Abstract
Microscopy, especially fluorescence microscopy, has proven to be a powerful method for studying biological processes. Unfortunately, some of the same features that make biological membranes powerful (for example, all of the action taking place across a narrow 4 nm film) also make it difficult to visualize by fluorescence. Over the past 30 years numerous tricks have been developed to narrow the plane over which data is collected. One approach is particularly well suited for studying membrane events: total internal reflection fluorescence microscopy. A key issue to address, when using TIR to tackle a new biological problem is: How can one judge whether the signals being observed are actually the biological phenomena that one wishes to study?
Introduction
Membranes, essential to the development of life, share three critical, unique features. First, by restricting various cellular components to a two-dimensional plane, they substantially increase the rates of reactions 1. Reaction rates are essential for life. If the same components were diluted into three-dimensions such rates would be substantially lower. Second, membranes do not let ions pass easily through their hydrocarbon core. If a transporter spanning the membrane can segregate an ion to one side or another, the membrane remains charged, like a capacitor, primed to deliver energy when required. This capability allows membranes to be the core of energy generation in the electron transport chain of bacteria, mitochondria and chloroplasts. Third, membranes act as barriers to diffusion, allowing cells to segregate and protect key components including their energy reserves (in the form of nucleoside triphosphates) and genetic information (also in the form of nucleoside triphosphates). By blocking diffusion, and regulating the kinds of molecules that can pass and the kinds of messengers that can be recognized, membranes, in essence, help define the cell.
As a consequence of their importance, membranes have been the subject of considerable study. Their ability to selectively modify their permeability was a focus of some of the leading physiologists and physicists of the nineteenth century. In the mid-twentieth century, the studies of the Hodgkin, Huxley and Katz on the propagation of the action potential and the transmission of signals across the synapse gave us our first look at molecular-level events as they demonstrated that they could quantitatively reconstitute biological events and even predict the existence and properties of single individual molecules and their actions 2-4. Subsequent reconstitutions of ion channels into planar lipid bilayers in the1960’s 5-8 and then the ability to patch clamp single ion channels in situ 9 opened up a new world of molecular physiology.
The study of individual ion channels revealed the power of studying single events. Significant insights could be gleaned from the study of single events that were inaccessible from studying macroscopic events. For example, sometimes a molecular event can exist in two states. In that case, the average activity – the mean – reports a state between the two: the mean is meaningless in the sense that it is a value that does not exist in nature. For example, ion channels exist in two states: open or closed. When a macroscopic current is used to calculate the average current per channel, it yields a value between the two – a value that does not exist for any individual channel. This kind of information is accessible from studying single events. Other times, the average event does represent the dominant state of a molecule. But in biology the important event is often a transient one localized in time or space. For a neuron that fires an action potential every second, its voltage-dependent sodium channels are closed most of the time, opening only for the 500 microseconds of the action potential. Thus, they are open 0.05% of the time. A recording of the average current might indicate that the sodium channels are basically quiescent. At 0.05%, frequency, it is a rare yet clearly critical event.
Thanks to the sensitivity of electrophysiology, the study of ion channels is most amenable to studying individual events. Alas, the same is not true for many other membrane-bound activities of the cell such as exocytosis, endocytosis, signal transduction, and viral assembly. A number of creative techniques have used electrophysiology to study these events, such as quantifying the capacitance of the cell membrane to determine when a vesicle fuses (exocytosis) or internalizes (endocytosis) 10. Such an assay could resolve when the event takes place. However, it lacks the spatial sensitivity to say where it occurs or the ability to study the molecules involved in the process.
Microscopy, especially fluorescence microscopy, has proven to be a powerful method for studying biological processes. Unfortunately, some of the same features that make biological membranes powerful (for example, all of the action taking place across a narrow 4 nm film) also make it difficult to visualize by fluorescence. The focal plane of most lenses is many hundreds of nanometers. This means that most of the fluorescence captured will be originated from somewhere other than in the plane of the membrane. This significantly compromises the ratio of the signal (what is happening at the 4 nm membrane) to noise (the other 500 nm that is producing fluorescence).
Over the past 30 years numerous tricks have been developed to narrow the optical volume over which data is collected. One approach is particularly well suited for studying membrane events: total internal reflection fluorescence microscopy (TIRF). This approach succeeds by limiting the extent of the excitation to a narrow region above the cover slip. When light propagating through glass is reflected off an interface with media of lower refraction index, such as water, at a steep enough angle, the light will completely reflect. No light will be propagated on the other side (see side box 1). However, while no light is propagated, there is a field of light above the coverslip that decays exponentially into the aqueous milieu. The depth of this field can be restricted to tens of nanometers. This narrows the volume of fluorophores that are excited and, as result, significantly improves the signal-to-noise for events right at the membrane. This improved signal-to-noise makes it particularly optimal for studying vesicles or viruses whose size is comparable to the space constant for decay. This technique has been used to study a myriad of problems including movement of polymers near an interface 11, cell substrate interactions 12, exocytosis 13, endocytosis 14,15 and viral assembly 16 . There are number of ways by which total internal reflection can be achieved (see Box 2 for a discussion of various approaches). These have been covered in a series of reviews by Dan Axelrod who has been developing and championing this technique for the past 30 years 17. Additionally there are hands-on guides to TIR microscopy 18.
BOX 1. Total Internal Reflection Fluorescence Microscopy.
When light travels from a media of higher to lower refractive index some of the light will reflect and some will be transmitted to the other side, albeit refracted. There is a critical angle (Θc, which is measured relative to the normal to the surface) at which the refracted beam is parallel to the surface: no light is propagated on the other side. This angle=arcsin (n2/n1) where n2 is the refractive index of the lower refractive index and n1 is the media of higher refractive index. For borosilicate glass with n1=1.515 if the media on the other side is water (1.33) the critical angle is 61.4°; A cell with refractive index 1.36 would yield a critical angle of 63.9°. Cells are not homogeneous and some regions may have a refractive index as high as 1.39 yielding a critical angle of 66.6°.
While no light energy is propagated on the other side, there is a field that decays exponentially on the other side. Since it decays to “nothingness” it is referred to as an evanescent field. The space constant, d, over which it decays is a function both of the refractive indices of the media and the wavelength (λ) of light.
For light propagating from glass (n=1.515) into water (1.33) at an angle of 66°, the space constant for decay is 100 nm.
Box 2. Obtaining the evanescent field excitation required for TIR microscopy.
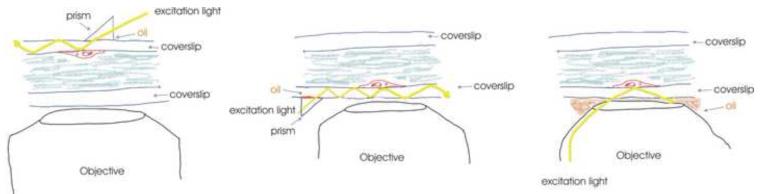
There are a number of ways of obtaining the evanescent field excitation required for TIR microscopy, three of which are illustrated in Figure I. A: The excitation wave can be via a prism on the opposite side of the cell from the objective. This allows tremendous freedom for adjusting the angle of light. However, it limits how close the objective can get to the sample, and thus limits the numerical aperture (NA) of the objective (how much light it can gather). B: A prism is used to the side of the objective. This allows the objective to be brought closer in to the sample and thus higher NA water immersion lens can be used. This approach is technically more challenging as any spread of oil on the coverslip surface will disrupt the illumination. C: A high NA objective (1.45 NA or higher) is sufficient to bring in a laser beam at a steep enough angle to achieve total internal reflection illumination. This is the easiest approach to setup. However, it limits the angle at which the light can be brought in. For further details see 18.
There has been a rapid increase in the implementation of TIR both due to the growing appreciation of the power of the technique and the availability of commercial instruments for TIR fluorescence microscopy. On a first pass, the theory and implementation of TIR fluorescence microscopy is straightforward. However, there are two major issues that should be attended to by any user. First, interpreting the magnitude of the measured fluorescence can be considerably more problematic for TIR than for other forms of fluorescence microscopy. For TIR the intensity of the fluorescence is the product of many factors other than the number of fluororphores (See Box 3 for further discussion of many of these factors). Second, since TIR is often used to detect an event that has not been previously studied, , a key issue to address is: How can one judge whether the signals being observed are actually the biological phenomena that one wishes to study. (or that you promised the study section/your department chair/your donors/your grandmother that you would study).
Box 3. What does the intensity of the fluorescence emission from TIR excitation report? (The greatest strengths of TIR are also its greatest limitations).
The power of TIR comes from the creation of an excitatory field that decays exponentially perpendicular to the cover slip over a distance that is very short, even relative to the size of the wavelength of light. This significantly reduces fluorescence from background fluorophores and improves the relative size of the signal. This also means that slight movements of the fluorophore – even on the scale of nanometers – will change the magnitude of the excitation and thus the observed fluorescent emission. If one is following a fluorophore one might suspect that you could ‘guesstimate’ the changes in distance of the fluorescent object as a function of the intensity of its fluorescence. This could potentially be used as a method for following the movement of a fluorescent object in z, perpendicular to the coverslip. However, there are number of other factors that can and often do affect the intensity of the fluorescent emission, some of these are specific to the excitation by the evanescent field. The following is a brief listing of some of the other factors that could affect the magnitude of the fluorescence observed in a particular pixel:
The number of fluorophores: If the number of fluorescent tags is increasing or decreasing in a particular voxel (a volume from which one is collecting photons), that would also alter the emission.
The orientation of the fluorophore: The polarization of the excitation beam will determine if the evanescent field is polarized parallel to the coverslip or perpendicular. The efficacy of excitation is affected by the angle between the polarization of the excitation and the excitation dipole of the fluorophore. A rotation of your fluorophore can significantly affect the excitation and thus the fluorescence emission.
The local environment: The presence of other macromolecular complexes, such as a membrane-bound vesicles, will locally change the refractive index. Immediately at a glass-solution interface where the incident angle is high enough, total internal reflection will occur. However, if within the evanescent field there are objects of higher refractive index, that could result in either “frustrated” total internal reflection, with the local generation of a propagated wave, or an evanescent field that decays further away from the coverslip. Either circumstance will increase the local excitation of fluorophores.
The proximity of the fluorophore to the coverslip: The evanescent field is narrow enough that the proximity to the coverslip will affect the capture of light. The efficiency of acquisition and therefore affect the brightness of the emission are affected by the distance of the fluorophore from the coverslip, the orientation of the fluorophore, and even the angle at which the emission light enters the coverslip30.
Some of the most useful advances in fluorescent microscopy have been the result of the increased use of quantification. Fluorescence microscopy is no longer treated simply as a “pretty picture” but instead as a valuable quantitative tool. Unfortunately, TIR, which gives such a strong signal relative to noise, can be much more problematic when quantifying fluorescence intensity. For the reasons given above, it is not trivial to determine if alterations in fluorescence intensity are the consequence of movements in z, of rotation of the fluorophore, of changes in the number of fluorophores, changes in the environment near the fluorophores or changes in the proximity to the coverslip. An important critical challenge for the future is to develop the protocols for analyzing the magnitude of fluorescent signals from TIR
The short answer is simple: One never knows. However, in a slightly longer form, the key is to establish a series of criteria to judge if a particular event will or will not be relevant. That way, as long as other experimenters use the same criteria, they should observe the same results. Others may disagree with some or all of one’s criteria. However, it becomes a platform for discussion. I will illustrate this approach using two different examples: imaging single viruses as they assemble and imaging single secretory vesicles as they exocytose to release their contents.
Assembly of HIV-1
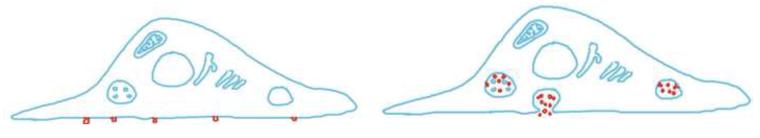
In infected cells, HIV-1 is observed both on the cell surface and in internal organelles, in particular multivesicular bodies. For a number of years there was a debate as to whether HIV-1 assembled within the multivesicular bodies, and was then secreted, similar to exosomes, or whether it assembled directly on the cell surface 19. The two hypotheses make very different predictions of what should be seen if one observes the surface of the cell with total internal reflection fluorescence microscopy. If the virus assembles on internal organelles, and is then released by secretion, one should observe a burst of viruses appearing on the surface with each secretory event (Fig 1, left). If the virus assembles on the surface of the cell, then new viruses should appear one at a time (Fig 1 right).
Fig 1. Two models for the assembly of HIV-1.
Left: Model 1: HIV buds off the plasma membrane. Prediction: Viruses should appear at the surface one-at-a-time. Right: Model 2: HIV buds into multivesicular bodies (CD63+ compartment) and they get released from the cells by exocytosis. Prediction: Multiple viruses should be delivered to the surface simultaneously.
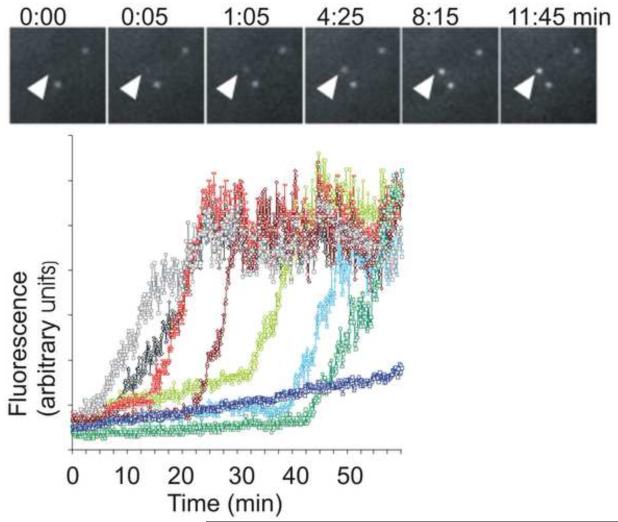
To observe the appearance of HIV-1, a GFP was inserted within the coding region of the HIV Gag protein. The Gag-GFP was either expressed on its own or from a plasmid that encodes the complete HIV genome. [Expression of Gag (which is subsequently cleaved to form a number of different proteins), is sufficient to form viral-like particles.] With either approach, when total internal reflection fluorescence microscopy was used to illuminate the basal surface, the GFP fluorescence was first detected as a diffuse glow in the cytosol and the surface of the cell. Then individual spots were observed to form and brighten on the plasma membrane. Each spot took 6-8 minutes until it reached a steady-state fluorescence (Fig 2). The spots only appeared one-at-a-time, they never appeared as a group of spots together. At first glance this might suggest that the virus only assembles on the plasma membrane surface and therefore resolves the debate as to the site of assembly of HIV-1. However, how would one judge which of these puncta of fluorescence were assembling virions of HIV-1? No one had observed a virus assemble before. How would one determine if these fluorescent spots are assembling viruses? Indeed, when a number of different proteins are expressed as a fusion to the fluorescent proteins, it is not unusual for them to form fluorescent aggregates of the expressed protein. The problem was perhaps further complicated by the observation that there were two different patterns of fluorescent spots: One population, as mentioned before, slowly appeared over a 5-6 minute period and a second population that appeared and disappeared with a time period of seconds. This is where it becomes important to establish criteria for judging the phenomena to be studied.
Fig 2. Gag-GFP fluorescence plots to track appearance of HIV-1 at the cell surface.
A. A series of images of three puncta over time. B. Quantification of the fluorescence intensity in seven different puncta over time. Each spot of Gag-GFP fluorescence takes a stereotypic amount of time to reach its steady state which is consistent across different cells. HIV-Gag-GFP synthesized in the cell accumulates at the plasma membrane. As a critical concentration is reached, the Gag forms into discrete puncta at the surface. [from: Jouvenet, 2008].
To determine if a particular fluorescence was formation of a virus we established the following criteria:
Criterion 1: If this was an assembling virus, then only a fixed number of molecules should be recruited. This establishes two experimentally testable predictions. First, the fluorescence should always reach the same steady-state at the same rate for each spot. Second, once assembled, no further molecules should be recruited.
Criterion 2: If this was an assembling virus, then the Gag molecules should be packing at molecular distances of nm. Thus, even if only a subset of the molecules were labeled with fluorescent proteins, there should be a considerable fluorescence resonance energy transfer (FRET) between the molecules.
Criterion 3: If this an assembling virus then it should septate off from the cytosol: The interior of the virus should not be continuous with the inside of the cell. Once fully assembled, no molecules, no matter how small, should be able to exchange from the inside of the virus and the inside of the virus.
Criterion 1: Recruitment of a fixed number of molecules
Each of the assembling spots always took a similar time to reach its steady-state fluorescence and each reached the same level of fluorescence. Neither of these observations is consistent with the fluorescent spots being aggregates of overexpressed protein. However, a steady-state could be the consequence of new molecules joining an aggregate at the same rate that others leave. On the other hand, if this was a virus that has assembled, there should be no further exchange of molecules. Thus, if a fluorescent spot was photobleached it should not recover. When spots of Gag that had reached their steady-state fluorescence were photobleached, they did not recover 16. This suggests that the steady level of fluorescence was the consequence of recruitment of a set number of fluorophores and not a steady-state with fluorophores partitioning in and out of the spot. In contrast, spots that were still in the process of increasing their fluorescence (still assembling), recovered the fraction of their fluorescence that they had not yet recruited, and no more 16. Together these results indicate a fixed number of fluorophores recruited per fluorescent spot.
Criterion 2: The Gag molecules should be packed at molecular distances
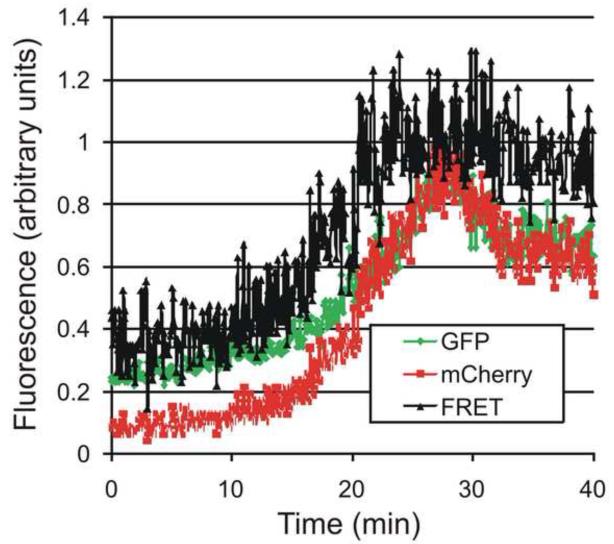
It is estimated that there are 5,000 Gag molecules in a 110 nm diameter virion, which yields an average distance between Gag molecules of a little over 2nm. This tight packing is consistent with the observation that it is problematic to make a virus in which all of the copies of Gag are tagged with a GFP 20: It has been necessary to use a mixture of Gag and Gag-GFP. At this tight packing, there should be a significant level of FRET between the fluorophores. This FRET should be further strengthened by the packing of Gag within a large two-dimensional matrix leading to many interactions. The high degree of FRET expected opens the possibility of using two fluorescent proteins that do not have as much overlap between the emission of the donor and the excitation of the acceptor as in the case of the fluorescent proteins frequently used, CFP and YFP. The use of two fluorescent proteins whose spectra are spread further apart would have the disadvantage of a lower FRET signal. However, it would also mean that less of the acceptor will be directly excited by the laser that is used to excite the donor (thereby lowering the non-specific signal) and there will be a decreased emission of the donor that is overlapping into the region of the emission of the acceptor (thereby also lowering the non-specific signal). For the experiment a mixture of gag was used that included wt GAG, Gag GFP : Gag mCherry. If only Gag-EGFP is expressed, with an excitation at 488nm, the green fluorescence increased, as observed previously. If only Gag-mCherry was expressed, with an excitation of 488 nm, there was no detectable change in fluorescence. However, when both Gag-EGFP and Gag-mCherry were expressed, with an excitation at 488nm, first there was an increase of the emission of the GFP fluorescence (see figure 3). Then a few minutes later the mCherry fluorescence starts to increase and it increases faster than the increase of GFP emission. This results in an increase of the ratio of the emission of mCherry:GFP. As an independent test for FRET, photobleaching the mCherry increased emission of the GFP. The FRET was observed even with Gag-GFP and Gag mCherry diluted with unlabeled Gag, indicating that the Gag is packing at molecular distances (< 10 nm).
Fig 3. Using FRET to show that Gag molecules are packed at molecular distances (< 10 nm).
With excitation at 488 nm, there is first an increase of fluorescence of the GFP-Gag, and then the fluorescence of the mCherry-Gag increases. (This figure adapted from 16,38 which includes additional controls for FRET).
Criterion 3: If this an assembling virion then these spots of fluorescence should septate off from the cytosol
The approach was to test if a small molecule could move between the cytosol and the inside of the presumed virion. As a probe, we used the smallest molecule we could access: a proton. The strategy was to label the Gag with a GFP called pHluorin that is sensitive to pH 21 and then acidify the cytosol. To preferentially acidify the cytosol, the partial pressure of CO2 above the cells was varied. The high cytosolic activity of carbonic anhydrase ensures that the reaction CO2 + 2 H20 ↔ H3O+ + HCO3 occurs more rapidly in the cytosol than outside. When the pCO2 was altered, the Gag-pHluorin that was still diffuse in the cytosol was rapidly altered in its fluorescence. In contrast, when virions, or viral like-particles, containing the Gag-pHluorin were collected from the supernatant, their fluorescence changed at only 20% of the rate of the Gag-pHluorin that was exposed to the cytosol. When the same test was applied to those spots that were still increasing in fluorescence, they were as responsive to the pCO2 as any of the Gag-pHluorin that was exposed to the cytosol. However, once these spots reached their steady-state fluorescence, their sensitivity to pCO2 dropped to the level of the virions that had been collected from the supernatant: Even though they had not moved, they had lost their connection to the cytosol. Protons in the cytosol were no longer accessible to the Gag-pHluorin in the virions: they had separated from the cell.
If it is accepted that these three criteria can be used to determine if a particular puncta of Gag-fluorescence represents an assembling virion, then it becomes possible to establish different benchmarks in the life-cycle of the virus: When is the genome recruited? When are various components of the host cellular machinery recruited? Does the HIV protease always cut its target sites in a described order, or are they cut stochastically? Is the protease activated before or after scission from the cell? These questions explore the biology of the virus which is the subject of a different article. What is relevant here is that the establishment of clear criteria (whether they are eventually accepted or not), allow a viral assembly event to be defined and examined in terms that can be critically examined and tested by different labs. What about the second population of fluorescent spots that were rapidly appearing and disappearing? All of those spots of Gag-GFP co-localized with the proteins CD63 and clathrin, markers for internal organelles. The Gag-GFP only enters these compartments after appearance at the cell surface. When endocytosis is blocked, the Gag-GFP does not go to the internal organelles and release of virions from the cell continues unaffected 19.
Exocytosis From Secretory Vesicles
The first application to which we had tried to apply total internal reflection fluorescence microscopy was secretion from exocytic vesicles. The experimental prediction seemed straightforward: The excitatory field created by TIR decays over the distance roughly the same thickness as a secretory vesicle. When a vesicle containing fluorescent cargo reached the cell surface, it should be observed to get brighter as it approaches the membrane (See Fig 4A, right hand cartoon). Upon fusion to the cell surface, there should be a rapid brightening as the cargo moves closer to the coverslip, and subsequent dimming as the cargo diffuses away.
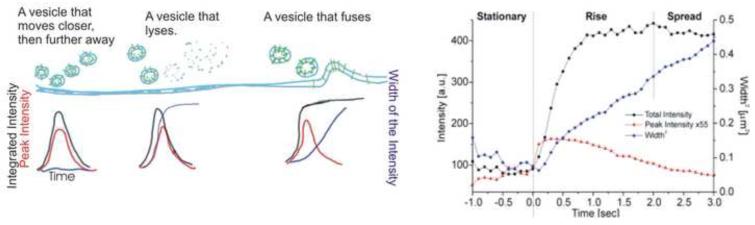
Fig 4. Models for secretion from exocytic vesicles.
A. When a fluorescently tagged vesicle approaches the membrane, it should increase in peak fluorescence as it enters the evanescent field used for TIR excitation. Subsequently, depending upon whether the vesicle simply approaches and moves away (left), approaches and lysed by photodamage (center) or fuses (right), there are specific predicted changes in the peak intensity of the vesicular fluorescence (red), intensity integrated locally (black) and area of the fluorescence (blue). B. The experimentally observed values. The total fluorecence (black) increases, and stays up, meaning that all of the fluorescent membarne proteins in the vesicle have been delivered to plasma membrane. The area of the fluorescence (blue) increases linearly with time indicating that the proteins are diffusing away in the plane of the membrane. Adapted from Schmoranzer 24.
In the first experiment cells were incubated with acridine orange: A fluorescent base which accumulates in the acidified lumen of secretory organelles. While illuminating the bottom surface of the cell, bright fluorescent spots were observed to slowly get brighter, then within a hundred milliseconds they got much brighter and then the fluorescence diffused laterally 22. One-by-one each vesicle flashed brightly, and then the fluorescence diffused laterally. This was one of the first times that an experiment had “worked” (meaning it gave the expected response) the very first time, so the experiment was repeated with a second group of cells, again successfully. And then a third group of cells, successfully. At this point a different cargo was chosen: daunorubicin. Daunorubicin is a chemotherapeutic drug which happens to be fluorescent. Since it is an alkaloid, a weak base, it also accumulates in the acidified secretory organelles 23. Once again, one-by-one each vesicle was observed to quickly “flash” (a brightening of its fluorescence), prior to a lateral spread. This was the way every experiment should work.
The problem with the approach was that we were looking for an experimental observation that was consistent with our prediction. However, a set of rigorous criteria had not been established to evaluate the signal that was being observed. With reflection, it was clear that all of the observations were consistent with an independent hypothesis: That as the vesicle, loaded with its fluorophore, approached the membrane, the light excitation was causing photodamage which compromised the integrity of the vesicle, thereby releasing the contents (see Figure 4A, middle panel). Once again it was important to establish criteria for judging the phenomena to be studied.
To determine whether a secretory vesicle was fusing or lysing we monitored the fluorescence of a membrane protein. If this was a vesicle that was fusing, then all of the vesicular membrane proteins should be delivered to and become membrane proteins in the plasma membrane. If this was a vesicle that ruptured, then the vesicular membrane proteins would not be delivered to the plasma membrane. This would be judged by the following criteria 22,24.
Criterion 1
If all of the vesicular membrane proteins were delivered to the plasma membrane, then if one integrated the vesicular membrane protein fluorescence over the region of fusion, the fluorescence should increase as the vesicle fuses, and then remain high. If the fluorophores were delivered to the plasma membrane, then the fluorescence should remain at a constant level not decrease (until the proteins diffused out of the area over which fluorescence was being integrated). If the vesicle was lysing or rupturing, the fluorophores would not be delivered to the plasma membrane.
Criterion 2
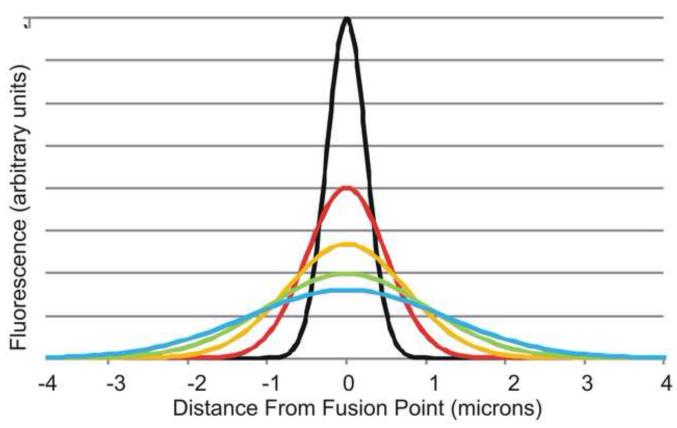
If all of the vesicular membrane proteins were delivered the plasma membrane, then the proteins should defuse laterally in the plane of the membrane. The fusion of the vesicle would be the equivalent of delivering a large number of fluorescent membrane proteins to a single spot (or at least, a single spot relative to the resolution of the microscope). These proteins would then diffuse laterally in the plane of the membrane giving a Gaussian distribution (Fig 5 at right) where the intensity of the fluorescence, I, is a function of the diffusion constant D, the time t and the radial distance,r, from the fusion point:
If one plots the area of the fluorescence (or more precisely, the (1/2 width)2, this should increase linearly with time and the slope should be four times the diffusion constant (See Fig 4A, right figure). Integral membrane proteins diffuse relatively slowly in the plane of the membrane (order of magnitude 10-9 cm2/sec) relatively to the faster diffusion of proteins in the cytosol.
Fig 5. Lateral diffusion of vesicular membrane proteins after delivery to the plasma membrane.
When a vesicle with fluorescently labeled membrane proteins first approaches the membrane, even if the vesicle is much smaller than the wavelength of light, the optical properties of the objective (its point-spread function) will result in a Gaussian shape for the fluorescence of the vesicle (the center black line). Upon fusion of the vesicle, the membrane proteins of the vesicle will diffuse laterally in the plane of the plasma membrane. Over time the proteins will diffuse laterally distributing first as the red line, then the orange, then the green and cyan until the proteins are evenly diffuse through the plasma membrane.
How well does this work in practice? The first test with a membrane protein used a viral membrane protein, the Vesicular Stomatitus Viral Glycoprotein fused to a GFP. In each case, when the fluorescence of the membrane protein was integrated locally in a region, the fluorescence increased, and then stayed high. All of the fluorophores were delivered to the surface and none were lost (black line in fig 4B). When the area (1/2 width)2, was plotted over time, it increased linearly and yielded a diffusion constant of 1.1*10-9 cm2/sec (blue line in Fig 4b). This experiment has been repeated with many membrane proteins including LDL receptor, NCAM, P75 (neurotropin receptor), transferrin receptor, Glut4 24-29. In all cases all of the fluorophores were delivered to the plasma membrane and in all cases the slope of the (1/2 width)2, as a function of time increased linearly and matched the known diffusion constant of the membrane protein. Thus, based on the two criteria stated above, all of these should represent fusion of a vesicle to the plasma membrane.
So what about the original experiments with acridine orange or daunomycin? Were they reporting fusion of the vesicle or rupture? To test this possibility, the experiments were repeated while simultaneously labeling the vesicles with a fluorescent membrane and one of the luminal probes (either acridine orange or daunomycin). The luminal cargo was observed to “flash brightly” and then spread laterally. However, in the presence of the luminal probes a significant fraction of the vesicles did not meet the criteria for fusion 22. First, the membrane probes were not delivered to the plasma membrane (there was no increase of the membrane reporter in the plane of the plasma membrane and the membrane reporters did not spread laterally, as predicted by diffusion in a two-dimensional plane). Second, the frequency of rupture was directly proportional the intensity of the excitation light, once again, not consistent with fusion. In retrospect, many of our original observations, fortunately never published, were likely of light-induced lysis of vesicles and not, as originally thought, vesicular fusion to the plasma membrane.
Concluding remarks
In recent years there have been rapid advances in imaging modalities and methods for labeling molecules. As result, many biological processes are being studied that were considered inaccessible just a short time ago. Total internal reflection fluorescence microscopy is one of the most powerful techniques for imaging events at the surface of a cell and its potential has yet to be fully explored (Box 4). With the added power of these techniques comes added caution. These results demonstrate the necessity of setting up clear criteria for evaluating whether a specific fluorescent event will be determined to represent the physiological event one hopes to study. There may always be other explanations that can account for the data. There may always be other assumptions for which we are not yet aware. As long as one carefully delineates the criteria to be used in evaluating data, one can rest assured that if anyone else repeats the experiment, as long as they use the same criteria they will get a similar answer.
Box 4. Imagi(ni)ng the future of TIR.
John Tyndal, who is accredited for explaining why the sky is blue, gave the earliest recorded demonstration of the phenomena of total internal reflection in 1854 31. It was not until 100 years later that Hirschfeld used it to study the interface between a solid and liquid 32. Starting with a seminal publication in 1981 12, Dan Axelrod has provided a series of papers that have both provided a firmer foundation for the theory of TIR as well as extend its capabilities 12,33-36.
Two potentials of total internal reflection microscopy that have yet to be fully exploited include structured illumination 37 and polarized illumination. One article of note from ten years ago presented an elegant example of using total internal reflection illumination with two different polarized fields (“s” and “p” polarization) to probe the orientation of molecules near membranes 33. This approach has the potential to reveal previously inaccessible fine structural dynamics of the molecules at the cell surface.
Figure I Box 2.
Ways to obtain the evanescent field excitation required for TIR microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Berg HC, Purcell EM. Physics of chemoreception. Biophys. J. 1977;20:193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodgkin AL, et al. Ionic currents underlying activity in the giant axon of the squid. Arch. Sci. Physiol. 1949;3:129–150. [Google Scholar]
- 3.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fatt P, Katz B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. (Lond. ) 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller P, et al. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature (London) 1962;194:979–980. doi: 10.1038/194979a0. [DOI] [PubMed] [Google Scholar]
- 6.Mueller P, Rudin DO. Action potential phenomena in experimental bimolecular lipid membranes. Nature (London) 1967;213:603–604. doi: 10.1038/213603a0. [DOI] [PubMed] [Google Scholar]
- 7.Bean RC, et al. Discrete conductance fluctuations in lipid bilayer protein membranes. J. Gen. Physiol. 1969;53:741–757. doi: 10.1085/jgp.53.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latorre R, et al. Ion transport through excitability-inducing material (EIM) channels in lipid bilayer membranes. The Journal of General Physiology. 1972;60:72–85. doi: 10.1085/jgp.60.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neher E, Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature (London) 1976;260:799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- 10.Neher E, Marty A. Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6712–6716. doi: 10.1073/pnas.79.21.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migler KB, et al. Slip transition of a polymer melt under shear stress. Phys. Rev. Lett. 1993;70:287–290. doi: 10.1103/PhysRevLett.70.287. [DOI] [PubMed] [Google Scholar]
- 12.Axelrod D. Cell-substrate contacts illuminated by total internal reflection fluorescence. J. Cell Biol. 1981;89:141–145. doi: 10.1083/jcb.89.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steyer JA, et al. Transport, docking and exocytosis of single secretory granules in live chromaffin cells. Nature (London) 1997;388:474–478. doi: 10.1038/41329. [DOI] [PubMed] [Google Scholar]
- 14.Merrifield CJ, et al. Imaging actin and dynamin recruitment during invagination of single clathrin-coated pits. Nat. Cell Biol. 2002;4:691–698. doi: 10.1038/ncb837. [DOI] [PubMed] [Google Scholar]
- 15.Rappoport JZ, Simon SM. Real-time analysis of clathrin-mediated endocytosis during cell migration. J. Cell Sci. 2003;116:847–855. doi: 10.1242/jcs.00289. [DOI] [PubMed] [Google Scholar]
- 16.Jouvenet N, et al. Imaging the biogenesis of individual HIV-1 virions in live cells. Nature (London) 2008;454:236–240. doi: 10.1038/nature06998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Axelrod D. Chapter 7: Total internal reflection fluorescence microscopy. Methods Cell Biol. 2008;89:169–221. doi: 10.1016/S0091-679X(08)00607-9. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal JK, Simon SM. Total Internal Reflection Fluorescence Microscopy for High Resolution Imaging of Cell Surface Events. In: Lippincott-Schwartz J, editor. Current Protocols in Cell Biology. John Wiley & Sons, Inc; 2003. pp. 4.12.1–4.12.15. [DOI] [PubMed] [Google Scholar]
- 19.Jouvenet N, et al. Plasma Membrane Is the Site of Productive HIV-1 Particle Assembly. PLoS. Biol. 2006;4:e435. doi: 10.1371/journal.pbio.0040435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larson DR, et al. Visualization of retrovirus budding with correlated light and electron microscopy. Proc. Natl. Acad. Sci. U. S. A. 2005;102:15453–15458. doi: 10.1073/pnas.0504812102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miesenböck G, et al. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature (London) 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 22.Jaiswal JK, et al. Resolving vesicle fusion from lysis to monitor calcium-triggered lysosomal exocytosis in astrocytes. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14151–14156. doi: 10.1073/pnas.0704935104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altan N, et al. Defective acidification in human breast tumor cells and implications for chemotherapy. J. Exp. Med. 1998;187:1583–1598. doi: 10.1084/jem.187.10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmoranzer J, et al. Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J. Cell Biol. 2000;149:23–32. doi: 10.1083/jcb.149.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampson MA, et al. Insulin-regulated Release from the Endosomal Recycling Compartment Is Regulated by Budding of Specialized Vesicles. Mol. Biol. Cell. 2001;12:3489–3501. doi: 10.1091/mbc.12.11.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreitzer G, et al. Three-dimensional analysis of post-Golgi carrier exocytosis in epithelial cells. Nat. Cell Biol. 2003;5:126–136. doi: 10.1038/ncb917. [DOI] [PubMed] [Google Scholar]
- 27.Schmoranzer J, et al. Migrating fibroblasts perform polarized, microtubule-dependent exocytosis towards the leading edge. J. Cell Sci. 2003;116:4513–4519. doi: 10.1242/jcs.00748. [DOI] [PubMed] [Google Scholar]
- 28.Schmoranzer J, Simon SM. Role of microtubules in fusion of post-Golgi vesicles to the plasma membrane. Mol. Biol. Cell. 2003;14:1558–1569. doi: 10.1091/mbc.E02-08-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaiswal JK, et al. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellen EH, Axelrod D. Fluorescence Emission at Dielectric and Metal-Film Interfaces. Journal of the Optical Society of America B-Optical Physics. 1987;4:337–350. [Google Scholar]
- 31.Pepper JH. The Boy’s Playbook of Science: Including the Various Manipulations and Arrangements of Chemical and Philosophical Apparatus Required For the Successful Performance of Scientific Experiments In Illustration of the Elementary Branches of Chemistry and Natural Philosophy. Routledge, Warne, and Routledge; 1860. [Google Scholar]
- 32.Hirschfeld T. Total reflection fluorescence. Canadian Journal of Spectroscopy. 1965;10:128. [Google Scholar]
- 33.Sund SE, et al. Cell membrane orientation visualized by polarized total internal reflection fluorescence. Biophys. J. 1999;77:2266–2283. doi: 10.1016/S0006-3495(99)77066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Axelrod D. Selective imaging of surface fluorescence with very high aperture microscope objectives. J. Biomed. Opt. 2001;6:6–13. doi: 10.1117/1.1335689. [DOI] [PubMed] [Google Scholar]
- 35.Axelrod D. Total internal reflection fluorescence microscopy in cell biology. Methods Enzymol. 2003;361:1–33. doi: 10.1016/s0076-6879(03)61003-7. [DOI] [PubMed] [Google Scholar]
- 36.Mattheyses AL, Axelrod D. Direct measurement of the evanescent field profile produced by objective-based total internal reflection fluorescence. J. Biomed. Opt. 2006;11:014006. doi: 10.1117/1.2161018. [DOI] [PubMed] [Google Scholar]
- 37.Kner P, et al. Super-resolution video microscopy of live cells by structured illumination. Nat. Methods. 2009;6:339–342. doi: 10.1038/nmeth.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Axelrod D, Wang MD. Reduction-of-dimensionality kinetics at reaction-limited cell surface receptors. Biophys. J. 1994;66:588–600. doi: 10.1016/s0006-3495(94)80834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]