Abstract
A randomized controlled efficacy trial targeting older adults with hypertension is providing a tailored education intervention with a Next Generation Personal Education Program (PEP-NG) in primary care practices in New England. Ten participating advanced practice registered nurses (APRNs) completed online knowledge and self-efficacy measures pre-onsite training and twice more after completing a continuing education program. Patient participants self-refer in response to study recruitment brochures and posters. Twenty-four participants from each APRN practice (total N = 240) are randomly assigned by the PEP-NG software to either control (data collection and four routine APRN visits) or tailored intervention (PEP-NG interface and four focused APRN visits) conditions. Patients access the PEP-NG interface via wireless tablet and use a stylus to answer demographic, knowledge, and self-efficacy questions as well as prescription and over-the-counter self-medication practice questions. The PEP-NG analyzes patient-reported information and delivers tailored educational content. Patients’ outcome measures are self-reported antihypertensive medication adherence, blood pressure, knowledge and self-efficacy concerning potential adverse self-medication practices, adverse self-medication behavior “risk” score and satisfaction with the PEP-NG and APRN provider relationship. APRN outcome measures are knowledge and self-efficacy concerning adverse self-medication practices, self-efficacy for communicating with older adults and satisfaction with the PEP-NG. Time–motion and cost–benefit analyses will be conducted.
Keywords: hypertension, self-medication, older adults, tailored intervention, computer-based education
Introduction
Despite frequent primary care visits, adults over the age of 60 years often do not achieve target blood pressure (BP) readings (<140/90; <130/80 for those with diabetes or chronic kidney disease).1–5 An estimated $100 billion is spent annually in the United States on health care for patients with poorly controlled BP in part due to poor antihypertensive medication adherence and other adverse self-medication behaviors.6–10 Failure of the health care system to identify and remediate poor adherence and adverse self-medication behaviors adds to the overall cost of treatment as providers typically intensify antihypertensive therapy and add additional agents to the regimen which further increases the risk of adverse drug side effects as well as cost.11,12
Recent trials aimed at improving patient adherence to antihypertensive therapy have yet to demonstrate large long-term improvements in either adherence or health outcomes.13 Intensive and frequent (monthly) counseling can greatly improve antihypertensive adherence, but adherence declines to baseline when the intervention is removed.14–16 Patient adherence to antihypertensives has been shown to be greatest five days prior and five days after health care visit and usually declines within 30 days to typify the so called “white coat adherence.”7
Inadequate patient education about adherence and safe medication use contributes to preventable adverse drug events.6 Over-the-counter (OTC) medications, supplements, and alcohol can interact with antihypertensives and contribute to poor BP control.6,10,17 For example, nonsteroidal anti-inflammatory drugs (NSAIDS such as ibuprofen) increase BP and antagonize the efficacy of antihypertensives and the anti-platelet effects of low-dose aspirin when taken concurrently.18–20 Many patients choose inappropriate OTC analgesics such as NSAIDs to self-medicate pain.21,22 In separate surveys of English- and Spanish-speaking older adults with hypertension, more than 85% reported two or more adverse self-medication practices.21,22 Older adults with hypertension also had large knowledge deficits regarding conflicts between prescription and OTC agents, as well as low confidence in their ability to avoid these conflicts.23 Addressing adverse self-medication practices is one step toward reducing the risk of potential adverse drug interactions (PADI).
A personal computer (PC)-based interactive Personal Education Program (PEP) was effective in improving knowledge and self-efficacy in addition to reducing adverse self-medication practices in older adults with hypertension.24,25 The PEP was upgraded to the “next generation” PEP (PEP-NG), a web-based program accessed wirelessly. The PEP-NG has the following attributes: 1) embedded measurement instruments to capture demographics, medication use, knowledge and self-efficacy about avoiding adverse self-medication behaviors, and satisfaction with the provider relationship and system-interface, and 2) a rules engine that analyzes patient reported information as the basis for delivering a tailored educational intervention to the individual patient.
This paper details the design and methods of a clinical trial testing the effectiveness of the PEP-NG in primary care practices. Specifically designed to increase medication adherence and reduce adverse self-medication behaviors in older patients with hypertension, this web-based intervention/education system is the first of its kind.
Trial design and methods
Overview
The PEP-NG profiles and analyzes patient risk levels by capturing individual self-medication behaviors and related knowledge and self-efficacy. Patients access the PEP-NG interface via a wireless tablet PCa and use a stylus to interact with a set of medication regimen (prescription and over-the-counter) and self-medication practice questions. The PEP-NG analyzes patient-entered information and delivers tailored educational content. A rules engine selects and addresses three adverse self-medication behaviors (with the highest risk scores) and offers the following: 1) “medicine facts;” 2) animations that illustrate the consequences of the adverse behaviors identified; 3) “what you can do” corrective strategies; and 4) realistic scenarios that allow the user to practice the information learned.
The PEP-NG prints summaries for both the patient and provider of patient-reported symptoms, medication use (including frequency/time), adverse self-medication behaviors (along with a thumbnail illustration) and corrective strategies. Prior to the patient-provider visit, providers enter the prescribed medication regimen and patient’s blood pressure, age, and health literacy scores on a separate provider interface on the PEP-NG. This allows the provider to review and contrast the provider-entered data against the patient printout prior to the primary care visit. The printout also supports the provider in reinforcing guidance and oversight of adherence behaviors. The patient takes a copy of the printout home for self-study.
Trial data collected are automatically transferred to a Microsoft Access database (Microsoft Corp., Redmond, WA) via Virtual-Private-Network, which meets or exceeds the HIPAA requirements26 and the European Union Directive 95/46/EC.27 The interface was developed in accordance with ISO 9100 international standards.28,29
The PEP-NG was designed through formative research and formal usability studies with advanced practice registered nurse (APRN) providers and older adults.30–33 The text, graphic elements and animation materials are programmed with Macromedia’s Flash ActionScript language (Adobe, San Jose, CA). Objects are large (3 cm high) and text is in a 20-point Arial Black font. The text and background colors, illumination level, and the graphic and animation style and speed meet the visual and cognitive processing capabilities of older adults.30 Wide scroll bars and dropdown-menus displayed in blocks of eight lines ease use for those with impaired hand mobility and/or fine tremor. An animated clock enables the accurate selection of time of medication and dosage (For a complete description of the database and interface, see Strickler and colleagues).31
Results from iterative usability testing suggest that the production version of the PEP-NG permits users to navigate with minimal errors and less “subject burden” (in relation to mental task load).32,33 The experience of using the PEP-NG did not affect BP measured immediately before and immediately after piloting its use. Additionally, both older adult users and APRNs rated the PEP-NG highly in terms of system usefulness and satisfaction with the program.32,33 The mean time for interface use by seven verification usability study participants (average age 82 years, range 60 to 93 years) was 33.08 ± 7.65 minutes.32
The PEP-NG was also evaluated with 11 older adults with hypertension in a time-series beta test conducted over a three-month period (four visits).34 The increases in knowledge and self-efficacy for avoiding adverse self-medication behaviors were statistically significant with large effect sizes. Behavior risk score did not change significantly, but the risk score was significantly correlated with systolic BP at visit 4. There was a significant decline in systolic BP (medium effect size) for the nine participants not at BP goal upon study entry.
The goal of the clinical trial is to reduce adverse self-medication practices in older adults with hypertension in active primary care settings through improved patient-provider communication about self-medication adherence and safety. Specific aims for older adults are to show that users of the PEP-NG will: 1) increase knowledge concerning potential adverse outcomes arising from self-medication practices; 2) increase self-efficacy as to how to avoid adverse self-medication practices; 3) reduce adverse self-medication behaviors; 4) improve prescription antihypertensive medication adherence; 5) achieve and maintain target blood pressure readings; 6) demonstrate satisfaction with the PEP-NG; and 7) enhance the APRN provider-patient relationship. Specific aims for APRN providers are to show that their use of the PEP-NG will: 1) increase knowledge concerning potential adverse outcomes arising from older adults’ self-medication practices; 2) increase self-efficacy for teaching older adults about adverse self-medication practices; 3) increase self-efficacy for communicating with older adults about self-medication adherence and safety; and 4) demonstrate satisfaction using the PEP-NG with clients.
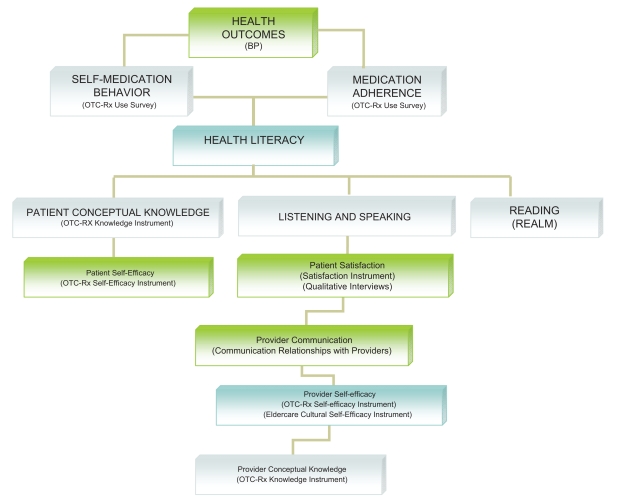
In summary, the PEP-NG trial will educate both patients with hypertension and APRN providers to improve patient self-medication literacy, which will result in improved medication adherence, reduced adverse self-medication behaviors, and improved BP control. As shown in Figure 1, increased patient self-efficacy and knowledge can increase patient health literacy. In addition, increased provider self-efficacy and conceptual knowledge will foster patient/provider communication and patient satisfaction, resulting in improved listening and speaking components of patient health literacy.
Figure 1.
Interplay of health literacy with project outcomes.
Design, setting, and recruitment
The study has approval from the University Institutional Review Board (IRB) and meets all HIPAA regulations. The clinical trial is in cooperation with two practice-based research networks (PBRN) in New England. APRNet is the only PBRN of APRNs and is funded by the Agency for Healthcare Research and Quality (AHRQ) through the Yale School of Nursing. The Connecticut Center for Primary Care (CCPC) is a PBRN and an independent, not-for-profit corporation established under CT law by ProHealth Physicians, Inc. The primary care practices have widely different patient demographics and practice characteristics. The practices are located in urban centers, small cities, suburbs, and rural areas; while some APRNs are salaried, others are paid by the number of patients seen.
Primary care practice owners and APRNs affiliated with each PBRN were contacted by letter inviting participation in the study. A member of the research team met with each APRN who responded with interest in learning more about the study. The APRNs received an illustrated brochure describing the study and an informed consent form and given a demonstration of the PEP-NG software. Participating practices were offered free installation of a wireless access node (meeting HIPAA requirements)26 and a second tablet PC unit (in addition to the unit used for the study) as incentives for participation. Practices associated with the PBRN networks entered the study in an ongoing basis. Ten APRNs in eight practice locations are enrolled in the study. Five other practices signed on to the study but withdrew soon after the installation of the wireless access nodes for reasons unrelated to the study (due to APRN illness, APRN job change, and practice location change).
Each participating APRN received a two-hour on-site training session with a member of the research team (who is a master’s prepared Registered Nurse). The APRNS were given a research notebook with a step-by-step study protocol, instruments for assessing study eligibility, record sheets for documenting each visit, grocery gift cards to compensate participants for their time, and PEP-NG study appointment cards. A supply of illustrated participant recruitment brochures and posters with the APRNs’ names and practice contact information were placed in waiting rooms and examination rooms.
APRNS were offered $80 to compensate for their time during the two-hour on-site PEP-NG training. They were also offered 10 continuing education units (CEUs) for reading 10 journal articles written by the principal investigator (PI) (aimed at practitioners and related to potential adverse outcomes stemming from patients’ adverse self-medication behaviors) and subsequently completing the APRN pre- and post-training knowledge and self-efficacy surveys. APRNs were also offered $55 (to be paid to either the APRN or the practice) for each participant enrolled (up to 24 participants) to compensate for spending approximately 40 minutes per participant to: 1) ascertain study eligibility; 2) conduct the informed consent process; 3) cover the online tutorial with the patient; 4) keep receipts for participant gift cards; and 5) file recruitment reports.
Older adults self-refer for the study by calling the practice and making an appointment with the APRN. The APRN meets with the prospective participant to review the consent form (written in a 14-point Arial font at a grade 6 reading level). Participants are requested not to participate in another research study related to their health while enrolled in the current study. If the patient consents to participate, the APRN then ascertains study eligibility with the following inclusion criteria: 1) not previously involved in a PEP study; 2) aged at least 60 years (by self-report); 3) a health literacy score of at least 44 (6th grade) as measured by the Rapid Estimate of Adult Literacy in Medicine (REALM) tool;35,36 4) takes prescribed antihypertensive medication; and 5) lives independently with independent physical and cognitive functioning. The last criterion assesses patient ability to: 1) independently manage the tasks of telephone communication, shopping, travel arrangements, medication taking, and personal finances, as assessed with the Instrumental Activities of Daily Living Scale;37 and 2) answer six of 10 items on the Short Portable Mental Status Questionnaire.38 Participants also need to demonstrate a visual acuity of at least 20/100 (with corrective lenses if needed).
The APRN selects a four-digit random number from a list (provided in advance by the study) as the login ID for each participant, another for the APRN login ID, and another for the site ID. The PEP-NG system randomly assigns participants to either the control or the intervention group. Patient randomization is carried out within each APRN practice in order to eliminate any possible confounding effects due to the heterogeneity among APRNs or their practices. APRNs mail study monitoring data monthly to the PI, including the numbers of patients: 1) screened for participation; 2) met and not met study criteria; 3) enrolled; 4) dropped out of the study; and 5) experienced adverse or unexpected effects.
Interventions
APRN provider intervention
As described above, APRNs received a two-hour on-site training session. Before training, they logged on to a dedicated website to complete pre-training knowledge and self-efficacy instruments. As part of their on-site training, they logged onto the PEP-NG and proceeded through the program, trying out both the patient and provider interfaces. They were given a packet of 10 articles documenting the evidence that underlies the specific adverse medication behaviors addressed by the PEP-NG. The APRNs read these articles on their own time over the following two weeks and then logged on to the APRN PEP-NG website to complete post-training knowledge and self-efficacy instruments. The APRNs completed the post-training instruments again after enrollment of their 6th participant (typically three months later) and again after enrollment of their 12th participant (typically 6 months later).
Patient intervention
Each participant (from both control and intervention groups) meets with the APRN four times over three months in a private examination room at the practice site. Participants are encouraged to bring all of their medications (including supplements) to each visit. At the beginning of visit 1, the tablet is connected to its detachable keyboard and the APRN records the participant’s age, gender, BP and health literacy score on the APRN provider screen. The APRN also enters each patient’s prescribed and provider recommended (eg, low-dose aspirin) medications, including, dose, timing, and any special instructions for taking the medication. The APRN then removes the tablet from the keyboard and sets it on a height/angle adjustable stand designed for study use. The APRN reads a script to the participant about how to use the PEP-NG while the participant practices using the stylus to interface with the tutorial program (that includes a sample question, a medication screen, a “clock” screen, and an interactive animation screen). When the participant expresses comfort with the PEP-NG interface, the APRN leaves the patient to continue with the PEP-NG to complete a demographic questionnaire, the knowledge and self-efficacy scales, the health care relationship scale as well as answer questions about medications they take.
On subsequent visits, before asking the patient to continue with the PEP-NG unassisted, the APRN reviews patient comfort with stylus use and the PEP-NG interface. The demographic questionnaire is omitted on subsequent visits. At the beginning of visit 4, participants complete the patient PEP-NG satisfaction instrument, in addition to the other scales and questions measured during visits 1–3. After each PEP-NG use, the participant meets with the APRN for approximately 15 minutes. The APRN takes the participant’s blood pressure and reviews the provider interface to review/update any changes in the medication regimen.
Participants in the intervention group receive tailored education. This process is triggered by the PEP-NG’s analysis of patient-entered data, which instantaneously generates and delivers intervention and education content tailored to the patient-reported behaviors (including the aforementioned “medicine facts,” animations dealing with the adverse behaviors identified, corrective strategies, and interactive questions that allow the user to apply information learned). A printout generated by the patient-reported data on the PEP-NG lists patient reported symptoms, three identified adverse self-medication behaviors (with the highest risk scores) and tailored corrective strategies suggested by the PEP-NG, along with a thumbnail illustration from the animations. In the case of fewer than three reported adverse behaviors, the PEP-NG delivers a set of up to three default statements dealing with medication adherence, OTC pain relievers (that can be safely taken with antihypertensives), and dangers of combining different types of pain relievers (prescription or OTC). An office assistant gives a copy of the printout to the patient and a second copy of the printout to the APRN to inform the patient visit.
Participants in the control group complete all questions via the PEP-NG, in addition to receiving a general education message and interactive animation as well as a question at the end that explains how BP medicines work and emphasizes how BP medications must be taken every day. Participants in the control group meet with the APRN for a care as usual BP visit without a printout.
Each participant in both groups is offered a $10 grocery gift card at the end of each of the first three visits and a $25 grocery gift card at the end of the 4th visit. At the end of the 4th visit, the patient is provided a card with a dedicated telephone number to call, if they wish to schedule a 20-minute post-PEP-NG qualitative interview at the practice site with a nurse from the research team. They are then given an additional $10 grocery gift card for participating in the post-trial qualitative interview. Each APRN will also be interviewed at the end of the study to elicit their experience with the PEP-NG study and will be given a $25 grocery gift card to reimburse them for their time.
Health care utilization will be compared between the control and intervention groups for a cost-benefit analysis (CBA). A time-motion study will also be conducted. The total number of provider visits, emergency room visits and hospitalizations will be tracked for 52 weeks following the participant’s entry to the study. This data will be obtained via medical record review by the participating APRNs at the practice sites.
Measures
Primary and secondary outcomes
The primary outcome is patient adverse self-medication behavior score. BP control at each visit is a secondary outcome for patients. Knowledge, self-efficacy, health care relationships, and satisfaction with the PEP-NG are secondary outcomes for both patients and APRNs. Eldercare cultural self-efficacy is a secondary outcome for APRNs.
Baseline
The APRN records the patient’s age (confirmed from the medical record), gender, health literacy (using the (REALM) tool)35,35 and blood pressure at visit 1. All questions are prepared at a 6th grade Flesch–Kincaid reading level39 and appear on the screen in a 20-point Arial Black font. The patient answers demographic questions concerning living arrangements (who they live with, type of residence), education, race and ethnicity, income (eg, whether their monthly income is above or below $1,500 per month) as well as health questions about current medical problems and symptoms. The patient then completes the self-efficacy and knowledge instruments. Finally, the patient responds to questions about what they take for hypertension and for common health problems.b
Blood pressure
BP measurements are taken by the APRN at each visit - at the beginning of PEP-NG use on visit 1 and post-PEP-NG use on subsequent visits. Inadequate BP control is defined as a SBP ≥ 140 mm Hg or a DBP ≥ 90 mm Hg for patients without diabetes and SBP ≥ 130 mm Hg or a DBP ≥ 80 mm Hg for patients with diabetes or chronic kidney disease according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) guidelines.1 A potential study limitation associated with this protocol is that the BP measurements are taken by the participating APRN and could be subject to observer bias. Also, since patients self-refer to the study; the percentage of participants with controlled BP may not be representative of the total patient population in the practice site.
Medication use
The instrument captures self-reported, patient-entered frequency and longitudinal use data on medications, supplements, and alcohol use. Adherence to antihypertensive medication is assessed from questions related to what, when, and how patients take their antihypertensive medication. Construct validity and concurrent validity of the self-report survey have been established in two past studies.24,25 In particular, concurrent validity was estimated from a survey of 25 married couples who completed the survey twice, once to self-report his or her medication use behavior and once to report his or her mate’s pattern of behavior. An 85% overall match rate across all items in each survey section was taken as supportive evidence of concurrent validity of self-report.40 There was no significant difference between the match rate for any section of the instrument or for the overall instrument. The high Kappa values (>0.75) among all of the specific categories (eg, anti-hypertensives, calcium supplements, pain relievers) provide further support of concurrent validity of the instrument.
Adverse self-medication behavior score (behavior risk score)
Following a modified Delphi method,41 data from the pilot test of the medication use survey were used to develop an adverse self-medication behavior score.24 Using a five-point scale from 1, “very unlikely” to 5, “very likely”, a five-member expert panel rated a list of self-medication behaviors associated with an adverse outcome. The importance weight for each behavior was the mean of the expert panel ratings. The total score is the weighted sum of the scores for the adverse behaviors identified. Due to nonnormality in the distribution of self-medication scores in the older adult population, the log of the total adverse self-medication behavior score will be used in parametric statistical analyses.24
OTC-Rx knowledge
The knowledge instrument is modified from a previously validated 17-item instrument23–25 and based on the PEP content outline. Items test both knowledge and application levels in the cognitive domain.42 These items are short scenarios presenting potential adverse medication behaviors in realistic, interesting settings and have one correct response and three distracters based upon common misconceptions about anti-hypertensives, OTC medicines, and alcohol. The total score is the percent of the items that are correct. The Cronbach’s α reliability coefficient from testing this instrument in a convenience sample with 52 older adults aged over 60 years was 0.68, an acceptable value as naïve individuals use random guessing to answer knowledge items.23 Difficulty indices were 0.25–0.75 and discrimination indices were all >0.20. The one-month test-retest reliability estimate was 0.50, acceptable for an instrument with heterogeneous content administered to a naïve group that uses random guessing to answer items.39 The mean percent score obtained from these 52 older adults meeting study criteria at a BP clinic was 43.1 ± 15.4.23 In the current study, three items unrelated to hypertension (ie, related to warfarin) are omitted.
Preliminary data derived from administration of the same knowledge instrument to 31 APRN graduate student nurses support use with an APRN sample. The Cronbach’s α reliability coefficient for this scale was 0.68 and the test–test reliability was 0.73. The mean percent score was 58.8 ± 13.2 for 20 experienced registered nurses practicing in community health settings.43
OTC-Rx self-efficacy
The scale is based on a previously validated 13-item instrument consisting of behavioral, task specific statements related to patient confidence in avoiding drug interactions arising from self-medication behaviors.23–25 The five-point self-report response categories range from 1, “not sure” to 5, “totally sure.” Responses are summed and divided by the number of items answered, so that the overall score is not affected by omitted items and is expressed in the original five-point metric. Previous data from 134 older adults subjected to a principal factor analysis (PFA) revealed that the structure of the instrument was unidimensional. The single internal consistency estimate (Cronbach’s α) was 0.95 and item loadings on the single factor solution were all >0.63. The one-month test-retest reliability estimate for this scale was 0.81 (P < 0.001).25 The mean score on the scale in the aforementioned convenience sample of 52 older adults attending a blood pressure clinic (who met study criteria) was 2.0 ± 0.8, indicating a low level of self-efficacy.23 In the current study, one item related to warfarin and OTC pain relievers is omitted.
Psychometric data were obtained with the same 31 APRN graduate student nurses who pilot tested the knowledge instrument. The self-efficacy instrument was also found to be a unidemensional scale with the Cronbach’s α for internal consistency at 0.95. The one-month test–retest reliability estimate for the scale was 0.84. For 20 experienced community health nurses,43 the mean rating on the scale was 3.5 ± 0.8 on the five-point scale.
Communication relationships with providers
The five-item instrument, based on two qualitative studies,44,45 addresses patient–provider communication (two questions), trust, decision-making related to care, and satisfaction with care. The scale was modified for use with older adults by changing the visual analog 10 cm response format to five-point Likert-type responses with two extremes (eg, “not at all easy” to “very easy”).
The Cronbach’s α for the modified scale was 0.81 (from a sample of 121 persons aged 60 years and over) and the test–retest reliability estimate for the scale (generated with 19 persons from the same sample) was r = 0.57, P = 0.014. Factor analysis via the principal component extraction method revealed one component accounting for 57% of the variance. All items loaded above 0.78, except for the item “How easy is it for you to talk with your primary care provider” which loaded as 0.47. From the sample of 121 persons, 90.4% reported high trust in their primary care provider and 86.3% felt very to completely satisfied with their care. Regarding the item “calling their primary care provider,” 82% felt very to completely comfortable and 77.5% found it was very to completely easy to speak with their primary care provider. In making decisions about their care, 79.2% reported being very to completely involved (Anderson et al 2009, unpublished data).
Eldercare cultural self-efficacy
This five-point Likert-type scale assesses APRN self-efficacy in communicating with older adults about their medications. The scale measures nurses’ perceptions of their own confidence in caring for older adults of diverse racial and ethnic backgrounds.46 Pilot testing of this scale with 275 senior student nurses revealed four principal factors following PFA with orthogonal rotation: 1) assessing lifestyle and social patterns; 2) determining cultural health practices; 3) determining cultural beliefs; and 4) dealing with grief and loss. The cut-off point for retaining factor loading was above 0.40, as recommended by Nunnally and Bernstein.40 The Cronbach’s α reliability coefficient for the subscales ranged between 0.83 and 0.92; the overall internal consistency α of the 28-item scale was 0.96. Average mean ratings on the four subscales ranged from 3.37 to 3.72. The group subscale mean for Factor 2: “Determining cultural health practices” (assessment of medication practices, use of health systems, dietary patterns) (seven items) was 3.52 ± 0.18 for 275 senior student nurses.46 The subscale mean generated from testing a group of 42 APRNs was 3.54 ± 0.54 and the one week test–retest correlation for 17 APRNs was 0.94 (Neafsey and Anderson 2009, unpublished data).
Satisfaction
The patient questionnaire is modified from a 14-item instrument previously validated with older adults using the first generation PEP.24 Eight items focus on the ease of program use, program content, and suitability of program content and the other six items address the perceived likelihood of behavior change following program use. The five-point Likert-type response format ranges from 1, “strongly disagree” to 5, “strongly agree.” Ratings are summed and divided by the number of items answered, so that the overall satisfaction scale is not affected by omitted items and is expressed in the original five-point metric. Data from a previous study of 83 older adults using the original PEP revealed a unidimensional measure with a single factor accounting for 70% of the covariance across scale items. The Cronbach’s α estimate for internal consistency was 0.89.24 In the current study, an additional item has been added “The advice in this program suited my special needs” to capture satisfaction with the tailored education delivered.
The APRN satisfaction instrument, a five-point Likert-type scale, contains 10 statements regarding use of computer-based technology for nursing education.43 Ratings are summed and divided by the number of items answered, so that the overall satisfaction scale is not affected by omitted items and is expressed in the original five-point metric. When this instrument was applied to a previous software program (on drug interactions) for APRNs, the results showed a unidimensional scale (by PFA) with a single factor accounting for 68% of the variance. The Cronbach’s α for the scale’s internal consistency was 0.91.47
Qualitative interviews
Patient participants and APRNs choosing to be interviewed regarding their experience in the PEP-NG study leave a message on a dedicated telephone number. A member of the research team arranges a convenient time for the interview. All interviews take place at the APRN’s practice site. An a priori set of 15 questions (developed following the method of Krippenddorff)48 is used to elicit information regarding what it was like to learn with the PEP-NG and their experience with the study. Interviews are tape-recorded and last between 15 minutes to one hour.
Sample size and power considerations
The study design involves three factors–ie, intervention (PEP-NG vs control), APRN (10 advanced practice nurses), and time (evaluation at baseline and at three subsequent time points). The design is factorial and balanced in that each factor is crossed with the other two factors and equal numbers of participants will be in each study “cell” defined by crossing the three factors.
When data collection is completed, the analysis plan is to employ repeated measures ANOVA as the basis for statistical testing. The primary study hypothesis concerns the possibility of differential change in the PEP-NG and control conditions between the baseline and final study assessments.
From our pilot data,25,34 we estimated that standardized effect sizes (d) range from 0.41 to 1.30, depending on which participant outcome measure was considered. Calculations using the smallest of these effect sizes (adverse self-medication risk score – the primary outcome variable) were used to determine the sample size. We believe that the pre-intervention mean value of the adverse self-medication risk score will be 21.5 among participants in both the intervention and control groups. We expect that, with intervention, this mean score will decrease to 16.5 by the end of follow-up; without intervention, the mean score will remain unchanged. Using data from the preliminary studies, we assume the standard deviation of the adverse self-medication risk score will be ±10.1 in both groups throughout follow-up. We also anticipate that the correlation between scores on individuals between the baseline and final assessments will be +0.20 or higher. Setting an objective to have 80% power to detect a difference in mean changes between groups, we determined a sample size estimate of 82 participants per group –ie, a total of 164. However, in order to maintain balance across all 10 APRNs, this estimate implied a need to recruit a minimum of 180 participants, ie, nine per group per APRN. Anticipating the potential for 20% loss to follow-up among study participants, the final recruitment goal was set at 240 participants, ie, 12 per group per APRN, in order to have complete data on a sample of 180 participants. In a crossed and balanced design, testing results for the study’s primary hypothesis will be asymptotically equivalent whether performed using a multi-factor model or by applying a two-sample t-test to contrast the mean levels of within-subject changes between groups. The sample size estimates were derived from formulas related to the t-test, assumed application of one-tailed testing at the 5% level of significance, and were calculated using PASS 2008 software (NCSS, LLC., Kaysville, UT).
A secondary outcome of special interest is blood pressure control. Accordingly, we also conducted power analyses relative to this variable to ensure that the sample size determined for the primary outcome would be sufficient to detect clinically meaningful differences between the intervention and control conditions in the maintenance or achievement of acceptable blood pressure levels. Based on the rates of hypertension control found by Chobanian and colleagues,1 we expect to find 35% of participants with controlled blood pressure at the time of study entry. Due to randomization, the percentage of participants with controlled blood pressure is expected to hold, on average, in both the intervention and control groups. During the study period, some patients’ blood pressures will move into the controlled range while other patients’ blood pressures move out of it. We expect that, in the control group, at most 30% of participants will experience a change in blood pressure control during the study period, that these changes will occur in both directions with equal probability, and that, by the end of the follow-up period, 35% of participants will have blood pressures in the controlled range (though not necessarily the same persons who had controlled blood pressure at study entry). In the intervention group, we also expect that up to 30% of participants will experience a change in blood pressure control during the study period, but that these changes will occur predominantly in the direction from the uncontrolled range to the controlled range. Under these assumptions, the sample size objective of 180 participants with complete data will provide 80% power to detect a 20% or greater increase in the percentage of participants with controlled blood pressure in the intervention group relative to the control group at the end of follow-up, ie, an expected frequency of 55% or more of intervention participants with controlled blood pressure by study completion. This power analysis accounted for random variation in the percentage of participants with controlled blood pressure at study entry in both the control and intervention groups (under the assumption of an expected frequency of 35%), for systematic differences and random variation in the percentages of participants who change blood pressure control status during follow-up (under the assumption of an expected frequency of 30% or less), and for correlations due to pairing of initial and final blood pressure control assessments within participants. Determination of the variance of the test statistic was based on formulas from Agresti.49
Data analyses
SAS software (v. 9.2; SAS Institute, Inc., Cary, NC) will be used for data analysis. Both univariate (skewness and kurtosis coefficient) and multivariate (Mahalanobis distance) data screening techniques will be used. Invalid multivariate outliers based on Mahalanobis distance values will be inspected for possible errors in coding and will be discarded if deemed erroneous or unrepresentative. Missing data at the item level will be imputed with regressed scores from available variables and the degrees of freedom in the error term reduced by the number of points estimated. Any case that has 30% or more errors will be deleted, rather than subjected to estimation.
Descriptive statistics will be derived by tabulating data recorded by the PEP-NG tracking software. Data will be analyzed as a repeated measures analysis of variance (ANOVA) with APRN and GROUP as between-subjects factors and TIME as the within-subjects factor. Because APRN can be considered a random factor, the appropriate analysis will be preceded by an assessment of the APRN × GROUP effect. If strong, bias in the ANOVA error terms introduced by this effect will be compensated by the use of quasi-F ratio tests or maximum likelihood techniques. Psychometric estimates for all instruments will be cross-validated. Cronbach’s α levels will be calculated for each scale and item analyses conducted on the knowledge scale. Standardized effect sizes will be calculated. Correlations between user age, education, and health literacy score with knowledge, self-efficacy, adverse self-medication behavior score, blood pressure, and satisfaction scores will be assessed.
Descriptive statistics organized by race, ethnicity and gender will be presented. With regard to race and ethnicity, because the number of participants in each minority group in our catchment area is relatively small, and our sample reflects those small percentages, we do not have the basis for a statistically powerful analysis of race unless the differences among Hispanics, Blacks, and Caucasians are very large and consistent. If such a situation occurs, the ANOVAs will incorporate race and/or ethnicity as a factor, or alternatively, separate analyses will be conducted for each minority group. The same approach will be used if gender proves to be important (contrary to our expectation).
Analysis of qualitative interviews
A graduate research assistant (trained in transcription) transcribes all the interviews. Krippenddorf’s content analysis approach will be used to guide data analysis.48 This qualitative research method involves identifying, categorizing and labeling the patterns and themes in the data. Transcriptions will be reviewed and examined as part of the coding process. Thematic distinctions will be identified through recurring patterns within the data.
Cost benefit analysis
As most of the patients enrolled in this study will have a number of co-morbid states, it would be difficult to determine whether a certain instance of health care utilization is solely due to inadequate/poor hypertension control. Consequently, the costs for any type of health care utilization, regardless of cause, will be included in the CBA. We will, however, also separately evaluate health care utilization thought to be related primarily to cardiac events. The total number of hospitalizations, emergency room and provider visits will be tracked for 52 weeks following study entry via queries of claims and billing databases and supplemented through medical record review by the APRN.
The cost of health care resources will be valued using Medicare reimbursment rates as described by Tumeh and colleagues.50 Prospective payment system coding for inpatient and outpatient services (utilizing diagnosis-related group [DRG] and Ambulatory Payment Classification [APC] codes) and the Medicare Physician Fee Schedule (utilizing the Health Common Procedure Coding System [HCPCS]/Current Procedural Terminology [CPT] codes) will be used to assign costs to hospitalizations, emergency room and provider office visits. A time-motion study will be conducted to determine APRN time required to obtain medical history information and complete the APRN visit in the presence (tailored) or absence (routine) of the PEP-NG.51,52 For both control and intervention groups, three to five observations will be made on at least three separate occasions. The APRN will record time to the nearest minute and will be based on activities required to collect and organize medical history data for an individual patient. The cost of personnel will be estimated by multiplying mean time spent by the average hourly wage rate.53 Due to the short-term nature of the study, discounting will not be performed.54 Costs will be adjusted to 2008 US dollars using the Consumer Price Index for Medical Care.55
Threshold (TSA) and probabilistic sensitivity analyses (Monte Carlo simulation) will be conducted to test the robustness of the results and conclusions of our cost-benefit analysis.51 The PEP-NG acquisition cost, personnel time and wage rate will be varied in the sensitivity analyses. For the TSA, if the tested variables do not vary outside the range of plausible values, confidence in the study results will be strengthened. For the Monte Carlo simulation, the acquisition cost of the PEP-NG will be varied by 50%, personnel time will be varied ±1 standard deviation, and wage rate will be varied using the minimum and maximum wage rate for APRNs employed in the United States.51,53
Discussion
The study described herein is an effectiveness trial in the “realistic setting” of 10 primary care practices. Participants self-refer to the study and may not reflect the general population of patients in the practice with respect to either demographic characteristics or degree of adherence to their antihypertensive regimen. While this aspect of the design will limit generalizability, we will derive findings as to the feasibility and cost/benefit of the intervention, as well as patient and provider satisfaction.
Gehi and colleagues56 found that the cardiovascular risk associated with self-reported nonadherence (ie, answering a single survey question, “In the last month, how often did you take your medications as your doctor prescribed?”) was as great as that from smoking or diabetes. While the simple self-report approach taken by the PEP-NG may underestimate adherence, it does identify nonadherence and may foster a subsequent discussion between patient and provider about the reasons for nonadherence as well as strategies for improved adherence to the medication regimen.
The risk of potential adverse drug interactions (PADI) is greatly increased in older adults having three or more chronic diseases, five or more medications per day, more than 12 medication doses taken per day, a history of nonadherence, or a drug requiring therapeutic monitoring.57 In a primary care environment where the median time of a visit is 14 minutes,58 the PEP-NG can use patient “waiting room time” to identify those patients with PADIs and deliver education tailored to the patient’s specific medication behaviors. The PEP-NG has the potential to allow providers to redirect time during the visit to answer patient questions and engage in patient teaching instead of querying patients about medication behaviors and looking up drug interaction information.
Conclusion
The PEP-NG is a self-directed computer-mediated communication program that provides a means for patients to become aware of their own adverse medication behaviors and learn to modify behavior for improving their own health. It empowers older adults with hypertension with a self-management mechanism that enables them to enhance their self-efficacy in self care, which is the corner stone of improving public health. The PEP-NG offers the provider advance knowledge of a patient’s self-medication behavior and literacy, which can be instrumental in facilitating patient–provider communication aimed at improving medication adherence and safety. If found effective in the current clinical trial, the PEP-NG has the potential for rapid adoption in the primary care setting and become a model for other self-management and medication adherence interventions directed at other serious chronic conditions (eg, diabetes, asthma, etc) that pose the major threats to public health.
Acknowledgments
The authors wish to thank Christian Rauh, MS and Yan Li, PhD for creating the computer code for the PEP interface, Zoe Strickler MDes for animation design, Jonathan Gill for wireless network configuration and server support and Sheri Peabody, MS, APRN, Jessica Planas, MPH, RN, and Karen Pasquale, MPH for their assistance in site recruitment, training and monitoring. The authors are grateful to Margaret Grey, RN, DrPH, Dean, Yale School of Nursing and Principal Investigator of APRNet and Jonathan Rosen, MD, Principal Investigator of the Connecticut Center for Primary Care for facilitating recruitment of primary care practices for this study from their Practice-Based Research Networks. This work is supported by funding from the National Heart, Lung and Blood Institute, grant number 5R01HL084208, Neafsey PJ, Principal Investigator.
Footnotes
The tablet PC (Motion LE 1600 Centrino) was manufactured by the Motion Computing, Inc. in 2006. Technical specifications for this model include: Intel Pentium® M Processor LV 778 (1.6 GHz), Integrated Intel PRO Wireless 2915ABG, 512MB RAM, 30GB HDD with View Anywhere Display (to eliminate glare from overhead fluorescent lights), 12.1” wide view XGA TFT display, convertible keyboard, 3-M privacy filter, and Genuine Windows® XP.
“Did you take something for ___ in the last month?” is asked with respect to the following problems: blood pressure, blood thinning, pain, cold or sinus, allergies, sleep, stomach problems such as indigestion or gas, and low thyroid. “Did you take ____ in the last month?” is asked with respect to: calcium pills, vitamins, minerals, herbs or supplements, and alcohol, wine or liquor.
Disclosure
The University of Connecticut granted an exclusive license for the PEP-NG to AdhereTx Corporation on August 25, 2009. PJ Neafsey and the University of Connecticut are stockholders of AdhereTx.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1256. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Berlowitz D, Ash A, Hickey E. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–1963. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 3.Knight EL, Bohn RL, Wang PS, Glynn RJ, Mogun H, Avorn J. Predictors of uncontrolled hypertension in ambulatory patients. Hypertension. 2002;38:809–814. doi: 10.1161/hy0901.091681. [DOI] [PubMed] [Google Scholar]
- 4.Düsing R. Adverse events, compliance, and changes in therapy. Curr Hypertens Rep. 2001;3:488–492. doi: 10.1007/s11906-001-0011-0. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder K, Fahey T, Ebraham S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? A systematic review of randomized controlled trials. Arch Intern Med. 2004;164:722–732. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz J, Field T, Harrold L, Rothschild J, Debellis K, Seger A, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003;289:1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 7.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 8.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 9.National Heart, Lung and Blood Institute. Healthy People 2010. Prevent and control America’s high blood pressure: Mission possible. Washington, DC: NHLBI; 2007. [Accessed November 21, 2007]. Available from: http://hp2010.nhlbihin.net/mission/abouthbp/abouthbp.htm. [Google Scholar]
- 10.Institute of Medicine. Preventing Medication Errors. Washington, DC: National Academies Press; [Accessed October 6, 2006]. Available from: http://www.nap.edu/ [Google Scholar]
- 11.Ho PM, Magid DJ, Shetterly SH, et al. Importance of therapy intensification and medication nonadherence for blood pressure control in patients with coronary disease. Arch Intern Med. 2008;168:271–276. doi: 10.1001/archinternmed.2007.72. [DOI] [PubMed] [Google Scholar]
- 12.Peterson ED. Is information the answer for hypertension control. Arch Intern Med. 2008;168:259–260. doi: 10.1001/archinternmed.2007.71. [DOI] [PubMed] [Google Scholar]
- 13.Haynes RB, Ackloo E, Sahota N, McDonald HP, Yao X. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2008;1:CD000011. doi: 10.1002/14651858.CD000011.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Bosworth HB, Olsen MK, Goldstein MK, et al. The veterans’ study to improve the control of hypertension (V-STITCH): Design and methodology. Contemp Clin Trials. 2005;26:155–168. doi: 10.1016/j.cct.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Roumie C, Elasy T, Greevy R, et al. Improving blood pressure control through provider education, provider alerts and patient education. A cluster randomized trial. Am J Intern Med. 2006;145:165–175. doi: 10.7326/0003-4819-145-3-200608010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: A randomized controlled trial. JAMA. 2006;296:2563–2571. doi: 10.1001/jama.296.21.joc60162. [DOI] [PubMed] [Google Scholar]
- 17.Wallsten S, Sullivan R, Hanlon J, Blazer D, Tyrey M, Westlund R. Medication taking behaviors in the high- and low-functioning elderly: MacArthur field studies of successful aging. Ann Pharmacother. 1995;29:359–364. doi: 10.1177/106002809502900403. [DOI] [PubMed] [Google Scholar]
- 18.Aw TJ, Haas SJ, Liew D, Kurm M. Meta-analysis of cyclooxygenase-2 inhibitors and their effects on blood pressure. Arch Intern Med. 2005;165:490–496. doi: 10.1001/archinte.165.5.IOI50013. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald T, Wei L. Effect of ibuprofen on cardioprotective effect of aspirin. Lancet. 2003;361:573–574. doi: 10.1016/s0140-6736(03)12509-3. [DOI] [PubMed] [Google Scholar]
- 20.Polonia J. Interaction of antihypertensive drugs with anti-inflammatory drugs. Cardiology. 1997;88(Suppl 3):47–51. doi: 10.1159/000177507. [DOI] [PubMed] [Google Scholar]
- 21.Neafsey PJ, Shellman J. Adverse self-medication practices of older adults with hypertension attending rural blood pressure clinics. [Accessed July 30, 2009];The Internet Journal of Advanced Nursing Practice. 2001 5(1) [about 7 p]. Available from: http://www.ispub.com/journal/the_internet_journal_of_advanced_nursing_practice/archive/volume_5_number_1_6.html. [Google Scholar]
- 22.Neafsey PJ, Jarrin O, Luciano S, Coffman M. Self-medication practices of Spanish-speaking older adults in Hartford, Connecticut. Hisp Health Care Int. 2007;5:169–179. [Google Scholar]
- 23.Neafsey PJ, Shellman J. Misconceptions of older adults with hypertension concerning OTC medications and alcohol. Home Healthcare Nurse. 2002;20:300–307. doi: 10.1097/00004045-200205000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Neafsey PJ, Strickler Z, Shellman J, Padula A. Use of touchscreen equipped computers to deliver health information about self-medication to older adults. J Gerontol Nurs. 2001;27:19–27. doi: 10.3928/0098-9134-20011101-08. [DOI] [PubMed] [Google Scholar]
- 25.Neafsey PJ, Strickler Z, Shellman J, Chartier V. An interactive technology approach to educate older adults about drug interactions arising from over-the-counter self-medication practices. Public Health Nurs. 2002;19:255–262. doi: 10.1046/j.1525-1446.2002.19405.x. [DOI] [PubMed] [Google Scholar]
- 26.Federal Register. Health insurance reform: Standards for electronic transactions; announcement of designated standard maintenance organizations; final rule and notice. 2000 Mar 17;:50312–50372. 45 CFR Parts 160 and 162. [Google Scholar]
- 27.de Meyer F, Lundgren P, de Moor G, Fiers T. Determination of user requirements for the secure communication of electronic medical record information. Int J Med Inform. 1998;49:125–130. doi: 10.1016/s1386-5056(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 28.International Organization for Standardization. ISO International Standards. [Accessed May 10, 2004]. Available from: http://www.iso.ch/iso/en/ISOOnline.frontpage.
- 29.Kelly M. What users want from a tool for analyzing and documenting electronic questionnaires. Soc Sci Comput Rev. 2000;18:407–420. [Google Scholar]
- 30.Strickler Z, Neafsey PJ. Visual design of interactive software for older adults: Preventing drug interactions in older adults. Visible Lang. 2002;36:4–28. [Google Scholar]
- 31.Strickler Z, Rauh C, Lin C, Neafsey PJ. Educating older adults to avoid harmful self-medication. J Comm Healthcare. 2008;1:110–128. [Google Scholar]
- 32.Lin C, Neafsey PJ, Strickler Z. Usability testing of a computer-mediated health communication program tailored for older adults with hypertension. J Health Comm. 2009;14:102–118. doi: 10.1080/10810730802659095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CA, Neafsey PJ, Anderson E. APRN usability testing of a tailored computer-mediated health communication program. Comput Inform Nurs. 2010 doi: 10.1097/NCN.0b013e3181c0484e. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neafsey PJ, Anderson E, Peabody S, Lin C, Strickler Z, Vaughn K. Beta testing of a network-based health literacy program tailored for older adults with hypertension. Comput Inform Nurs. 2008;26:311–319. doi: 10.1097/01.NCN.0000336466.17811.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis T, Long W, Jackson R, et al. Rapid estimate of adult literacy in medicine: A shortened screening instrument. Fam Med. 1993;25:391–395. [PubMed] [Google Scholar]
- 36.Davis T, Michielutte R, Askov E, Williams M, Weiss B. Practical assessment of adult literacy in health care. Health Educ Behav. 1998;25:613–624. doi: 10.1177/109019819802500508. [DOI] [PubMed] [Google Scholar]
- 37.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 38.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1974;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 39.Flesch RA. A readability formula that saves time. J Read. 1968;11:129–228. [Google Scholar]
- 40.Nunnally J, Berstein L. Psychometric Theory. 3rd ed. New York, NY: McGraw-Hill; 1995. [Google Scholar]
- 41.McKillip J. Need Analysis: Tools for the human services and education. Newbury Park, CA: Sage Publications; 1987. [Google Scholar]
- 42.Bloom B. Taxonomy of Educational Objectives. New York, NY: David McKay; 1956. [Google Scholar]
- 43.Neafsey PJ, Shellman J. Knowledge and self-efficacy of community nurses concerning interactions of prescription medicines with over-the-counter agents and alcohol. J Gerontol Nurs. 2002;28:30–39. doi: 10.3928/0098-9134-20020901-07. [DOI] [PubMed] [Google Scholar]
- 44.Anderson EH, Spencer MH. Cognitive representations of AIDS: a phenomenological study. Qual Health Res. 2002;12:1338–1352. doi: 10.1177/1049732302238747. [DOI] [PubMed] [Google Scholar]
- 45.DeGeest S, Abraham I, Gemoets H, Evers G. Development of the long-term medication behavior self-efficacy scale: Qualitative study for item development. J Adv Nurs. 1994;19:233–238. doi: 10.1111/j.1365-2648.1994.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 46.Shellman J. Reliability and validity testing of the Eldercare Cultural Self-Efficacy Scale. [Accessed June 24, 2007];Internat J Nurs Ed Scholarship. 2006 3(1) doi: 10.2202/1548-923X.1156. Article 9. Available from: http://www.bepress.com/ijnes/vol3/iss1/art9. [DOI] [PubMed] [Google Scholar]
- 47.Neafsey PJ. Computer-assisted instruction for home study: A new venture for continuing education programs in nursing. J Continuing Ed Nurs. 1997;28:3–12. doi: 10.3928/0022-0124-19970701-06. [DOI] [PubMed] [Google Scholar]
- 48.Krippendorff K. Content Analysis: An introduction to its methodology. 2nd ed. Thousand Oaks, CA: Sage Publications; 2004. [Google Scholar]
- 49.Agresti A. Categorical Data Analysis. New York, NY: John Wiley & Sons; 1990. [Google Scholar]
- 50.Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res. 2005;5:153–162. doi: 10.1586/14737167.5.2.153. [DOI] [PubMed] [Google Scholar]
- 51.Gold MR, Luce BR, Manning WG, Siegel JE, Lipscomb J. Estimating costs in cost-effectiveness analysis. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. pp. 176–212. [Google Scholar]
- 52.Hitt CM, Nightingale CH, Quintiliani R, et al. Cost comparison of single daily iv doses of ceftriaxone versus continuous infusion of cefotaxime. Am J Health Syst Pharm. 1997;54:1614–1618. doi: 10.1093/ajhp/54.14.1614. [DOI] [PubMed] [Google Scholar]
- 53.American Academy of Nurse Practitioners. AANP National compensation survey. 2008. [Accessed February 17, 2009]. Available from: http://www.aanp.org/AANPCMS2/ResearchEducation/Research/2008+AANP+NP+Compensation+Report.htm. [DOI] [PubMed]
- 54.Eisenberg JM. Clinical Economics: A guide to the economic analysis of clinical practices. JAMA. 1989;262:2879–2886. doi: 10.1001/jama.262.20.2879. [DOI] [PubMed] [Google Scholar]
- 55.US Bureau of Labor Statistics. CPI Detailed Report. Washington, DC: Department of Labor; [Accessed March 2, 2009]. Available from: http://www.bls.gov/cpi/cpid0901.pdf. [Google Scholar]
- 56.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease. The Heart and Soul Study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Isaksen SF, Jonassen J, Malone DC, Billups SJ, Carter BL, Sintek CD. Estimating risk factors for patients with potential drug-related problems using electronic pharmacy data. Ann Pharmacother. 1999;33(4):406–412. doi: 10.1345/aph.18268. [DOI] [PubMed] [Google Scholar]
- 58.Hing E, Cherry DK, Woodwell DA. National Ambulatory Medical Care Survey: 2004 Summary. Advance data from vital and health Statistics. Hyattsville, MD: National Center for Health Statistics; 2006. No. 374. [PubMed] [Google Scholar]