Abstract
Since the discovery and synthesis of a novel DNA mimic, peptide nucleic acid (PNA) in 1991, PNAs have attracted tremendous interest and have shown great promise as potential antisense drugs. They have been used extensively as tools for specific modulation of genes expression by targeting translation or transcription processes. This review discusses the present and future therapeutic potential of this class of compound as anti-HIV-1 drugs.
1. INTRODUCTION
In AIDS patients, genetic variation of the HIV-1 genome poses a major challenge to treatment regimens. The most commonly used anti-HIV-1 drugs target two key enzymes, protease and reverse transcriptase. The highly active anti-retroviral therapy (HAART), which also targets these key enzymes, has dramatically decreased AIDS-related mortality, lengthened the life expectancy, and improved the quality of life for infected individuals. Although these drugs have had great success in reducing the viral load in AIDS patients, the emergence of strains that are resistant to these drugs has made management of this disease particularly difficult.
Drug-resistant strains are usually recognized when the viral load does not fall even after the administration of combination therapies such as HAART. Furthermore, these therapies do not act on reservoirs of HIV in latently infected cells and therefore keep patients vulnerable to fresh eruptions of viral titer whenever the drug regimen is discontinued[1].
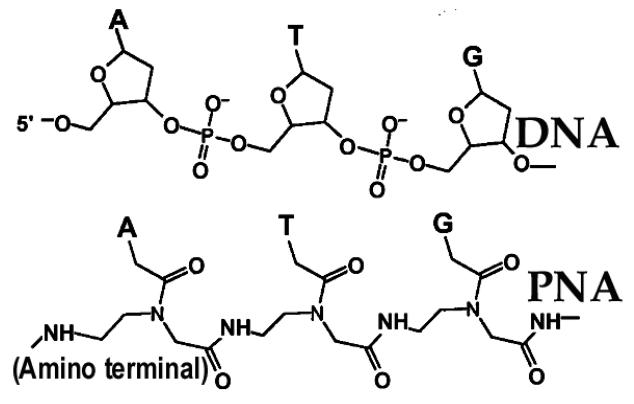
The major effort to establish a stable drug line to combat HIV-1 has focused on the accessory and regulatory proteins of HIV-1 as new drug targets. However, these targets have not yet been sufficiently explored; their functions in the cell and their modes of action in virus production and maturation are not well understood at the molecular level. Another major strategy, which has been worked on extensively, is aimed at inhibiting HIV-1 replication by targeting regulatory conserved and immutable sequences on the viral genome using antisense technology. These regulatory regions on the viral genome, which are essential for viral replication and gene expression, could be, individually or collectively, potential targets for the next generation of drug design and development. In this context, peptide nucleic acids (PNAs) have recently been explored as potential antisense agents for targeting these regulatory regions on the HIV-1 genome. PNAs are DNA mimics in which sugar phosphate backbone (Fig. 1) is replaced with N-(2- amino ethyl) glycine (aeg) units [2]. This modification makes them highly stable in the cellular compartment. In addition, PNAs show stronger binding affinity to their complementary RNA or DNA than do the respective RNA/DNA or DNA/DNA hybridizations [3].
Fig.1.
Comparison of Chemical Structure for PNA and DNA: In the structure of PNA, bases (A, T, G, C) are linked with polyamide backbone as against sugar phosphate backbone in DNA.
2. The HIV-1 LTR and U5-PBS regions as attractive therapeutic targets of PNA
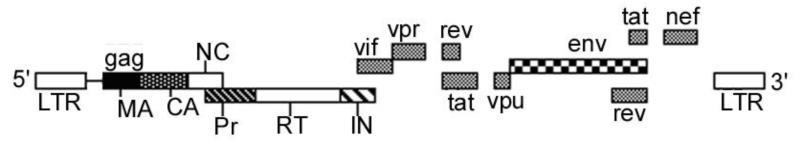
The first 1-333 nucleotides of HIV-1 genomic RNA comprise the nontranslated region, which contains several domains that are essential for viral replication (Fig. 2). The long terminal repeat (LTR) spans nucleotides 1-182 and consists of a unique 3′ region (U3), a repeat region (R), and a unique 5′ region (U5). This U3-R-U5 segment is located at both the ends of the viral genome. An 18-nucleotide-long domain that is required for the initiation of reverse transcription is located downstream of the U5 region of the 5′LTR; it spans nucleotides 183-201. This domain, the primer binding site (PBS), is complementary to the 3′ terminal 18 nucleotides of tRNA3Lys, which are involved in the initiation of reverse transcription [4, 5]. Another important domain downstream of the PBS region is the dimerization initiation site, which spans nucleotides 235-281.This domain has been shown to have a dual role in genomic RNA packaging and in the second-strand transfer reaction during reverse transcription [6].
Fig.2.
Organization of HIV-1 genome
The 5′LTR of the proviral genome contains multiple cis-acting DNA target sequences for cellular transcription factors [7-9]. Also present in the LTR are various DNA elements, including the binding sites for nuclear factor NF-kB, NF-AT, and the stimulatory factor USF-1, which are required for stimulation of transcription in activated lymphocytes [10-12]. These cis-acting sequences can thus be successfully targeted to arrest viral multiplication. Other promising targets for drug intervention are the RNA binding regions for Rev and Tat regulatory proteins. The HIV-1 LTR contains a unique region, the TAR element, which spans nucleotides 1-60. The HIV protein Tat, which binds to the TAR region, is a potent transactivator of transcription that is required for virus infectivity [8, 9, 13-17]. The 3′ LTR, which functions as the transcriptional terminator, contains signals for polyadenylation of viral mRNA [18-20]. The DNA sequences, known as att sites, are important for the integration of proviral DNA into the host genome; they lie at the ends of the linear proviral DNA [21, 22]. Mutation at the att site makes virus replication defective [23, 24]. The att sites are located in the U3 and U5 region of LTR. Although the DNA sequences of the att site vary among different retroviruses, all of them have a CA dinucleotide located 2 bases from the 3′ end of both U3 and U5. Substitution of the conserved CA dinucleotide abolishes the integration reaction [25-27]. Another conserved sequence in the gag coding region at the site of initiation codon is an excellent target to block the translation of gag mRNA. This site has been effectively targeted by phosphorothioate oligonucleotides (GEM-91, GEM-92) complementary to this region [28-30]. These important sites on the viral genome are expected to be extremely stable since viruses with mutational changes in these regions are at risk of being eliminated from the viral population. PNAs targeting these sites on the viral genome could be excellent candidates for drug intervention.
3. PNA as a potential anti-HIV agent
The success of the anti-HIV activity of PNA depends on two major factors. The first factor is selection of a conserved region on the viral genome; the second is that the target region should be crucial to the HIV life cycle. Various studies have targeted the HIV-1 TAR region, a relatively conserved 59-mer (+1 to +59 nt) regulatory or enhancer sequence at the 5′ terminal of the HIV-1 genome. The viral protein Tat binds to this region and facilitates the recruitment of cellular factors cyclin T1 and CDK9, which phosphorylate the C-terminal domain of cellular RNA Pol II and facilitates its processivity - so that the complete viral genome is transcribed [31]. Inhibition of this transactivation process results in decreased viral production due to premature termination of transcription process. Therefore, HIV-1 TAR RNA is a potential antisense drug target. The use of PNA against the TAR region has shown encouraging results [32-38].
Another relatively conserved and crucial region in the HIV genome is the U5 primer binding site (PBS), an 18-mer (+183 to +201 nt) sequence where the tRNAlys anneals and primes the synthesis of negative-strand strong-stop DNA. A 19-mer PNA targeted against the HIV-1 PBS blocked in vitro reverse transcription by binding to a preprimed RNA primer duplex [39]. An 18-mer PNA targeted to the PBS region blocked the initiation of reverse transcription by displacing the tRNALys3 primer from the viral genome [40]. Interruption of this interaction blocks viral replication [41]. Similarly, PNA targeted against the polypurine tract (PPT) region of the viral genome could inhibit initiation of positive-strand strong-stop DNA synthesis [42]. Another conserved sequence near the 3′ end of the HIV-1 gag-pol transframe domain, when challenged by PNA, inhibited the production of virus up to 98.4% in chronically infected H-9 cells [43]. Another nonconserved region of the viral genome that has been targeted by PNA is the HIV-1 gag gene, which, upon binding to its target, completely blocked in-vitro transcription [44].
It is obvious from these studies that significant inhibition of viral replication was achieved by the antisense PNA targeted against different conserved regions of the viral genome, although none of them, individually, could inhibit 100% of viral replication. However, the PNA-based approach is still promising in that a cocktail of multiple PNAs can be used to target different conserved regions of the viral genome simultaneously, building up an additive antiviral effect. It is pertinent that PNA does not bind only to cognate mRNA, as do other antisense molecules or siRNAs, but can complex with the DNA duplex even in nondividing cells or the reservoir of cells with latent infection and exert an antigene effect [45]. Therefore, PNA can serve as an antisense as well as antigene agent against HIV-1.
4. Biodelivery of anti-HIV PNA
Since PNA/RNA hybrids are not substrates for RNase H [39, 46], the antisense effect of PNA occurs mainly through blocking the function of its target by irreversible binding to the targeted sequences. Therefore, proficient biodelivery of PNA at a stoichiometric concentration to its target in the cell is of the utmost importance for its strong antisense effects. Different strategies for biodelivery have used naked or modified forms of PNA in cell culture systems or in vivo [47, 48]. The major advance in biodelivery of PNA as therapeutic antisense was made by conjugating cell-transducing agents, including cell penetrating peptide (CPP). A number of reviews on CPP and their therapeutic application have recently been published [49-52]. A triphenyl-phosphonium-tagged PNA targeted to HIV-1 TAR was found to be efficiently taken up by cells and to block HIV-1 replication in cell culture [53]. In one unique study, PNA complementary to the HIV-1 TAR region was conjugated to neamine, a polycationic aminoglycoside. This conjugate was efficiently taken up by the cells and inhibited HIV-1 replication [36]. The PNA-Neamine conjugate targeted to HIV-1 TAR region displayed an endonuclease property that cleaved the targeted RNA sequence at the binding site [36, 54].
The most efficient cellular uptake of anti-HIV-1 PNA was noted with the use of cell-penetrating peptides, including penetratin, transportan, and HIV-1 Tat-derived peptide as vehicle to deliver anti-HIV-1 PNA into cells. Uptake of CPP carrying bulky cargos has been shown via endosomal pathway specially with cationic CPP such as Tat and oligo-Arg [48, 52, 55], similar pathway has not been clearly established for less cationic CPP cargos such as PNA-penetratin conjugates. A number of devices for endosomal escape have been suggested including the use of lysosomotropic agents, membrane disruptive polymers and peptides [55]. When penetratin, a 16 residue peptide derived from the Drosophila homeodomain transcription factor antennapedia, is conjugated to PNA, its biological uptake is tremendously enhanced over that of naked PNA [35, 38, 56]. Another CPPs, a chimeric transportan and 13-mer HIV-1 Tat peptide, has also been used successfully to improve the cellular uptake of anti-HIV-1 PNA [38, 41, 57, 58]. Transportan is a 27-residue chimeric cell-permeating peptide containing 13 amino acids from the amino terminus of the neuropeptide galanin and 14 amino acids from the amino terminus of the wasp venom toxin mastoparan [59]. When transportan was conjugated to a PNA analog targeted against the HIV-1 TAR region, the conjugate was rapidly internalized by the CEM cells, as well as chronically HIV-1 infected H-9 cells, and substantially inhibited HIV production [35, 38, 57]. This conjugate was distributed throughout the cell as observed by microscopy using a fluorescent TAMRA-tagged PNA [57]. This study was extended to evaluate the optimal length of the transportan residue essential for PNA uptake and its effective intracellular antisense activity. It was established that the full-length (27-mer) residue of transportan-PNA conjugate manifested maximum inhibition of viral replication in a cell culture system, although the uptake behavior of 27,22 and 21-mer Transportan-PNA conjugate was very close to that level [38]. The antiviral activity of anti-TAR PNA conjugated to different peptides had respective IC50 values of 400nM, 800nM, and 720nM for PNA transportan, PNA penetratin, and PNA tat in HIV-producing CEM cells. This clearly shows that PNA-Transportan conjugate had a better antiviral property in the cell culture system than did the other PNA conjugates. The exact reason for this difference in antiviral property of PNA conjugated to three different peptides is not well understood, but their uptake properties may be the major reason for this difference. Although the mechanisms of cellular uptake of PNA mediated through these peptide conjugates is not well explained, they are projected to adopt a cell-receptor-free mode of uptake [35, 60, 61].
4.1 Anti-HIV-1 PNAs conjugated to cell-penetrating peptides are potent antiviral and virucidal agents against HIV-1
An anti HIV-1 TAR PNA (PNATAR) conjugated with transportan peptide was shown to be rapidly taken up by cells, block Tat-mediated transactivation of HIV-1 transcription, and strongly inhibit HIV-1 production in chronically HIV-1-infected H9 cells [57]. It also has been found that the PNATAR-Transportan conjugate has potent virucidal activity. This conjugate rendered HIV-1 virions noninfectious on brief exposure, suggesting that it may have penetrated the host-derived viral membrane [35]. The anti-HIV-1 virucidal activity of this conjugate may be useful either in topical formulations to block HIV-1 infection or as a prophylactic agent for inactivation of HIV-1 in circulating plasma before its attachment and entry. Similar results were reported with anti-HIV-1 PNA conjugated with penetratin and tat-peptide [38].
The conjugates with penetratin, transportan and Tat peptides were shown to be the most effective as anti-HIV virucidal agents, with IC50 values in the range of 28 nM-37 nM [38]. The IC50 for inhibition of HIV-1 replication was lowest with PNATAR-Transportan-27 (0.4 µM), followed by PNATAR-Tat (0.72 μM) and PNATAR-Penetratin (0.8 μM). These results indicate that anti-HIV-1 PNAs conjugated with CPP not only inhibit HIV-1 replication in vitro, but also are potent virucidal agents.
4.2 Dual function of anti-TAR PNA-CPP conjugates
Besides blocking Tat-TAR interaction and down-regulation of HIV-1 gene expression [32, 34, 57], anti-TAR PNA aborts the reverse transcription process catalyzed by viral reverse transcriptase [38]. HIV-1 RT is unable to displace the upstream PNATAR bound to the loop region of TAR RNA. Thus, the anti-TAR PNAs serve two purposes: preventing Tat- mediated transactivation of HIV-1 LTR, resulting in down-regulation of viral RNA production and gene expression, and interrupting reverse transcription by blocking the strand displacement reaction. Earlier, it was shown that the RNA strand in the PNA: RNA duplex is resistant to RNase H-mediated cleavage [39, 46]. The loop region of TAR bound with PNATAR is also resistant to such cleavage. The inhibition of RNase-H by the anti-HIV-1 PNAs and the strand displacement activities of HIV-1 RT should enhance the potential inhibitory effect of these molecules on RT and thereby halt viral replication. In fact, oligomeric PNAs targeted to multiple sites can be expected to have additive effects. In theory, this approach is an appealing way to adapt these molecules, not only as specific HIV inhibitors, but also as general anti-retroviral agents.
4.3 HIV-1 virions pretreated with PNATAR-CPP are blocked from carry out endogenous reverse transcription
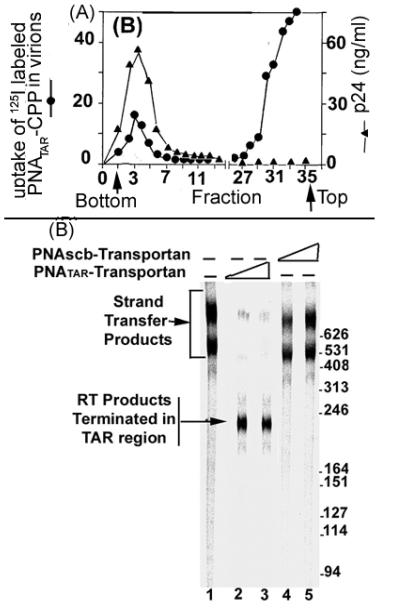
It has been reported that PNATAR-CPP efficiently internalizes in the HIV-1 virion particle, binding to its target on the viral genome and blocking the synthesis of full-length (−) strand strong-stop DNA. When purified HIV-1 virions were incubated in the presence of 125I-labeled conjugate and ultracentrifuged in discontinuous sucrose density gradient (10%-40%), a portion of the radioactivity sedimented at the bottom, corresponding to the sedimentation position of HIV-1 virions (Fig 3A;[35]. This indicates that the PNA-CPP conjugates are able to penetrate the membrane barrier of the virions. Moreover, the presence of 10% fetal calf serum in the incubation medium had no influence on internalization of the labeled conjugate in virions, indicating the absence of its nonspecific binding to serum proteins. When virions pretreated with PNATAR-CPP conjugate and separated from the conjugate on a 20% sucrose cushion were examined for endogenous reverse transcription activity, it was found that the majority of the RT products were blocked at the site of PNATAR-CPP binding in the TAR region (Fig. 3B, lanes 2 and 3). In controls without the conjugate or with scrambled PNA-Transportan conjugate, products larger than 400 bases were seen (lanes 1, 4, and 5). The predicted size of the tRNA3Lys derived (−) ssDNA is 253 nucleotides. The two major bands migrating above the 400-nucleotide position represent products of strand transfer reaction.
Fig 3.
(A) Internalization of PNATAR-CPP in HIV-1 virions. The HIV-1 virions incubated with I125-labeled PNATAR-CPP conjugate was subjected to discontinuous sucrose density gradient ultracentrifugation. Fractions collected from the bottom and analyzed for p24 antigen and for radioactivity [35].
(B) Abortive endogenous reverse transcription in HIV-1 virions pretreated with PNATAR-CPP conjugate [35]. Lane1, untreated HIV-1 virions; lanes 2 and 3, pre-treated with 1 μM and 2.5 μM of PNATAR Transportan conjugate, respectively; lanes 4 and 5, pre-treated with scrambled PNA-Transportan conjugate at 2 μM and 5 μM concentrations, respectively.
4.4 PNA-CPP conjugates targeting the primer binding site (PBS) and upstream A-loop region inhibit initiation of endogenous reverse transcription
Among retroviruses, the synthesis of proviral DNA occurs by multi-step reverse transcription. HIV-1 exclusively uses tRNA3Lys as the initiating primer; it anneals among the naturally occurring cellular tRNAs at the PBS and the upstream A-loop region in the 5′-UTR [62]. Both PNAPBS and PNAA-loop efficiently displace the tRNA3Lys-U5-PBS complex and inhibit the initiation of reverse transcription [40, 63]. Since HIV-1 virions pretreated with CPP conjugates of PNAPBS and PNAA-loop were rendered noninfectious, it was postulated that these conjugates may be readily internalized into the virion particles and bind to their target sequences on the viral genome. This hypothesis was tested by pretreating virion particles with varying concentrations of individual conjugates and then ultracentrifuging them through a 20% sucrose cushion.
When virion pellets were disrupted and assayed for endogenous reverse transcription activity, it was noted that initiation of reverse transcription was completely blocked in virion particles pretreated with both PNAPBS-CPP and PNAA-loop-CPP conjugates [41]. The endogenous reverse transcription products from untreated virions or virions treated with scrambled PNA-CPP conjugate were larger than 490 nucleotides, indicating that strand transfer had taken place after synthesis of (−) strand strong-stop DNA. This suggested that the conjugates had permeated the virus envelope and bound to their cognate sequences on the viral RNA, thus preventing the initiation of reverse transcription. When HIV-1 virions were incubated with I125-labeled PNATAR-CPP conjugate, the radioactive PNA-CPP conjugate co-sedimented with the virion particles [35], indicating that conjugates are internalized in the virion particles and render them noninfectious. As expected, both conjugates displayed potent virucidal activity against HIV-1 with IC50 values of ~50 nM. The virucidal property of these conjugates suggests that a cocktail of anti-HIV-1 PNA-CPP conjugates targeting critical regions of the HIV-1 genome could serve as a prophylactic agent for inactivating HIV-1 virions after exposure to HIV-1 [41].
5. Neamine as an effective and stable vehicle to biodeliver Anti-HIV-1 PNAs
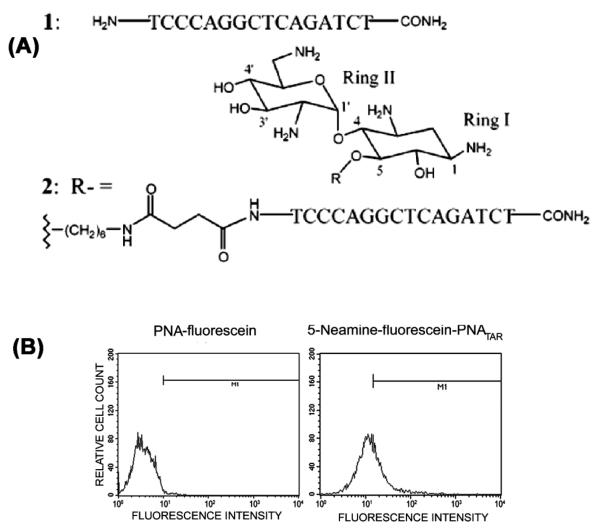
Neamine is a constituent of aminoglycoside antibiotics. These hydrophilic pseudo-oligosaccharides have several amino functions that are mostly protonated under physiological conditions. Neomycin B, an example of this class of antibiotics, contains a polycationic neamine moiety that not only binds the rev response element and TAR regions in HIV-1, but also blocks interaction of these regions with their respective transacting HIV-1 proteins, Rev and Tat [64, 65];. The conjugation of anti-HIV-1 TAR PNA with neamine monomer at the 5-position of the neamine ring 1 enhanced its cellular uptake [36] (Fig. 4) and antiviral efficacy when supplemented in HIV-1 infected cell culture. It was also demonstrated that neamine-PNA conjugates are not toxic to cells and that the antiviral effect was due to inhibition of TAR-Tat interaction.
Fig. 4.
(A). Structure of the anti-TAR PNA- neamine conjugate and the aminoglycosides neamine.(B) Cellular uptake of PNA-Neamine conjugate. Lymphocytes incubated with 2 μM conjugate at 37°C in complete RPMI medium and uptake of the conjugate in the cells was examined by FACscan [33, 47].
5.1 Anti-HIV-1 PNA-neamine conjugates display sequence-specific RNA cleavage activity
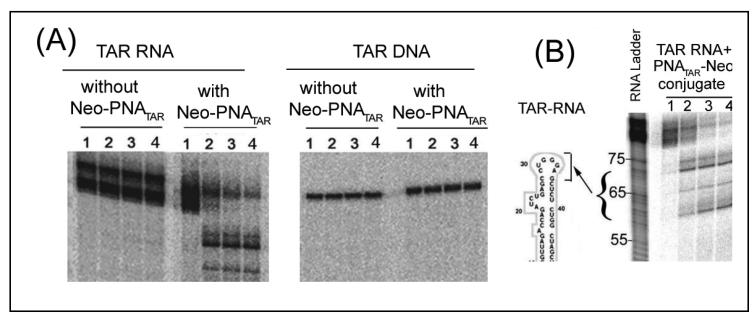
Since aliphatic diamines and polyamines catalyze RNA hydrolysis [66], we examined whether the neamine core of the conjugate carrying, at pH 7, protonated and unprotonated amino groups [67], could catalyze similar cleavage of target RNA in the vicinity of PNATAR binding. Neomycin B has been shown to bind various RNAs [68] and to accelerate the hydrolysis of an RNA dinucleoside phosphate, ApA [69]. When anti-TAR PNA-Neamine conjugate was incubated with its target RNA and DNA, the neamine moiety of the conjugate, besides being sequence-specific, specifically cleaved the target RNA but left corresponding DNA uncleaved (Fig. 5A). Optimum RNA cleavage occurred in the pH range of 7.0 to 7.4 [54]. No cleavage was seen with naked RNATAR or neamine alone under similar conditions. Significantly, this cleavage activity was unaffected at Mg2+ concentrations lower than 2 mM, but was strongly inhibited at higher concentrations of the divalent metal ion [36]. The cleavage site was mapped near the nucleotides at positions 30-39 in the stem-loop region of HIV-1 TAR in the proximity of the neamine core at the end of the PNA-RNA duplex, finding a cleavage product of 60-70 mer (Fig. 5B).
Figure 5.
(A) PNATAR.Neamine specifically cleaves the target RNA but not the corresponding DNA. Internally 32P-labeled TAR RNA or 5′ 32P-labeled DNA incubated in the presence or absence of 400 nM 5-neamine-PNATAR conjugate at 25°C. Lanes 1 through 4 in each panel show the results after incubation periods of 0, 1, 3, and 5 h, respectively [54].
(B). PNATAR-Neamine Conjugate cleaves HIV-1 TAR RNA in the vicinity of its binding site. The internally 32P-labeled HIV-1 TAR RNA incubated without or with 400 nM of the conjugate at room temperature. Lanes 1-4 represent incubation for 0, 1, 3 and 5 h, respectively. The arrow indicates the possible cleavage site on TAR RNA generating approximately 60-70 nucleotide long products [33].
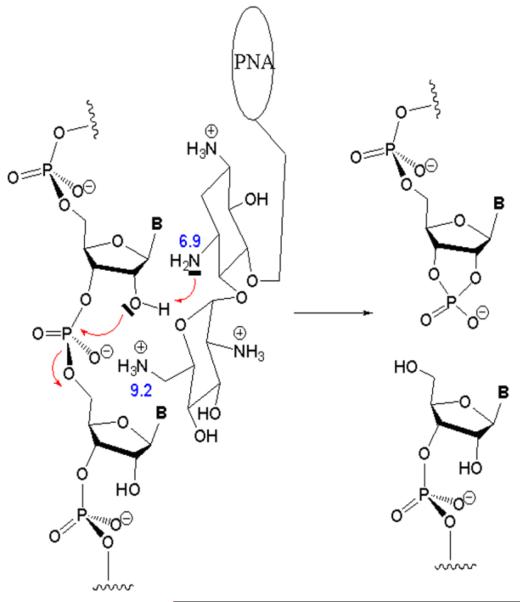
A model of was proposed for the underlying mechanism of RNA cleavage involving amino groups in rings I and II of the neamine core (Fig. 6). Binding of the PNA moiety of the conjugate to the target RNA sequences brings the neamine moiety into close proximity to the phosphodiester backbone, so that the unprotonated amino group at the 3-position of ring I is within interacting distance with the 2 -OH of the ribose moiety. This interaction may increase the nucleophilic character of the vicinal 2 -oxygen atom through abstraction of a hydrogen atom by the amino group at the 3-position of ring I. The activated 2 -O may carry out a nucleophilic attack on the phosphorus atom of the phosphodiester group. If so, the protonated amino group at the 6-position in ring II may favor this attack by decreasing electron density in the phosphodiester group and donating its proton to the 5-oxygen atom of the following sugar, resulting in cleavage of the phosphodiester bond. This mechanism is supported by the finding that PNA attached to the 4-position in ring II was unable to cleave the target RNA [54]. This indicates that the position of conjugation of PNA with neamine is crucial for the observed ribonuclease activity.
FIG. 6.
Proposed mechanism of PNA-Neamine conjugate-mediated cleavage of transactivation response element (TAR) RNA. The PNA attached to the 5-position of ring I of the neamine moiety through an 11-atom linker is shown as a circle. The numbers shown are the pKa value of amino groups at positions 3 and 6 of ring 1 and ring 6 of the neamine moiety. The deprotonated amino group at position 3 of ring I is shown extracting a proton from the 2 OH group of the ribose moiety of the RNA backbone. The activated 2 O is shown interacting intra-molecularly with a phosphorus atom, while the protonated amino group at the 6 -position in ring II is shown interacting with the negatively charged oxygen atom of the phosphate group. The arrows indicate the direction of interaction leading to final cleavage of the phosphodiester bond [54].
6. Small lipophilic triphenylphosphonium (TPP) cation as an effective strategy for biodelivery of anti-HIV-1 PNA
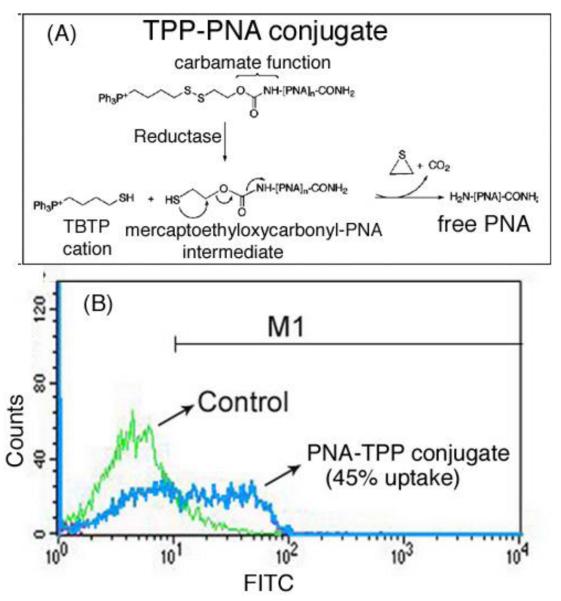
In addition to using CPP and neamine as carriers, an easy-to-perform anti-HIV-1PNA biodelivery protocol was designed (Fig. 7A) based on attachment of PNA to the lipophilic triphenylphosphonium (TPP) cation through an intracellularly biodegradable carbamate linker containing a disulfide bridge [53]. The TPP moiety was selected for its ability to pass through a lipid bilayer, driven by membrane potential [70, 71]. Once in the cytosol, the intracellular glutathione pool rapidly reduces the disulfide bond, giving rise to the thiobutyltriphenyl cation (TBTP) and an unstable mercaptoethyloxycarbamate-PNA intermediate, leading to the release of free PNA (Fig. 7A).
Figure 7.
(A) Synthesis of PNA-TPP conjugate and mechanism of intracellular release of free PNA following uptake. (B) Flow-cytometry of uptake of TPP-PNA conjugate [53].
This strategy has some similarities to that for promoting the intracellular release of anti-HIV active nucleoside monophosphates (Nu-MP) from phosphotriesters incorporating an enzymatically biodegradable protective agent [72]. In this new protocol, the releasable TPP carrier system and the N-extremity of PNA are linked via a carbamate group, which is stable in acidic or basic media and is relatively insensitive to enzymatic hydrolysis. It was found that the cellular uptake of fluo-PNATAR-TPP conjugate evaluated in CEM cells was 45%-50% within 6 h of incubation in RPMI medium containing 2% FCS (Fig. 7B). The IC50 for antiviral activity of PNATAR-TPP conjugate was 1.0 □M.
7. Pharmacokinetic properties and toxicity of anti-HIV PNA-CPP conjugates
PNATAR-CPP conjugate in male Balb/C mice was pharmokinetically analyzed after administration through intraperitoneal or gavage routes [73]. The clearance profiles of the test compound from different organs and tissues as a function of time displayed a biphasic exponential pathway whereby part of the radioactivity cleared rapidly, while a significant portion of it was slowly released over a prolonged period.
The acute toxicity of this class of compounds was evaluated in mice by administering single IP doses of 100, 300, and 600 mg/kg of body weight. The 600 mg dose was lethal, killing all injected mice within 72 h. However, death did not occur after single doses of 100 and 300 mg/kg, although mice experienced initial and transitory diarrhea, as well as loss of agility, which they quickly recovered. Thus, the LD50 of the PNATAR-penetratin conjugate was determined to be ≤ 600-mg/kg body weight. Autopsies on dead mice revealed no gross findings that could be correlated with the lethal dose of the conjugate. It was presumed that the death of these mice might have been the result of diarrhea-induced dehydration caused by the high dose of the conjugate.
Repeat daily doses of 10 mg, 30 mg, and 100-mg/kg body weight were well tolerated by mice during 8 days of treatment. Daily doses of 100 mg/kg caused diarrhea during the first 4 days of treatment, after which mice showed distinct recovery from day 5 onward. During 8 weeks of follow-up, mice fully recuperated. Necropsies on mice given daily doses of 100 mg showed serenities in the spleen, liver, and kidney at the 9th day of treatment, but not after the follow-up period. Necropsies, clinical chemistry studies, and hematological parameters demonstrated normal function of the major organs and no irreversible damage to the mice. Therefore, the PNA-peptide conjugate should be completely nontoxic at therapeutic doses [74].
Clinical blood chemistry analyses on the 9th and 60th days of the study showed normal levels of all values except those for ALT, which were marginally elevated (1.5-1.25-fold) in mice given a daily dose of 100 mg/kg body weight. Normal levels of alkaline phosphatase implied that there had been no significant damage to liver cells. Overall, these findings suggest the maintenance of normal cellular function at repeated high doses of the PNATAR-Penetratin conjugate. Most of the hematological parameters determined on the 9th and 60th days were within normal limits in all mice, suggesting that the anti-HIV-1 PNA-CPP conjugate had no deleterious effects on the health and functions of mouse organs. Similarly, the examination of bone-marrow aspirate revealed no abnormality in any mice. Differences in the ratio of myeloid to erythroblasts (M:E) were observed in all groups of mice only in 9th-day aspirate, suggesting that the PNATAR-Penetratin conjugate had no significant influence on the immune system [74].
7.1 Naked PNA is immunologically inactive, while PNA-Penetratin conjugates are moderately immunogenic
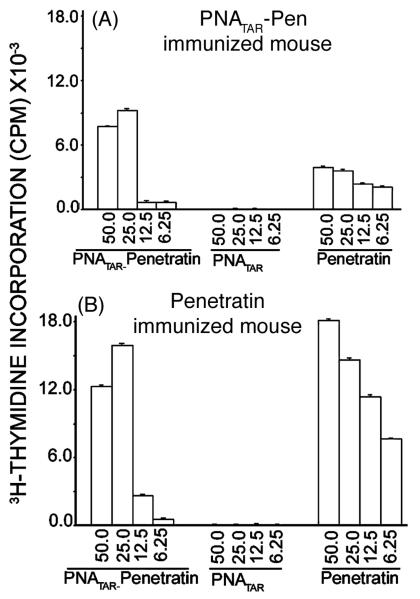
The proliferation responses of splenocytes and lymph node cells from mice immunized with naked PNATAR. PNATAR-Penetratin conjugate or penetratin were determined by challenge with the same immunizing antigen. The responses obtained with naked PNATAR as an immunizing and challenging antigen were negligible [75]. Other studies have also suggested that, irrespective of the PNA sequences, naked PNAs are immunologically inert molecules [68, 69]. However, the low proliferation responses noted with PNATAR-Penetratin conjugate could be due to the penetratin component of the conjugate. This premise was supported when lymph-node cells from mice immunized with penetratin or PNATAR-Penetratin conjugate were individually challenged with conjugate or its constituents, naked PNATAR and penetratin (Fig. 8). The proliferation responses were significant when challenge was done with either penetratin or PNA-Penetratin conjugate, but not when it was done with naked PNA.
Figure 8.
Proliferation responses of lymph node cells from the conjugate-immunized (A) or penetratin immunized (B) mouse challenged with naked PNA, penetratin and the conjugate [67].
Analysis of the cytokine secretion profiles of lymph node cells from mice immunized with either PNA-Penetratin conjugate or penetratin and then challenged by the immunizing antigen yielded interesting results. The secretion of interleukin-2 (IL-2) by lymph node cells was the highest when they were challenged with either penetratin or PNA-Penetratin conjugate. Since the candidate compound, PNATAR-Penetratin conjugate, is a potent virucidal agent against HIV-1 and displays strong HIV-1 antiviral activity, the immunological response elicited by the conjugate with respect to the elevated production of IL-2 is expected to be beneficial to patients. Although IL-2 exerts its effects on many cell types, its most prominent effect is on the activation of T-lymphocytes [76-79];. IL-2 promotes proliferation of T lymphocytes and increases the count of mature T cells; it also slows the death of CD4+ T cells. HIV-1 infection not only decreases the CD4+ cell count and endogenous levels of IL-2, but also causes an increase in the dysfunction of existing CD4+ cells. These three deleterious effects of HIV-1 infection are potentially reversible with the administration of recombinant IL-2 [80]. Clinical trials have shown that HIV-1 patients undergoing antiretroviral therapy with IL-2 supplement show both significant increases in the number of their CD4+ cells and significant decreases in HIV/AIDS-related morbidity as compared with patients receiving the same therapy without IL-2 supplement [81-88].
In addition to IL-2, interleukin-12 (IL-12), interferon-gamma (IFN-γ;), and tumor necrosis factor-alpha (TNF-α) were produced by lymph node cells from immunized mice. IL-12 stimulates the growth and function of T cells and the production of IFN-γ and TNF-α. Both IL-12 and IL-2 are important cytokines in the regulation of a cell-mediated immune response, with IL-2 being responsible for stimulating the proliferation of T cells while IL-12 promotes differentiation of the antigen-activated CD4+ T cells into the TH1 cell type [89]. The production of pro-inflammatory cytokines (IL-2, IFN-γ, TNF-α), but not anti-inflammatory cytokines (IL-4, IL-10) from lymph node cells stimulated with penetratin and PNATAR-Penetratin conjugate is consistent with TH1 cell differentiation, which supports cell-mediated immune responses (e.g., cytotoxic T lymphocytes, natural killer cells, activated macrophages) that would benefit patients with HIV-1 infection.
8. Current approach to developing virucidal vaginal gel formulations of anti-HIV-1 PNA-CPP conjugates
The majority of HIV-1 infections are due to heterosexual contact [90], although the highest risk for HIV-1 transmission occurs with receptive anal intercourse [91]. The semen from HIV-1 infected men [92, 93] and cervical mucus from HIV-1 infected women contain both cell-free virus and HIV-1 infected cells [94]. Therefore, sexual transmission of HIV-1 can occur by both cell-free HIV-1 virions and HIV-1 infected cells. However, infection can be prevented by using either mechanical barriers such as condoms or an effective virucidal vaginal gel formulation. As of July 2008, six anti-HIV-1 vaginal gel formulations were undergoing clinical trials in different countries: PRO 2000/5, Tenofovir, SPL7013 (VivaGel), Dapivirine (TMC120), HEC/CS/N-9, Emollient, and UC-781. Of these, four are undergoing phase 1 clinical trials; two (PRO2000/5 and Tenofovir) are in phase 2/3 clinical trials.
Efforts are under way to develop a potential vaginal gel formulation using a cocktail of nontoxic anti-HIV-1 PNA-CPP conjugates, which have been shown to be potently virucidal against HIV-1. The most important pharmacological properties of anti-HIV-1 PNA-CPP conjugates are their long biological half-life and nontoxic characteristics, even at high unphysiological doses of 100-300 mg/kg body weight [74]. Moreover, these conjugates are potently virucidal even in subnanomolar concentrations. Because these conjugates are capable of efficient internalization in virion particles and in uninfected or infected cells, they are expected to block sexual transmission of HIV-1 by inactivating virion particles in semen and by interfering with virus replication or production in HIV-1-infected cells in both semen and cervical mucus from HIV-1-infected individuals. A virucidal gel can be formulated with a cocktail of anti-HIV-1 PNA-CPP conjugates targeting HIV-1 TAR, the primer-binding site, and its upstream A-loop region, the dimerization site of HIV-1 RNA. It therefore should offer a multipronged effect, blocking every phase of the HIV-1 life cycle.
9. Future strategies for enhancing the selective biodelivery of ani-HIV1 PNA to CD4+ T lymphocytes and macrophages
The clinical potential of current anti-HIV-1 drugs has been limited not only by their toxicity and the development of drug-resistant strains of the virus, but also by nonspecific systemic delivery of the drugs. The therapeutic potential of these drugs could be greatly increased if they could be selectively delivered into T-lymphocytes and macrophages, which are, respectively, the targets and reservoirs for HIV-1 infection and replication. Although anti-HIV-1 PNA-CPP conjugates display potent antiviral and virucidal activities, they penetrate all organs and cell types and distribute within them with equal efficiency. Effective strategies for biodelivery of anti-HIV-1 PNAs specifically into T-lymphocytes and macrophages would be an ideal approach to block HIV-1 infection and replication.
9.1 The use of glycoconjugates containing mannose clusters as potent vehicles to deliver anti-HIV-1 PNA to macrophages
The mannose receptor (MR) is an endocytic receptor of macrophages and endothelial cell subsets that selectively bind and internalize both endogenous and foreign glycoconjugates [95, 96]. These carbohydrates, which are present on the surface of many microorganisms, enable the MR to bind and internalize them during phagocytosis. MRs, which are expressed on human macrophages and cultured dendritic cells, are attractive points for specific drug delivery to these cells. Therefore, designing cell-recognizable glycoconjugates of anti-HIV-1 PNA having a cluster of mannose will be highly desirable for their specific delivery to macrophages.
9.2 Formylated oligopeptide as a potent chemoattractant for selective delivery of anti-HIV-1 PNA to macrophages
The movement of monocytes or macrophages to various tissue sites is greatly influenced by various chemotactic substances, including lymphokines and bacterially derived factors, which are responsible for macrophage migration toward a site of bacterial invasion [97]. It has long been known that small synthetic N-formylated oligopeptides, which are structurally related to bacterially derived products, are potent chemoattractants for macrophages [97]. A small formylated peptide, N-formyl-Nle-Leu-Phe-Nle-Tyr-Lys, is a potent chemoattractant with high receptor-binding affinity. The number of receptors on each macrophage was determined to be 120,000, with a dissociation constant of 1.3 nM [98, 99]. Binding of chemoattractant peptides to macrophages at physiological temperature is highly specific, rapid, and saturable. After time- and temperature-dependent binding, the receptor ligand complex is altered and is no longer reversible since the ligand is rapidly internalized into the cells [98, 99]. Use of this unique and specific interaction between chemoattractant formylated peptides and macrophages can be exploited to deliver anti-HIV-1 PNA selectively into macrophages.
9.3 Use of the V3-loop peptide of gp120 as the navigator for anti-HIV-1 PNA to ferret out lymphocytes and macrophages
The V3-loop region of HIV-1 gp120 is critical for HIV-1 infection. In addition to interacting with the CD4 receptor and coreceptor, gp120 interacts specifically with certain surface glycosphingolipids (GSL) such as the globotriaosylceramide (Gb3) and monosialoganglioside (GM3) of lymphocytes and macrophages. These interactions are crucial for viral entry and fusion [100, 101]. CD4-independent HIV-1 infection of human colonic epithelial cells (HT-29) involves the use of galactosylceramide (GalCer) as an alternate receptor for gp120 [102]. A small peptide corresponding to the central 15-amino-acid region of the V3-loop (RIQRGPGRAFVTIGK) of HIV-1 gp120 exhibits specific binding to host cell membrane GSL-like GalCer, GM3, and Gb3, and effectively competes with gp120 from various HIV-1 strains [103]. This binding specificity of the V-3 loop peptide can be an attractive strategy to navigate anti-HIV-1 PNA specifically to T-lymphocytes, macrophages, and colonic epithelial cells.
9.5 A synthetic peptide corresponding to variable regions of an anti-CD4 monoclonal antibody specifically binds to extracellular domain D1 of CD4
The CD4 receptor is a transmembrane glycoprotein on thymocytes, T-cells, and macrophages. CD4, a member of the immunoglobulin gene superfamily, consists of four extracellular domains (D1-D4) [104]. The loop region of the D1 domain of CD4 has been identified as the primary binding site for HIV-1 gp120, which, in association with chemokine receptors, facilitates HIV-1 entry into cells [105-107]. A peptide derived from the variable region of anti-CD4 monoclonal antibody (GGVLWSRRGDFD) specifically binds to the D1 loop of CD4 with an affinity of 1.6 nM Kd [108]. Attaching this peptide to anti-HIV-1 PNAs should greatly enhance their specificity to targeted cells.
10. Conclusion
The sequestering of critical regulatory sequences in the viral genome by sequence-specific PNA conjugated with specially designed peptide and mannose clusters that specifically target T-lymphocytes, macrophages, and rectal and vaginal epithelial cells has great potential to block HIV infection and replication. This is an important new strategy for therapeutic intervention. The armamentarium of antiretroviral agents targeting one or more steps in the replicative cycle of HIV is likely to grow in the near future. It is possible that multiple steps in the viral life cycle can be targeted for new antiretroviral therapy. In this context, the virucidal and antiviral activities of PNA-CPP conjugates targeting critical regions of the HIV-1 genome hold enormous promise with regard to the development of effective virucidal vaginal gel formulations to stop HIV-1 transmission at the earliest stages of infection. The most compelling aspect of this approach is that, upon exposure to PNA-CPP conjugates targeted against critical regions of the viral genome, HIV-1 in the plasma can be inactivated before its entry into cells. Penetrating the virion particles, the PNA-CPP conjugates render them replication-incompetent. These conjugates have great potential as prophylactic agents to block HIV-1 infection. Studies on the pharmacokinetics and toxicity of these compounds have clearly demonstrated that they are nontoxic and have prolonged clearance profiles. These attributes should form the basis for determining their appropriate doses for preclinical evaluation.
11. Expert Opinion
PNAs have been in the news since their discovery in 1991. However, the initial excitement about the potential of this new class of compounds as potential antisense molecules has been dampened by their poor cellular uptake. Many strategies have now been developed to improve their cellular uptake, with cell-penetrating peptides proving to be the most effective biodelivery vehicle. Although efficient cellular uptake occurs with anti-HIV PNA-CPP in cell culture systems, a detailed study of their uptake efficacy in primary cells and in-vivo systems remains to be determined. The CPP conjugated with PNA via an amide bond is most stable in cells and does not interfere with target specificity. One drawback of this approach is expected to be the route of administration. PNA-CPP conjugates must be administered either intraperitoneally or intravenously since gavage of PNA-CPP conjugate is not efficient due to cleavage of peptide in the gut. The D form of CPP peptide should enhance the stability of anti-HIV-1 PNA-CPP conjugates in the gut. Gavage of naked PNA is not encouraging because the PNA remains in the stomach for a long time. A strategy to enhance oral biodelivery of PNAs will certainly improve their therapeutic potential. In this context, anti-HIV PNA-Neamine conjugates are promising, being stable in the gut environment and relatively effectively taken up when administered orally (unpublished results).
One detailed toxicity study of an anti-HIV-1 PNA CPP conjugate is encouraging because it showed that this conjugate is almost nontoxic to mice at multiple doses of 100 mg/kg of body weight, with an LD50 between 300-600 mg/kg body weight [74]. Because the body-to-surface area of a mouse (0.0066m2:0.02kg) is 12-fold higher than that of humans (1.6 m2/60kg) [109], the effective human dosing concentrations of PNA-CPP conjugates are expected to be 10-12-fold lower than those for mice. These small mammals also eliminate drugs faster than large mammals do [110]. One point of concern is that cellular uptake of PNA-CPP conjugates or PNA-Neamine conjugates is not cell-specific. The clinical potential of anti-HIV-1 PNA could be enhanced several-fold if it were selectively delivered into CD4-positive T cells and macrophages, which are, respectively, the targets and reservoirs for HIV-1 infection and replication. Therefore, alternate strategies are needed for their specific delivery into T-lymphocytes and macrophages, which support CD4-dependent HIV infection, and into human colonic epithelial cells, which use GalCer, a major glycosphingolipid expressed by these cells, for HIV-1 infection. Optimization of their biodelivery by conjugating these anti-HIV-1 PNAs to specially designed peptide and mannose clusters aimed specifically at T-lymphocytes, macrophages, and human colonic epithelial cells would offer a logical and promising means of blocking HIV replication in its sanctuaries.
Women, who now account for nearly 60% of newly HIV-infected adults worldwide, most often acquire HIV through heterosexual exposure [111]. The semen from HIV-1 infected men [92, 93, 112] and cervical mucus from HIV-1 infected women contain both cell-free virus and HIV-1 infected cells [94]. Therefore, sexual transmission of HIV-1 can occur by both cell-free HIV-1 virions and HIV-1 infected cells. Targeting the earliest stages of infection offers the best prospect for preventing or containing infection. The virucidal and antiviral properties of anti-HIV-1 PNA-CPP conjugates could be exploited in the development of a potent virucidal vaginal gel formulation with components known to have excellent rectal and vaginal tolerance and strong bioadhesive properties.
REFERENCES
- 1.Kress KD. HIV update: emerging clinical evidence and a review of recommendations for the use of highly active antiretroviral therapy. Am J Health Syst Pharm. 2004 Oct 1;61(Suppl 3):S3–14. doi: 10.1093/ajhp/61.suppl_3.S3. quiz S5-6. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991 Dec 6;254(5037):1497–500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 3.Demidov VV, Potaman VN, Frank-Kamenetskii MD, Egholm M, Buchard O, Sonnichsen SH, et al. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem Pharmacol. 1994 Sep 15;48(6):1310–3. doi: 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 4.Wakefield JK, Kang SM, Morrow CD. Construction of a type 1 human immunodeficiency virus that maintains a primer binding site complementary to tRNA(His) J Virol. 1996 Feb;70(2):966–75. doi: 10.1128/jvi.70.2.966-975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang SM, Zhang Z, Morrow CD. Identification of a sequence within U5 required for human immunodeficiency virus type 1 to stably maintain a primer binding site complementary to tRNA(Met) J Virol. 1997 Jan;71(1):207–17. doi: 10.1128/jvi.71.1.207-217.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paillart JC, Berthoux L, Ottmann M, Darlix JL, Marquet R, Ehresmann B, et al. A dual role of the putative RNA dimerization initiation site of human immunodeficiency virus type 1 in genomic RNA packaging and proviral DNA synthesis. J Virol. 1996 Dec;70(12):8348–54. doi: 10.1128/jvi.70.12.8348-8354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaynor R. Cellular transcription factors involved in the regulation of HIV-1 gene expression. Aids. 1992 Apr;6(4):347–63. doi: 10.1097/00002030-199204000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Harrich D, Ulich C, Gaynor RB. A critical role for the TAR element in promoting efficient human immunodeficiency virus type 1 reverse transcription. J Virol. 1996 Jun;70(6):4017–27. doi: 10.1128/jvi.70.6.4017-4027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrich D, Ulich C, Garcia-Martinez LF, Gaynor RB. Tat is required for efficient HIV-1 reverse transcription. Embo J. 1997 Mar 17;16(6):1224–35. doi: 10.1093/emboj/16.6.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crabtree GR. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–61. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- 11.Peterlin BM. Transcriptional Regulation of HIV. New York: 1991. [Google Scholar]
- 12.Waterman ML, Sheridan PL, Milocco LH, Sheline CT, Jones KA. Nuclear Protein Implicated In HIV-1 Transcriptional Control. New York: 1991. [Google Scholar]
- 13.Sodroski J, Patarca R, Rosen C, Wong-Staal F, Haseltine W. Location of the trans-activating region on the genome of human T-cell lymphotropic virus type III. Science. 1985 Jul 5;229(4708):74–7. doi: 10.1126/science.2990041. [DOI] [PubMed] [Google Scholar]
- 14.Arya SK, Gallo RC. Three novel genes of human T-lymphotropic virus type III: immune reactivity of their products with sera from acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2209–13. doi: 10.1073/pnas.83.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arya SK, Gallo RC. Human T-cell growth factor (interleukin 2) and gamma-interferon genes: expression in human T-lymphotropic virus type III- and type I-infected cells. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8691–5. doi: 10.1073/pnas.82.24.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feinberg MB, Baltimore D, Frankel AD. The role of Tat in the human immunodeficiency virus life cycle indicates a primary effect on transcriptional elongation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4045–9. doi: 10.1073/pnas.88.9.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marciniak RA, Sharp PA. HIV-1 Tat protein promotes formation of more-processive elongation complexes. Embo J. 1991 Dec;10(13):4189–96. doi: 10.1002/j.1460-2075.1991.tb04997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherrington J, Ganem D. Regulation of polyadenylation in human immunodeficiency virus (HIV): contributions of promoter proximity and upstream sequences. Embo J. 1992 Apr;11(4):1513–24. doi: 10.1002/j.1460-2075.1992.tb05196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eggermont J, Proudfoot NJ. Poly(A) signals and transcriptional pause sites combine to prevent interference between RNA polymerase II promoters. Embo J. 1993 Jun;12(6):2539–48. doi: 10.1002/j.1460-2075.1993.tb05909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weichs an der Glon C, Ashe M, Eggermont J, Proudfoot NJ. Tat-dependent occlusion of the HIV poly(A) site. Embo J. 1993 May;12(5):2119–28. doi: 10.1002/j.1460-2075.1993.tb05860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vink C, Plasterk RH. The human immunodeficiency virus integrase protein. Trends Genet. 1993 Dec;9(12):433–8. doi: 10.1016/0168-9525(93)90107-s. [DOI] [PubMed] [Google Scholar]
- 22.Goff SP. Genetics of retroviral integration. Annu Rev Genet. 1992;26:527–44. doi: 10.1146/annurev.ge.26.120192.002523. [DOI] [PubMed] [Google Scholar]
- 23.Cannon PM, Wilson W, Byles E, Kingsman SM, Kingsman AJ. Human immunodeficiency virus type 1 integrase: effect on viral replication of mutations at highly conserved residues. J Virol. 1994 Aug;68(8):4768–75. doi: 10.1128/jvi.68.8.4768-4775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engelman A, Englund G, Orenstein JM, Martin MA, Craigie R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J Virol. 1995 May;69(5):2729–36. doi: 10.1128/jvi.69.5.2729-2736.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chow SA, Brown PO. Substrate features important for recognition and catalysis by human immunodeficiency virus type 1 integrase identified by using novel DNA substrates. J Virol. 1994 Jun;68(6):3896–907. doi: 10.1128/jvi.68.6.3896-3907.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaFemina RL, Schneider CL, Robbins HL, Callahan PL, LeGrow K, Roth E, et al. Requirement of active human immunodeficiency virus type 1 integrase enzyme for productive infection of human T-lymphoid cells. J Virol. 1992 Dec;66(12):7414–9. doi: 10.1128/jvi.66.12.7414-7419.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherman PA, Dickson ML, Fyfe JA. Human immunodeficiency virus type 1 integration protein: DNA sequence requirements for cleaving and joining reactions. J Virol. 1992 Jun;66(6):3593–601. doi: 10.1128/jvi.66.6.3593-3601.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanova G, Arzumanov AA, Turner JJ, Reigadas S, Toulme JJ, Brown DE, et al. Anti-HIV activity of steric block oligonucleotides. Ann N Y Acad Sci. 2006 Oct;1082:103–15. doi: 10.1196/annals.1348.033. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal S, Tang JY. GEM 91--an antisense oligonucleotide phosphorothioate as a therapeutic agent for AIDS. Antisense Res Dev. 1992 Winter;2(4):261–6. doi: 10.1089/ard.1992.2.261. [DOI] [PubMed] [Google Scholar]
- 30.Zheng R. Technology evaluation: GEM-92, Hybridon Inc. Curr Opin Mol Ther. 1999 Aug;1(4):521–3. [PubMed] [Google Scholar]
- 31.Gunnery S, Green SR, Mathews MB. Tat-responsive region RNA of human immunodeficiency virus type 1 stimulates protein synthesis in vivo and in vitro: relationship between structure and function. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11557–61. doi: 10.1073/pnas.89.23.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayhood T, Kaushik N, Pandey PK, Kashanchi F, Deng L, Pandey VN. Inhibition of Tat-mediated transactivation of HIV-1 LTR transcription by polyamide nucleic acid targeted to TAR hairpin element. Biochemistry. 2000 Sep 26;39(38):11532–9. doi: 10.1021/bi000708q. [DOI] [PubMed] [Google Scholar]
- 33.Arzumanov A, Walsh AP, Liu X, Rajwanshi VK, Wengel J, Gait MJ. Oligonucleotide analogue interference with the HIV-1 Tat protein-TAR RNA interaction. Nucleosides Nucleotides Nucleic Acids. 2001 Apr-Jul;20(4-7):471–80. doi: 10.1081/NCN-100002321. [DOI] [PubMed] [Google Scholar]
- 34.Kaushik N, Basu A, Pandey VN. Inhibition of HIV-1 replication by anti-transactivation responsive polyamide nucleotide analog. Antiviral Res. 2002 Oct;56(1):13–27. doi: 10.1016/s0166-3542(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 35.Chaubey B, Tripathi S, Ganguly S, Harris D, Casale RA, Pandey VN. A PNA-transportan conjugate targeted to the TAR region of the HIV-1 genome exhibits both antiviral and virucidal properties. Virology. 2005 Jan 20;331(2):418–28. doi: 10.1016/j.virol.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 36.Riguet E, Tripathi S, Chaubey B, Desire J, Pandey VN, Decout JL. A peptide nucleic acid-neamine conjugate that targets and cleaves HIV-1 TAR RNA inhibits viral replication. J Med Chem. 2004 Sep 23;47(20):4806–9. doi: 10.1021/jm049642d. [DOI] [PubMed] [Google Scholar]
- 37.Terreux R, Pairot S, Cabrol-Bass D, Patino N, Condom R. Interaction of new PNA-based molecules with TAR RNA of HIV-1: molecular modelling and biological evaluation. J Mol Graph Model. 2001;19(6):579–85. doi: 10.1016/s1093-3263(01)00095-x. 614-5. [DOI] [PubMed] [Google Scholar]
- 38.Tripathi S, Chaubey B, Ganguly S, Harris D, Casale RA, Pandey VN. Anti-HIV-1 activity of anti-TAR polyamide nucleic acid conjugated with various membrane transducing peptides. Nucleic Acids Res. 2005;33(13):4345–56. doi: 10.1093/nar/gki743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee R, Kaushik N, Modak MJ, Vinayak R, Pandey VN. Polyamide nucleic acid targeted to the primer binding site of the HIV-1 RNA genome blocks in vitro HIV-1 reverse transcription. Biochemistry. 1998 Jan 20;37(3):900–10. doi: 10.1021/bi972197m. [DOI] [PubMed] [Google Scholar]
- 40.Kaushik N, Pandey VN. PNA targeting the PBS and A-loop sequences of HIV-1 genome destabilizes packaged tRNA3(Lys) in the virions and inhibits HIV-1 replication. Virology. 2002 Nov 25;303(2):297–308. doi: 10.1006/viro.2002.1630. [DOI] [PubMed] [Google Scholar]
- 41.Tripathi S, Chaubey B, Barton BE, Pandey VN. Anti HIV-1 virucidal activity of polyamide nucleic acid-membrane transducing peptide conjugates targeted to primer binding site of HIV-1 genome. Virology. 2007 Jun 20;363(1):91–103. doi: 10.1016/j.virol.2007.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boutimah-Hamoudi F, Leforestier E, Senamaud-Beaufort C, Nielsen PE, Giovannangeli C, Saison-Behmoaras TE. Cellular antisense activity of peptide nucleic acid (PNAs) targeted to HIV-1 polypurine tract (PPT) containing RNA. Nucleic Acids Res. 2007;35(12):3907–17. doi: 10.1093/nar/gkm374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sei S, Yang QE, O'Neill D, Yoshimura K, Nagashima K, Mitsuya H. Identification of a key target sequence to block human immunodeficiency virus type 1 replication within the gag-pol transframe domain. J Virol. 2000 May;74(10):4621–33. doi: 10.1128/jvi.74.10.4621-4633.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koppelhus U, Zachar V, Nielsen PE, Liu X, Eugen-Olsen J, Ebbesen P. Efficient in vitro inhibition of HIV-1 gag reverse transcription by peptide nucleic acid (PNA) at minimal ratios of PNA/RNA. Nucleic Acids Res. 1997 Jun 1;25(11):2167–73. doi: 10.1093/nar/25.11.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen PE. Peptide nucleic acid (PNA) from DNA recognition to antisense and DNA structure. Biophys Chem. 1997 Oct;68(1-3):103–8. doi: 10.1016/s0301-4622(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 46.Knudsen H, Nielsen PE. Antisense properties of duplex- and triplex-forming PNAs. Nucleic Acids Res. 1996 Feb 1;24(3):494–500. doi: 10.1093/nar/24.3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koppelhus U, Nielsen PE. Cellular delivery of peptide nucleic acid (PNA) Adv Drug Deliv Rev. 2003 Feb 10;55(2):267–80. doi: 10.1016/s0169-409x(02)00182-5. [DOI] [PubMed] [Google Scholar]
- 48.Shiraishi T, Nielsen PE. Photochemically enhanced cellular delivery of cell penetrating peptide-PNA conjugates. FEBS Lett. 2006 Feb 20;580(5):1451–6. doi: 10.1016/j.febslet.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 49.Abes S, Ivanova GD, Abes R, Arzumanov AA, Williams D, Owen D, et al. Peptide-based delivery of steric-block PNA oligonucleotides. Methods Mol Biol. 2009;480:85–99. doi: 10.1007/978-1-59745-429-2_6. [DOI] [PubMed] [Google Scholar]
- 50.Wagstaff KM, Jans DA. Protein transduction: cell penetrating peptides and their therapeutic applications. Curr Med Chem. 2006;13(12):1371–87. doi: 10.2174/092986706776872871. [DOI] [PubMed] [Google Scholar]
- 51.Futaki S. Membrane permeable peptide vectors: chemistry and functional design for the therapeutic applications. Adv Drug Deliv Rev. 2008 Mar 1;60(4-5):447. doi: 10.1016/j.addr.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 52.Gait MJ. Peptide-mediated cellular delivery of antisense oligonucleotides and their analogues. Cell Mol Life Sci. 2003 May;60(5):844–53. doi: 10.1007/s00018-003-3044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehiri M, Upert G, Tripathi S, Di Giorgio A, Condom R, Pandey VN, et al. An efficient biodelivery system for antisense polyamide nucleic acid (PNA) Oligonucleotides. 2008 Fall;18(3):245–56. doi: 10.1089/oli.2008.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaubey B, Tripathi S, Desire J, Baussanne I, Decout JL, Pandey VN. Mechanism of RNA cleavage catalyzed by sequence specific polyamide nucleic acid-neamine conjugate. Oligonucleotides. 2007 Fall;17(3):302–13. doi: 10.1089/oli.2007.0085. [DOI] [PubMed] [Google Scholar]
- 55.El-Sayed A, Futaki S, Harashima H. Delivery of macromolecules using argininerich cell-penetrating peptides: ways to overcome endosomal entrapment. Aaps J. 2009 Mar;11(1):13–22. doi: 10.1208/s12248-008-9071-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koppelhus U, Awasthi SK, Zachar V, Holst HU, Ebbesen P, Nielsen PE. Cell-dependent differential cellular uptake of PNA, peptides, and PNA-peptide conjugates. Antisense Nucleic Acid Drug Dev. 2002 Apr;12(2):51–63. doi: 10.1089/108729002760070795. [DOI] [PubMed] [Google Scholar]
- 57.Kaushik N, Basu A, Palumbo P, Myers RL, Pandey VN. Anti-TAR polyamide nucleotide analog conjugated with a membrane-permeating peptide inhibits human immunodeficiency virus type 1 production. J Virol. 2002 Apr;76(8):3881–91. doi: 10.1128/JVI.76.8.3881-3891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pooga M, Langel U. Targeting of cancer-related proteins with PNA oligomers. Curr Cancer Drug Targets. 2001 Nov;1(3):231–9. doi: 10.2174/1568009013334142. [DOI] [PubMed] [Google Scholar]
- 59.Pooga M, Soomets U, Hallbrink M, Valkna A, Saar K, Rezaei K, et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat Biotechnol. 1998 Sep;16(9):857–61. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 60.Pooga M, Soomets U, Bartfai T, Langel U. Synthesis of cell-penetrating peptide-PNA constructs. Methods Mol Biol. 2002;208:225–36. doi: 10.1385/1-59259-290-2:225. [DOI] [PubMed] [Google Scholar]
- 61.Thoren PE, Persson D, Isakson P, Goksor M, Onfelt A, Norden B. Uptake of analogs of penetratin, Tat(48-60) and oligoarginine in live cells. Biochem Biophys Res Commun. 2003 Jul 18;307(1):100–7. doi: 10.1016/s0006-291x(03)01135-5. [DOI] [PubMed] [Google Scholar]
- 62.Wakefield JK, Wolf AG, Morrow CD. Human immunodeficiency virus type 1 can use different tRNAs as primers for reverse transcription but selectively maintains a primer binding site complementary to tRNA(3Lys) J Virol. 1995 Oct;69(10):6021–9. doi: 10.1128/jvi.69.10.6021-6029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushik N, Talele TT, Monel R, Palumbo P, Pandey VN. Destabilization of tRNA3(Lys) from the primer-binding site of HIV-1 genome by anti-A loop polyamide nucleotide analog. Nucleic Acids Res. 2001 Dec 15;29(24):5099–106. doi: 10.1093/nar/29.24.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Houng-Yau Mei, AAG, Halim Nadia S., Mack David P., Moreland David W., Sanders Kathryn B., Truong Hoa N., Czarnik Anthony W. Inhibition of an HIV-1 Tat-derived peptide binding to TAR RNA by aminoglycoside antibiotics. Bioorganic & Medicinal Chemistry Letters. 1995;5(22):2755–60. [Google Scholar]
- 65.Zapp ML, Stern S, Green MR. Small molecules that selectively block RNA binding of HIV-1 Rev protein inhibit Rev function and viral production. Cell. 1993 Sep 24;74(6):969–78. doi: 10.1016/0092-8674(93)90720-b. [DOI] [PubMed] [Google Scholar]
- 66.Oivanen M, Kuusela S, Lonnberg H. Kinetics and Mechanisms for the Cleavage and Isomerization of the Phosphodiester Bonds of RNA by Bronsted Acids and Bases. Chem Rev. 1998 May 7;98(3):961–90. doi: 10.1021/cr960425x. [DOI] [PubMed] [Google Scholar]
- 67.Botto RaC B. Nitrogen-15 Nuclear Magnetic Resonance spectroscopy of Neomycin B and Related Aminoglycosides. J Am Chem Soc. 1983;105:1021–8. [Google Scholar]
- 68.Moazed D, Noller HF. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987 Jun 4-10;327(6121):389–94. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 69.Kirk SaT Y. Hydrolysis of an RNA dinucleoside mono-phosphate by neomycin B. Chem Commun. 1998;1:147–8. [Google Scholar]
- 70.Filipovska A, Eccles MR, Smith RA, Murphy MP. Delivery of antisense peptide nucleic acids (PNAs) to the cytosol by disulphide conjugation to a lipophilic cation. FEBS Lett. 2004 Jan 2;556(1-3):180–6. doi: 10.1016/s0014-5793(03)01403-0. [DOI] [PubMed] [Google Scholar]
- 71.Muratovska A, Lightowlers RN, Taylor RW, Turnbull DM, Smith RA, Wilce JA, et al. Targeting peptide nucleic acid (PNA) oligomers to mitochondria within cells by conjugation to lipophilic cations: implications for mitochondrial DNA replication, expression and disease. Nucleic Acids Res. 2001 May 1;29(9):1852–63. doi: 10.1093/nar/29.9.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lefebvre I, Perigaud C, Pompon A, Aubertin AM, Girardet JL, Kirn A, et al. Mononucleoside phosphotriester derivatives with S-acyl-2-thioethyl bioreversible phosphate-protecting groups: intracellular delivery of 3′-azido-2′,3′-dideoxythymidine 5′-monophosphate. J Med Chem. 1995 Sep 29;38(20):3941–50. doi: 10.1021/jm00020a007. [DOI] [PubMed] [Google Scholar]
- 73.Ganguly S, Chaubey B, Tripathi S, Upadhyay A, Neti PV, Howell RW, et al. Pharmacokinetic analysis of polyamide nucleic-acid-cell penetrating peptide conjugates targeted against HIV-1 transactivation response element. Oligonucleotides. 2008 Fall;18(3):277–86. doi: 10.1089/oli.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaubey B, Tripathi S, Pandey VN. Single acute-dose and repeat-doses toxicity of anti-HIV-1 PNA TAR-penetratin conjugate after intraperitoneal administration to mice. Oligonucleotides. 2008 Spring;18(1):9–20. doi: 10.1089/oli.2007.0088. [DOI] [PubMed] [Google Scholar]
- 75.Upadhyay A, Ponzio NM, Pandey VN. Immunological response to peptide nucleic acid and its peptide conjugate targeted to transactivation response (TAR) region of HIV-1 RNA genome. Oligonucleotides. 2008 Dec;18(4):329–35. doi: 10.1089/oli.2008.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kremer IB, Hilkens CM, Sylva-Steenland RM, Koomen CW, Kapsenberg ML, Bos JD, et al. Reduced IL-12 production by monocytes upon ultraviolet-B irradiation selectively limits activation of T helper-1 cells. J Immunol. 1996 Sep 1;157(5):1913–8. [PubMed] [Google Scholar]
- 77.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–53. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 78.Wang KS, Frank DA, Ritz J. Interleukin-2 enhances the response of natural killer cells to interleukin-12 through up-regulation of the interleukin-12 receptor and STAT4. Blood. 2000 May 15;95(10):3183–90. [PubMed] [Google Scholar]
- 79.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998 Nov;10(11):1593–8. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 80.Conrad A. Interleukin-2--where are we going? J Assoc Nurses AIDS Care. 2003 Nov-Dec;14(6):83–8. doi: 10.1177/1055329003255620. [DOI] [PubMed] [Google Scholar]
- 81.Abrams DI, Bebchuk JD, Denning ET, Davey RT, Fox L, Lane HC, et al. Randomized, open-label study of the impact of two doses of subcutaneous recombinant interleukin-2 on viral burden in patients with HIV-1 infection and CD4+ cell counts of > or = 300/mm3: CPCRA 059. J Acquir Immune Defic Syndr. 2002 Mar 1;29(3):221–31. doi: 10.1097/00126334-200203010-00002. [DOI] [PubMed] [Google Scholar]
- 82.Arno A, Ruiz L, Juan M, Jou A, Balague M, Zayat MK, et al. Efficacy of low-dose subcutaneous interleukin-2 to treat advanced human immunodeficiency virus type 1 in persons with </=250/microL CD4 T cells and undetectable plasma virus load. J Infect Dis. 1999 Jul;180(1):56–60. doi: 10.1086/314831. [DOI] [PubMed] [Google Scholar]
- 83.Davey RT, Jr., Murphy RL, Graziano FM, Boswell SL, Pavia AT, Cancio M, et al. Immunologic and virologic effects of subcutaneous interleukin 2 in combination with antiretroviral therapy: A randomized controlled trial. Jama. 2000 Jul 12;284(2):183–9. doi: 10.1001/jama.284.2.183. [DOI] [PubMed] [Google Scholar]
- 84.Emery S, Capra WB, Cooper DA, Mitsuyasu RT, Kovacs JA, Vig P, et al. Pooled analysis of 3 randomized, controlled trials of interleukin-2 therapy in adult human immunodeficiency virus type 1 disease. J Infect Dis. 2000 Aug;182(2):428–34. doi: 10.1086/315736. [DOI] [PubMed] [Google Scholar]
- 85.Kovacs JA, Vogel S, Albert JM, Falloon J, Davey RT, Jr., Walker RE, et al. Controlled trial of interleukin-2 infusions in patients infected with the human immunodeficiency virus. N Engl J Med. 1996 Oct 31;335(18):1350–6. doi: 10.1056/NEJM199610313351803. [DOI] [PubMed] [Google Scholar]
- 86.Levy Y, Capitant C, Houhou S, Carriere I, Viard JP, Goujard C, et al. Comparison of subcutaneous and intravenous interleukin-2 in asymptomatic HIV-1 infection: a randomised controlled trial. ANRS 048 study group. Lancet. 1999 Jun 5;353(9168):1923–9. doi: 10.1016/s0140-6736(98)07345-0. [DOI] [PubMed] [Google Scholar]
- 87.Losso MH, Belloso WH, Emery S, Benetucci JA, Cahn PE, Lasala MC, et al. A randomized, controlled, phase II trial comparing escalating doses of subcutaneous interleukin-2 plus antiretrovirals versus antiretrovirals alone in human immunodeficiency virus-infected patients with CD4+ cell counts >/=350/mm3. J Infect Dis. 2000 May;181(5):1614–21. doi: 10.1086/315430. [DOI] [PubMed] [Google Scholar]
- 88.Ruxrungtham K, Suwanagool S, Tavel JA, Chuenyam M, Kroon E, Ubolyam S, et al. A randomized, controlled 24-week study of intermittent subcutaneous interleukin-2 in HIV-1 infected patients in Thailand. Aids. 2000 Nov 10;14(16):2509–13. doi: 10.1097/00002030-200011100-00013. [DOI] [PubMed] [Google Scholar]
- 89.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–9. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 90.Chirgwin KD, Feldman J, Dehovitz JA, Minkoff H, Landesman SH. Incidence and risk factors for heterosexually acquired HIV in an inner-city cohort of women: temporal association with pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Mar 1;20(3):295–9. doi: 10.1097/00042560-199903010-00013. [DOI] [PubMed] [Google Scholar]
- 91.Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999 Aug 1;150(3):306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- 92.Vernazza PL, Eron JJ, Fiscus SA. Sensitive method for the detection of infectious HIV in semen of seropositive individuals. J Virol Methods. 1996 Jan;56(1):33–40. doi: 10.1016/0166-0934(95)01899-9. [DOI] [PubMed] [Google Scholar]
- 93.Tachet A, Dulioust E, Salmon D, De Almeida M, Rivalland S, Finkielsztejn L, et al. Detection and quantification of HIV-1 in semen: identification of a subpopulation of men at high potential risk of viral sexual transmission. Aids. 1999 May 7;13(7):823–31. doi: 10.1097/00002030-199905070-00012. [DOI] [PubMed] [Google Scholar]
- 94.Hart CE, Lennox JL, Pratt-Palmore M, Wright TC, Schinazi RF, Evans-Strickfaden T, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999 Apr;179(4):871–82. doi: 10.1086/314656. [DOI] [PubMed] [Google Scholar]
- 95.East L, Isacke CM. The mannose receptor family. Biochim Biophys Acta. 2002 Sep 19;1572(2-3):364–86. doi: 10.1016/s0304-4165(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 96.Linehan SA, Martinez-Pomares L, Gordon S. Mannose receptor and scavenger receptor: two macrophage pattern recognition receptors with diverse functions in tissue homeostasis and host defense. Adv Exp Med Biol. 2000;479:1–14. doi: 10.1007/0-306-46831-X_1. [DOI] [PubMed] [Google Scholar]
- 97.Snyderman R, PM, Altman LC. Abnormalities of leukocyte chemotaxis in human disease. Ann N Y Acad Sci. 1975 Jun 13;256:386–401. doi: 10.1111/j.1749-6632.1975.tb36065.x. [DOI] [PubMed] [Google Scholar]
- 98.Niedel JE, Kahane I, Cuatrecasas P. Receptor-mediated internalization of fluorescent chemotactic peptide by human neutrophils. Science. 1979 Sep 28;205(4413):1412–4. doi: 10.1126/science.472759. [DOI] [PubMed] [Google Scholar]
- 99.Niedel J, Wilkinson S, Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–6. [PubMed] [Google Scholar]
- 100.Hammache D, Yahi N, Maresca M, Pieroni G, Fantini J. Human erythrocyte glycosphingolipids as alternative cofactors for human immunodeficiency virus type 1 (HIV-1) entry: evidence for CD4-induced interactions between HIV-1 gp120 and reconstituted membrane microdomains of glycosphingolipids (Gb3 and GM3) J Virol. 1999 Jun;73(6):5244–8. doi: 10.1128/jvi.73.6.5244-5248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Puri A, Hug P, Jernigan K, Barchi J, Kim HY, Hamilton J, et al. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14435–40. doi: 10.1073/pnas.95.24.14435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yahi N, Baghdiguian S, Moreau H, Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992 Aug;66(8):4848–54. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nehete PN, Vela EM, Hossain MM, Sarkar AK, Yahi N, Fantini J, et al. A post-CD4-binding step involving interaction of the V3 region of viral gp120 with host cell surface glycosphingolipids is common to entry and infection by diverse HIV-1 strains. Antiviral Res. 2002 Dec;56(3):233–51. doi: 10.1016/s0166-3542(02)00130-4. [DOI] [PubMed] [Google Scholar]
- 104.Leahy DJ. A structural view of CD4 and CD8. FASEB J. 1995 Jan;9(1):17–25. doi: 10.1096/fasebj.9.1.7821755. [DOI] [PubMed] [Google Scholar]
- 105.Arthos J, Deen KC, Chaikin MA, Fornwald JA, Sathe G, Sattentau QJ, et al. Identification of the residues in human CD4 critical for the binding of HIV. Cell. 1989 May 5;57(3):469–81. doi: 10.1016/0092-8674(89)90922-7. [DOI] [PubMed] [Google Scholar]
- 106.Clayton LK, Sieh M, Pious DA, Reinherz EL. Identification of human CD4 residues affecting class II MHC versus HIV-1 gp120 binding. Nature. 1989 Jun 15;339(6225):548–51. doi: 10.1038/339548a0. [DOI] [PubMed] [Google Scholar]
- 107.Mizukami T, Fuerst TR, Berger EA, Moss B. Binding region for human immunodeficiency virus (HIV) and epitopes for HIV-blocking monoclonal antibodies of the CD4 molecule defined by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9273–7. doi: 10.1073/pnas.85.23.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monnet C, Laune D, Laroche-Traineau J, Biard-Piechaczyk M, Briant L, Bes C, et al. Synthetic peptides derived from the variable regions of an anti-CD4 monoclonal antibody bind to CD4 and inhibit HIV-1 promoter activation in virus-infected cells. J Biol Chem. 1999 Feb 5;274(6):3789–96. doi: 10.1074/jbc.274.6.3789. [DOI] [PubMed] [Google Scholar]
- 109.Bast RC, HJ, Frei E. Cancer Medicine. (5th ed) 2000 [Google Scholar]
- 110.Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci. 1986 Nov;75(11):1028–40. doi: 10.1002/jps.2600751104. [DOI] [PubMed] [Google Scholar]
- 111.Annan KA. Africa, AIDS Has a Woman's Face. The New York Times; Dec 29, 2002. 2002;Sect. 9. [Google Scholar]
- 112.Pilcher CD, Tien HC, Eron JJ, Jr., Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004 May 15;189(10):1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]