Numbers of T or B lymphocytes (a), CD4+/CD8+ ratio (b), CD8+/CD19+ ratio (c), antigen-presenting cells (APC) (d), TNP-specific immunoglobulin (Ig)M antibody secreting cells (ASC) (e), interleukin (IL)-4/interferon (IF)-γ ratio (f) or IL-4 and IFN-γ levels (g, h) were determined by flow cytometry of live cells in suspension, enzyme-linked immunospot assay or enzyme-linked immunosorbent assay. Data are expressed as total cells, ASC/lymph node, or as pg/ml, respectively. Each bar represents mean values ± standard error of the mean of five mice per group. Similar studies were performed twice with similar results.
Effects on antigen presenting cells (APC)
Treatment with HE2000 led to significant increases in numbers of B cells (CD19+) and other (non-B) APC (defined as major histocompatibility complex (MHC)II+, CD11c+ and CD19- cells) in the PLN (Fig. 5d). CD86-bearing cells were also increased significantly, whereas CD80-expressing cells were not (data not shown).
Effects on T cell-dependent (IgG1) and independent (IgM) antibody secreting cells (ASC)
The formation of TNP-specific IgM and IgG1 ASC in the PLNA is a measure for the drug's capacity to induce T cell-independent and/or T cell-dependent stimulation. HE2000 was able to induce the formation of IgM ASC (Fig. 5e). HE2000 neither increased nor decreased the number of IgG1 ASC (data not shown).
Effects on cytokine production (IL-4 and IFN-γ)
PLN cells were stimulated in culture overnight with Con A for IL-4 and IFN-γ measurements. Interestingly, when compared to control (vehicle), HE2000 treatment shifted the balance of IL-4/IFN-γ towards IFN-γ (Th1) (Fig. 5f). It was found that HE2000 treatment increased both IL-4 and IFN-γ levels in culture supernatants compared to control (Fig. 5g, h).
HE2000 reduces bacterial burden in the CFTR mouse
At 24 h after bacterial challenge, HE2000 treatment did not induce frank toxicity in these animals, and there were no significant differences between groups (anova) with respect to body weight or BAL cell counts (data not shown). There were significantly greater numbers of bacteria in vehicle-treated mice compared to mice treated with either 120 mg or 12 mg/kg HE2000 (P < 0·05) (Fig. 6). Similar results were obtained in this model when similar doses of HE2000 were given by buccal administration or oral gavage (data not shown).
Fig. 6.
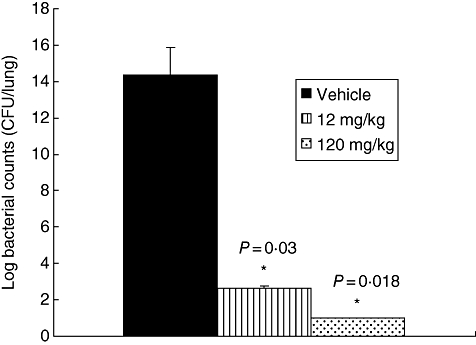
Effect of HE2000 on bacterial burden in lungs of cystic fibrosis transmembrane conductance regulator mice. Mice (nine per group) were inoculated with a 1 : 35 dilution of Pseudomonas aeruginosa-laden agarose beads. HE2000 (12 or 120 mg/kg) or vehicle was delivered subcutaneously (0·1 ml) to mice 24 h before and 1 h after challenge with bacteria-laden beads. Bacterial colony counts were performed on whole lung homogenates taken 24 h after the final challenge. Data are expressed as natural log of colony-forming units per lung.
Monotherapy with HE2000 does not exacerbate H1N1 infection in mice
HE2000 treatment (up to 100 mg/kg), as monotherapy, did not alter survival in this model, although at the lowest dose (1 mg/kg) a significant (P < 0·05) effect on prolongation of mean day to death was observed (Table 1). HE2000 treatment did not alter SaO2 decline, although a significant (P < 0·05) inhibition of increasing lung weight was observed, again with the lowest dose of HE2000 tested. No effects were observed on lung virus titres. As expected, Ribavirin was markedly effective. All infected mice treated with Ribavirin survived, and significant inhibition of all lung parameters was observed.
Table 1.
Effect of subcutaneous HE2000 treatment on an influenza A (H1N1) virus infection in mice.
| Tox controls |
Infected, treated mice |
|||||
|---|---|---|---|---|---|---|
| Compound | Dose (mg/kg/day) | Surv/total | Mean host weight change† (g) | Surv/total | Mean day to death‡ ± s.d. | Mean day 11 SaO2 (% ± s.d.) |
| HE2000 | 100 | 3/3 | 1·7 | 0/10 | 8·8 ± 0·6 | 75·0 ± 0·0 |
| 10 | 3/3 | 0·6 | 0/10 | 8·4 ± 0·7 | 75·0 ± 0·0 | |
| 1 | 3/3 | 1·1 | 0/10 | 9·1 ± 0·3* | 75·0 ± 0·0 | |
| Ribavirin | 75 | 3/3 | 0·2 | 10/10*** | > 21·0 ± 0·0*** | 88·4 ± 2·5*** |
| Vehicle | – | – | – | 0/20 | 8·7 ± 0·5 | 75·0 ± 0·0 |
| Normal controls | – | 3/3 | 1·5 | – | – | 89·0 ± 1·0 |
P < 0·05;
P < 0·001 compared to saline-treated controls.
Difference between initial weight and weight 18 h after final treatment.
Mean day to death of mice dying prior to day 21; s.d.: standard deviation.
Discussion
Several findings stand out from the results reported here. HE2000 inhibited nitric oxide production by LPS-stimulated RAW 264·7 cells with an IC50 of 1 µM. HE2000 also reduced neutrophils and exudate volumes as well as IL-6 levels in the murine model of carrageenan-induced pleurisy. A similar effect was observed in the murine model of LPS-induced lung inflammation. In the RA-PLN assay, HE2000 enhanced elements associated with protective immunity, including antigen-specific IgM ASC, and increased IFN-γ signalling. HE2000 reduced bacterial burden in an acute model of opportunistic bacterial lung infection, independent of route of administration. HE2000 treatment as a monotherapy had no effect on survival in a murine model of H1N1 influenza virus infection. To our knowledge, HE2000 is the first synthetic steroidal analogue to be shown to limit non-productive inflammation while enhancing effective immune responses in animals and humans.
A successful phase I/II, placebo-controlled, randomized study of HE2000 in treatment-naive HIV-1-infected individuals in South Africa was concluded. In that study, HE2000 treatment was found to be safe and to increase activated CD8+ T cells and CD11c+ and CD123+ dendritic cells. The treatment was associated with an increase in some haematopoietic elements, including neutrophils, monocytes and platelets. Decreased inflammatory mediators (TNF-α, IL-1β, IL-4, IL-6, IL-8) and greater than one-half log decreases in viral load were also reported. In a subsequent study in patients with AIDS, HE2000 treatment reduced both the incidence of tuberculosis co-infection by 42% and the cumulative incidence of opportunistic infections [2]. Additionally, phase II studies of HE2000 have been completed in patients with malaria. Results showed that 41/42 HE2000-treated malaria patients met the primary end-point of 50% clearance of parasitized erythrocytes and 31/42 infested patients cleared completely within 7 days [1]. Several publications also report in vitro activity of HE2000 against malaria [10,11]. These studies established that treatment of humans with HE2000 results in attenuation of non-productive inflammation and enhancement of protective immunity. The present studies reflected the ability of HE2000 to limit non-productive inflammation in the murine model of carrageenan-induced pleurisy [12,13] and LPS-induced lung injury [14], in which the inflammatory signals occur in the absence of infection. This was confirmed further by our observations with RAW cells, which implied that treatment with HE2000 could limit tissue damage associated with excess NO production in response to non-productive inflammatory signals.
The ability of HE2000 to enhance effective immunity was reflected by reduced bacterial burden in the CFTR−/− mouse model of acute opportunistic bacterial lung infection and in the PLN assay, which was developed primarily to evaluate the immune toxic effects of drugs and environmental pollutants on autoimmune responses [6,15]. Applied to HE2000, the PLN assay was predictive of the immune enhancing ability of this molecule observed in animal models and in clinical trials. Consistent with this was the observed increase in the ratio of CD8+/CD19+ cells and increased IFN-γ signalling, in the form of a decrease in the IL-4/IFN-γ ratio. HE2000 appeared to enhance elements of innate immunity, including increased numbers of activated APC and antigen-specific IgM secreting cells. These observations are in agreement with the anti-malarial properties of the compound in rats [10,11] and with studies in HIV-1-infected individuals, in which HE2000 treatment reduced opportunistic infections and was associated with increased activated CD8+ T cells, and CD11c+ and CD123+ dendritic cells [3].
HE2000 treatment was found to be safe and well tolerated in humans, and our observations in the mouse model of H1N1 influenza infection imply that treatment with HE2000 should not exacerbate virulence in H1N1-infected patients. Although prolongation of mean day to death was observed, the failure of HE2000 as a monotherapy to provide a survival benefit in the H1N1 influenza mouse model does not support the use of HE2000 alone as an anti-viral therapy. This is in keeping with our observations in HIV-infected patients, where HE2000 treatments reduced viral load only mildly [3]. However, in both the mouse H1N1 model and in HIV-infected patients, HE2000-mediated anti-viral activity may be achievable by different doses or treatment schedules. It should also be noted that the H1N1-infected mice were not co-infected with opportunistic bacteria, as is often the case with high-risk influenza patients. However, it is not our studies in the H1N1 mouse model that support the use of H2000 in influenza, but rather the ability of the compound to reduce non-productive inflammation, enhance IFN-γ signalling and clear opportunistic infections in lungs. Further, this mechanism makes development of viral resistance unlikely. Discrepancies between the H1N1 mouse data and our previous HIV/FIV results may be due to variations in receptors and/or mechanisms used by the different viruses.
The mechanism of action and target(s) of HE2000 remain unknown. Unpublished observations by HollisEden Pharmaceuticals suggest that, in vivo, HE2000 is metabolized rapidly, and in vitro as much as 60% of HE2000 is metabolized by RAW 264·7 cells at the 24-h time-point. This suggests the generation of multiple HE2000 metabolites with different biological activities and potencies. This would be in agreement with studies of Schmitt and colleagues, who showed conversion of dehydroepiandosterone (DHEA) to downstream steroid hormones in macrophages [16]. This, in turn, raises the possibility that specific therapeutic aspects of HE2000, for example anti-inflammatory properties versus enhancement of immune function, may relate to different chemical structures, and it may not be possible to capture the full range of HE2000 activities in any single metabolite or synthetic derivative or in any single animal model or clinical trial.
Influenza kills as many as 40 000 Americans annually [17,18]. Outbreaks such as H5N1 avian flu and the most recent variation of the H1N1 swine flu virus, even when causing relatively few deaths, lead to confusion, economic disruption and retain the potential for pandemic outbreaks, which have in the past killed upwards of 50 million people worldwide. Three pandemic outbreaks of influenza have been recognized since 1900. The most recent, the Hong Kong influenza pandemic, was detected first in Hong Kong early in 1968. Since then, globalization in general, and the exponential growth in commercial air travel in particular, have vastly increased the potential lethal impact of casual human–human transmission to cataclysmic proportions. The development of effective therapeutic agents to mitigate mortality of influenza infection is therefore a high priority. Mechanisms thought to be associated with mortality of influenza infection include an insufficient anti-viral immune response, excessive lung inflammation and secondary bacterial infection. However, recent work distinguished non-productive from excessive inflammation in the lung as driving mortality and associated enhanced innate immunity and IFN-γ signalling with survival [19].
Fatalities among H1N1 influenza patients correlate with secondary opportunistic infection and a non-productive inflammation characterized by reduced IFN-γ signalling [19]. An important complication of influenza is secondary bacterial pneumonia, which accounts for approximately 25% of all influenza-related deaths [20]. This complication occurs most frequently in patients with underlying conditions that include immune suppression, chronic illnesses and older age. Our observations that treatment with HE2000 can reduce non-productive inflammation and opportunistic bacterial burden while increasing IFN-γ signalling suggest that as an adjunct therapy in high-risk patients admitted to the hospital for treatment of influenza, HE2000 may reduce mortality related to excessive lung inflammation and secondary bacterial infection.
The ability of HE2000 treatment to provide benefit to patients suffering from a broad spectrum of infectious diseases probably relates to the unique ability of this compound to enhance innate and adaptive immune responses, while at the same time limiting non-productive inflammation. The development of HE2000 and/or synthetic derivatives of natural adrenal steroid hormones may presage a platform from which new pharmaceutical agents could be discovered, that can effectively treat inflammatory, infectious and neoplastic diseases.
Acknowledgments
The authors wish to acknowledge Mr Kevin Liu for help in creating Tables and Figures and formating.
Disclosure
Authors may hold equity interest in Hollis-Eden Pharmaceuticals.
References
- 1.Frincke J, Stickney D, Onizuka-Handa N, et al. Reduction of parasite levels in patients with uncomplicated malaria by treatment with HE2000. Am J Trop Med Hyg. 2007;76:232–6. [PubMed] [Google Scholar]
- 2.Stickney DR, Noveljic Z, Garsd A, Destiche DA, Frincke JM. Safety and activity of the immune modulator HE2000 on the incidence of tuberculosis and other opportunistic infections in AIDS patients. Antimicrob Agents Chemother. 2007;51:2639–41. doi: 10.1128/AAC.01446-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reading C, Dowding C, Schramm B, et al. Improvement in immune parameters and HIV-1 viral response in individuals treated wtih 16alpha-bromoepiandrosterone (HE2000) Clin Microbiol Infect. 2006;12:1082–8. doi: 10.1111/j.1469-0691.2006.01520.x. [DOI] [PubMed] [Google Scholar]
- 4.Pedersen NC, North TW, Rigg R, et al. 16alpha-Bromo-epiandrosterone therapy modulates experimental feline immunodeficiency virus viremia: initial enhancement leading to long-term suppression. Vet Immunol Immunopathol. 2003;94:133–48. doi: 10.1016/s0165-2427(03)00081-3. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez-Pando R, Aguilar Leon D, Orozco H, et al. 16alpha-Bromoepiandrosterone restores T helper cell type 1 activity and accelerates chemotherapy-induced bacterial clearance in a model of progressive pulmonary tuberculosis. J Infect Dis. 2005;191:299–306. doi: 10.1086/426453. [DOI] [PubMed] [Google Scholar]
- 6.Pieters R, Albers R. Assessment of autoimmunogenic potential of xenobiotics using the popliteal lymph node assay. Methods. 1999;19:71–7. doi: 10.1006/meth.1999.0829. [DOI] [PubMed] [Google Scholar]
- 7.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L944–52. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- 8.van Heeckeren AM, Schluchter MD. Murine models of chronic Pseudomonas aeruginosa lung infection. Lab Anim. 2002;36:291–312. doi: 10.1258/002367702320162405. [DOI] [PubMed] [Google Scholar]
- 9.van Heeckeren AM, Tscheikuna J, Walenga RW, et al. Effect of Pseudomonas infection on weight loss, lung mechanics, and cytokines in mice. Am J Respir Crit Care Med. 2000;161:271–9. doi: 10.1164/ajrccm.161.1.9903019. [DOI] [PubMed] [Google Scholar]
- 10.Ayi K, Giribaldi G, Skorokhod A, Schwarzer E, Prendergast PT, Arese P. 16alpha-bromoepiandrosterone, an antimalarial analogue of the hormone dehydroepiandrosterone, enhances phagocytosis of ring stage parasitized erythrocytes: a novel mechanism for antimalarial activity. Antimicrob Agents Chemother. 2002;46:3180–4. doi: 10.1128/AAC.46.10.3180-3184.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freilich D, Ferris S, Wallace M, et al. 16alpha-bromoepiandrosterone, a dehydroepiandrosterone (DHEA) analogue, inhibits Plasmodium falciparum and Plasmodium berghei growth. Am J Trop Med Hyg. 2000;63:280–3. [PubMed] [Google Scholar]
- 12.Di Rosa M. Biological properties of carrageenan. J Pharm Pharmacol. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 13.Vinegar R, Truax JF, Selph JL. Some quantitative temporal characteristics of carrageenin-induced pleurisy in the rat. Proc Soc Exp Biol Med. 1973;143:711–4. doi: 10.3181/00379727-143-37397. [DOI] [PubMed] [Google Scholar]
- 14.Asti C, Ruggieri V, Porzio S, Chiusaroli R, Melillo G, Caselli GF. Lipopolysaccharide-induced lung injury in mice. I. Concomitant evaluation of inflammatory cells and haemorrhagic lung damage. Pulm Pharmacol Ther. 2000;13:61–9. doi: 10.1006/pupt.2000.0231. [DOI] [PubMed] [Google Scholar]
- 15.Pieters R. The popliteal lymph node assay in predictive testing for autoimmunity. Toxicol Lett. 2000;112:453–9. doi: 10.1016/s0378-4274(99)00231-3. 113. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M, Kreutz M, Loffler G, Scholmerich J, Straub RH. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J Endocrinol. 2000;164:161–9. doi: 10.1677/joe.0.1640161. [DOI] [PubMed] [Google Scholar]
- 17.Lamb RA, Takeda M. Death by influenza virus protein. Nat Med. 2001;7:1286–8. doi: 10.1038/nm1201-1286. [DOI] [PubMed] [Google Scholar]
- 18.Regan SF, Fowler C. Influenza. Past, present, and future. J Gerontol Nurs. 2002;28:30–7. doi: 10.3928/0098-9134-20021101-08. quiz 52–3. [DOI] [PubMed] [Google Scholar]
- 19.Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PLoS One. 2009;4:e4176. doi: 10.1371/journal.pone.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17(Suppl. 1):S3–10. doi: 10.1016/s0264-410x(99)00099-7. [DOI] [PubMed] [Google Scholar]


