Abstract
Purpose
Although the retinoid cycle is essential for vision, all-trans-retinal and the side products of this cycle are toxic. Delayed clearance of all-trans-retinal causes accumulation of its condensation products, A2E, and all-trans-retinal dimer (RALdi), both associated with human macular degeneration. The protective roles were examined of the all-trans-RDHs, Rdh8 and Rdh12, and the ATP-binding cassette transporter Abca4, retinoid cycle enzymes involved in all-trans-retinal clearance.
Methods
Mice genetically engineered to lack Rdh8, Rdh12, and Abca4, either singly or in various combinations, were investigated because all-trans-retinal clearance is achieved by all-trans-RDHs and Abca4. Knockout mice were evaluated by spectral-domain optical coherence tomography (SD-OCT), electroretinography, retinal morphology, and visual retinoid profiling with HPLC and MS. ARPE19 cells were examined to evaluate A2E and RALdi oxidation and toxicity induced by exposure to UV and blue light.
Results
Rdh8−/−Abca4−/− and Rdh8−/−Rdh12−/−Abca4−/− mice displayed slowly progressive, severe retinal degeneration under room light conditions. Intense light-induced acute retinal degeneration was detected by SD-OCT in Rdh8−/−Rdh12−/− Abca4−/− mice. Amounts of A2E in the RPE correlated with diminished all-trans-retinal clearance, and the highest A2E amounts were found in Rdh8−/−Rdh12−/−Abca4−/− mice. However oxidized A2E was not found in any of these mice, and A2E oxidation was not induced by blue light and UV illumination of A2E-loaded ARPE19 cells. Of interest, addition of all-trans-retinal did activate retinoic acid receptors in cultured cells.
Conclusions
Rdh8, Rdh12, and Abca4 all protect the retina and reduce A2E production by facilitating all-trans-retinal clearance. Delayed all-trans-retinal clearance contributes more than A2E oxidation to light-induced cellular toxicity.
A major intermediate of the retinoid (visual) cycle, the ocular vitamin A recycling system required for vision, is all-trans-retinal.1 Regeneration of the chromophore, 11-cis-retinal, is essential for continuous renewal of light-sensitive visual pigments in the vertebrate retina. Whereas inadequate 11-cis-retinal production leads to congenital blindness in humans, accumulation of the photoisomerized chromophore all-trans-retinal also can be detrimental. Such is the case when this reactive aldehyde is not efficiently cleared from the internal membranes of retinal outer segment discs.2,3 Clearance of all-trans-retinal consists of two steps: (1) translocation of all-trans-retinal across the photoreceptor disc membranes by ATP-binding cassette transporter 4 (ABCA4), and (2) reduction of all-trans-retinal to all-trans-retinol by retinol dehydrogenase 8 (RDH8) expressed in the outer segments of photoreceptors and RDH12 located in photoreceptor inner segments.4–6
In humans, lipofuscin fluorophores accumulate with age in the retinal pigmented epithelium (RPE), especially in RPE cells underlying the macula.7,8 Such accumulation has been considered to constitute one of the major risk factors for age-related macular degeneration (AMD), the predominant cause of legal blindness in developed countries.9 Lipofuscin fluorophores also are especially abundant in Stargardt disease, the most common juvenile form of macular degeneration.10 Di-retinoid-pyridinium-ethanolamine (A2E), the major fluorophore of lipofuscin, is formed by condensation of phosphatidylethanolamine with two molecules of all-trans-retinal followed by oxidation and hydrolysis of the phosphate ester.11 Various mechanisms have been proposed to explain the toxicity of A2E. These include properties of A2E as a cationic detergent,12 physiologic interference with RPE function,13,14 and radical reactions induced by light-dependent A2E oxidation.15
Recently, we reported that genetic ablation of Rdh8 and Abca4, two important enzymes responsible for all-trans-retinal clearance from photoreceptors, caused severe retinal degeneration in mice.16 Even though this disease was associated with accumulation of all-trans-retinal condensation products such as A2E and all-trans-retinal dimer (RALdi), the mechanisms of RPE and photoreceptor death remain to be clarified. Although Rdh8 is the major dehydrogenase responsible for clearing all-trans-retinal from outer segments of photoreceptor cells,17 Rdh12 also contributes to this process in the inner segments of photoreceptor cells, and mutations in Rdh12 are associated with congenital blindness.18 Therefore, we used genetically engineered mice lacking Rdh8, Rdh12, and/or Abca4 alone or in various combinations together with cell culture experiments to investigate the roles of these enzymes in all-trans-retinal clearance and induction of progressive light-dependent severe retinal degeneration.
Materials and Methods
Animals
Rdh8−/−Rdh12−/−Abca4−/− triple-knockout and Rdh12−/−Abca4−/− double-knockout mice were generated by cross-breeding Rdh8−/− Abca4−/− double-knockout mice with Rdh8−/− Rdh12−/− double-knockout mice, and Rdh12−/− mice with Abca4−/− mice, respectively. Rdh8−/−, Rdh12−/−, and Abca4−/− single-knockout mice and Rdh8−/− Rdh12−/− and Rdh8−/−Abca4−/− double-knockout mice were generated as previously described, and all knockout animals were genotyped by established methods.2,6,17 Only mice with the leucine variation at amino acid 450 of Rpe65 were used. All mice were housed in the animal facility at the School of Medicine, Case Western Reserve University, where they were maintained either under complete darkness or in a 12-hour light (∼10 lux)/12-hour dark cyclic environment. Manipulations in the dark were performed under dim red light transmitted through a filter (transmittance > 560 nm; No. 1 Safelight; Eastman Kodak, Rochester, NY). All animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committees and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanatization and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction and Analysis of Light Damage
Before exposure to light, 4-week-old mice were dark-adapted for 48 hours. Damage to the retina was induced by exposure of mice with dilated pupils by 1% tropicamide to 10,000 lux of diffuse, white fluorescent light (150-W spiral lamp; Commercial Electric, Cleveland, OH) for 30 minutes. Mice were kept in the dark after light exposure and before evaluation by SD-OCT.
Ultrahigh-Resolution Spectral-Domain Optical Coherence Tomography Imaging
SD-OCT (Bioptigen, Durham, NC) was used for in vivo imaging of mouse retinas. Mice were anesthetized by intraperitoneal injection of 20 μL/g body weight of 6 mg/mL ketamine and 0.44 mg/mL xylazine diluted with 10 mM sodium phosphate (pH 7.2) containing 100 mM NaCl. Pupils were dilated with 1% tropicamide. The right eye was used for in vivo SD-OCT imaging. Four pictures acquired in the B-scan mode were used to construct each final averaged image.
Materials and Chemical Synthesis
All-trans-retinoic acid was purchased from Sigma-Aldrich (St. Louis, MO), and all-trans-retinal was obtained from Toronto Research Chemicals Inc. (Toronto, ON, Canada). A2E and all-trans-retinal dimer (RALdi) were synthesized and detected by previously described methds 16,17
Light Exposure of A2E and RALdi
Retinoids (0.5 mL of 30 μM samples) in 75% acetonitrile in water were exposed to UV (365 nm; 8-W UV lamp from UVP, Inc., Upland, CA) or blue light (405 and 440 nm; X-Cite120 Fluorescence illumination system; Exfo Mississauga, ON, Canada with 405- or 440-nm filters) for 1, 5, and 10 minutes at room temperature.
For in vitro assays, ARPE19 (human RPE) cells were preincubated with equal amounts of retinoids for 16 hours at 37°C and then maintained in medium (Dulbecco's Modified Eagle's Medium [DMEM] with 10% fetal calf serum) without retinoids for 3 days. The cells were either kept in the dark (control) or exposed to UV or blue light for 1, 5, and 15 minutes. Retinoids were dissolved in 100% DMSO, and the final concentration of DMSO in the medium was 2%. A2E precipitated from the cell culture medium if the DMSO concentration was less than 2%.
Retinoid and A2E Analyses
All experimental procedures related to extraction, derivatization, and separation of retinoids from dissected mouse eyes were performed as formerly described.17 A2E was extracted twice from two eyes with 1 mL of acetonitrile in a glass/glass homogenizer. After evaporation of solvent, extracts were dissolved in 150 μL acetonitrile with 0.1% TFA and then filtered through a Teflon syringe filter (National Scientific Company, Rockwood, TX). Samples (100 μL) were loaded on C18 columns (Phenomenex, Torrance, CA) and analyzed by normal-phase HPLC with mobile-phase gradients of acetonitrile-H2O, 100:0, and acetonitrile-H2O, 80:20 with 0.1% TFA for 30 minutes. Quantification of A2E by HPLC was achieved by comparison with known concentrations of pure synthetic A2E prepared as described previously.17 Oxidized A2E was quantified with a mass spectrometer (LXQ; Thermo Scientific, Waltham, MA, equipped with an APCI source and combined with an 1100 series HPLC; Agilent Technologies, Santa Clara, CA).16
Histology
Histologic and immunohistochemical procedures used were previously described.17 Eye cups for histology were fixed in 2% glutaraldehyde/4% paraformaldehyde, and processed for embedding in Epon. Sections were cut at 1 μm and stained with toluidine blue.
Electroretinograms
All ERG procedures were performed by published methods.17 For single-flash recording, the duration of white light flash stimuli (from 20 μs to 1 ms) was adjusted to provide a range of illumination intensities (from −3.7 to 1.6 log cd · m−2). Three to five recordings were made at sufficient intervals between flash stimuli (from 10 seconds to 10 minutes) to allow recovery from photobleaching effects. For evaluation of dark adaptation, retinas of dark-adapted mice were bleached with the background light of a Ganzfeld chamber (500 cd · m−2) for 3 minutes. After this bleaching, a single-flash ERG at −0.2 log cd · s · m−2 was used to monitor recovery of a-wave amplitudes every 5 minutes for 60 minutes in the dark. The recovery ratio was calculated by normalizing single flash a-wave amplitude responses at various times after bleaching to the dark-adapted a-wave response at the identical flash intensity of −0.2 log cd · s · m−2. The recovery ratio versus time after bleaching was plotted and fit by a linear regression algorithm (Sigma Plot 2002 ver. 8.02; Systat, Richmond, CA).
RAR Activation Assay
RAR activation was assayed by methods previously reported.19 The RAR element reporter cell line, F9-RARE-lacZ (SIL15-RA), was a kind gift from Michael Wagner (Downstate Medical Center, SUNY, Brooklyn, NY) and Peter McCaffery (E. K. Shriver Center, University of Massachusetts Medical School, Worcester, MA). The cells were grown in L15-CO2 medium containing N-3 supplements and antibiotics. They were stimulated for 24 hours in the dark at 37°C and 100% humidity with all-trans-retinoic acid, A2E, or all-trans-retinal dissolved in DMSO at indicated concentrations. Then they were lysed and assayed for expression of β-galactosidase with a β-galactosidase enzyme assay system (Promega, Madison, WI).
Statistical Analyses
Data representing the means ± SD for results of at least three independent experiments were compared by the one-way ANOVA-test.
Results
Age-Related Retinal Degeneration in Rdh8−/−Rdh12−/−Abca4−/− Mice
Rdh8−/−Rdh12−/−Abca4−/− mice were generated to learn how loss of Rdh8, Rdh12, and Abca4 together affects all-trans-retinal clearance and retinal health. Decreased numbers of photoreceptor nuclei (outer nuclear layer; ONL) and shorter photoreceptor outer segments (OS) with RPE death were evident in Rdh8−/−Rdh12−/−Abca4−/− mice by 6 weeks of age (Fig. 1A). However, such retinal degeneration was evident only in the inferior retina, a finding similar to that in Rdh8−/− Abca4−/− mice.16 Reduced amounts of total retinoids found in Rdh8−/−Rdh12−/−Abca4−/− eyes also were consistent with retinal degeneration in these mice (Rdh8−/−Rdh12−/− Abca4−/− versus WT; 569.0 ± 21.6 vs. 716.2 ± 33.3 picomoles/eye). Amounts of 11-cis-retinal, all-trans-retinal, and all-trans-retinyl esters in the eyes were quantified by HPLC after illumination that bleached ∼40% of visual chromophore in 6-week-old Rdh8−/−Rdh12−/−Abca4−/− and WT mice. Regenerated amounts of 11-cis-retinal were comparable in Rdh8−/−Rdh12−/−Abca4−/− and WT mice, but the triple knockout animals exhibited both a slower disappearance of all-trans-retinal and an increased retention of all-trans-retinyl esters than the WT control (Fig. 1D).
Figure 1.
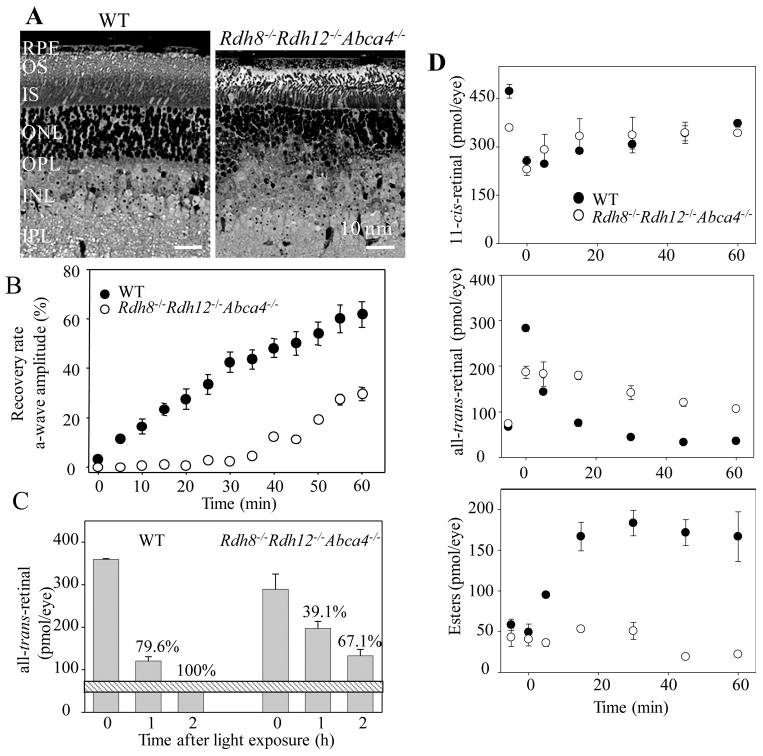
Characterization of Rdh8−/− Rdh12−/− Abca4−/− mice. (A) Retinal histology of 6-week-old Rdh8−/− Rdh12−/− Abca4−/− mice (right) and WT mice (left). Representative images are shown (n > 10). RPE, retinal pigment epithelium; OS, outer segment; IS, inner segment; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer. (B) Rates of dark-adaptation were assessed in 6-week-old Rdh8−/−Rdh12−/−Abca4−/− and WT mice by monitoring ERG a-wave amplitudes after 3 minutes of exposure to light in a Ganzfeld chamber (500 cd · m−2). Bars, SD (n > 6). (C) Amounts of all-trans-retinal in eyes of 6-week-old Rdh8−/−Rdh12−/− Abca4−/− and WT mice exposed for 3 minutes to light in a Ganzfeld chamber (500 cd · m−2). 0, immediately after light exposure; 1, 1-hour dark adaptation after light exposure; and 2, 2-hour dark adaptation after light exposure. Bars, SD (n > 3) Horizontal bar: the amount of all-trans-retinal found in dark-adapted WT mice. Numbers are the percentages of all-trans-retinal cleared relative to the WT dark-adapted level set at 100%. (D) Kinetics of 11-cis-retinal formation (top), all-trans-retinal disappearance (middle), and all-trans-retinyl ester accumulation (bottom) in 6-week-old Rdh8−/−Rdh12−/−Abca4−/− and WT mice. Retinoids were quantified by HPLC in eye samples collected at different time points after a flash that bleached ∼40% of visual pigments. Bars, SD (n > 3). At 6 weeks of age Rdh8−/−Rdh12−/−Abca4−/− mice exhibited mild retinal degeneration with a ∼25% loss of rhodopsin.
Effects of all-trans-Retinal Accumulation on Photoreceptor Function
Effects of all-trans-retinal accumulation on photoreceptor sensitivity, assessed by the dark-adaptation rate after a 60% rhodopsin bleaching induced by a 3-minute light exposure in a Ganzfeld chamber (500 cd · m−2), was examined by recording ERG a-waves. Compared with WT mice, 6-week-old Rdh8−/− Rdh12−/−Abca4−/− mice exhibited a marked delay in dark-adaptation kinetics (Fig. 1B) associated with reduced clearance of all-trans-retinal (Fig. 1D). Amounts of all-trans-retinal had returned to dark-adapted levels between 1 and 2 hours after light exposure in WT mice, whereas a delayed recovery (only 391% at 1 hour and 67.1% at 2 hours versus the dark-adapted level at 100%) was noted in Rdh8−/−Rdh12−/−Abca4−/− mice (Fig. 1C).
Effect of Intense Illumination on Retinal Damage in Rdh8−/−Rdh12−/−Abca4−/− Mice
To learn more about acute retinal damage caused by light, the retinas of 4-week-old Rdh8−/−Rdh12−/−Abca4−/− and WT mice without a sign of retinal degeneration were examined at 0 and 24 hours and 3 and 7 days after a 30-minute exposure to bright light (10,000 lux) by ultrahigh-resolution SD-OCT. This technique has several advantages, including the capability of imaging the retina in vivo and observing changes in the same eye over time. Acute retinal degeneration was evident in Rdh8−/−Rdh12−/−Abca4−/− mice after this exposure, whereas no damage was observed in WT retinas (Fig. 2). Repeated imaging by SD-OCT revealed that the most prominent degenerative changes occurred in the ONL. Structures of the outer plexiform layer (OPL), ONL, and photoreceptor layer were poorly delineated 24 hours after light exposure, but images of these layers were better defined 3 and 7 days after this insult. As shown in cryosection images in Figure 2, scattered photoreceptor nuclei in areas of the OPL/ONL, and reduced thickness of the photoreceptor layer were evident 7 days after illumination, and SD-OCT images at the same time exhibited these characteristic changes as well.
Figure 2.
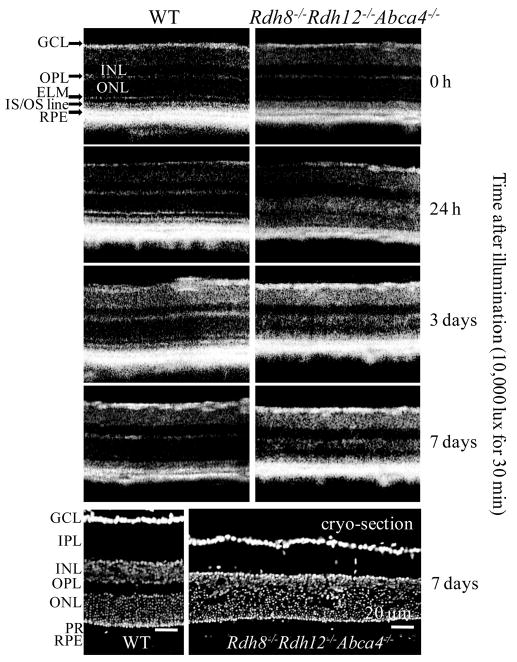
Retinal changes after intense light illumination in Rdh8−/− Rdh12−/−Abca4−/− and WT mice. Normal-appearing retinas of 4-week-old Rdh8−/−Rdh12−/−Abca4−/− and WT mice were exposed to bright light (10,000 lux) for 30 minutes and then at 0 and 24 hours and 3 and 7 days after being kept in the dark were examined by ultrahigh-resolution SD-OCT. Representative SD-OCT images (n = 4) of the same eye are shown (top). Cryosections of retinas were prepared 7 days after illumination, and nuclei were stained with 4′-6-diamidino-2-phenylindole (DAPI) (bottom). Representative images of the same eye imaged by SD-OCT are shown (n = 4). Bars, 20 μm. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ELM, external limiting membrane; IS, inner segment; OS, outer segment; RPE, retinal pigment epithelium; PR, photoreceptor.
Retinoid Flow in Mice with Deletions of Rdh8, Rdh12, and Abca4
Retinoid flow after intense illumination was examined further in 6-week-old mice lacking various combinations of Rdh8, Rdh12, and Abca4. Rdh8−/− mice demonstrated the greatest delay in all-trans-retinal clearance of all the single-knockout mice (Fig. 3A). Among the double-knockout mice, Rdh8−/−Rdh12−/− and Rdh8−/−Abca4−/− mice evidenced a more delayed clearance than either Rdh12−/−Abca4−/− or WT mice. These data and previously reported results2 indicate that Rdh8 is the most critical enzyme for all-trans-retinal removal from mouse eyes and that Rdh8, Rdh12, and Abca4 together account for this activity in these animals.
Figure 3.
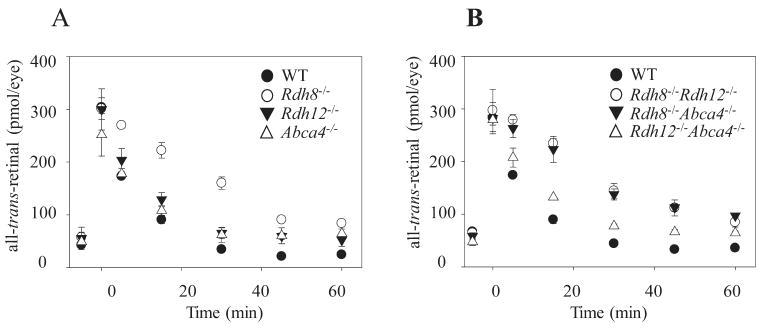
All-trans-retinal reduction in 6-week-old mice with either a single- or a double-knockout combination of Rdh8, Rdh12, and/or Abca4. Kinetics of all-trans-retinal disappearance in 6-week-old single-knockout (A) and double-knockout (B) mice lacking functional Rdh8, Rdh12, and Abca4 genes. Retinoids were quantified by HPLC in eyes collected at different time points after a flash that bleached ∼40% of visual pigments. Bars, SD (n > 3).
A2E Fluorophore in Eyes of Mice with Deletions of Rdh8, Rdh12, and/or Abca4, either Singly or in Various Combinations
A2E was quantified in eyes of mice at 3 and 6 months of age to learn how loss of Rdh8, Rdh12, and/or Abca4 affects A2E accumulation (Fig. 4). Mice with all knockout genotypes demonstrated more A2E accumulation than did WT mice, suggesting that Rdh8, Rdh12, and Abca4 all are associated with formation of A2E. Abca4−/− mice accumulated the greatest amount of A2E among the single-knockout animals, indicating that Abca4 is the most effective enzyme in preventing A2E accumulation. More A2E was detected in Rdh8−/−Abca4−/− mice than in Rdh12−/−Abca4−/− mice among the double-knockout animals suggesting that slower all-trans-retinal removal due to Rdh8 ablation is also an important factor in A2E acquisition. Consistent with these findings, Rdh8−/−Rdh12−/−Abca4−/− mice exhibited the greatest A2E accumulation among the tested animals.
Figure 4.
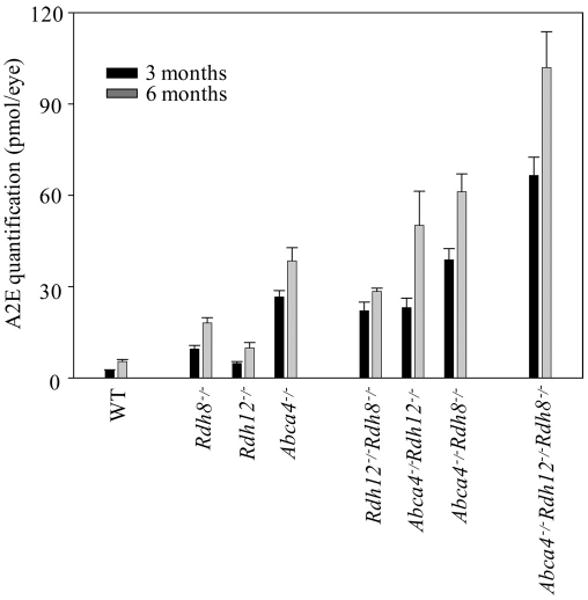
A2E quantities in the eyes of mice with single, double, and triple knockouts of Rdh8, Rdh12, and Abca4. A2E amounts in the whole eye were quantified by HPLC in 3- and 6-month-old mice with single, double, and triple knockouts of Rdh8, Rdh12, and Abca4. Mice were reared under 12-hour light (∼10 lux)/12-hour dark conditions. Bars, SD (n > 3). All knockout mice showed significant age-dependent increases in A2E compared with WT animals (P < 0.01).
Retinal Degeneration in Mice with Combined Deletions of Rdh8, Rdh12, and Abca4
Percentages of age-related retinal degeneration found in mice of various ages lacking Rdh8, Rdh12, and/or Abca4 are summarized in Table 1. All mice were maintained in a 12-hour light (∼10 lux)/12-hour dark cycle before testing. Retinal degeneration was observed only in Rdh8−/−Abca4−/− and Rdh8−/− Rdh12−/−Abca4−/− mice, suggesting that loss of both Rdh8 and Abca4 are necessary for retinal degeneration in these animals when they are maintained in this typical light-dark cyclic environment. At 4 and 6 weeks of age Rdh8−/− Rdh12−/− Abca4−/− mice exhibited a higher incidence of retinal degeneration than did Rdh8−/−Abca4−/− mice. This finding implies that decreased all-trans-RDH activity in photoreceptor inner segments due to loss of Rdh12 activity promotes retinal degeneration in Rdh8−/−Abca4−/− mice. Of importance, dark-maintained Rdh8−/−Rdh12−/−Abca4−/− mice did not manifest retinal degeneration at 6 weeks of age. Retinal degeneration was strongly associated with a delay in all-trans-retinal clearance and increased levels of condensation products of all-trans-retinal, including A2E.
Table 1.
Incidence of Retinal Degeneration in Wild-Type and Genetic Knockout Mice of Various Ages
| Genotype* | 4-week-old | 6-week-old | 3-month-old | 6-month-old |
|---|---|---|---|---|
| WT | –† | 0% (0/10)‡ | 0% (0/10) | 0% (0/10) |
| Rdh8−/− | – | 0% (0/10) | 0% (0/16) | 0% (0/10) |
| Rdh12−/− | – | 0% (0/16) | 0% (0/16) | 0% (0/10) |
| Abca4−/− | – | 0% (0/10) | 0% (0/10) | 0% (0/10) |
| Rdh8−/−Rdh12−/− | – | 0% (0/10) | 0% (0/20) | 0% (0/20) |
| Rdh8−/−Abca4−/− | 25% (5/20) | 90% (18/20) | 97.5% (39/40) | 100% (18/18) |
| Rdh12−/−Abca4−/− | – | 0% (0/10) | 0% (0/20) | 0% (0/16) |
| Rdh8−/−Rdh12−/−Abca4−/− | 56% (5/9) | 100% (10/10) | – | – |
| Rdh8−/−Rdh12−/−Abca4−/− (dark)§ | – | 0% (0/9) | 10% (1/10) | – |
Mice with the L450 variant of RPE65 were analyzed. Mice were maintained in a 12-hour light (∼10 lux)/12-hour dark cyclic environment.
Not determined.
The number of mice with retinal degeneration divided by the total number of analyzed mice is indicated in parentheses.
Mice maintained in the dark.
Effects of Exposure to UV and Blue Light on A2E and RALdi
Because A2E toxicity reportedly is associated with generation of light-induced oxidation products,15 we quantified A2E and RALdi after exposure to light. Initially, A2E and RALdi in solution were exposed to UV light, a well-known radical reaction inducer, and then their amounts were compared with those in unexposed samples. RALdi was rapidly degraded whereas A2E withstood UV light exposure (Fig. 5). Because A2E oxidation has been well studied after exposure to blue light (400 – 480 nm),15 we also exposed both A2E and RALdi in solution to 405 and 440 nm blue light. Again, RALdi was readily broken down but A2E was stable in a 75% aqueous acetonitrile solution (Figs. 6A, 6B, data not shown).
Figure 5.
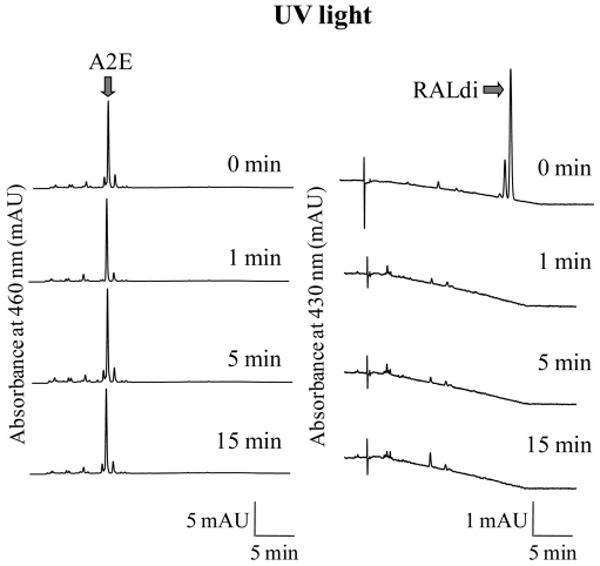
Effect of UV light exposure on A2E and RALdi. A2E and RALdi in 75% aqueous acetonitrile were exposed to UV light (365 nm) for 1, 5, or 15 minutes, and the amounts of A2E and RALdi were analyzed by HPLC. A2E eluted slightly after 5 minutes and remained stable after exposure to UV light, whereas amounts of RALdi eluting at 20 minutes had virtually disappeared by 1 minute after exposure. Representative chromatograms are shown (n > 3)
Figure 6.
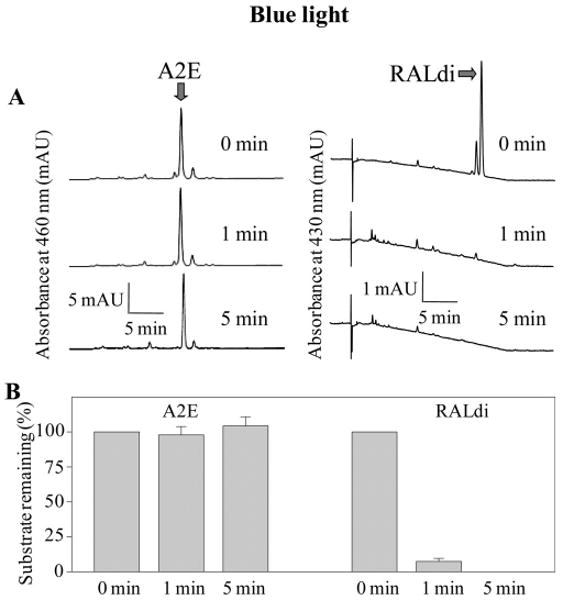
A2E and RALdi disappearance after exposure to blue light. A2E and RALdi in 75% aqueous acetonitrile were exposed to blue light (440 nm) at room temperature for 1 minute or 5 minutes, and amounts of A2E and RALdi were determined by HPLC at 460 and 430 nm, respectively. Representative chromatograms are shown (n > 3) Amounts of A2E or RALdi after blue light illumination were compared to those in identical nonilluminated samples of A2E or RALdi (A), and indicated as percentages of the original substrate (B). Bars, SD (n > 3) The amount of A2E remained unchanged, whereas RALdi decreased markedly after exposure.
Because we did not detect A2E depletion in the acetonitrile/water solvent, we co-cultured ARPE19 cells with 30 μM A2E for 16 hours to load them with A2E. The A2E-laden ARPE19 cells were then cultured without A2E for 3 days in the dark before they were exposed to light in a series of experiments. Culture supernatants containing A2E also were collected and stored at −80° C before analysis. Both A2E-laden ARPE19 cells and thawed supernatants containing A2E were exposed to UV or blue light. Blue (440 nm) light exposure for 5 minutes at room temperature did not deplete amounts of A2E in these cells, whereas it did reduce A2E content in the culture medium (Figs. 7A-C). Similar results were obtained after a 5-minute exposure to UV light (365 nm; Fig. 7C). Most of the increased product in the medium was identified as a single oxidized form of A2E (MW 608.5) by MS analyses (Fig. 7D). Ratios of oxidized A2E to A2E quantified by HPLC were 0.214 ± 0.035 with blue light and 0.150 ± 0.026 with UV light compared with 0.089 ± 0.008 in medium from unexposed samples. Thus, exposure to blue light produced the highest amounts of the single oxidized form of A2E in the medium of ARPE19 cells loaded with A2E. Surprisingly, no oxidized A2E was detected in blue- and UV light-exposed A2E-laden ARPE19 cells. We therefore assessed levels of oxidized A2E products in the eyes of Rdh8−/− Abca4−/− and Rdh8−/−Rdh12−/−Abca4−/− mice at the ages of 3, 6, and 12 months that were maintained in a 12-hour light (∼10 lux)/12-hour dark environment. But again, no A2E oxidation products were detected (data not shown).
Figure 7.
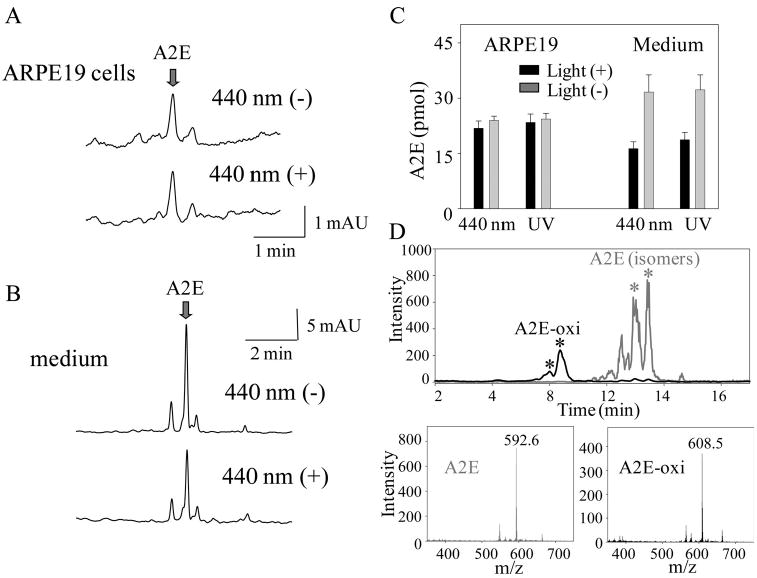
A2E oxidation by ARPE19 cells after exposure to blue light. (A) A2E-laden ARPE19 cells were produced by incubating them for 16 hours at 37°C in medium with 30 μM A2E in 2% DMSO followed by 3 days' incubation in media without A2E. Cells were exposed to blue light (440 nm) for 5 minutes and placed in the dark for 16 hours at 37°C. After collection of cells by centrifugation, A2E content was analyzed by HPLC. Representative chromatograms are shown (n > 5). (B) A2E in the ARPE19 cell culture medium (100 μL) was exposed to blue light (440 nm) for 5 minutes and then quantified by HPLC. Representative chromatograms are shown (n > 5). (C) Quantification of A2E in A2E-laden ARPE19 cells and medium with and without exposure to blue (440 nm) or UV (365 nm) light for 5 minutes. Bars, SD (n > 5). Exposure to blue and UV light decreased the amounts of A2E in the medium but not in the ARPE19 cells. (D) Oxidized products of A2E in medium after exposure to blue light detected by MS. Top: Representative chromatogram of an A2E peak showing several iso-forms (stereoisomers; asterisk) of A2E (gray trace). Representative chromatogram of a light-induced oxidized A2E peak (black trace). These peaks corresponded to masses of 592.6 (bottom left; A2E, gray line) and 608.5 (bottom right; oxidized A2E [A2E-oxi], black line).
Other than A2E which was reported to cause light-dependent cell death,20 RALdi, consisting of two molecules of all-trans-retinal, has been proposed to cause light-induced toxicity. As shown in Figures 5 and 6, RALdi was rapidly destroyed by blue and UV light exposure, and MS failed to reveal any oxidized derivatives of RALdi with an increased mass (data not shown). Of note, rapid oxidation of RALdi did occur under physiological room light conditions, and multiple oxidation products of RALdi were found (Fig. 8).
Figure 8.
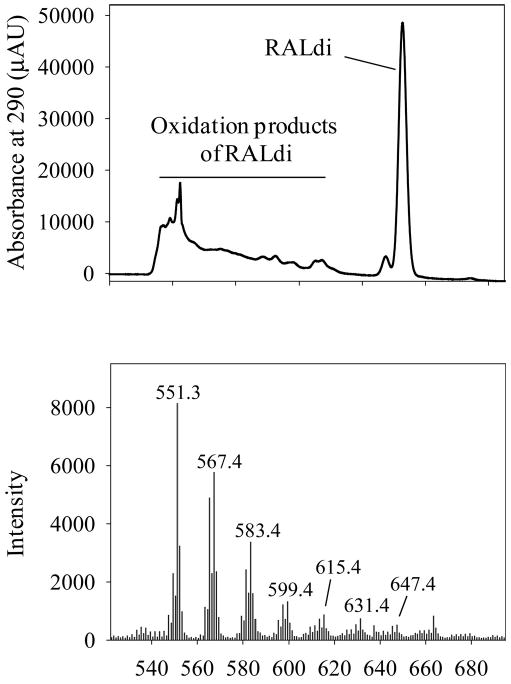
Oxidation of RALdi in 75% aqueous acetonitrile under room light. RALdi in 75% aqueous acetonitrile was kept under room light (<100 lux) for 16 hours at 25°C, and samples were analyzed by MS. Top: A HPLC chromatogram at 290 nm. Bottom: oxidation products of RALdi appeared as multiple peaks with increasing masses. The mass of RALdi is 551.3.
Activation of Retinoic Acid Receptor (RAR) by Ocular Retinoids
Modulation of cell survival through retinoic acid receptor (RAR) activation was examined in a cell culture system, because this is one factor critical to cell survival.21 A2E, all-trans-retinoic acid, RALdi, oxidized RALdi and all-trans-retinal were all evaluated as substrates for RAR activation (Fig. 9). Of these tested compounds, all-trans-retinoic acid exhibited strong RAR activity, as previously reported.19 At 10−7 M, all-trans-retinal also strongly activated the RAR but this activity may have resulted from its oxidation to all-trans-retinoic acid. The decreased activity of all-trans-retinal observed at 10−6 M resulted from decreased cell survival (Fig. 9)22 A2E showed weak activity at 10−6 M but this was substantially less than that observed with all-trans-retinal. Retinoic acids were not detected in the eyes of light-exposed and nonexposed triple, double- and single-knockout mice (data not shown).
Figure 9.
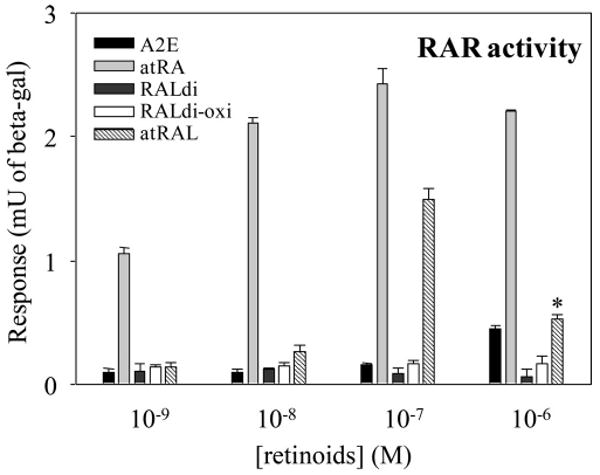
Retinoid activation of RAR. RAR activation was assessed by the RAR element reporter cell line F9-RARE-lacZ (SIL15-RA) assay.19 All-trans-retinoic acid activated this receptor at concentrations of 10−9 M and higher, whereas all-trans-retinal activated it at 10−7 M. Decreased activity of all-trans-retinal at 10−6 M was caused by its cytotoxicity.22 Bars, SD (n > 5)
Discussion
Delayed Clearance of all-trans-Retinal in Retinal Degeneration
Impaired removal of all-trans-retinal from photoreceptors has been suggested as an important mechanism involved in retinal degeneration. This perception is supported by retinal phenotypes of Rdh12 mutations in Leber's congenital amaurosis,18,23 Abca4 mutations in Stargardt disease and AMD,24,25 and Rdh8−/−Abca4−/− and Rdh8−/−Rdh12−/− mutations in mice.2,16 The importance of all-trans-retinal in retinal degeneration is also highlighted because it serves as a precursor of A2E, a possibly toxic molecule that accumulates with age.12 Clearance of all-trans-retinal from photoreceptor cells involves two steps: transport of all-trans-retinal from the inside to the outside of ROS disc membranes by Abca4 and reduction of all-trans-retinal to all-trans-retinol in the cytosolic lumen of the ROS by all-trans-RDHs.4 Although Rdh8 accounted for most of the all-trans-RDH effect2,17 (Fig. 1D, Fig. 3A), loss of Abca4 facilitated accumulation of A2E as well3 (Fig. 4). High accumulation of A2E was well associated with the incidence of retinal degeneration under room light in Rdh8−/−Abca4−/− and Rdh8−/−Rdh12−/−Abca4−/− mice (Table 1), whereas the severity of intense light-induced retinal degeneration related to a delayed clearance of all-trans-retinal (Fig. 2, Ref22). These data indicate that all-trans-retinal accumulation is more critical than A2E in causing light-induced acute retinal degeneration.
Minor Roles of A2E and RALdi Oxidation in Retinal Degeneration
Because oxidized forms of A2E were reported to cause RPE cell death in cultured cell lines,26 we examined A2E oxidation profiles in both in vivo and in vitro models. Blue light and UV light exposure facilitated A2E oxidation in cell culture medium but not in A2E-laden ARPE19 cells (Fig. 7) or in Rdh8−/− Abca4−/− and Rdh8−/−Rdh12−/−Abca4−/− mouse retinas, even after intense (10,000 lux) illumination (data not shown).22 Nor was A2E in 75% acetonitrile solution oxidized after exposure to UV and blue light (Figs. 5, 6). Although any effect of acetonitrile on scavenging or quenching radicals involved in A2E oxidation should be considered, these findings indicate that although some A2E oxidation occurs in a hydrophilic environment, A2E is more stable in a more hydrophobic cellular environment. A2E accumulates inside the cell membranes11 and may localize to hydrophobic sites in vivo that are unfavorable to its oxidation. That the amounts of A2E found did not relate to the incidence and severity of light-induced retinal degeneration in Rdh8−/−Abca4−/− mice22 provides further evidence that light-induced oxidation of A2E does not play a major role in this disease.
RAR Activation by all-trans-Retinal
Surprisingly, this study revealed that free all-trans-retinal induced substantial activation of the retinoic acid receptor (RAR) at a 10−7-M concentration as well as substantial cytotoxicity at 10−6 M (Fig. 9, Ref. 22). Although 1 μM A2E showed weak RAR activation, no activation was detected with RALdi and its oxidative products at this concentration. Activation of RAR initiates many biological reactions related to development and survival,27 and thus continuous production of all-trans-retinal in the light could be a critical factor for photoreceptor cell survival. Previous studies suggested a role for A2E in RPE cell death and retinal degeneration.28 But most of these studies used A2E synthesized from all-trans-retinal, and so it is difficult to exclude the possibility that residual amounts of all-trans-retinal was responsible. Moreover, some all-trans-retinal may be oxidized to the highly potent biologically active all-trans-retinoic acid. This explanation may also account for a conflicting report that A2E activates RAR similarly to all-trans-retinoic acid.29
Unique Roles of Rdh8, Rdh12, and Abca4 in all-trans-Retinal Clearance
Although amounts of accumulated all-trans-retinal were similar in Rdh−/−, Rdh8−/−Abca4−/−, and Rdh8−/−Rdh12−/− mice (Fig. 1D, Fig. 3A), the retinal phenotypes were different. Under room light, Rdh8−/−Abca4−/− retinas developed progressive severe degeneration,16 whereas Rdh8−/−Rdh12−/− retinas showed slow, mild rod/cone dystrophy.2 No retinal degeneration was observed in Rdh8−/− mice17 (Table 1). When mice were illuminated with intense light for 30 minutes, Rdh8−/− retinas exhibited mild changes,16 Rdh8−/− Abca4−/− retinas showed punctate retinal degeneration with rosette formation,16,22 and Rdh8−/−Rdh12−/− retinas manifested severe widespread destruction.16 These observations indicate that loss of Rdh8 enhances retinal damage induced by light, and is intimately involved in the progression of retinal degeneration. Once free, all-trans-retinal accumulates in the impaired eye, retinal degeneration may accelerate because of the acute toxicity conferred by this aldehyde.
Total amounts of all-trans-retinal accumulation in the eye are currently measurable by precise analytical methods but local concentrations of this reactive aldehyde in different compartments of photoreceptor cells are unknown. All-trans-retinal could exert different pathologic effects, depending on where it accumulates (e.g., either within the intradiscal space free to bind to amino-containing phospholipids located within the inner leaflets of the plasma membrane surrounding rod and cone outer segments, or within the mitochondria-rich inner segments). Thus, differences in the intracellular distribution of all-trans-retinal may account for variations in the onset, progression, and severity of retinal degeneration noted in Table 1.
In conclusion, the experimental results described herein for genetically engineered mice lacking Rdh8, Rdh12, and Abca4, either singly or in various combinations, along with in vitro findings in cell culture systems indicate a central role of all-trans-retinal in the pathogenesis of retinal degeneration. This study also provides new evidence that all-trans-retinal's condensation products, including A2E and RALdi, are surrogate markers for delayed clearance of all-trans-retinal. These findings support the hypothesis that all-trans-retinal is a key contributor to retinal degeneration induced by light.30
Acknowledgments
The authors thank Leslie T. Webster (Case Western Reserve University) for comments on the manuscript, Alexander R. Moise (Case Western Reserve University) for his help with retinoic acid receptor activation analyses, Kerrie H. Lodowski (Case Western Reserve University) for her help with the light exposure experiments, Kiichiro Okano (Case Western Reserve University) for his help with SD-OCT study, and Melissa S. Matosky, Satsumi Roos, Madeline Singer, and Hiroko Matsuyama (Case Western Reserve University) for technical support.
Supported by National Institutes of Health Grants K08EY019031, EY09339, P30EY11373, and EY08123, the Research to Prevent Blindness Foundation, and the Ohio Lions Eye Research Foundation.
Footnotes
Disclosure: A. Maeda, None; M. Golczak, None; T. Maeda, None; K. Palczewski, None
References
- 1.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maeda A, Maeda T, Sun W, Zhang H, Baehr W, Palczewski K. Redundant and unique roles of retinol dehydrogenases in the mouse retina. Proc Natl Acad Sci USA. 2007;104:19565–19570. doi: 10.1073/pnas.0707477104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 4.Molday RS, Zhong M, Quazi F. The role of the photoreceptor ABC transporter ABCA4 in lipid transport and Stargardt macular degeneration. Biochim Biophys Acta. 2009;1791(7):573–583. doi: 10.1016/j.bbalip.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275:11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 6.Maeda A, Maeda T, Imanishi Y, et al. Retinol dehydrogenase (RDH12) protects photoreceptors from light-induced degeneration in mice. J Biol Chem. 2006;281:37697–37704. doi: 10.1074/jbc.M608375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wing GL, Blanchard GC, Weiter JJ. The topography and age relationship of lipofuscin concentration in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1978;17:601–607. [PubMed] [Google Scholar]
- 8.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci. 2001;42:1855–1866. [PubMed] [Google Scholar]
- 9.Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat Rev. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- 10.Delori FC, Staurenghi G, Arend O, Dorey CK, Goger DG, Weiter JJ. In vivo measurement of lipofuscin in Stargardt's disease: fundus flavimaculatus. Invest Ophthalmol Vis Sci. 1995;36:2327–2331. [PubMed] [Google Scholar]
- 11.Sparrow JR, Parish CA, Hashimoto M, Nakanishi K. A2E, a lipofuscin fluorophore, in human retinal pigmented epithelial cells in culture. Invest Ophthalmol Vis Sci. 1999;40:2988–2995. [PubMed] [Google Scholar]
- 12.Eldred GE, Lasky MR. Retinal age pigments generated by self-assembling lysosomotropic detergents. Nature. 1993;361:724–726. doi: 10.1038/361724a0. [DOI] [PubMed] [Google Scholar]
- 13.Yasukawa T, Wiedemann P, Hoffmann S, et al. Glycoxidized particles mimic lipofuscin accumulation in aging eyes: a new age-related macular degeneration model in rabbits. Graefes Arch Clin Exp Ophthalmol. 2007;245:1475–1485. doi: 10.1007/s00417-007-0571-z. [DOI] [PubMed] [Google Scholar]
- 14.Vives-Bauza C, Anand M, Shirazi AK, et al. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008;283:24770–24780. doi: 10.1074/jbc.M800706200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41:1981–1989. [PubMed] [Google Scholar]
- 16.Maeda A, Maeda T, Golczak M, Palczewski K. Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem. 2008;283:26684–26693. doi: 10.1074/jbc.M804505200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda A, Maeda T, Imanishi Y, et al. Role of photoreceptor-specific retinol dehydrogenase in the retinoid cycle in vivo. J Biol Chem. 2005;280:18822–18832. doi: 10.1074/jbc.M501757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janecke AR, Thompson DA, Utermann G, et al. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36:850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 19.Moise AR, Kuksa V, Imanishi Y, Palczewski K. Identification of all-trans-retinol:all-trans-13,14-dihydroretinol saturase. J Biol Chem. 2004;279:50230–50242. doi: 10.1074/jbc.M409130200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SR, Jang YP, Jockusch S, Fishkin NE, Turro NJ, Sparrow JR. The all-trans-retinal dimer series of lipofuscin pigments in retinal pigment epithelial cells in a recessive Stargardt disease model. Proc Natl Acad Sci USA. 2007;104:19273–19278. doi: 10.1073/pnas.0708714104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schug TT, Berry DC, Shaw NS, Travis SN, Noy N. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–733. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeda A, Maeda T, Golczak M, et al. Involvement of all-trans-retinal in acute light-induced retinopathy of mice. J Biol Chem. 284(22):15173–15183. doi: 10.1074/jbc.M900322200. 200929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun W, Gerth C, Maeda A, et al. Novel RDH12 mutations associated with Leber congenital amaurosis and cone-rod dystrophy: biochemical and clinical evaluations. Vision Res. 2007;47:2055–2066. doi: 10.1016/j.visres.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 25.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 26.Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K. Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci. 2002;43:1222–1227. [PubMed] [Google Scholar]
- 27.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6:793–810. doi: 10.1038/nrd2397. [DOI] [PubMed] [Google Scholar]
- 28.Sparrow JR, Boulton M. RPE lipofuscin and its role in retinal pathobiology. Exp Eye Res. 2005;80:595–606. doi: 10.1016/j.exer.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Iriyama A, Fujiki R, Inoue Y, et al. A2E, a pigment of the lipofuscin of retinal pigment epithelial cells, is an endogenous ligand for retinoic acid receptor. J Biol Chem. 2008;283:11947–11953. doi: 10.1074/jbc.M708989200. [DOI] [PubMed] [Google Scholar]
- 30.Rozanowska M, Sarna T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]


