Abstract
Clinical studies using omega-3 polyunsaturated fatty acids (ω3-PUFA) to Crohn's disease (CD) are conflicting. Beneficial effects of dietary ω3-PUFA intake in various experimental inflammatory bowel disease (IBD) models have been reported. However, animal models of large intestinal inflammation have been used in all previous studies, and the effect of ω3 fat in an animal model of small intestinal inflammation has not been reported. We hypothesized that the effects of ω3 fat are different between large and small intestine. The aim of this study was to determine whether the direct effect of ω3 fat is beneficial for small intestinal inflammation. Senescence accelerated mice (SAM)P1/Yit mice showed remarkable inflammation of the terminal ileum spontaneously. The numbers of F4/80-positive monocyte–macrophage cells as well as β7-integrin-positive lymphocytes in the intestinal mucosa were increased significantly compared with those in the control mice (AKR-J mice). The area of mucosal addressin cell adhesion molecule-1 (MAdCAM-1)-positive vessels was also increased. The degree of expression levels of monocyte chemoattractant protein-1 (MCP-1), interleukin (IL)-6 and interferon (IFN)-γ mRNA were increased significantly compared with those in the control mice. The feeding of two different kinds of ω3 fat (fish-oil-rich and perilla-oil-rich diets) for 16 weeks to SAMP1/Yit mice ameliorated inflammation of the terminal ileum significantly. In both the ω3-fat-rich diet groups, enhanced infiltration of F4/80-positive monocytes/macrophages in intestinal mucosa of SAMP1/Yit mice cells and the increased levels of MCP-1, IL-6 and IFN-γ mRNA expression were ameliorated significantly compared with those in the control diet group. The results suggest that ω3 fat is beneficial for small intestinal inflammation by inhibition of monocyte recruitment to inflamed intestinal mucosa.
Keywords: ileum, inflammation, mice, omega-3 fatty acids
Introduction
Crohn's disease (CD) is a chronic inflammatory bowel disease (IBD) of unknown aetiology[1]. CD can occur in any part of the gastrointestinal tract, but occurs frequently in the small intestine [2]. Although genetic, immunological and environmental factors have been proposed, the mechanism remains unclear [1,3–5].
The incidence of CD has been shown to be related to dietary intake of total fat, suggesting that CD is a lifestyle-related disease [6]. Many studies have been carried out to determine whether dietary fat intake modulates intestinal inflammation [6–13]. There is also considerable evidence implicating dietary fat as a modulator of different immune functions [14–16]. We have reported previously that fatty acid modulates cytokine production from intestinal epithelial cells, intraepithelial lymphocytes and dendritic cells [14,15]. In addition, we have reported the effect of fatty acid on the migration of T lymphocytes to Peyer's patches [16]. Two factors determine the role of lipid nutrition in health and disease: (i) the composition and (ii) the total amount of fat in the diet. A high fat intake has been reported to be associated with an increased risk of CD. Several epidemiological studies in Inuit people have revealed a low incidence of IBD compared with that in western populations, indicating that dietary intake of ω3 polyunsaturated fatty acids (ω3-PUFA) [17] has a protective role. It has also been reported that patients with IBD had lower plasma levels of ω3-PUFA [18]. In addition, beneficial effects of ω3-PUFA have been shown in different experimental models of intestinal inflammation induced by trinitrobenzene sulphonic acid (TNBS), dextran sulphate sodium (DSS) and acetic acid [19–21]. However, results of clinical studies using ω3-PUFA are conflicting [7,8]. We hypothesized that fat has a greater effect on CD in the small intestine than on CD in the large intestine, because the small intestinal mucosa is exposed to a much higher concentration of fat. However, in most studies, the effect of ω3-PUFA has been investigated using colitis models as intestinal inflammation models. Therefore, it is necessary to investigate the effect of ω3-PUFA using small intestinal inflammation.
Until recently, progress in understanding the pathogenesis of CD had been hindered seriously by a lack of animal models that closely resemble human disease. With the advent of new technologies, several animal models of IBD have been established recently [22–24]. In most cases, the large bowel rather than the small intestine is affected, and IBD models are generated artificially by immunological, genetic or chemical manipulation. Although these models have provided useful information on the basic mechanisms of intestinal inflammation, intestinal inflammation does not develop spontaneously and lacks many of the important characteristics of CD. Therefore, these models may have limited value for investigating the precise aetiopathogenesis of human CD [25]. Senescence-accelerated mice (SAM) were derived from AKR/J mice established by Takeda et al.[26]. The SAMP1/Yit mouse strain, a subline of the SAMP1 strain, develops chronic ileitis spontaneously, with virtually 100% penetrance after 30 weeks of age under specific pathogen-free conditions [25,27,28]. In this model, spontaneous and chronic ileitis that closely resembles human CD develops in the absence of chemical, immunological or genetic manipulation [27]. We have reported previously that the blockade of mucosal addressin cell adhesion molecule-1 (MAdCAM-1) or P-selectin glycoprotein ligand-1 (PSGL-1) attenuates T lymphocyte or monocyte recruitment in the intestinal mucosa and ameliorates ileitis in this model [29,30].
In this study, we first investigated whether the direct effect of ω3-PUFA is beneficial for intestinal inflammation using the SAMP1/Yit ileitis model.
Materials and methods
Animals
Male SAMP1/Yit mice were kindly donated by the Yakult Central Institute for Microbiological Research (Tokyo, Japan) and were maintained in an animal colony at the National Defense Medical College (NDMC; Saitama, Japan). AKR-J mice (Japan Clea Co., Tokyo, Japan), from which SAMP1/Yit mice were derived and did not have ileitis, were purchased and maintained in an animal colony at NDMC. SAMP1/Yit mice and AKR-J mice (as control mice) were used for this study. The mice were maintained on a diet of standard laboratory chow (Oriental Yeast Manfacturing Ltd, Tokyo, Japan) in specific pathogen-free conditions. Mice were housed four to five per cage, allowed free access to food and tap water and were maintained according to the guidelines for laboratory animals at NDMC. Body weight of the mice was measured every 4 weeks from 14 to 30 weeks of age. There was no significant difference in diet consumption among all groups. This study protocol was approved by Animal Ethical Committee of NDMC (No. 05093).
Omega-3 fat feeding protocol
We fed SAMP1/Yit mice with ω3 fat for 16 weeks. Fat feeding treatment was performed from 14 weeks (when ileitis began to occur) to 30 weeks (when ileitis was completely established). Each high fat-containing diet consisted of fat-free AIN76 (Japan Clea Co.) diet and two kinds of 8% w/w ω3-PUFA. We chose fish oil (containing 25–30% eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA); Sigma, St Louis, MO, USA) or perilla oil (containing 55–60% α-linolenic acid; Ota Yushi, Aichi, Japan) as ω3-PUFA. Standard chow diet, CE-2 (Japan Clea Co.) was used as control. The diets were stored at 4°C room temperature to prevent fatty acid oxidation.
Assessment of ileitis
The terminal ileum was removed under pentobarbital anaesthesia. After mechanical cleaning, the weight of the terminal 7 cm section of the ileum was determined. The ileum was cut in half longitudinally and one of the segments was fixed in 10% buffered formalin. Tissues were embedded in paraffin, cut into 4 µm longitudinal sections and stained with haematoxylin and eosin (H&E).
One parameter for intestinal inflammation was quantified as the weight of terminal ileum per cm. Another parameters were quantified as thickness of ileum and propria muscularis using Image J software (National Institute of Health, Bethesda, MD, USA).
Immunohistochemistry
Another section of the removed intestine was fixed for 12 h at 4°C in periodate–lysine–paraformaldehyde (PLP). Subsequently these specimens were washed with phosphate-buffered saline (PBS) and dehydrated for 12 h with PBS containing 10, 15 and 20% sucrose. After fixation, they were embedded in optical cutting temperature compound (Tissue-Tek, Miles Inc., Pittsburgh, PA, USA), frozen in liquid nitrogen and stored at −70°C. Cryostat sections of frozen tissue were cut at 7 µm. Immunohistochemistry was performed using the labelled streptavidin–biotin technique (LSAB). Primary antibodies used in immunostaining were monoclonal antibodies that react to β7-integrin [rat immunoglobulin (Ig)G2a; PharMingen, San Diego, CA, USA], CD4 (rat IgG2a; PharMingen), F4/80 (rat IgG; Cosmo Bio. Co. Ltd, Tokyo, Japan) and MAdCAM-1 (rat IgG2a; PharMingen). The tissue sections were incubated with adequately diluted primary antibodies overnight at 4°C and treated with subclass- and host-matched biotinylated antibodies for 1 h at room temperature. They were visualized by streptavidin–fluorescein isothiocyanate (FITC). Rinsing with PBS containing 1% bovine serum albumin was performed for each group. A coverslip was applied using glycerol jelly. These sections were observed under a fluorescent microscope (Carl Zeiss Inc., Overkohen, Germany).
The MAdCAM-1-positive vessels in lamina propria were quantified as an area of positively stained vessels per mm muscularis mucosa using Image J software. The infiltrated cells were expressed as the number of β7-integrin, CD4 and F4/80-positive cells per mm muscularis mucosa in the same manner.
Quanitative reverse transcription polymerase chain reaction (RT-PCR)
The intestinal mucosa was removed after mice were killed. Total messenger RNA (mRNA) was extracted by using the RNeasy Mini isolation kit (Qiagen, CA, USA). TaqMan RT-PCR was performed in duplicate for each sample using the ABI PRISM 7000 Sequence Detector (Applied Biosystems, CA, USA). Primer and probes used in this study were purchased from Applied Biosystems, as follows: monocyte chemoattractant protein-1 (MCP-1; Mm00441242), interleukin (IL)-6 (Mm00446190) and interferon gamma (IFN-γ; Mm00801778).
Statistical analysis
Data are expressed as mean ± standard error. Differences between groups were examined for statistical significance using one-way factorial analysis of variance (anova) and Fisher's protected least-significant difference test. P values of 0·05 or less were considered to be statistically significant. Statistical analyses were performed using the Statcel2 software (Addinsoft, OMS, Tokyo, Japan).
Results
Effect of ω3-PUFA on ileal inflammation
Figure 1 shows the time-course body weight change. SAMP1/Yit mice fed the control diet had lost their body weight from 22 weeks and continued to decrease as ileitis advanced. However, both SAMP1/Yit mice fed the fish-oil-rich diet and SAMP1/Yit mice fed the perilla-oil-rich diet had gained body weight during this study. The body weight of both groups was significantly higher than SAMP1/Yit mice fed the control diet. Figure 2a–d shows representative H&E sections in all groups. A remarkable increase in villous height, extension of submucosa and increase in thickness of the muscular layer were observed in SAMP1/Yit mice fed the control diet compared with those in the control AKR-J group. These features of ileitis were ameliorated in SAMP1/Yit mice fed the fish-oil-rich or perilla-oil-rich diet. Figure 2e–g shows weight, total thickness and muscular thickness of the ileum. The weight of the ileum in the SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. Feeding fish-oil-rich or perilla-oil-rich diet to the SAMP1/Yit mice reduced significantly the increase in ileum weight. Total thickness of ileum in the SAMP1/Yit mice fed the control diet was also increased significantly compared with that in the control AKR-J group, and feeding fish-oil-rich or perilla-oil-rich diets to SAMP1/Yit mice reduced significantly the increase in the total ileum thickness. Muscular thickness of the ileum in SAMP1/Yit mice fed the control diet was increased, although the increase was not significant compared with that in the control AKR-J group. Feeding the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice reduced the increase in ileum muscular thickness, and the degree of reduction was significantly greater in the perilla-oil-rich diet group than in the fish-oil-rich diet group.
Fig. 1.
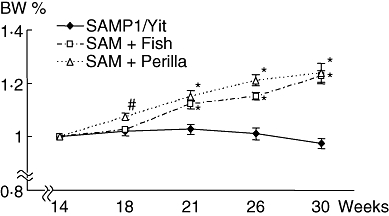
Time-course change of body weight. Senescence accelerated mice (SAM)P1/Yit, SAMP1/Yit mice fed control diet; SAM+Fish: SAMP1/Yit mice fed fish-oil-rich diet; SAM+Perilla: SAMP1/Yit mice fed perilla-oil-rich diet. Data are expressed as mean ± standard error. #P < 0·05 versus SAMP1/Yit mice fed control diet; *P < 0·01 versus SAMP1/Yit mice fed control diet.
Fig. 2.
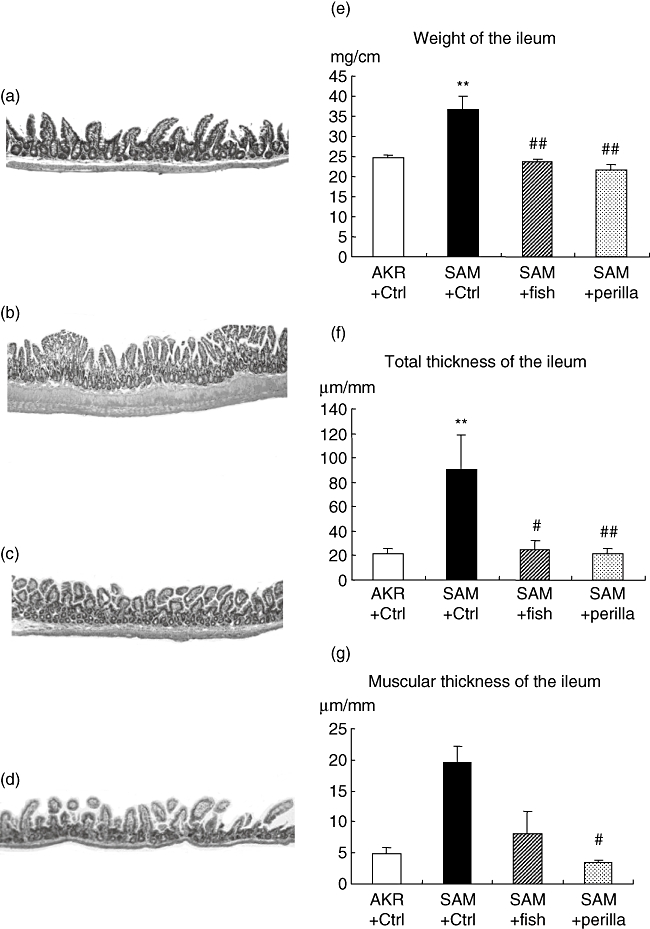
Representative haematoxylin and eosin sections (a–d). (a) AKR-J group. (b) Senescence accelerated mice (SAM)P1/Yit mice fed control diet. (c) SAMP1/Yit mice fed fish-oil-rich diet. (d) SAMP1/Yit mice fed perilla-oil-rich diet. (e) Weight of the ileum in all groups. (f) Total thickness of the ileum in all groups. (g) Muscular thickness of the ileum in all groups. Data are expressed as mean ± standard error. **P < 0·01 versus AKR-J group; #P < 0·05 versus SAMP1/Yit mice fed control diet; ##P < 0·01 versus SAMP1/Yit mice fed control diet.
Infiltration of monocytes and levels of related molecules
Figure 3a–e shows immunohistochemistry for F4/80 and the number of F4/80-positive cells per mm muscularis mucosa in all groups. The number of F4/80-positive cells in the SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. Feeding the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice significantly lowered the increase in the number of positive cells; the degree of decrease was greater in the perilla-oil-rich diet group than in the fish-oil-rich diet group.
Fig. 3.
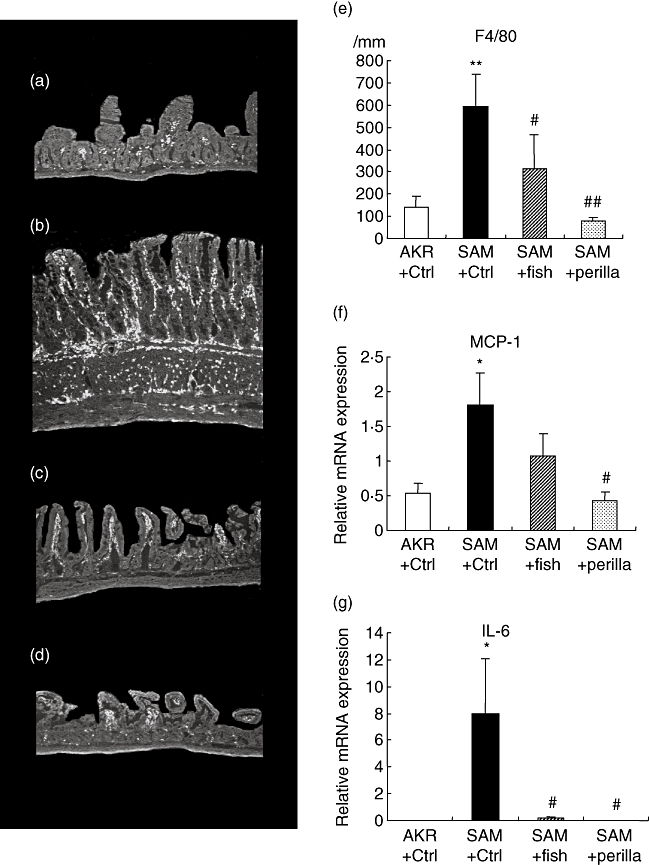
Immunohistochemistry for F4/80 (a–d). (a) AKR-J group. (b) Senescence accelerated mice (SAM)P1/Yit mice fed control diet. (c) SAMP1/Yit mice fed fish-oil-rich diet. (d) SAMP1/Yit mice fed perilla-oil-rich diet. (e) Number of F4/80 positive cells in all groups. (f) Relative mRNA expression level of monocyte chemoattrantant protein-1 (MCP-1) in all groups. (g) Relative mRNA expression level of interleukin (IL)-6 in all groups. Data are expressed as mean ± standard error. *P < 0·05 versus AKR-J group; **P < 0·01 versus AKR-J group; #P < 0·05 versus SAMP1/Yit mice fed control diet. ##P < 0·01 versus SAMP1/Yit mice fed control diet.
Figure 3f–g shows mRNA expression levels of MCP-1 and IL-6 in the terminal ileum in all groups. The expression level of MCP-1 in SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. Feeding the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice reduced the increased level of MCP-1, and the degree of reduction was significantly greater in the perilla-oil-rich diet group than in the fish-oil-rich diet group. The expression level of IL-6 in SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. Feeding either the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice reduced the increased level of IL-6 significantly.
MAdCAM-1-positive vessels and β-7-positive cells
Figure 4a–e shows immunohistochemistry for MAdCAM-1 and the area of MAdCAM-1-positive vessels per mm muscularis mucosa in all groups. MAdCAM-1-positive vessels in SAMP1/Yit mice fed the control diet were increased significantly compared with those in the control AKR-J group. Feeding the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice lessened the increase in positive vessels significantly; among the ω3-fat-rich diet groups, the degree of decrease was greatest in the perilla-oil-rich diet group.
Fig. 4.
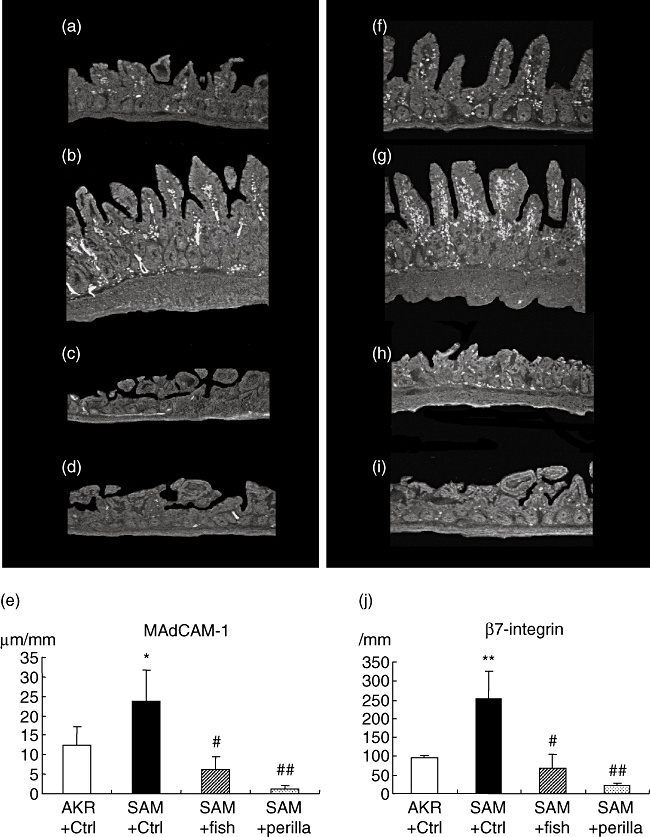
Immunohistochemistry for mucosal addressin cell adhesion molecule-1 (MAdCAM-1) (a–d) and β7-integrin (f–i). (a) AKR-J group. (b) Senescence accelerated mice (SAM)P1/Yit mice fed control diet. (c) SAMP1/Yit mice fed fish-oil-rich diet. (d) SAMP1/Yit mice fed perilla-oil-rich diet. (e) Area of MAdCAM-1-positive vessels in the all groups. (f) AKR-J group. (g) SAMP1/Yit mice fed control diet. (h) SAMP1/Yit mice fed fish-oil-rich diet. (i) SAMP1/Yit mice fed perilla-oil-rich diet. (j) Number of β7-integrin positive cells in all groups.
Figure 4f–j shows immunohistochemistry for β7-integrin and the number of β7-integrin-positive cells per mm muscularis mucosa in all groups. The number of β7-integrin-positive cells in SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. Feeding the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice lowered the increase in number of positive cells significantly; among the ω3-fat-rich diet groups, the degree of decrease was greatest in the perilla-oil-rich diet group.
Infiltration of lymphocytes and levels of related molecules
Figure 5a–e shows immunohistochemistry for CD4 and the number of CD4-positive cells per mm muscularis mucosa in all groups. The number of CD4-positive cells in SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. SAMP1/Yit mice fed the fish-oil-rich or perilla-oil-rich diet showed a significant decrease in the increased number of positive cells; among the ω3-fat-rich diet groups, the degree of decrease was greatest in the perilla-oil-rich diet group.
Fig. 5.
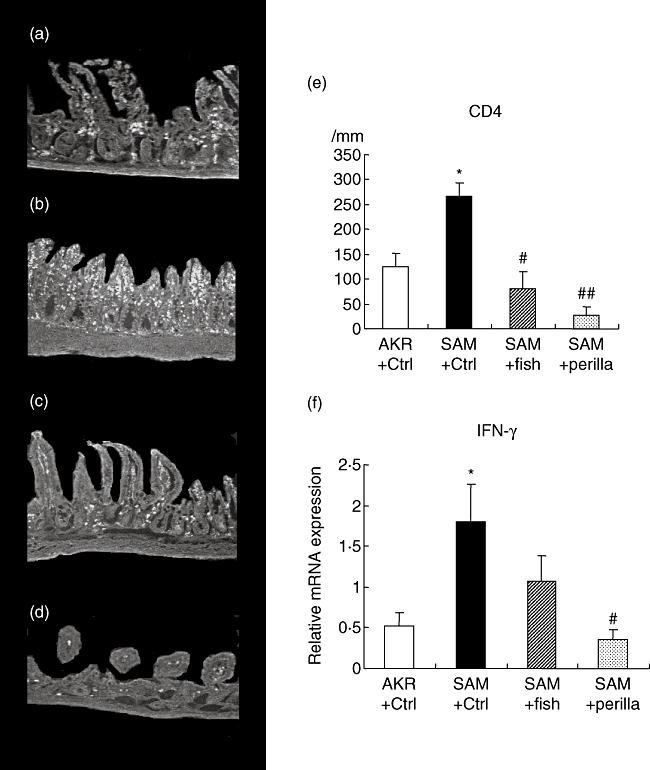
Immunohistochemistry for CD4 (a–d). (a) AKR-J group. (b) Senescence accelerated mice (SAM)P1/Yit mice fed control diet. (c) SAMP1/Yit mice fed fish-oil-rich diet. (d) SAMP1/Yit mice fed perilla-oil-rich diet. (e) Number of CD4 positive cells in all groups. (f) Relative mRNA expression level of interferon (IFN)-γ in all groups. Data are expressed as mean ± standard error. *P < 0·05 versus AKR-J group; # P < 0·05 versus SAMP1/Yit mice fed control diet; ## P < 0·01 versus SAMP1/Yit mice fed control diet.
Figure 5f shows the mRNA expression level of IFN-γ in the terminal ileum in all groups. The expression level of IFN-γ in SAMP1/Yit mice fed the control diet was increased significantly compared with that in the control AKR-J group. Feeding the fish-oil-rich or perilla-oil-rich diet to SAMP1/Yit mice lowered the increased level of MCP-1; the effect was greater in the perilla-oil-rich diet group than in the fish-oil-rich diet group.
Discussion
In this study, SAMP1/Yit mice showed remarkable inflammation of the terminal ileum compared with that in AKR-J mice. Feeding the ω3-fat-rich diet to SAMP1/Yit mice ameliorated the terminal ileal inflammation significantly. Many studies have shown the effect of ω3 fat on intestinal inflammation; however, in all those studies, animal models of large intestinal inflammation were used. On the other hand, the effect of ω3 fat in Crohn's disease is controversial, because ω3 fat was shown to have an anti-inflammatory effect in many [19–21] but not in all studies [7,8]. Thus, it is possible that the effects of ω3 fat in the small intestine and colon are different. However, the effect of ω3 fat in an animal model of small intestinal inflammation has never been reported. We have reported for the first time the effect of ω3 fat on small intestinal inflammation and our results suggest that ω3 fat has an anti-inflammatory effect on small intestinal inflammation as well as on large intestinal inflammation.
SAMP1/Yit mice ileitis is mediated by a T helper type 1 (Th1) response [31]. Blocking the recruitment of lymphocytes by anti-MAdCAM-1 antibody ameliorated the ileitis, supporting the idea that excessive lymphocyte migration through increased expression of adhesion molecules in the intestinal mucosa is involved in this murine ileitis [30]. In addition, using an intravital microscope in a previous study, we found by observation that monocyte recruitment is increased in the terminal ileum of SAMP1/Yit mice [29]. We also found that the monocyte–macrophage lineage plays a key role in this ileitis model. Blocking monocyte recruitment ameliorated the ileitis, suggesting that excessive migration of monocytes is involved in the pathophysiology of this mouse model [29]. These studies suggest that (i) excessive recruitment of monocytes to the intestinal mucosa results in the production of proinflammatory cytokines and an increase in the expression levels of adhesion molecules, such as MAdCAM-1; and (ii) aberrant migration of lymphocytes is induced by enhanced expression of adhesion molecules, and infiltrating lymphocytes mediate the Th1-type response. In this study, we found a significant increase in the number of F4/80-positive cells infiltration, a significant increase in the IL-6 mRNA expression level, an increase in the expression of MAdCAM-1-positive vessels and an increase in the number of β7-integrin-positive and CD4-positive cells, and an increase in IFN-γ mRNA expression level in the intestinal mucosa of SAMP1/Yit mice, supporting our proposed scenario. We focused upon the initial step of ileal inflammation, i.e. monocyte recruitment. It is generally accepted that MCP-1 plays a key role in monocyte recruitment [32,33] and increased expression of MCP-1 has been shown to be involved in the pathophysiology of some monocyte-macrophage-related diseases such as atherosclerosis [34,35], as well as in Crohn's disease [36,37]. We measured MCP-1 expression and found that the MCP-1 mRNA expression level was increased significantly. This increase was reduced significantly by the ω3 fat diet. In addition, it decreased the number of CD4-positive cells, the levels of MAdCAM-1, IFN-gamma and IL-6 expression, the number of F4/80-positive cells and level of MAdCAM-1 expression, all of which are regarded as downstream mechanisms of monocyte recruitment. These results suggest that ω3 fat diet treatment ameliorated ileitis in the initial step, i.e. monocyte recruitment, by decreasing MCP-1 expression. The mechanism by which MCP-1 expression was decreased was not clarified in this study. However, it has been reported that oxidized ω3 fatty acids inhibit MCP-1 expression in human umbilical vein endothelial cells (HUVEC) [38]. Expression of MCP-1 in human mononuclear cells was reduced by 4 weeks of ω3 fat diet treatment [39]. The ω3 fat diet is beneficial in cardiovascular disease involving atherosclerosis, another lifestyle-related disease [40], and the blocking of monocyte recruitment to atherosclerosis plaque by decreasing the MCP-1 expression level is regarded as one of the possible mechanisms. In this study, we consider that the effect of ω3 fatty acids on ileitis is due mainly to its inhibitory effect on leucocyte recruitment. However, as it is reported that ω3 fatty acids inhibited lipopolysaccharide-induced proinflammatory cytokines such as IL-6, tumour necrosis factor (TNF)-α and IL-1β, it is possible that the ω3 fat diet ameliorates inflammation directly through decreasing proinflammatory cytokines from macrophages [41].
We used fish oil and perilla oil as the ω3 fatty acids in this study. The magnitude of decrease in mucosa thickness, muscular thickness, F4/80-positive cells, MCP-1 mRNA expression level, MAdCAM-1-positive vessels, β7-integrin-positive cells, CD4-positive cells and IFN-γ mRNA expression level were much greater in the perilla-oil-rich group than in the fish-oil-rich group. Fish oil is rich in EPA and DHA, and perilla oil is rich in α-linolenic acids. Shoda et al.[19] reported that α-linolenic acids have greater anti-inflammatory effects than do EPA or DHA in TNBS colitis mice. Our findings in ileitis mice also suggest that α-linolenic acids have a stronger effect on intestinal inflammation than do DHA or EPA. Most of the effect of α-linolenic acids on the immune system is considered to be carried out after metabolism into EPA/DHA by delta-6-desaturase [42]. In addition, α-linolenic acid itself carries out some functions by competitive inhibition of the 5-lipoxygenase pathway. It is possible that this additional effect may be important in small intestinal inflammation.
In conclusion, we propose that ω3 fat ameliorates intestinal inflammation in the small intestine as well as in the large intestine. This is due possibly to a decrease in innate immunity by inhibition of monocyte recruitment to the inflamed small intestinal mucosa.
Acknowledgments
Special thanks to Dr S. Matsumoto for SAMP1/Yit mice support, the Yakult Central Institute for Microbiological Research.
Disclosure
The authors declare that they have no conflict of interest related to the publication of this manuscript.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Podolsky DK. Inflammatory bowel disease (1) N Engl J Med. 1991;325:928–37. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- 3.Satsangi J, Welsh KI, Bunce M, et al. Contribution of genes of the major histocompatibility complex to susceptibility and disease phenotype in inflammatory bowel disease. Lancet. 1996;347:1212–7. doi: 10.1016/s0140-6736(96)90734-5. [DOI] [PubMed] [Google Scholar]
- 4.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–6. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 5.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 6.Shoda R, Matsueda K, Yamato S, Umeda N. Epidemiologic analysis of Crohn disease in Japan: increased dietary intake of n-6 polyunsaturated fatty acids and animal protein relates to the increased incidence of Crohn disease in Japan. Am J Clin Nutr. 1996;63:741–5. doi: 10.1093/ajcn/63.5.741. [DOI] [PubMed] [Google Scholar]
- 7.Lorenz-Meyer H, Bauer P, Nicolay C, et al. Omega-3 fatty acids and low carbohydrate diet for maintenance of remission in Crohn's disease. A randomized controlled multicenter trial. Study Group Members (German Crohn's Disease Study Group) Scand J Gastroenterol. 1996;31:778–85. doi: 10.3109/00365529609010352. [DOI] [PubMed] [Google Scholar]
- 8.MacLean CH, Mojica WA, Newberry SJ, et al. Systematic review of the effects of n-3 fatty acids in inflammatory bowel disease. Am J Clin Nutr. 2005;82:611–9. doi: 10.1093/ajcn.82.3.611. [DOI] [PubMed] [Google Scholar]
- 9.Bamba T, Shimoyama T, Sasaki M, et al. Dietary fat attenuates the benefits of an elemental diet in active Crohn's disease: a randomized, controlled trial. Eur J Gastroenterol Hepatol. 2003;15:151–7. doi: 10.1097/00042737-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Miura S, Tsuzuki Y, Hokari R, Ishii H. Modulation of intestinal immune system by dietary fat intake: relevance to Crohn's disease. J Gastroenterol Hepatol. 1998;13:1183–90. [PubMed] [Google Scholar]
- 11.French MA, Parrott AM, Kielo ES, et al. Polyunsaturated fat in the diet may improve intestinal function in patients with Crohn's disease. Biochim Biophys Acta. 1997;1360:262–70. doi: 10.1016/s0925-4439(97)00012-4. [DOI] [PubMed] [Google Scholar]
- 12.Alzoghaibi MA, Walsh SW, Willey A, Yager DR, Fowler AA, III, Graham MF. Linoleic acid induces interleukin-8 production by Crohn's human intestinal smooth muscle cells via arachidonic acid metabolites. Am J Physiol. 2004;286:G528–37. doi: 10.1152/ajpgi.00189.2003. [DOI] [PubMed] [Google Scholar]
- 13.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 14.Hara Y, Miura S, Komoto S, et al. Exposure to fatty acids modulates interferon production by intraepithelial lymphocytes. Immunol Lett. 2003;86:139–48. doi: 10.1016/s0165-2478(03)00007-5. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Miura S, Kishikawa H, et al. Fatty acids enhance GRO/CINC-1 and interleukin-6 production in rat intestinal epithelial cells. J Nutr. 2001;131:2943–50. doi: 10.1093/jn/131.11.2943. [DOI] [PubMed] [Google Scholar]
- 16.Tsuzuki Y, Miura S, Kurose I, et al. Enhanced lymphocyte interaction in postcapillary venules of Peyer's patches during fat absorption in rats. Gastroenterology. 1997;112:813–25. doi: 10.1053/gast.1997.v112.pm9041243. [DOI] [PubMed] [Google Scholar]
- 17.Belluzzi A, Boschi S, Brignola C, Munarini A, Cariani G, Miglio F. Polyunsaturated fatty acids and inflammatory bowel disease. Am J Clin Nutr. 2000;71:339S–42S. doi: 10.1093/ajcn/71.1.339s. [DOI] [PubMed] [Google Scholar]
- 18.Siguel EN, Lerman RH. Prevalence of essential fatty acid deficiency in patients with chronic gastrointestinal disorders. Metabolism. 1996;45:12–23. doi: 10.1016/s0026-0495(96)90194-8. [DOI] [PubMed] [Google Scholar]
- 19.Shoda R, Matsueda K, Yamato S, Umeda N. Therapeutic efficacy of N-3 polyunsaturated fatty acid in experimental Crohn's disease. J Gastroenterol. 1995;30(Suppl. 8):98–101. [PubMed] [Google Scholar]
- 20.Camuesco D, Galvez J, Nieto A, et al. Dietary olive oil supplemented with fish oil, rich in EPA and DHA (n-3) polyunsaturated fatty acids, attenuates colonic inflammation in rats with DSS-induced colitis. J Nutr. 2005;135:687–94. doi: 10.1093/jn/135.4.687. [DOI] [PubMed] [Google Scholar]
- 21.Campos FG, Waitzberg DL, Habr-Gama A, et al. Impact of parenteral n-3 fatty acids on experimental acute colitis. Br J Nutr. 2002;87(Suppl. 1):S83–8. doi: 10.1079/bjn2001460. [DOI] [PubMed] [Google Scholar]
- 22.Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999;11:648–56. doi: 10.1016/s0952-7915(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 23.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 24.Bhan AK, Mizoguchi E, Smith RN, Mizoguchi A. Colitis in transgenic and knockout animals as models of human inflammatory bowel disease. Immunol Rev. 1999;169:195–207. doi: 10.1111/j.1600-065x.1999.tb01316.x. [DOI] [PubMed] [Google Scholar]
- 25.Pizarro TT, Arseneau KO, Cominelli F. Lessons from genetically engineered animal models XI. Novel mouse models to study pathogenic mechanisms of Crohn's disease. Am J Physiol. 2000;278:G665–9. doi: 10.1152/ajpgi.2000.278.5.G665. [DOI] [PubMed] [Google Scholar]
- 26.Takeda T, Hosokawa M, Takeshita S, et al. A new murine model of accelerated senescence. Mech Ageing Dev. 1981;17:183–94. doi: 10.1016/0047-6374(81)90084-1. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut. 1998;43:71–8. doi: 10.1136/gut.43.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strober W, Nakamura K, Kitani A. The SAMP1/Yit mouse: another step closer to modeling human inflammatory bowel disease. J Clin Invest. 2001;107:667–70. doi: 10.1172/JCI12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue T, Tsuzuki Y, Matsuzaki K, et al. Blockade of PSGL-1 attenuates CD14+ monocytic cell recruitment in intestinal mucosa and ameliorates ileitis in SAMP1/Yit mice. J Leukoc Biol. 2005;77:287–95. doi: 10.1189/jlb.0204104. [DOI] [PubMed] [Google Scholar]
- 30.Matsuzaki K, Tsuzuki Y, Matsunaga H, et al. In vivo demonstration of T lymphocyte migration and amelioration of ileitis in intestinal mucosa of SAMP1/Yit mice by the inhibition of MAdCAM-1. Clin Exp Immunol. 2005;140:22–31. doi: 10.1111/j.1365-2249.2005.02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kosiewicz MM, Nast CC, Krishnan A, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graves DT, Jiang YL, Williamson MJ, Valente AJ. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989;245:1490–3. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- 33.Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–93. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- 34.Joris I, Zand T, Nunnari JJ, Krolikowski FJ, Majno G. Studies on the pathogenesis of atherosclerosis. I. Adhesion and emigration of mononuclear cells in the aorta of hypercholesterolemic rats. Am J Pathol. 1983;113:341–58. [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz CJ, Sprague EA, Kelley JL, Valente AJ, Suenram CA. Aortic intimal monocyte recruitment in the normo and hypercholesterolemic baboon (Papio cynocephalus). An ultrastructural study: implications in atherogenesis. Virchows Arch. 1985;405:175–91. doi: 10.1007/BF00704370. [DOI] [PubMed] [Google Scholar]
- 36.Mazzucchelli L, Hauser C, Zgraggen K, et al. Differential in situ expression of the genes encoding the chemokines MCP-1 and RANTES in human inflammatory bowel disease. J Pathol. 1996;178:201–6. doi: 10.1002/(SICI)1096-9896(199602)178:2<201::AID-PATH440>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 37.Grip O, Janciauskiene S, Lindgren S. Circulating monocytes and plasma inflammatory biomarkers in active Crohn's disease: elevated oxidized low-density lipoprotein and the anti-inflammatory effect of atorvastatin. Inflamm Bowel Dis. 2004;10:193–200. doi: 10.1097/00054725-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Mishra A, Chaudhary A, Sethi S. Oxidized omega-3 fatty acids inhibit NF-kappaB activation via a PPARalpha-dependent pathway. Arterioscler Thromb Vasc Biol. 2004;24:1621–7. doi: 10.1161/01.ATV.0000137191.02577.86. [DOI] [PubMed] [Google Scholar]
- 39.Baumann KH, Hessel F, Larass I, et al. Dietary omega-3, omega-6, and omega-9 unsaturated fatty acids and growth factor and cytokine gene expression in unstimulated and stimulated monocytes. A randomized volunteer study. Arterioscler Thromb Vasc Biol. 1999;19:59–66. doi: 10.1161/01.atv.19.1.59. [DOI] [PubMed] [Google Scholar]
- 40.Colussi G, Catena C, Baroselli S, et al. Omega-3 fatty acids: from biochemistry to their clinical use in the prevention of cardiovascular disease. Recent Pat Cardiovas Drug Discov. 2007;2:13–21. doi: 10.2174/157489007779606158. [DOI] [PubMed] [Google Scholar]
- 41.Weldon SM, Mullen AC, Loscher CE, et al. Docosahexaenoic acid induces an anti-inflammatory profile in lipopolysaccharide-stimulated human THP-1 macrophages more effectively than eicosapentaenoic acid. J Nutr Biochem. 2007;18:250–8. doi: 10.1016/j.jnutbio.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Horrobin DF. Fatty acid metabolism in health and disease: the role of delta-6-desaturase. Am J Clin Nutr. 1993;57:732S–36S. doi: 10.1093/ajcn/57.5.732S. [DOI] [PubMed] [Google Scholar]


