SUMMARY
There is an urgent need for the discovery and development of new antitubercular agents that target novel biochemical pathways and treat drug-resistant forms of the disease. One approach to addressing this need is through high-throughput screening of drug-like small molecule libraries against the whole bacterium in order to identify a variety of new, active scaffolds that will stimulate additional biological research and drug discovery. Through the Molecular Libraries Screening Center Network, the NIAID Tuberculosis Antimicrobial Acquisition and Coordinating Facility tested a 215,110-compound library against M. tuberculosis strain H37Rv. A medicinal chemistry survey of the results from the screening campaign is reported herein.
Keywords: MLSCN, TAACF, antitubercular, high-throughput screening, medicinal chemistry analysis, small molecule chemical library, tuberculosis
Tuberculosis (TB) represents one of the top public health concerns worldwide. One-third of the world’s population is infected with Mycobacterium tuberculosis (M. tb), the etiological agent of TB, resulting in 9.2 million new cases and 1.7 million deaths in 2006.1 Multidrug-resistant TB, defined as resistance to at least the first-line drugs isoniazid and rifampin, and extensively drug-resistant (XDR-) TB, defined as resistance to rifampin, isoniazid, fluoroquinolones, and to at least one of the injectable second-line drugs, have contributed to the resurgence of the disease.2-3 Estimates for the global prevalence of drug-resistant TB (including XDR-TB, currently estimated at 40,000 cases per year) are likely a lower bound of the real case burden.4 Forty-nine countries have now reported XDR-TB infections as of June 2008, according to the WHO Stop TB Department.5 Consequently, the need for novel, more effective drugs is evident. Treatment of active disease needs to be shortened, simplified, and not interfere with the administration of antiretroviral agents. It is also highly desirable to identify new types of TB drugs acting on novel drug targets with no cross-resistance to existing therapeutics.
The Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF) was established by the National Institute of Allergy and Infectious Diseases (NIAID) in 1994 to allow researchers access to high quality screening services in order to encourage antituberculosis drug discovery research. The functions of the TAACF are described more fully in a recent publication.6 Unique to the program is the involvement of medicinal chemists in order to recruit high quality, medicinally relevant compounds into the screening program. More recently, large libraries of drug-like molecules were designed and purchased to supplement those screening samples being donated by the larger research community. Modern high-throughput screening (HTS) systems provide an immensely powerful strategy to identify new lead compounds in a relatively short amount of time. The results of the screening of one such library, a 100,997-compound set obtained by NIAID from ChemBridge, was described in the previous paper in this issue.7 Here we summarize the results of a second complementary HTS campaign resulting from a collaboration between the TAACF and an NIH Roadmap initiative, the Molecular Libraries Screening Center Network (MLSCN).
The MLSCN was established in 2005 as a pilot program to assemble a large library of biologically relevant small molecules and make them available through a network of HTS laboratories to researchers worldwide through a competitive assay submission process. Acceptance of the TAACF assay into the MLSCN program made available the unique resources of the NIH Small Molecule Repository (SMR), significantly expanding the spectrum of molecules tested for activity against TB. For this screen, a 215,110-compound library from the SMR was examined for anti-TB activity using the assay described previously,7 with the only change to the screening protocol being the elimination of the polyethylene incubator bags, resulting in the identification of a number of novel chemical scaffolds. Moreover, even for classes of compounds identified earlier during testing of the NIAID ChemBridge library,7 additional examples emerged that further clarified the structure-activity picture. Since the compounds in the SMR have been examined in scores of diverse assays undertaken by the MLSCN, and the results published on the NIH PubChem website,8 another motivation for conducting the MLSCN campaign is the ability to correlate antituberculosis activity of the hits with other biological activities that these compounds may possess, potentially providing information about possible mechanisms of action or toxicity. The raw screening results upon which the structural analysis below is based are now publicly available on PubChem (assay AIDs 1332 and 1626).
MATERIALS AND METHODS
MLSCN TB Compound Selection and Cluster Analysis
A total of 215,110 compounds from the MLSCN SMR library was screened against M. tb in a single-dose assay at a concentration of 10 μM. Of these compounds, 5,839 displayed >80% inhibition of growth of the organism. As described more fully in the previous paper,7 growth inhibition was determined by measurement of Alamar blue fluorescence relative to untreated inoculated control wells. Cell viability was determined by luminescence using CellTiter-Glo reagent (Promega). Because of limitations imposed by MLSCN compound resupply rules, only a subset of these hits were able to advance to confirmatory dose-response (D-R) testing. Removal of analogs of known TB drugs, and of compounds with hydrazide, thiono, and other reactive or undesirable functional groups, yielded a set of 3,817 hit compounds; further removal of compounds possessing close similarity to the scaffolds identified as active in a previous HTS campaign7 left 3,753 compounds. From this smaller set, a structurally maximally diverse 2,500 compounds were selected on the basis of Tanimoto dissimilarity using a pairwise comparison algorithm in the Tripos SYBYL software package. These 2,500 compounds were evaluated in a D-R assay against M. tb and in a cell cytotoxicity assay using VERO cells as previously described.7 The D-R assay generated valid TB IC90 data for 2,273 unique compounds, of which 610 compounds possessed TB IC90 values of <10 μM. In order to identify potentially privileged scaffolds, a clustering analysis was performed on the set of 2,273 compounds using a hierarchical clustering method as implemented in Leadscope. The clustering analysis led to the identification of 22 major scaffolds with significant enrichment ratios for the actives as compared to their distribution in the overall library. Based on activity and selectivity considerations, several scaffolds of interest were identified and are discussed in the following section.
RESULTS AND DISCUSSION
Substituted Quinolones
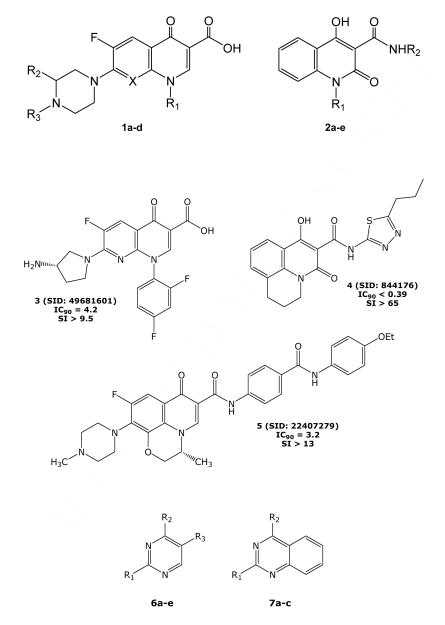
The screening library contained a small set of 6-fluoroquinolones and 6-fluoronaphthyridinones that are structurally related to established inhibitors of bacterial DNA gyrases and topoisomerase IV.9 It is well established that M. tb is very sensitive to the newer generation quinolones.10 Table 1 presents four of the representative actives (1a-d) from the screening set that are very similar to known bacterial inhibitors. These compounds have all the hallmarks of newer generation DNA gyrase inhibitors including the 3-COOH group, a 6-F substitution, as well as a basic piperazine moiety at the 7-position.9,10
Table 1.
| Cpd. | PubChem1 SID |
R1 | R2 | R3 | X | IC902 (μM) |
SI3 |
|---|---|---|---|---|---|---|---|
| 1a | 861394 | cyclopropyl | Me | H | C-methoxy | <0.19 | >204 |
| 1b | 855596 | cyclopropyl | H | ethyl | CH | <0.39 | >102 |
| 1c | 24837296 | cyclopropyl | H | acetyl | CH | 1.6 | >26 |
| 1d | 855614 | ethyl | H | H | CH | 3.1 | >13 |
| 2a | 26660376 | 1-heptyl | 2-pyrazinyl | --- | --- | <0.19 | >12 |
| 2b | 26660314 | 1-hexyl | 4- methylcarboxyethylthiazol- 2-yl |
--- | --- | <0.19 | >23 |
| 2c | 24784170 | 3-methyl-1- butyl |
4- methylcarboxyethylthiazol- 2-yl |
--- | --- | 0.22 | 38 |
| 2d | 852129 | ethyl | benzimidazol-2-yl | --- | --- | 0.42 | >95 |
| 2e | 24807142 | 1-butyl | 5-iso-propyl-1,3,4- thiadiazol-2-yl |
--- | --- | 0.84 | >48 |
PubChem Substance Identifier; see http://pubchem.ncbi.nlm.nih.gov
Antituberculosis activity, defined as the concentration of drug inhibiting 90% growth relative to untreated controls as measured fluorometrically
Selectivity index, defined as CC50/IC90
The table also shows a number of interesting quinolones that diverge from the typical structure associated with DNA gyrase inhibition (2a-e), and the basic scaffold shows high, selective activity that should be pursued. Structures 3-5 (see Figures) give other examples from the screen that are related to the compounds given in Table 1. In particular, 3 is an example of a newer generation naphthyridone DNA gyrase inhibitor (cf. gemifloxacin and trovaloxacin). Both 4 and 5 are alternative active amides related to inhibitors in Table 1, although it is not clear if these are prodrugs for a typical 3-COOH quinolone antibiotic or whether they are acting through an alternative mechanism. Quinolone carboxamides have been reported to show antibacterial activity, although the mechanism of action was not clarified.11
FIGURES.
Substituted Pyrimidines
The SMR screening set contained over 10,000 variously substituted simple and fused pyrimidines. Approximately 190 of these compounds showed good-to-modest activity in the bacterial growth assay, and only a small number of these samples were deemed both active and selective; for the most part, the active compounds also showed significant toxicity yielding a selectivity ratio (SI) < 10. Several of the active and selective analogs are depicted in structures 6 and 7 with specific substituents and activity presented in Table 2.
Table 2.
| Cpd. | PubChem SID |
R1 | R2 | R3 | IC90 (μM) |
SI |
|---|---|---|---|---|---|---|
| 6a | 860872 | 3,5- dimethylpyrazol- 1-yl |
N-(4- dimethylaminophenyl) amino |
COOEt | <0.19 | >120 |
| 6b | 26661470 | 3,5- dimethylpyrazol- 1-yl |
cyclopentylamino | COOEt | 0.86 | 18 |
| 6c | 861934 | 3,5- dimethylpyrazol- 1-yl |
phenethylamino | COOEt | 1.1 | >38 |
| 6d | 14741571 | 3,5- dimethylpyrazol- 1-yl |
N-(4-methoxyphenyl) amino |
COOEt | 1.3 | >32 |
| 6e | 861838 | 3,5- dimethylpyrazol- 1-yl |
(S)-(-)-α- methylbenzylamino |
COOEt | 2.1 | 17 |
| 7a | 864349 | 2-pyridyl | N-hexamethyleneimino | --- | 0.32 | 13 |
| 7b | 860397 | 2-pyridyl | N-4-phenylpiperazino | --- | 1.6 | 16 |
| 7c | 861193 | 2-pyridyl | (S)-(+)- tetrahydrofurfurylamino |
--- | 3.2 | >12 |
The small numbers of samples in these focused sets do not lend themselves to a thorough structure-activity relationship (SAR) discussion. There are, however, interesting trends in terms of activity/toxicity that are notable. For example, the combination of a 2-(3,5-dimethyl-pyrazol-1-yl) group with various 4-phenylamino and 4-cycloakylamino and 5-carboxyethyl substituents (6a-e) appears to show higher activity/selectivity ratios than closely related pyrimidines that are substituted with 2-(2-pyridyl), 4-phenylthio, and 5-methoxy groups (data not shown); the latter set being less active and more toxic, yielding selectivity ratios on the order of 1.0. Interestingly, quinazolines with a 2-pyridyl substitution (7a-c) as well as a 4-cycloalkyl or 4-arylmethyl group show good activity and selectivity ratios as compared to closely related 2-(2-pyridyl) pyrimidines that are mentioned above. This trend does not hold for other similar 2-substituted quinazolines (2-methyl, 2-cyclobutyl, or 2-(thiophen-2-yl)) that show relatively similar toxicity, but have relatively poor TB IC90 activity values.
Substituted pyrimidines12,13 and quinazolines have been reported to show a variety of antibacterial activities including antimycobacterial activity. Derivatives of 2-aryl-3-aminoquinazoline-4(3H)-ones have been reported that show good antibacterial and antitubercular activity.14-17 There are reports of antibacterial quinazolines that are similar to the hits found in our screen (see structures 8,18 9,19 and 1020).
There is no indication from the literature reports as to a potential target for these compounds, and the small number of actives in each set impacts the ability to define a structure –activity (SAR) profile. The good activity, however, of these drug-like small molecule scaffolds suggests that further work to identify a mode of action and prepare analogs to develop a clear SAR would be worthwhile.
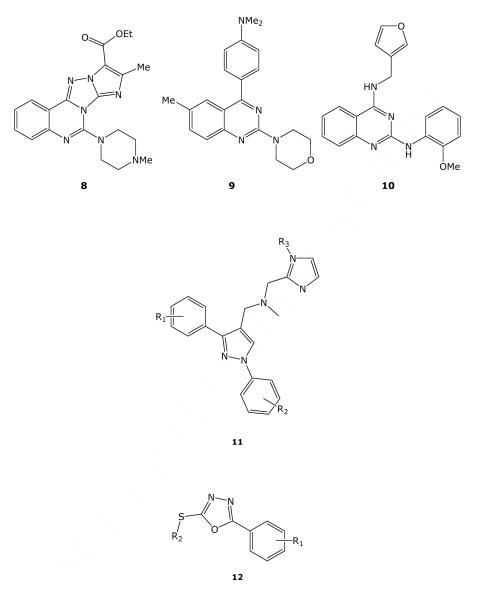
1,3-Diaryl-4-substituted Pyrazoles
Of the seven examples containing scaffold 11, three had TB IC90 ≤10 μM, while only one compound, 11a, the most active in this cluster, had an SI (defined as CC50/IC90, the ratio of the Vero cell cytotoxicity to the anti-TB activity) of >10 (Table 3). A search of the literature for this specific scaffold did not show any previous reports of TB activity. However, phenylpyrazoles have previously been evaluated for biological activity. For example, a phenylpyrazole has been evaluated as an inhibitor of indoleamine 2,3-dioxygenase, but found not to be active.21 Also, a series of 3-(4-phenyoxyphenyl) pyrazoles was studied as a novel class of sodium channel blocker in the rat Chung neuropathy paradigm.22
Table 3.
| Cpd. | PubChem SID |
R1 | R2 | R3 | IC90 (μM) |
SI |
|---|---|---|---|---|---|---|
| 11a | 24798067 | 3-methyl | 4-methyl | ethyl | 3.6 | >11 |
| 11b | 24784374 | 3-F | 3-methyl | ethyl | 4.2 | >9.6 |
| 11c | 24800363 | 4-F | 4-methyl | ethyl | 6.7 | >6.0 |
| 11d | 24783789 | H | 2, 5-dimethyl | ethyl | 10.3 | >3.9 |
| 11e | 24800267 | 3-F | 3-methyl | methyl | 12.1 | >3.3 |
| 11f | 24800597 | 3-methyl | 4-methyl | H | 13.5 | >3.0 |
| 11g | 24800814 | 3-methyl | 4-methyl | methyl | 15.9 | >2.5 |
1,3,4-Oxadiazoles
1,3,4-oxadiazoles are known from the literature to possess antimycobacterial activity,23-26 and two novel series emerged from this analysis. Of 25 2-substituted thio-5-aryl-1,3,4-oxadiazoles (12; Table 4), there were 18 active examples with TB IC90 ≤10 μM. D-R testing of these compounds showed that eight had at least modest selectivity with SI >10. The most active was compound 12a with SI of 38.
Table 4.
| Cpd. | PubChem SID |
R1 | R2 | IC90 (μM) |
SI |
|---|---|---|---|---|---|
| 12a | 843463 | 3,4-dimethoxy |  |
1.0 | >38 |
| 12b | 851931 | 3,4,5-trimethoxy |  |
1.8 | >23 |
| 12c | 7972059 | 3,4,5-trimethoxy | n-PrOC(O)CH2 | 1.8 | >22 |
| 12d | 24788973 | 4-ethoxy |  |
1.9 | >21 |
| 12e | 847334 | 2,4-dimethoxy | EtO2CCH2 | 2.4 | >1 |
| 12f | 4246679 | 3,4-dioxoethylene |  |
2.5 | >16 |
| 12g | 3714049 | 2-bromo |  |
3.4 | >12 |
| 12h | 24808448 | 4-nitro | EtOC(O)CH2 | 3.8 | >10 |
| 12i | 24805084 | 3,4,5-trimethoxy | EtOC(O)CH2 | 4.4 | >9 |
| 12j | 852638 | 2,4-dimethoxy | MeOC(O)CH2 | 4.5 | >9 |
| 12k | 851695 | 4-methyl benzenesulfonami do |
EtOC(O)CH2 | 5.3 | >8 |
| 12l | 24804266 | 3,4-dimethoxy | MeOC(O)CH2 | 5.5 | >7 |
| 12m | 848919 | benzenesulfonami do |
MeOC(O)CH2 | 5.7 | >7 |
| 12n | 24837552 | 4-methoxy |  |
6.2 | >7 |
| 12o | 4246792 | 3,5-dimethoxy |  |
7.0 | >6 |
| 12p | 4247581 | 3-methoxy |  |
8.5 | >5 |
| 12q | 851619 | 2,4-dichloro | i-PrOC(O)CH2 | 9.2 | >4 |
| 12r | 852877 | 2,4-dichloro | EtOC(O)CH2 | 9.4 | >4 |
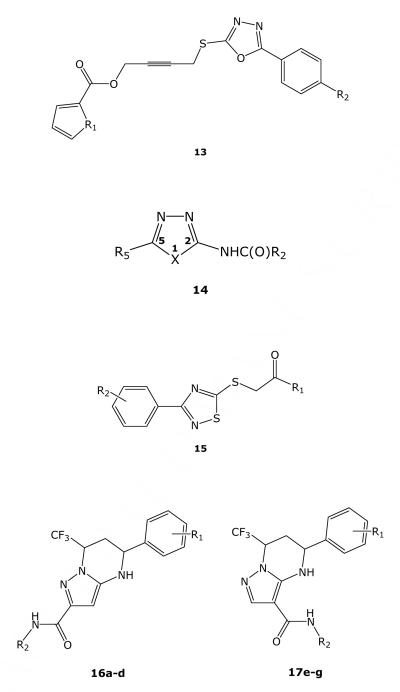
There were four additional 2-alkylthio-5-aryl-1,3,4-oxadiazoles containing the general structure depicted in 13, all of which had TB IC90 < 4 μM and SI > 10 (Table 5). The most active was compound 13a. Interestingly, the two most active examples also had the same thiophene-containing 2-alkythio side chain while the two least active representatives shared the analogous furan-containing 2-alkylthio side chain. Unfortunately, since each compound differed structurally in their respective 5-aryl substituent, it is not possible to definitively conclude whether their differing activities are due to the thiophene-furan modification. A search of the literature for this specific scaffold did not show any previous reports of TB activity.
Table 5.
| Cpd. | PubChem SID |
R1 | R2 | IC90 (μM) |
SI |
|---|---|---|---|---|---|
| 13a | 14735971 | S | NO2 | 1.3 | >31 |
| 13b | 14734336 | S | Br | 2.4 | >16 |
| 13c | 14723706 | O | methyl | 3.4 | >12 |
| 13d | 843601 | O | H | 3.9 | >10 |
2-Carboxamido-1,3,4-oxadiazoles and Related Compounds
The screening set contained 243 acylated 2-amino-1,3,4-oxadiazoles (14; Table 6, X = O), of which 44 showed enough activity in the single-dose primary assay to warrant further evaluation in the dose-response format. Of the latter, 20 were confirmed active with a TB IC90 ≤ 20 μM. Though some compounds possessed unacceptable toxicity, many displayed an SI >20. In these compounds the 5-position was invariably substituted, and good activity was observed with a diversity of alkyl, aryl, and heterocyclic groups (14a-e).
Table 6.
| Cpd. | PubChem SID |
R2 | R5 | IC90 (μM) |
SI |
|---|---|---|---|---|---|
| 14a | 14736804 | 2,4-dichlorophenyl | 1,2,3,4-tetrahydronaphth-6-yl | 1.0 | >41 |
| 14b | 3715915 | β-naphthyl | methyl | 1.5 | >27 |
| 14c | 24816541 | 4-methylphenyl | 4-pyridyl | 7.0 | >6 |
| 14d | 7971249 | 3-phenoxyphenyl | 2-thienyl | 2.7 | >13 |
| 14e | 17515772 | 4-chlorophenyl | 2,3-dihydro-1,4-dioxin-2-yl | 4.4 | >9 |
| 14f | 14743883 | quinolin-2-yl | 3-pentyl | 1.5 | 24 |
The closely related 2-amino-1,3,4-thiadiazoles (14, X = S; Table 6) were highly represented in the screening set, but were much less active in general. Of 2,277 such compounds, only 23 made the activity cutoff for dose-response screening, but of those, 14 were found active with an TB IC90 <= 20 μM. In general the thiadiazoles appeared to be more toxic than their oxygen counterparts, but some were marginally selective (for example, 14f). The range of 5-substituents was much smaller among the thiadiazole actives, generally restricted to small alkyl or alkylthio moieties.
It should be noted that similar compounds have previously been reported to possess antituberculosis and other antibacterial activities.27
1,2,4-Thiadiazoles
Of the six 3-aryl-5-thioacetamide-substituted 1,2,4-thiadiazoles that were tested in D-R (15; Table 7), five possessed TB IC90 <10 μM. Two compounds (15a and 15b) also displayed SI >10, and thus can be considered potential leads. A search of the literature for this specific scaffold did not identify previous reports of TB activity. However, similar compounds have been reported as bactericidal against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, using a paper-disk diffusion method.28
Table 7.
| Cpd. | PubChem SID |
R1 | R2 | IC90 (μM) |
SI |
|---|---|---|---|---|---|
| 15a | 4244687 |  |
3-methyl | 0.8 | >48 |
| 15b | 24816149 | NHCH2CO2Et | H | 3.2 | >13 |
| 15c | 24816198 |  |
3-methyl | 4.2 | >10 |
| 15d | 24816150 |  |
4-methoxy | 5.2 | >8 |
| 15e | 24816621 | NHCH2CO2Et | 3-methyl | 8.3 | >5 |
Tetrahydropyrazolo[1,5-a]pyrimidines
There were a total of 84 compounds containing 5-phenyl-4,5,6,7-tetrahydro[1,5-a]pyrimidine framework in the SMR collection. From this set, seven compounds listed in Table 8 were evaluated in the D-R assay. All of these compounds contained an aryl group at the 5-position and a trifluoromethyl group at the 7-position. In addition, a carboxamide function is present at the 2-position in four compounds (16a–d) and at the 3-position in three compounds (17e–g). Of these seven compounds, five compounds displayed TB IC90 values <10 μM. Interestingly, in a recent publication, compounds that are structurally related to 17e-g have been reported to display weak inhibitory activity against Staphylococcous aureus methionyl-tRNA synthetase.29
Table 8.
| Cpd. | PubChem SID |
R1 | R2 | IC90 (μM) |
SI |
|---|---|---|---|---|---|
| 16a | 846634 | 4-methyl | 2-furanylmethyl | 1.8 | >22 |
| 16b | 858272 | H | n-butyl | 4.1 | >10 |
| 16c | 4257312 | 3,4-methylenedioxy | 2-hydroxy-5-chlorophenyl | >100 | |
| 16d | 849999 | 3,4-dichlorophenyl | 2-furanylmethyl | >100 | |
| 17e | 7976388 | 4-bromo | 2-furanylmethyl | 1.7 | >23 |
| 17f | 844088 | 4-methyl | 2-furanylmethyl | 3.6 | >11 |
| 17g | 4260736 | 4-bromo | (1,5-dimethylpyrazol-4- yl)methyl |
6.5 | >6 |
Low Selectivity Scaffolds
Several classes of compounds were identified that possess good anti-TB activity, but had poor selectivity. In general for these scaffolds, not enough representatives were present in the screening set to fully elucidate the SAR. Consequently, it is possible that through further synthetic work the non-selective toxicities can be disentangled from the desired activity through iterative synthesis/testing, and so these classes should not be dismissed as potential leads. Some of the more interesting of these non-selective scaffolds are discussed below.
3-Phenylpyrazolo[1,5-a]pyrimidines
Among the compounds possessing a pyrazolo[1,5-a]pyrimidine template, a group of compounds possessing a phenyl substituent at the 3-position and an amine or hydroxyl substituent at the 7-position displayed moderate activity against M. tb. Most of these compounds also displayed significant cytotoxicity against VERO cells thus leading to poor SI values (<7.2). One compound in each series did display SI values >10 and both are depicted below (18,19).
2,5-Disubstituted Thiazolidin-4-ones
Three of four 4-thiazolidinones possessed TB IC90 of <10 μM (Table 9). Follow-up D-R testing showed that of these three, only 20a was modestly selective. The literature shows numerous examples of 4-thiazolidinones being studied as antitubercular agents. These include one report that some 2-imino-4-thiazolidinones showed antitubercular activity comparable to streptomycin or phthivazid.30 A few derivatives were reported to inhibit the growth of H37Rv at a concentration of 12.5 μg/mL,31 and related analogs were reported by others.32
Table 9.
| Cpd. | PubChem SID |
R1 | IC90 (μM) |
SI |
|---|---|---|---|---|
| 20a | 14744789 | 4-ethyl | 3.6 | >11.2 |
| 20b | 24832975 | 4-methoxy-3-hydroxy | 7.6 | 3.3 |
| 20c | 14744896 | 3-methyl | 11.6 | 2.5 |
| 20d | 14744668 | 2-methyl | >100 |
4(5)-Phenylacetylimidazole-5(4)-carboxamides
Two compounds from this series, 21a-b (Table 10), had TB IC90 <10 μM. Since neither had SIs >10 adequate selectivity, additional work is required before assessing the potential of this class of compounds.
Table 10.
| Cpd. | PubChem SID | R1 | R2 | IC90 (μM) |
SI |
|---|---|---|---|---|---|
| 21a | 26732680 | H |  |
5.6 | >7.1 |
| 21b | 24833778 | 4-methyl |  |
6.4 | >6.2 |
| 21c | 26732664 | 3-methyl |  |
>100 | |
| 21d | 26732673 | 3-methyl |  |
>100 |
Imidazo[1,2-a]pyridine-3-amines
The MLSCN library contained 27 imidazo[1,2-a]pyridines of generic structure 22a wherein R1 is a phenyl or pyridyl ring system. Of these 27 compounds, 12 compounds had the 2-pyridyl ring system (22b) as the aryl substituent. In the D-R assay, seven agents from this set displayed TB IC90 values in the range of 1.5–4.4 μM. All of these compounds, however, also displayed significant cytotoxic effects against VERO cells, resulting in poor SI values between 0.4–2.5.
5-Nitrofuran-2-carboxamides
Amides derived from 5-nitrofuran-2-carboxylic acids have emerged as a class of compounds that display potent antitubercular activity.7,33 In conformity with this, a group of such compounds that were present in the MLSCN screening deck were found active. A total of 14 compounds were tested in the D-R assay, resulting in eight compounds possessing TB IC90 <10 μM (23; Table 11).
Table 11.
| Cpd. | PubChem SID |
R1 | IC90 (μM) |
SI |
|---|---|---|---|---|
| 23a | 24808856 | 4-[(4-butanoyl)piperazinyl]-3-chlorophenyl | <0.19 | >9 |
| 23b | 24796288 | 4-[(4-benzoyl)piperazinyl]phenyl | <0.19 | >8 |
| 23c | 24793086 | 4-[4-(4-chlorobenzoyl)piperazinyl]phenyl | <0.19 | >2 |
| 23d | 24809040 | 4-[(4-butanoyl)piperazinyl]phenyl | 2.2 | 1.4 |
| 23e | 17403602 | 4-methylbenzyl | 2.9 | 2.9 |
| 23f | 24808888 | 3-methylphenyl | 6.2 | 0.6 |
| 23g | 24808199 | 5-chloro-2pyridyl | 7.2 | 1.2 |
| 23h | 24789398 | 3,4-dimethylphenyl | 8.6 | >4.6 |
Amides of 3-(Trifluoromethyl)-4-(piperazinylmethyl)aniline
A set of 22 amides derived from 3-(trifluoromethyl)-4-(piperazinylmethyl)aniline by acylation with 3-(acylamino)benzoic acids possessing the generic structure 24 emerged from the assay. Five of these 22 compounds displayed TB IC90 values between 2.8–6.7 μM. These compounds, however, displayed significant cytotoxicities against VERO cells, with SI values in the range of 0.8–4.6.
Individual Compounds
A large number of individual compounds or groups of two or three similar compounds within this library had significant potency and excellent selectivity on the basis of moderate cytoxicity. Some of these compounds are of structural types that are covered by our initial publication.7 Table 12 below provides the structures and activities of several of the most interesting of these compounds.
Table 12.
| Select Individual Compounds [IC90 (μM), SI] |
|---|
 |
 |
 |
 |
 |
ACKNOWLEDGEMENTS
The screening of this assay was supported by National Institutes of Health grant 1U54-HG-003917, Southern Research Molecular Libraries Screening Center. The data analysis was supported by the NIH TAACF contract, N01-AI-95364. The complete data sets for the HTS campaign have been deposited in PubChem (http://pubchem.ncbi.nlm.nih.gov/; assay AIDs 1332 and 1626). The authors thank Dr. Scott Franzblau who provided his protocol for the Alamar blue M. tuberculosis assay at the beginning of the TAACF program, and which was adapted for the MLSCN assay. We also thank Dr. William J. Suling for his assistance and insight during the analysis, and Ms. Sara McKellip for her assistance with the assay.
Abbreviations used in the text
- NIAID
National Institutes of Allergy and Infectious Disease
- MLSCN
Molecular Libraries Screening Center Network
- TAACF
Tuberculosis Antimicrobial Acquisition and Coordinating Facility
- TB
tuberculosis
- XDR
extensively drug-resistant
- WHO
World Health Organization
- HTS
high-throughput screening
- NIH
National Institutes of Health
- SMR
Small Molecule Repository
- AID
Assay Identifier
- D-R
dose-response
- SI
selectivity index
- SAR
structure-activity relationships
Footnotes
CONFLICT OF INTEREST STATEMENT Competing interests: Dr. Goldman is a NIAID staff member who either in the past or currently provides oversight for the project that generated the data used as the basis for this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.WHO: World Health Organization Global Tuberculosis Control 2008. Surveillance, Planning, Financing. 2008
- 2.Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83:44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Mondal R, Jain A. Extensively drug-resistant Mycobacterium tuberculosis, India. Emerg Infect Dis. 2007;13:1429–31. doi: 10.3201/eid1309.070443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen T, Murray M. Modeling epidemics of multidrug-resistant M. tuberculosis of heterogeneous fitness. Nat Med. 2004;10:1117–21. doi: 10.1038/nm1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. http://www.who.int/entity/tb/challenges/xdr/xdr_map_june08.pdf.
- 6.Goldman RC, Laughon BE, Reynolds RC, Secrist JA, III, Maddry JA, Guie M-A, Poffenberger AC, Kwong CA, Ananthan S. Programs to facilitate tuberculosis drug discovery: The Tuberculosis Antimicrobial Acquisition and Coordinating Facility. Infect Disord – Drug Targets. 2007;7:92–104. doi: 10.2174/187152607781001790. [DOI] [PubMed] [Google Scholar]
- 7.Ananthan S, Faaleolea ER, Goldman RC, Hobrath JV, Kwong CD, Laughon BE, Maddry JA, Mehta A, Rasmussen L, Reynolds RC, Secrist JA, III, Shindo N, Showe DN, Sosa MI, Suling WJ, White EL. High-throughput screening for inhibitors of Mycobacterium tuberculosis H37Rv. Tuberculosis. 2009 doi: 10.1016/j.tube.2009.05.008. In press. doi:10.1016/j.tube.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. http://pubchem.ncbi.nlm.nih.gov/
- 9.Takahashi H, Hayakawa I, Akimoto T. The history of the development and changes of quinolone antibacterial agents. Yakushigaku Zasshi. 2003;38:161–79. [PubMed] [Google Scholar]
- 10.Mduli K, Ma Z. Mycobacterium Tuberculosis DNA gyrase as a target for drug discovery. Infect Disord – Drug Targets. 2007;7:159–68. doi: 10.2174/187152607781001763. [DOI] [PubMed] [Google Scholar]
- 11.Reddy GV, Kanth SR, Maitraie D, Narsaiah B, Rao PS, Kishore KH, Murthy USN, Ravi B, Kumar AB, Parthasarathy T. Design, synthesis, structure-activity relationship and antibacterial activity series of novel imidazo fused quinolone carboxamides. Eur J Med Chem. 2009;44:1570–8. doi: 10.1016/j.ejmech.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Morgan J, Haritakul R, Keller PA. Anilinopyrimidines as novel antituberculosis agents. Bioorg Med Chem Lett. 2003;13:1755–7. doi: 10.1016/s0960-894x(03)00241-5. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Srivastava K, Puri SK, Sinha S, Chauhan PM. A small library of trisubstituted pyrimidines as antimalarial and antitubercular agents. Bioorg Med Chem Lett. 2005;15:5218–21. doi: 10.1016/j.bmcl.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis, antitubercular and anticancer activities of substituted furyl-quinazolin-3(4H)-ones. Arch Pharm. 2007;340:635–41. doi: 10.1002/ardp.200700096. [DOI] [PubMed] [Google Scholar]
- 15.Nanda AK, Ganguli S, Chakraborty R. Antibacterial activity of some 3-(arylideneamino)-2-phenylquinazoline-4(3H)-ones: Synthesis and preliminary QSAR studies. Molecules. 2007;12:2413–26. doi: 10.3390/12102413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis and antimicrobial activities of some novel substituted 2-imidazolyl-N-(4-oxoquinazolin-3(4H)-yl)-acetamides. Chem Pharm Bull. 2007;55:1615–19. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 17.Alagarsamy V, Rajasolomon V, Meena R, Ramseshu KV. Synthesis, analgesic, anti-inflammatory and antibacterial activities of some novel 2-butyl-3-substituted quinazolin-4-(3H)-ones. Biol Pharm Bull. 2005;28:1091–4. doi: 10.1248/bpb.28.1091. [DOI] [PubMed] [Google Scholar]
- 18.Nasr MN, Gineinah MM, El-Bendary ER. Synthesis and in vitro antibacterial evaluation of novel imidazo[2′,1′:5,1]-1,2,4,-triazolo[4,3-c]-quinazoline derivatives of 5-thioxo-1,2,4-triazole, 4-oxothiazolidine, and their open-chain counterparts. Arch Pharm. 2003;336:560–6. doi: 10.1002/ardp.200300809. [DOI] [PubMed] [Google Scholar]
- 19.Bedi PMS, Kumar V, Mahajan MP. Synthesis and biological activity of novel antibacterial quinazolines. Bioorg Med Chem Lett. 2004;14:5211–3. doi: 10.1016/j.bmcl.2004.07.065. [DOI] [PubMed] [Google Scholar]
- 20.De La Fuente R, Sonawane ND, Arumainayagam D, Verkman AS. Small molecules with antimicrobial activity against E. coli and P. aeruginosa identified by high-throughput screening. Br J Pharmacol. 2006;149:551–9. doi: 10.1038/sj.bjp.0706873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar S, Jaller D, Patel B, LaLonde JM, DuHadaway JB, Malachowski WP, Prendergast GC, Muller AJ. Structure based development of phenylimidazole-derived inhibitors of indoleamine 2,3-dioxygenase. J Med Chem. 2008;51:4968–77. doi: 10.1021/jm800512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Gharagozloo P, Yao J, Ilyin VI, Carter RB, Nguyen P, Robledo S, Woodward RM, HogenKamp DJ. 3-(4-Phenoxyphenyl)pyrazoles: A novel class of sodium channel blockers. J Med Chem. 2004;47:1546–52. doi: 10.1021/jm030498q. [DOI] [PubMed] [Google Scholar]
- 23.Mamolo MG, Zampieri D, Vio L, Fermeglia M, Ferrone M, Pricl S, Scialino G, Banfi E. Antimycobacterial activity of new 3-substituted 5-(pyridin-4-yl)-3H-1,3,4-oxadiazol-2-one and 2-thione derivatives. Preliminary molecular modeling investigations. Bioorg Med Chem. 2005;13:3797–809. doi: 10.1016/j.bmc.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Foks H, Ancechowska-Ksepko D, Janowiec M, Zwolska Z, Augustynowicz-Kopec E. Synthesis and tuberculostatic activity of some (4-phenylpiperazin-1-ylmethyl)-1,3,4-oxadiazole and (4-phenylpiperazin-1-ylmethyl)-1,2,4-triazole derivatives. Acta Pol Pharm. 2004;61:473–6. [PubMed] [Google Scholar]
- 25.Pancechowsak-Ksepko D, Foks H, Janowiec M, Zwolska-Kwiek Z. Synthesis and tuberculostatic activity of reaction products 5-(2-,3- and 4-pyridil)-1,3,4-oxadiazol-2-thione with amines. Acta Pol Pharm. 1993;50:259–67. [PubMed] [Google Scholar]
- 26.Yar MS, Siddiqui AA, Ali MA. Synthesis and anti tuberculostatic activity of novel 1,3,4-oxadiazole derivatives. J Chin Chem Soc. 2007;54:5–8. [Google Scholar]
- 27.Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole, and pyrrole ring systems: A novel class of potential antibacterial and antitubercular agents. Eur J Med Chem. 2008;43:1989–96. doi: 10.1016/j.ejmech.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Yousif NM, Mandour AH, Omar MT. Synthesis and reactions of some 1,2,4-thiadiazole derivatives for biological evaluation. Egypt Chem. 1991;32:607–14. (actual volume date 1989) [Google Scholar]
- 29.Finn J, Stidham M, Hilgers M, Kedar GC. Indentification of novel inhibitors of methionyl-tRNA synthetase (MetRS) by virtual screening. Bioorg Med Chem Lett. 2008;18:3932–7. doi: 10.1016/j.bmcl.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 30.Litvinchuk MD. Pharmacological assays of new tuberculostatic drugs. Farmakoli Toxicol. 1963;26:725–9. [PubMed] [Google Scholar]
- 31.Fujikawa F, Hirai K, Hirayama T, Yoshikawa T, Nakagawa T, Naito M, Tsukuma S, Kamada M, Ohta Y. Chemotherapeutics for Mycobacterium tuberculosis. XXII.: Synthesis and antibacterial activity on Mycobacterium tuberculosis of N2-(2-pyridyl)-3-(p-ethoxyphenyl)pseudothiohydantoin and N2-(2-benzothiazolyl)-3-allylpseudothiohydantoin derivatives. Yakugaku Zasshi. 1969;89:1099–103. doi: 10.1248/yakushi1947.89.8_1099. [DOI] [PubMed] [Google Scholar]
- 32.a) Zubenko VG, Ladnaya LY, Turkevich NM, Tatchin-kapustyak SM. Synthesis and tuberculostatic activity of azolidines. Farm Zh. 1974;29:78–82. [PubMed] [Google Scholar]; b) Danila G, Radu C. Derivatives of 2-thioxo-3-isonicotinylaminothiazolid-4-one with tuberculostatic activity. Rev Med-Chir. 1978;82:127–30. [PubMed] [Google Scholar]
- 33.Tangallapally RP, Yendapally R, Daniels AJ, Lee RE. Nitrofurans as novel anti-tuberculosis agents: Identification, development and evaluation. Curr Topics Med Chem. 2007;7:509–26. doi: 10.2174/156802607780059772. [DOI] [PubMed] [Google Scholar]